Introduction
Chronic obstructive pulmonary disease (COPD) is a
common chronic inflammatory disease of the airways (1,2) that is
characterized by poorly reversible airflow limitation and
structural changes in the airway components, known as remodeling
(3). Airway remodeling is strongly
associated with the progression of COPD. Current therapy for COPD
predominantly focuses on the relief of respiratory symptoms and
cannot alleviate the long-term deteriorating pulmonary function
effectively due to airway remodeling (4). Therefore, novel therapeutic strategies
and medications to prevent airway remodeling for COPD are highly
demanded.
The major pathological features of airway remodeling
in COPD include goblet cell hyperplasia, collagen deposition and
smooth muscle hyperplasia, as well as airway wall fibrosis
(4,5). Previous studies have demonstrated that
chronic airway inflammation was closely associated with airway
remodeling and is involved in airway injury and repair in COPD
(6–8). Chronic inflammation causes airway
structural changes and narrowing of the small airways (7,8).
Transforming growth factor (TGF)-β1, a key profibrotic mediator,
serves a critical role in inflammatory injury and repair,
contributing to the development of airway remodeling in COPD
(9,10). TGF-β1 has been indicated to be
increased in airway epithelium, airway smooth muscle, fibroblasts
and macrophages in lungs of patients with COPD (11,12).
These observations suggest that TGF-β signaling has an impact on
the development and progression of COPD. Research has indicated
that the TGF-β1/Smad signaling pathway notably impacts respiratory
disease and induces a series of pathological reactions related to
airway remodeling (13,14). Following the binding of TGF-β to its
receptors, Smad2 or Smad3 phosphorylates and binds to Smad4,
resulting in the alteration of TGF-β target genes, including
α-smooth muscle actin (SMA) and collagen I (13,15,16).
This whole process is essential for the pathogenesis of COPD airway
remodeling. Smad7 exerts a negative effect on TGF-β/Smad signaling
(17,18); therefore, inhibiting the TGF-β1/Smad
signaling pathway may lead to novel therapeutic strategies against
COPD.
Cordyceps sinensis has been used as a type of
traditional Chinese natural drug that has been demonstrated to
possess various therapeutic functions, including anti-cancer,
-diabetic, -inflammatory, immunomodulatory and anti-oxidant effects
(19,20). Due to the rarity of wild C.
sinensis fruiting bodies, the artificial cultivation of C.
sinensis has emerged as an attractive substitute for the
preparation of health supplements (21,22).
Previous studies have indicated that this medicine may also have a
protective effect against lung diseases (23,24). In
a previous study conducted by the present research group, it was
revealed that C. sinensis was able to significantly inhibit
senescence via the reactive oxygen species and phosphoinositide
3-kinase/AKT/mechanistic target of rapamycin signaling pathways in
cigarette smoke extract (CSE)-induced 16 human bronchial epithelial
cells (HBEs) (25). Previous reports
have suggested that C. sinensis may have anti-fibrotic
effects (24,26,27). It
was therefore hypothesized that C. sinensis may inhibit
airway remodeling in COPD. To test this hypothesis, the present
study investigated the effect of C. sinensis on airway
remodeling in vivo and explored its underlying mechanisms in
COPD.
Materials and methods
Rat model of COPD and C
sinensis treatment. A total of 50 male Wistar
rats (body weight, 200±20 g; age, 8–10 weeks) were purchased from
the Shandong University Experimental Animal Center (Jinan, China),
and the animal experiments were performed in accordance with and
approved by the Institutional Animal Care and Use Committee of
Shandong University (Jinan, China). The rats were housed in 24±1°C
with humidity of 50±10%, a 12 h light/dark cycle and had access to
a standard diet and water ad libitum. The rats were randomly
divided into five groups as follows: Control group (CON); COPD
group (COPD); COPD + low dose of C. sinensis group (LOW; 2.5
g/kg/day C. sinensis); COPD + moderate dosage of C.
sinensis group (MOD; 5 g/kg/day C. sinensis); and COPD +
high dose of C. sinensis group (HIG; 7.5 g/kg/day C.
sinensis) (n=10/group).
The rat model of COPD was established by cigarette
smoking and an intratracheal injection of lipopolysaccharide (LPS),
according to a previous report (28). Briefly, intratracheal injection of 1
mg/ml LPS (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
conducted on day 1 (0.2 ml) and 14 (0.1 ml). Except for days 1 and
14, rats were exposed to smoke generated by 8 cigarettes
(Hademen®Filter tip cigarette; Jinan Cigarette Factory
of China Tobacco Shandong Industrial Co., Ltd., Jinan, China; tar,
10 mg; nicotine content, 1.0 mg; carbon monoxide, 12 mg) in a
sealed box connected to the smoke source for 30 min, twice daily
for the first 2 weeks; then 15 cigarettes per treatment, three
times daily for 10 weeks. Artificially cultured C. sinensis
powder was obtained from Hangzhou Zhongmei Huadong Pharmaceutical
Co., Ltd. (Hangzhou, China). C. sinensis powder was
dissolved in normal saline (500 mg/ml) to prepare the turbid liquid
suspension, and applied to the LOW, MOD and HIG groups (2.5, 5 or
7.5 g/kg/day, respectively) intragastrically following cigarette
smoke exposure once a day for 12 weeks. Rats in the CON group were
exposed to air and treated with PBS. After 12 weeks, rats were
sacrificed with an intraperitoneal injection of pentobarbital
sodium (150 mg/kg; Sigma-Aldrich; Merck KGaA).
Bronchoalveolar lavage fluid
(BALF)
BAL was performed in the left lung through a
tracheal cannula under anesthesia using 1 ml sterile isotonic
saline three times in each rat. The BALF was immediately
centrifuged at 200 × g for 10 min at 4°C. The supernatant was
stored at −80°C for cytokine measurements. Total cell counts in
BALF were performed using a hemocytometer. Differential cell counts
were performed on cytospin preparations with Wright-Giemsa stain.
Briefly, the cells were evenly coated on clean glass slides and
fixed with absolute methanol after drying at room temperature. The
slides were stained with Wright's-Giemsa solution for 10 min at
room temperature, then washed, dried and observed by light
microscope (original magnification, ×1,000; Olympus Corporation,
Tokyo, Japan). At least 200 cells/sample were scored.
ELISA
The levels of interleukin (IL)-8 (cat. no.
MBS7606869; MyBioSource, Inc., San Diego, CA, USA), tumor necrosis
factor (TNF)-α (cat. no. RTA00; R&D Systems, Inc., Minneapolis,
MN, USA) and TGF-β1 (cat. no. MB100B; R&D Systems, Inc.) were
determined using a sandwich ELISA method, according to the
manufacturer's protocol.
Histopathological analysis
Tissue samples from the left lung of euthanized rats
were fixed with 4% paraformaldehyde at room temperature for 24 h
and processed for paraffin embedding. Lung paraffin sections were
sliced to 5-µm-thick sections and then stained with hematoxylin (3
min) and eosin (1 min) at room temperature using a staining kit
(cat. no. G1120; Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) under a light microscope (original
magnification, ×200; Olympus Corporation).
The lung sections were also examined with Masson's
trichrome stain to assess the deposition of peribronchial collagen.
Following dewaxing and hydration with xylene and a gradient
concentration of ethanol (100, 95, 80 and 70%). The slides were
stained with Weigert iron hematoxylin for 8 min and then
differentiated with 1% hydrochloric acid alcohol. Subsequently,
slides were stained with Ponceau fuchsin for 8 min. Following
staining with phosphomolybdic acid solution for ~5 min, the slides
were stained with aniline blue for 5 min, differentiated in 1%
acetic acid for 1 min, dehydrated in an ascending series of ethanol
(95 and 100%), washed in xylene and sealed with neutral gum. All
steps were conducted at room temperature. Masson's trichrome
staining was used to differentiate collagen from other fibers,
staining nuclei in black, cytoplasm and muscles in red and collagen
in blue. The sections were visualized using a light microscope at a
magnification of ×200.
Additionally, three random microscope fields in
which the bronchiole diameters were <100 µm (shortest path/lumen
diameter, cope fields in which the brox200 were observed to
determine the changes of thickness and area of the tube wall. The
wall area/total bronchiole area (MA%) and the wall
thickness/bronchiole diameter (MT%) were then calculated.
In the present study, it was found that the moderate
(5 g/kg/day) and high (7.5 g/kg/day) dose of C. sinensis
group had a marked inhibitory effect on airway remodeling
parameters compared with the low (2.5 g/kg/day) dose group, but
there was no significance between 5 and 7.5 g/kg/day treatments.
Therefore, the 5 g/kg/day dose of C. sinensis was selected
for pathological evaluation in following experiments.
Immunohistochemistry
Immunohistochemistry was performed by a two-step
immunoperoxidase protocol. Lung tissue was fixed at room
temperature in 4% paraformaldehyde for 24 h and embedded in
paraffin an sliced into a 4-µm-thick sections. Slides were
deparaffinized, rehydrated with xylene and ethanol of gradient
concentration (100, 95, 80 and 70%) and antigen retrieval was
performed by microwave (95°C, 15–20 min). Endogenous peroxidases
were blocked by soaking slides in 3% H2O2 for
30 min at room temperature. Subsequently, the slides were incubated
with anti-α-SMA (1:200; cat. no. sc-53142), anti-collagen I (1:100;
cat. no. sc-59772; both Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-TGF-β1 (1:100; cat. no. ab92486), anti-TGF-β receptor
(TβR) I (1:100; cat. no. ab31013) and anti-TβR II (1:100; cat. no.
ab186838; all Abcam, Cambridge, MA, USA) antibodies overnight at
4°C in a humidified chamber. Following this, the sections were
incubated with the secondary horseradish peroxidase-conjugated goat
anti-rabbit (cat. no. ZB-2301) and goat anti-mouse (cat. no.
ZB-2305) antibodies (both 1:500; OriGene Technologies, Inc.,
Beijing, China) for 1 h at room temperature. Sections were stained
with diaminobenzidine for 2–5 min and counterstained with 10%
hematoxylin for 3 min at room temperature. Stained areas of the
sections were visualized using a light microscope at magnification
of ×200. Positive areas were quantified by densitometry using
Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.,
Rockville, MD, USA).
Western blotting
Total proteins in right lung tissue samples from
each group were extracted prior to western blotting. Lung tissue
was mixed with RIPA lysate buffer (P0013K; Beyotime Institute of
Biotechnology, Haimen, China) with a ratio of 100 mg/ml. The
mixture was centrifuged (12,000 × g) at 4°C for 5 min to collect
the supernatant. The total protein was quantitated using a Pierce
BCA Protein Assay Kit. The protein (20 µg/lane) was loaded and
separated using 10% SDS-PAGE, and subsequently transferred onto
polyvinylidene difluoride (PVDF) membranes (Immobilon; EMD
Millipore, Billerica, MA, USA) at 4°C. The PVDF membranes were
blocked with 5% skim milk in Tris-buffered saline with Tween 20
(TBST) for 60 min at room temperature. The membranes were incubated
with the following antibodies: Anti-TGF-β1 (1:500), anti-TβR I
(1:500), anti-TβR II (1:500), anti-collagen I (1:1,000) anti-α-SMA
(1:1,000), anti-phosphorylated (p)-Smad2 (1:1,000; cat. no. 3104,
Cell Signaling Technology, Inc., Danvers, MA, USA), anti-p-Smad3
(1:1,000; cat. no. 9520, Cell Signaling Technology, Inc.)
anti-Smad7 (1:1,000; cat. no. sc-365846) and GAPDH (1:1,000; cat.
no. sc-47724; both Santa Cruz Biotechnology, Inc.) overnight at
4°C. The membrane was washed three times for 5 min each with 1X TBS
containing 0.15 Tween-20 followed by incubation with secondary
horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse
antibodies (both 1:5,000) at 37°C for 1 h. The immunoreactive bands
were visualized by a enhanced chemiluminescence kit (cat. no.
K820-500; BioVision, Inc. Milpitas, CA, USA). GAPDH was used for
normalization. The quantification of protein expression was
analyzed by densitometry using ImageJ software (version 1.46;
National Institutes of Health, Bethesda, MD, USA).
In the present study, it was found that C.
sinensis at a dose of 5 g/kg/day efficiently decreased the
protein and mRNA expression levels of TGF-β1, TβR I and TβR II, but
there was no significant difference between 5 and 7.5 g/kg/day
treatments. Therefore, the optimal dose was 5 g/kg/day of C.
sinensis, which was selected for following experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the right lung tissue
of rats using TRIzol reagent (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's protocol. Total RNA was extracted
from the right lung tissue of rats using TRIzol reagent (Takara
Bio, Inc., Otsu, Japan) according to the manufacturer's protocol. A
total of 1 µg total RNA was used for reverse transcription with
PrimeScript RT Master Mix (TaKaRa, Bio, Inc) according to the
manufacturer's protocol. qPCR was performed by using the
SYBR® Premix Ex Taq™ II (Takara Bio, Inc.)
according to the manufacturer's protocol on a LightCycler 480
System (Roche Diagnostics, Basel, Switzerland). The qPCR reaction
mixture consisted of 2 µl cDNA, 10 µl SYBR Premix Ex Taq II (2X),
0.8 µl of 10 uM forward primer, 0.8 µl of 10 uM reverse primer, and
6.4 µl double distilled H2O to a final volume 20 µl. The
thermocycling conditions were as follows: 95°C for 30 sec (initial
denaturation), followed by 40 cycles of 95°C for 5 sec
(denaturation) and 60°C for 30 sec (annealing). Next, a melting
curve was performed at 95°C for 5 sec, 60°C for 1 min and then
continuously at 95°C, followed by 50°C for 30 sec (cooling).
Relative quantification of mRNA was calculated using the
2−ΔΔCq method (29). The
sequences of primers used for RT-qPCR in the present study are
demonstrated in Table I. GAPDH gene
was used as an internal control.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
|
| Primer
sequence |
|---|
|
|
|
|---|
| Target gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| Transforming growth
factor-β1 |
CAACAATTCCTGGCGTTACCTTGG |
GAAAGCCCTGTATTCCGTCTCCTT |
| α-smooth muscle
actin |
CCACCGCAAATGCTTCTAAGT |
GGCAGGAATGATTTGGAAAGG |
| Collagen I |
TGCCGTGACCTCAAGATGTG |
CACAAGCGTGCTGTAGGTGA |
| TβR I |
GAGGTGTCAGACTGATTTTCAGGAG |
ATGTCACAAAGGAAATTCATAAAGC |
| TβR II |
GGCTCTGGTACTCTGGGAAA |
AATGGGGGCTCGTAATCCT |
| GAPDH |
GTGGCAAAGTGGAGATTGTT |
CTCGCTCCTGGAAGATGG |
Statistical analyses
All data are presented as the mean ± standard error
of the mean. All experiments in the present study were repeated at
least three times. One-way analysis of variance followed by
Newman-Keuls post hoc tests were used to compare measurements of
individual groups. The statistical analysis was conducted using
SPSS software (version 19.0; IBM Corp, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of C. sinensis on
histopathology in rats with COPD
To detect the airway structural changes in rats with
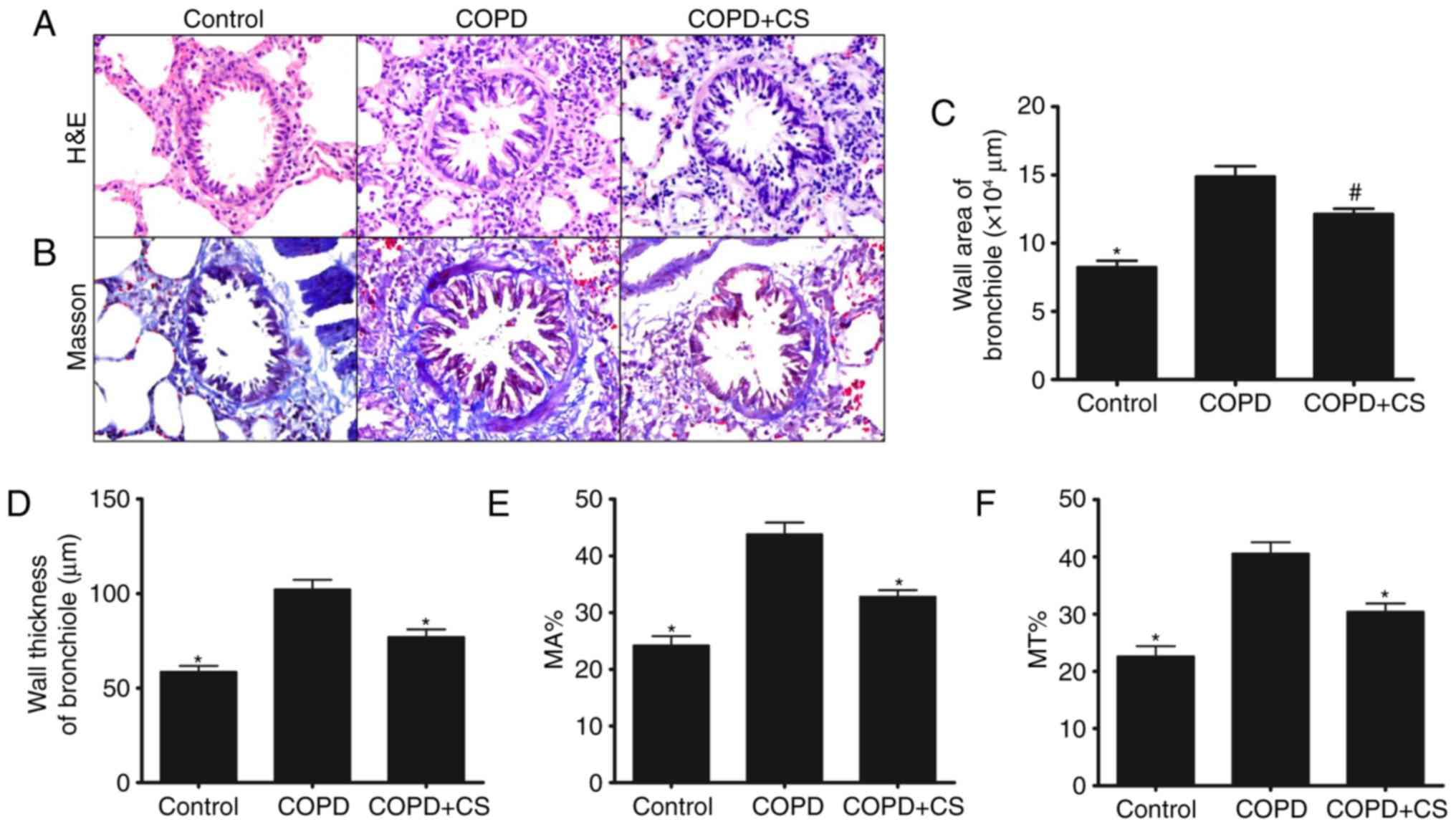
COPD, the slides from each group were stained with H&E. As
demonstrated in Fig. 1A, airway
structural changes, including airway wall thickening, numerous
inflammatory cell infiltration, subepithelial fibrosis, increased
smooth muscle mass and epithelial metaplasia, were observed in the
COPD group. However, C. sinensis treatment markedly
ameliorated the aforementioned changes. In addition, Masson's
trichrome staining was used to reveal the deposition of collagen
and proliferation of smooth muscle hypertrophy in airway areas of
the COPD group, while these changes were effectively decreased in
rats treated with C. sinensis (Fig. 1B). Additionally, a significantly
larger bronchiole tube area, thicker tube wall surrounded by
proliferative connective tissue and increased MA% and MT% were
detected in the COPD compared with that observed in the control
group (P<0.01). These characteristics were significantly
improved by C. sinensis treatment (P<0.05; Fig. 1C-F).
C. sinensis attenuates lung
inflammatory responses in a rat model of COPD
As inflammation serves an important role in the
development of airway remodeling in COPD, the effect of C.
sinensis treatment on lung inflammation was evaluated. BALF
cell counts were performed to examine the effect of C.
sinensis on the inflammatory response induced by cigarette
smoke. Smoking treatment induced extensive infiltration of
inflammatory cells, as demonstrated by the significant increase in
the numbers of total cells, macrophages, neutrophils and
lymphocytes in the COPD group compared with the numbers in the CON
group (P<0.01). C. sinensis significantly reversed all
types of cell accumulation in BALF of the COPD rats (P<0.05;
Fig. 2A-D). In addition, the
inflammatory cytokines, including TNF-α, IL-8 and TGF-β1, in BALF
were significantly increased in the rats with COPD compared with
the levels observed in the CON group (P<0.01; Fig. 2E-G), and the production of TNF-α,
IL-8 and TGF-β1 were significantly reduced in rats treated with
C. sinensis at all treatment doses (P<0.01; Fig. 2E-G).
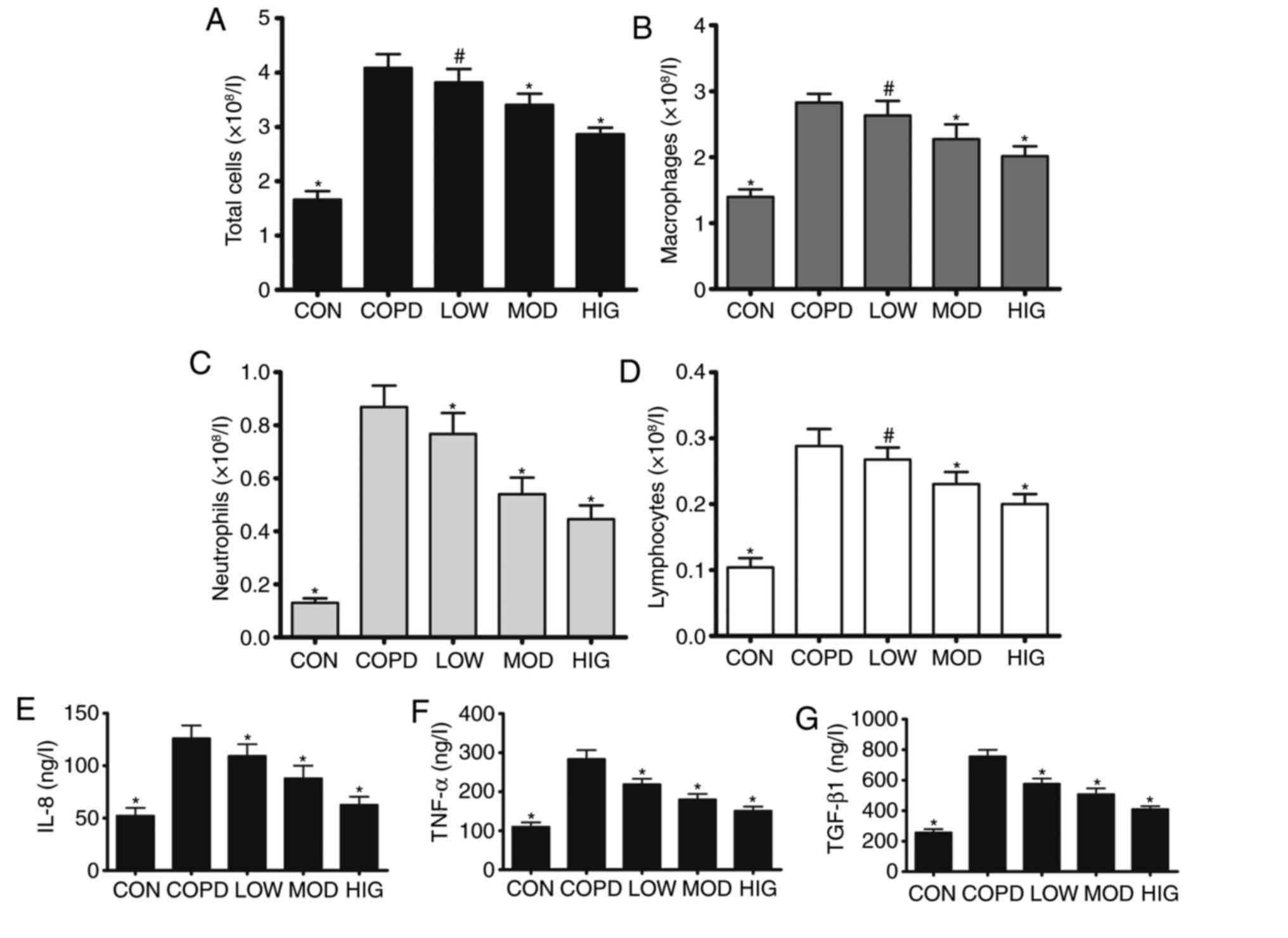 | Figure 2.C. sinensis reduces lung inflammation
in rats with COPD. BALF was collected to quantify (A) total cells,
(B) macrophages, (C) neutrophils and (D) lymphocytes. ELISA was
used to determine the production of (E) IL-8, (F) TNF-α and (G)
TGF-β1 in BALF. Data are expressed as mean + standard error of the
mean. Each result represents at least three independent
experiments. #P<0.05 and *P<0.01 vs. COPD group.
C. sinensis, Cordyceps sinensis; COPD, chronic
obstructive pulmonary disease; BALF, bronchoalveolar lavage fluid;
TNF-α, tumor necrosis factor-α; IL-8, interleukin-8; TGF-β1,
transforming growth factor-β1; CON, control group; LOW, COPD + low
dose of C. sinensis (2.5 g/kg/day); MOD, COPD + moderate
dose of C. sinensis (5 g/kg/day); HIG, COPD + high dose of
C. sinensis (7.5 g/kg/day). |
C. sinensis inhibits the expression of
collagen I and α-SMA in rats with COPD
To detect whether C. sinensis could inhibit
airway remodeling, the effect of C. sinensis on the
expression of collagen I and α-SMA in rats with COPD was examined
by immunohistochemistry, western blotting and RT-qPCR. The present
study revealed that the protein and mRNA expression levels of
collagen I and α-SMA were significantly elevated in rats with COPD
compared with the levels observed in the CON group (P<0.05;
Fig. 3A-C). However, the collagen I
and α-SMA protein and mRNA expression levels were significantly
decreased in rats treated with C. sinensis (P<0.05;
Fig. 3A-C).
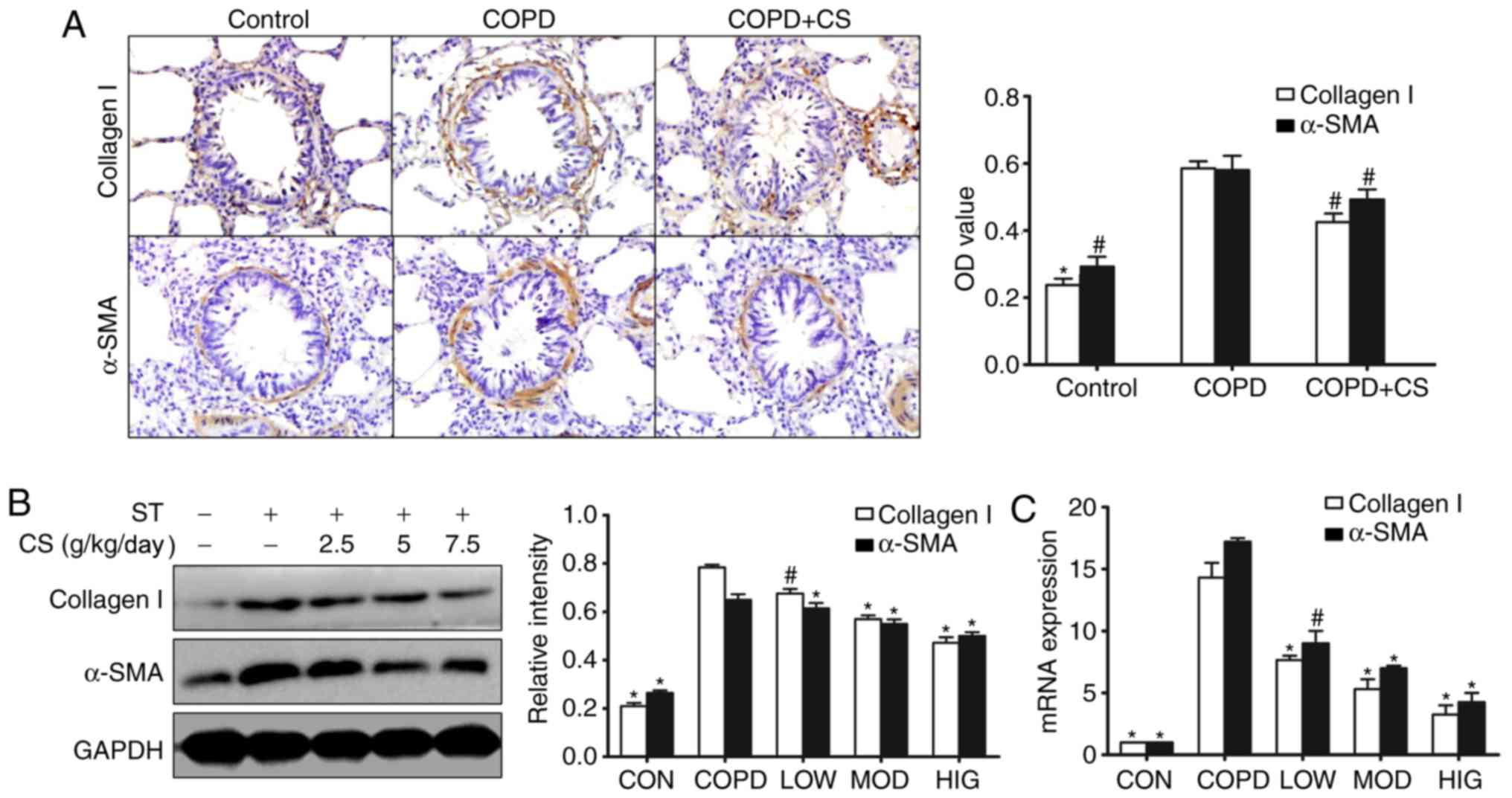 | Figure 3.CS reduces the markers of airway
remodeling in rats with COPD. (A) The expression of α-SMA and
collagen I were detected by immunohistochemistry (magnification,
×200). α-SMA and collagen I immunoreactive cells were stained brown
by immunohistochemical staining. (B) The production of α-SMA and
collagen I were analyzed by western blotting. (C) The mRNA
expression levels of collagen I and α-SMA were detected by reverse
transcription-quantitative polymerase chain reaction. Data are
expressed as mean + standard error of the mean. Each result
represents at least three independent experiments.
#P<0.05 and *P<0.01 vs. COPD group. CS,
Cordyceps sinensis; COPD, chronic obstructive pulmonary
disease; ST, smoking treatment; α-SMA, α-smooth muscle actin; CON,
control group; LOW, COPD + low dose of CS (2.5 g/kg/day); MOD, COPD
+ moderate dose of CS (5 g/kg/day); HIG, COPD + high dose of CS
(7.5 g/kg/day); OD, optical density. |
C. sinensis reduces the expression of
TGF-β1 and its receptors in the airway
A role for TGF-β1 has been proposed in airway
remodeling of COPD (6,9,10). To
explore the mechanisms by which C. sinensis inhibits airway
remodeling, the expression levels of TGF-β1, TβR I and TβR II were
assessed by immunohistochemistry, western blotting and RT-qPCR. As
demonstrated in Fig. 4, the protein
and mRNA expression levels of TGF-β1 were significantly increased
in rats with COPD compared with the levels observed in the CON
group (P<0.01). Meanwhile, the protein expression levels of TβR
I and TβR II were also significantly increased in the COPD group
compared with the levels observed in the CON group (P<0.01). The
administration of C. sinensis significantly attenuated
TGF-β1, TβR I and TβR II expression (P<0.05; Fig. 4A-C).
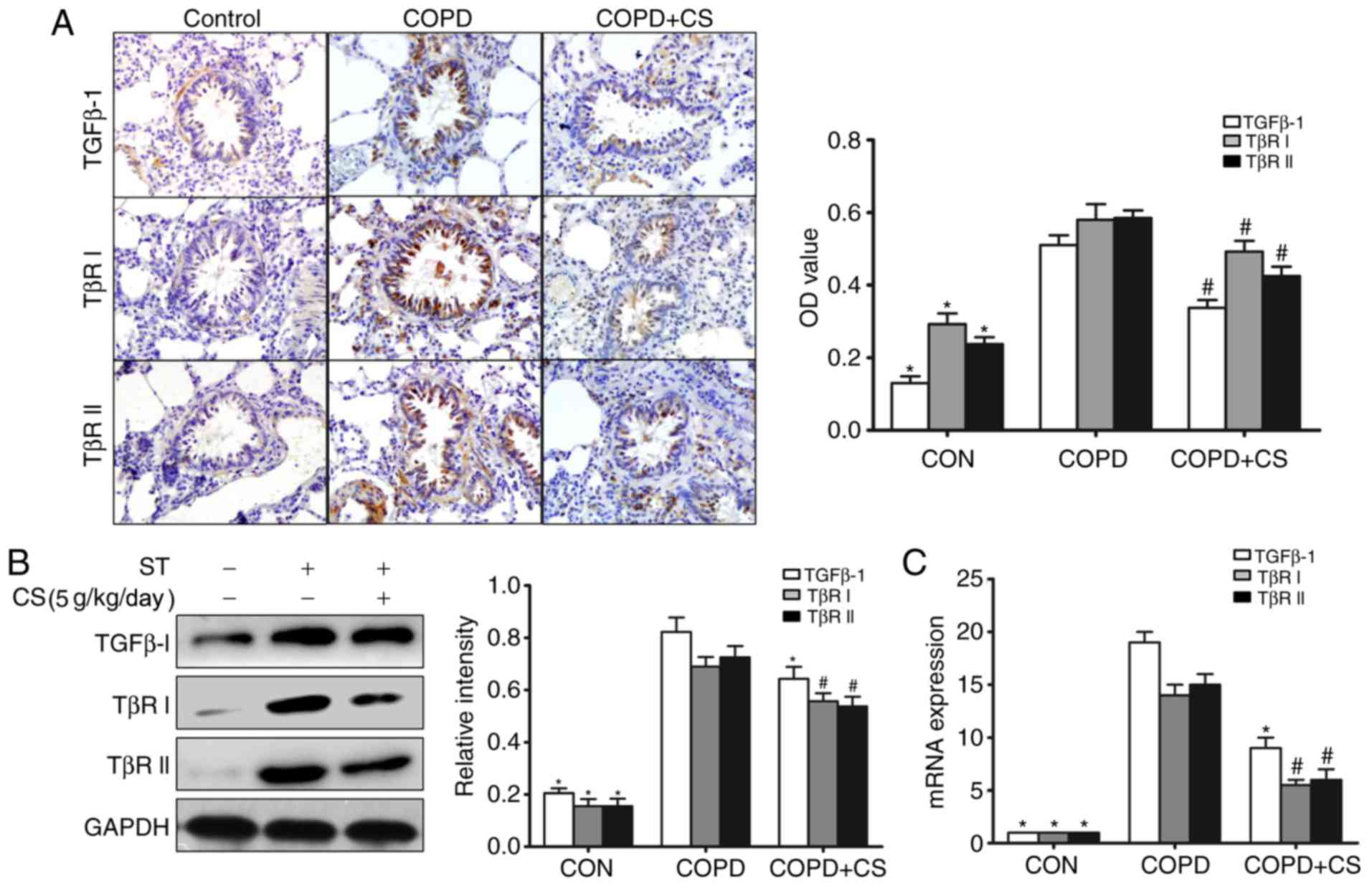 | Figure 4.CS reduces expression of TGF-β1 and
its receptors in the airways. (A) Immunohistochemistry of TGF-β1,
TβR I and TβR II. TGF-β1, TβR I and TβR II immunoreactive cells
were stained brown by immunohistochemical staining. (B) Western
blot analysis of TGF-β1, TβR I and TβR II protein expression
levels. (C) The mRNA expression levels of TGF-β1, TβR I and TβR II
were analyzed by reverse transcription-quantitative polymerase
chain reaction. Data are expressed as mean + standard error of the
mean. Each result represents at least three independent
experiments. #P<0.05 and *P<0.01 vs. COPD group.
CS, Cordyceps sinensis; COPD, chronic obstructive pulmonary
disease; ST, smoking treatment; TGF-β1, transforming growth
factor-β1; TβR, TGF-β receptor; OD, optical density. |
Effect of C. sinensis on the
TGF-β1/Smad signaling pathway
The TGF-β1/Smad signaling pathway is crucial in the
regulation of the transcription of genes involved in airway
remodeling in COPD (13). As
demonstrated in the present study, the expression levels of signal
proteins of p-Smad2 and p-Smad3 were significantly upregulated in
the COPD group compared with the levels observed in the CON group
(P<0.01; Fig. 5A-C). The
expression of Smad7 was significantly decreased in the COPD group
compared with the level observed in the CON group (P<0.01).
Administration of C. sinensis for 12 weeks significantly
decreased the high expression of p-Smad2 and p-Smad3 (P<0.05),
and increased the protein expression level of Smad7 (P<0.05) in
rats with COPD to different degrees (Fig. 5A-C).
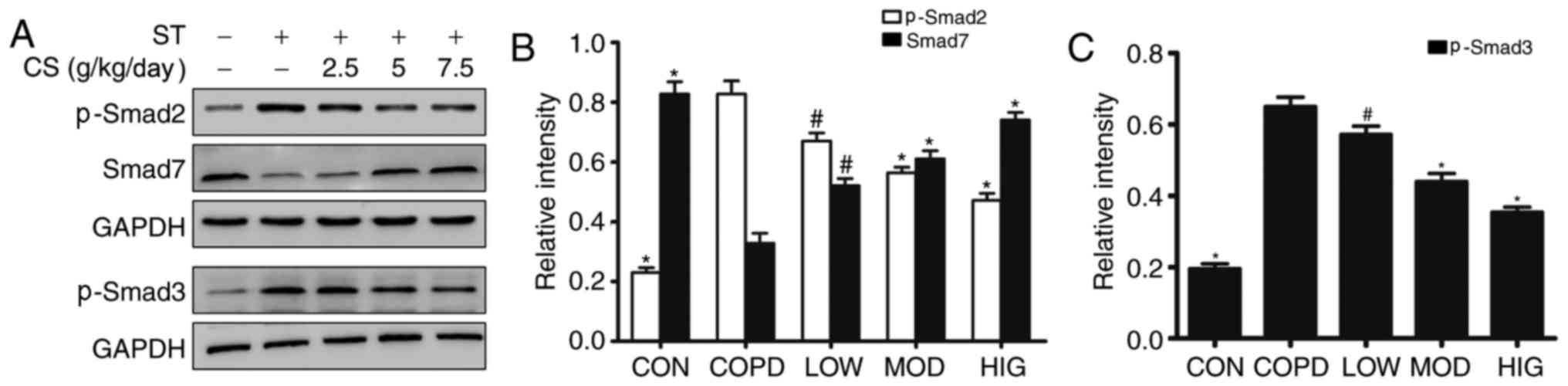 | Figure 5.Effect of CS on the TGF-β1/Smad
signaling pathway in rats with COPD. (A) CS treatment induced the
phosphorylation of Smad2 and Smad3 and upregulation of Smad7 in
rats with COPD, according to western blotting. Quantitation of (B)
p-Smad2 and Smad7, and (C) p-Smad3. Data are expressed as mean +
standard error of the mean. #P<0.05 and *P<0.01
vs. COPD group. CS, Cordyceps sinensis; COPD, chronic
obstructive pulmonary disease; ST, smoking treatment; p,
phosphorylated; CON, control group; LOW, COPD + low dose of CS (2.5
g/kg/day); MOD, COPD + moderate dose of CS (5 g/kg/day); HIG, COPD
+ high dose of CS (7.5 g/kg/day). |
Discussion
C. sinensis has been used in traditional
Chinese medicine to treat lung disease for centuries due to its
pharmacological and therapeutic effects (30). There has been an increased interest
in the anti-fibrotic effect of C. sinensis (24,26,27). The
present study investigated the inhibitory effect of C.
sinensis on airway remodeling in rats with COPD. It was
demonstrated that C. sinensis inhibited the accumulation of
inflammatory cells and production of cytokines in BALF of rats with
COPD. Meanwhile, C. sinensis treatment also reduced the
protein and mRNA expression levels of collagen I, α-SMA, TGF-β1 as
well as its receptors. Additionally, the present study revealed
that C. sinensis regulated the expression of key signal
proteins of the TGF-β1/Smad pathway, which may influence the
regulation of airway remodeling.
Various studies have indicated that inflammation
serves an important role in COPD airway remodeling (31–33).
Chronic inflammation could initiate the frequent occurrence of
injury and repair, which contributes to airway structural changes
and airway remodeling. Previous studies have demonstrated that
various proinflammatory cytokines and profibrotic growth factors
are involved in airway injury and repairing (34–36).
C. sinensis has been indicated to have effects on
anti-inflammatory and immunomodulatory activities in vitro
and in vivo (37,38). C. sinensis may suppress IL-1β,
TNF-α, IL-6 and IL-8 cytokine production in BALF (39). In the present study, it was observed
that the numbers of total cells, macrophages, neutrophils and
lymphocytes were significantly increased in BALF of the model rats
with COPD, and these numbers could be alleviated by C.
sinensis treatment. Consistent with the previous observation,
it was observed that the production of cytokines, including TNF-α,
IL-8 and TGF-β1, in rats with COPD were reduced by C.
sinensis treatment. These findings suggest that C.
sinensis has anti-inflammatory activities in vivo, as
shown by reduced inflammatory cell counts, and decreased expression
levels of IL-8, TNF-α and TGF-β1 in BALF of rats with COPD.
Overall, the present results support that suppression of airway
inflammation may contribute to the protective effect of C.
sinensis in COPD.
TGF-β1 is an important profibrotic cytokine, which
has been widely implicated in the pathogenesis of airway repair and
remodeling (9,10). TGF-β1 promotes collagen deposition,
airway smooth muscle cell and fibroblast proliferation, and
epithelial-to-mesenchymal transition (10,40).
TGF-β serves multiple biological effects through Smad-dependent and
Smad-independent signaling pathways (13,18).
Smad signaling is recognized as the most critical signaling pathway
of airway remodeling (10,13). Previous research has suggested that
specific inhibitor of Smad3 may inhibit TGF-β1-induced expression
of type I collagen in normal fibroblasts and scleroderma
fibroblasts (41). A study by Le
et al (42) demonstrated that
airway remodeling was alleviated in Smad3-deficient mice, with a
decrease in collagen deposition and the smooth muscle layer. Based
on these previous studies, it was hypothesized that inhibiting the
TGF-β1/Smad signaling pathway may be a useful therapeutic method
against airway remodeling.
In COPD, various factors have been revealed to
activate the TGF-β1/Smad signaling pathway (43–45).
Rats exposed to cigarette smoke highly express TGF-β and upregulate
downstream signature protein p-Smad2, accompanied by an increase in
collagen deposition (14). In the
present study, it was identified that the expression levels of
TGF-β1, TβR I, TβR II, p-Smad2 and p-Smad3 were upregulated in the
lungs of rats with COPD. This suggests that cigarette smoke may
activate the TGF-β1/Smad signaling pathway. Previous studies have
indicated that C. sinensis attenuated liver and renal
fibrosis by inhibition of the TGF-β1/Smad signaling pathway and
downregulation of α-SMA, collagen and TGF-β1 (26,27). In
the present study, C. sinensis treatment was also
demonstrated to decrease TGF-β1, TβR I and TβR II expression, and
suppress phosphorylation of Smad2 and Smad3 while increasing the
expression of Smad7 in rats with COPD. Additionally, the
biochemical and histological signs of airway remodeling, including
collagen I and α-SMA in the airway, were also markedly reduced in
rats treated with C. sinensis. Therefore, reduction of
phosphorylation of Smad2 and Smad3, and upregulation of Smad7
expression may directly lead to an attenuation of airway remodeling
in COPD. These results suggest that C. sinensis may inhibit
the TGF-β1/Smad signaling pathway to improve airway remodeling in
COPD.
In conclusion, the present study demonstrated that
C. sinensis attenuated airway remodeling in rats with COPD
by inhibiting airway inflammation and the TGF-β1/Smad signaling
pathway. These findings suggest C. sinensis may be a useful
approach for COPD therapy.
Acknowledgements
The present research was supported by the National
Nature Science Foundation of China (grant no. 81270072 for LD), the
Natural Science Funding Committee of Shandong province (grant no.
ZR2015PH022) and the Key Research Project of Shandong province
(grant no. 2016GSF201028).
References
|
1
|
Global Initiative for Chronic Obstructive
Lung Disease 2016. Global strategy for the diagnosis, management
and prevention of chronic obstructive pulmonary disease. https://goldcopd.org2016
|
|
2
|
Caramori G, Casolari P, Giuffrè S, Barczyk
A, Adcock I and Papi A: COPD pathology in the small airways.
Panminerva Med. 53:51–70. 2011.PubMed/NCBI
|
|
3
|
Decramer M, Janssens W and Miravitlles M:
Chronic obstructive pulmonary disease. Lancet. 379:1341–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirota N and Martin JG: Mechanisms of
airway remodeling. Chest. 144:1026–1032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoshiba K and Nagai A: Differences in
airway remodeling between asthma and chronic obstructive pulmonary
disease. Clin Rev Allergy Immunol. 27:35–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barnes PJ: The cytokine network in chronic
obstructive pulmonary disease. Am J Respir Cell Mol Biol.
41:631–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hogg JC and Timens W: The pathology of
chronic obstructive pulmonary disease. Annu Rev Pathol. 4:435–459.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hogg JC, Chu F, Utokaparch S, Woods R,
Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson
HO and Paré PD: The nature of small-airway obstruction in chronic
obstructive pulmonary disease. N Engl J Med. 350:2645–2653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Churg A, Tai H, Coulthard T, Wang R and
Wright JL: Cigarette smoke drives small airway remodeling by
induction of growth factors in the airway wall. Am J Respir Crit
Care Med. 174:1327–1334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartram U and Speer CP: The role of
transforming growth factor beta in lung development and disease.
Chest. 125:754–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Boer WI, van Schadewijk A, Sont JK,
Sharma HS, Stolk J, Hiemstra PS and van Krieken JH: Transforming
growth factor beta1 and recruitment of macrophages and mast cells
in airways in chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 158:1951–1957. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takizawa H, Tanaka M, Takami K, Ohtoshi T,
Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K and Umeda A:
Increased expression of transforming growth factor-beta1 in small
airway epithelium from tobacco smokers and patients with chronic
obstructive pulmonary disease (COPD). Am J Respir Crit Care Med.
163:1476–1483. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang YC, Zhang N, Crombruggen K, Hu GH,
Hong SL and Bachert C: Transforming growth factor-beta1 in
inflammatory airway disease: A key for understanding inflammation
and remodeling. Allergy. 67:1193–1202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung A, Tai H, Coulthard T, Wang R and
Wright JL: Cigarette smoke drives small airway remodeling by
induction of growth factors in the airway wall. Am J Respir Crit
Care Med. 174:1327–1334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qu ZH, Yang ZC, Chen L, Lv ZD, Yi MJ and
Ran N: Inhibition airway remodeling and transforming growth
factor-β1/Smad signaling pathway by astragalus extract in asthmatic
mice. Int J Mol Med. 29:564–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao GX, Li QM and Shen HH: Effect of
Astragali-Cordyceps Mixtura on TGF-beta/Smad signal pathway in the
lung of asthma airway remodeling. J Ethnopharmacol. 13:68–74. 2009.
View Article : Google Scholar
|
|
17
|
Nakao A, Afrakhte M, Morén A, Nakayama T,
Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH
and ten Dijke P: Identification of Smad7, a TGFbeta-inducible
antagonist of TGF-beta signalling. Nature. 389:631–635. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weiss A and Attisano L: The TGFbeta
superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol.
2:47–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Sun H, Qin F, Pan Y and Sun C:
Effect of various extracts and a polysaccharide from the edible
mycelia of Cordycepssinensis on cellular and humoral immune
response against ovalbumin in mice. Phytother Res. 20:646–652.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou Y, Liu Y, Ruan M, Feng X, Wang J, Chu
Z and Zhang Z: Cordyceps sinensis oral liquid prolongs the lifespan
of the fruit fly, Drosophila melanogaster, by inhibiting oxidative
stress. Int J Mol Med. 36:939–946. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou X, Gong Z, Su Y, Lin J and Tang K:
Cordyceps fungi: Natural products, pharmacological functions and
developmental products. J Pharm Pharmacol. 61:279–291. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Das SK, Masuda M, Sakurai A and Sakakibara
M: Medicinal uses of the mushroom Cordyceps militaris: Current
state and prospects. Fitoterapia. 81:961–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
An X, Zhang AL, May BH, Lin L, Xu Y and
Xue CC: Oral Chinese herbal medicine for improvement of quality of
life in patients with stable chronic obstructive pulmonary disease:
A systematic review. J Altern Complement Med. 18:731–743. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang TT, Lai HC, Ko YF, Ojcius DM, Lan
YW, Martel J, Young JD and Chong KY: Hirsutella sinensis mycelium
attenuates bleomycin-induced pulmonary inflammation and fibrosis in
vivo. Sci Rep. 5:152822015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu A, Wu J, Li A, Bi W, Liu T, Cao L, Liu
Y and Dong L: The inhibitory mechanism of Cordyceps sinensis on
cigarette smoke extract-induced senescence in human bronchial
epithelial cells. Int J Chron Obstruct Pulmon Dis. 11:1721–1731.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng J, Li X, Feng Q, Chen L, Xu L and Hu
Y: Anti-fibrotic effect of Cordyceps sinensis polysaccharide:
Inhibiting HSC activation, TGF-β1/Smad signalling, MMPs and TIMPs.
Exp Biol Med. 238:668–677. 2013. View Article : Google Scholar
|
|
27
|
Pan MM, Zhang MH, Ni HF, Chen JF, Xu M,
Phillips AO and Liu BC: Inhibition of TGF-β1/Smad signal pathway is
involved in the effect of Cordyceps sinensis against renal fibrosis
in 5/6 nephrectomy rats. Food Chem Toxicol. 58:487–494. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi Y, Shang JY, Ma LJ, Sun BB, Hu XG, Liu
B and Zhang GJ: Inhibition of AMPK expression in skeletal muscle by
systemic inflammation in COPD rats. Respir Res. 15:1562014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bensky D, Clavey S and Stöger E: Chinese
Herbal Medicine: Materia Medica. Eastland Press; Seattle, Wash,
USA: 2004
|
|
31
|
Caramori G, Kirkham P, Barczyk A, Di
Stefano A and Adcock I: Molecular pathogenesis of cigarette
smoking-induced stable COPD. Ann N Y Acad Sci. 1340:55–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Durham AL, Caramori G, Chung KF and Adcock
IM: Targeted anti-inflammatory therapeutics in asthma and chronic
obstructive lung disease. Transl Res. 167:192–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barnes PJ, Shapiro SD and Pauwels RA:
Chronic obstructive pulmonary disease: Molecular and cellular
mechanisms. Eur Respir J. 22:672–688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ganesan S and Sajjan US: Repair and
remodeling of airway epithelium after injury in chronic obstructive
pulmonary disease. Curr Respir Care Rep. 2:2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Camara J and Jarai G:
Epithelial-mesenchymal transition in primary human bronchial
epithelial cells is Smad-dependent and enhanced by fibronectin and
TNF-alpha. Fibrogenesis Tissue Repair. 3:22010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Doerner AM and Zuraw BL: TGF-beta1 induced
epithelial to mesenchymal transition (EMT) in human bronchial
epithelial cells is enhanced by IL-1beta but not abrogated by
corticosteroids. Respir Res. 10:1002009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang TT, Chong KY, Ojcius DM, Wu YH, Ko
YF, Wu CY, Martel J, Lu CC, Lai HC and Young JD: Hirsutella
sinensis mycelium suppresses interleukin-1β and interleukin-18
secretion by inhibiting bothcanonical and non-canonical
inflammasomes. Sci Rep. 3:13742013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SY, Jung SJ, Ha KC, Sin HS, Jang SH,
Chae HJ and Chae SW: Anti-inflammatory effects of Cordyceps
mycelium (Paecilomyces hepiali, CBG-CS-2) in Raw264.7 murine
macrophages. Orient Pharm Exp Med. 15:7–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuo YC, Tsai WJ, Wang JY, Chang SC, Lin CY
and Shiao MS: Regulation of bronchoalveolar lavage fluids cell
function by the immunomodulatory agents from Cordycepssinensis.
Life Sci. 68:1067–1082. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leask A and Abraham DJ: TGF-beta signaling
and the fibrutic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jinnin M, Ihn H and Tamaki K:
Characterization of SIS3, a novel specific inhibitor of smad3 and
its effect on transforming growth factor-β1-induced extracellular
matrix expression. Mol Pharmacol. 69:597–607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Le AV, Cho JY, Miller M, McElwain S,
Golgotiu K and Broide DH: Inhibition of allergen-induced airway
remodeling in Smad 3-deficient mice. J Immunol. 178:7310–7316.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guan S, Liu Q, Han F, Gu W, Song L, Zhang
Y, Guo X and Xu W: Ginsenoside Rg1 ameliorates cigarette
smoke-induced airway fibrosis by suppressing the TGF-β1/Smad
pathway in vivo and in vitro. Biomed Res Int. 2017:65101982017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu X, Hao B, Ma A, He J, Liu X and Chen
J: The expression of NOX4 in smooth muscles of small airway
correlates with the disease severity of COPD. Biomed Res Int.
2016:28918102016.PubMed/NCBI
|
|
45
|
Aschner Y and Downey GP: Transforming
growth factor-β: Master regulator of the respiratory system in
health and disease. Am J Respir Cell Mol Biol. 54:647–655. 2016.
View Article : Google Scholar : PubMed/NCBI
|