Introduction
Cancer-associated mortality rates are the highest
worldwide. It was found that the lethal rate among cancer patients
remains large due to the insufficient capabilities of existing
cancer therapies (1). Among the
different cancer types, lung cancer is considered as the largest
contributor to cancer-associated mortality and exhibits a serious
health issues (2). Furthermore, lung
cancer is the second most diagnosed type of cancer and accounts for
30% of cancer-associated mortality rates (3). In Europe, frequency rates of lung
cancer have reduced but the rate of survival remains below 20%
(4). Statistics reported in United
States during 2015 indicate that lung cancer and bronchus cancer
are responsible for a mortality rate of ~158,080 individuals
(5). Although there have been
high-tech advancements, no convincing increase in 5-year survival
rates has been observed for lung cancer during the past decade
(5). This is due to a lack of
sufficient knowledge of the biological functions that maintain lung
carcinogenesis. Among all other types of cancers, the mortality
rates are markedly higher for lung cancer and non-small cell lung
cancer is the predominant lung cancer type. It is well-known that
patients with metastasis have a lower survival period (6,7) and
hence, the aim of our treatment was to suppress metastasis.
Epithelial-mesenchymal transition (EMT) has been
demonstrated to be an essential process not only during embryonic
progression but also as a powerful mechanism for cancer growth. EMT
refers to a process in which cells were subjected to the transition
from an epithelial phenotype to a mesenchymal phenotype. Cells
alter their morphology to have advanced motility. Furthermore, EMT
has been demonstrated to have a crucial function in metastasis
(8). EMT can be well distinguished
by its high association between the gain and loss of
mesenchymal-like markers and epithelial-like cell junction
proteins, including N-cadherin or vimentin and E-cadherin
respectively (9). Transcriptional
suppression in E-cadherin is activated via regulators of EMT, such
as Snail and Slug; induction of cancer metastasis is mediated by
the same process (10–12).
To date, usage of herbal compounds has increased
along with the use of antibiotic drugs, particularly in Western
countries. The purpose of using herbal medicines is to suppress
side effects when combined with modern medicines or to afford
interdependent pharmacological properties (13). Generally, it is believed that herbal
medicines are secure for human intake since they are naturally
occurring; however, numerous studies have revealed that different
elements of these natural compounds can lead to severe modulations
in pharmacological characteristics of co-governed drugs, thus
altering their power and security (14–16).
Phytoestrogens, commonly known as dietary
isoflavones, occur extensively in the Leguminosae family. These
secondary metabolite plants display protective effects against
several diseases, including osteoporosis, cancers and heart-related
diseases (17,18). Among them, Biochanin A, which is an
O-methylated isoflavone present in chickpea, red clover, alfalfa
and cabbage, has been documented to have anti-tumor, anti-viral,
and anti-inflammatory properties (19,20).
Inhibition of tumor necrosis factor (TNF)-α and interleukin (IL)-6
production in lipopolysaccharide-induced macrophages was observed
with biochanin A treatment in a previous study (21). Previous reports of biochanin A have
identified several biological characteristics, such as
anti-proliferation, anti-oxidation, anticancer and hypoglycemic
properties (22–26). Suppression of ovalbumin-mediated
airway hyper-responsiveness was also noted with biochanin A
supplementation (27).
In a previous study, biochanin A exhibited
beneficial anti-oxidant activity in mice, which indicated its
promise for treating Alzheimer's disease (20). Meanwhile, anti-apoptotic (28) and anti-fibrotic (29) properties of biochanin A have also
been revealed. However, the anticancer effect of biochanin A on
A427 lung cancer cell lines remains unclear. The aim of this study
was to determine the anticancer properties of biochanin A on lung
cancer cell lines and to illustrate the potential anticancer
mechanism.
Materials and methods
Chemicals and reagents
Biochanin A, dimethyl sulfoxide (DMSO),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
reagent for MTT assay, antibodies for β-actin (cat. no. A5441),
E-Cadherin (cat. no. 07-697) and Snail (cat. no. SAB1306281), TNF-α
(cat. no. T0813) and IL-6 (cat.no. MABF41) were acquired from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Cell culture
medium, including Dulbecco's modified Eagle medium (DMEM) and
RPMI-1640 medium, and the other supplements required for cell
culture methods, including fetal bovine serum, penicillin and
streptomycin, were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA).
Cell culture and methods
Human lung adenocarcinoma cell line (A427) and human
monocytic leukemia cell line (AML-193) were obtained from American
Type Culture Collection (Manassas, VA, USA). A427 cell lines were
cultured in DMEM, whereas AML-193 cells were cultured in RPMI
medium. Both the media were supplemented with 10% fetal bovine
serum, 100 U/ml penicillin and 100 mg/ml streptomycin under a
humidified atmosphere at 37°C with 5% CO2. Biochanin A
was dissolved using dimethyl sulfoxide to prepare a stock solution
and was stored at 4°C for subsequent experiments.
MTT assay
The sub-lethal dosage of biochanin A on A427 cells
was examined to assess cell viability using an MTT assay. In order
to conduct this experiment, cells at a density of 3×103
cells/well were seeded into 96-well plates and incubated for 24 h.
Following culturing, various concentrations of biochanin A (5, 20
and 40 µM) were added by dissolving the compound in DMSO and
incubating a further 24 h. After this, the media were removed and
0.1 mg/ml MTT solution was added followed by 6-h incubation in the
dark. This was followed by the addition of DMSO of equal volume to
solubilize the formazan crystals and their absorbance at 560 nm was
recorded using a microplate reader (Sunrise; Tecan, Männedorf,
Switzerland). Cells treated without biochanin A in DMSO acted as
vehicle controls and the experiment was performed in
triplicate.
Coculture method, biochanin A
treatment and acquiring conditioned medium
A427 human lung cancer cell lines were subjected to
coculture with human monocytic leukemic AML-193 cells. Ratios (1:1,
1:10, 1:15, 1:25 and 1:50) were chosen to illustrate the role of
biochanin A in a potential pro-inflammation coculture method. In
brief, A427 cells at a density of 3×103 cells/well were
seeded into 96-well plates and maintained overnight in DMEM
supplemented with fetal bovine serum (10%) in order to grow the
cells. This was followed by the addition of various biochanin A
sub-lethal concentrations (5, 20 and 40 µM) and incubation for 24
h. Following incubation, PBS solution was used to wash the cells to
remove any minor amount of biochanin A. Consequently, A427 cells
were cocultured with AML-193 cells in different ratios using low
protein serum-free medium for 8 h at 37°C. A427 cells cocultured
with AML-193 and A427 cells cocultured with AML-193 in dimethyl
sulfoxide served as the control and vehicle control, respectively.
Conditioned medium was collected after 8 h of coculture and the
dead cells were removed by centrifugation at 3,000 × g for 30 min
and maintained at −80°C for further use. Conditioned media with and
without biochanin A treatment were represented as TCM and NTCM,
respectively.
Quantification of cytokines
Supernatants of the culture media were used to
quantify the amount of TNF-α (cat. no. 550610) and IL-6 (cat. no.
550799) levels using commercially purchased ELISA kits (BD
Biosciences Franklin Lakes, NJ, USA) by following the instructions
supplied by the manufacturer. Measurements were carried out in
triplicate for all assays.
Wound healing assay
A427 cell lines were seeded in 6-well plates until
95% confluency in DMEM. After removing the media, the cell
monolayer was wounded using a sterile needle and was subsequently
washed using PBS to remove the debris. Images were captured (0 h).
Cell lines were subjected to treatment with both the treated and
non-treated conditioned media (TCM and NTCM) and incubated for a
further 24 h. Images were captured after the wound formation (24
h). Three independent assays were performed and a percentage were
calculated from wound closures with the help of a formula as
previously outlined (30).
Invasion assay
Inserts of poly-carbonate filters (pore size, 8 mm)
were pre-polished using Matrigel and pre-incubated in DMEM for ~3 h
prior to plating the cells. The upper compartment was loaded with
250 ml TCM/NTCM cocultured conditioned media of A427 cells at a
density of 4×105, whereas the lower compartment was
loaded with DMEM (500 ml), which can function as a chemotactic
element, and incubated for 24 h. Following this, cotton buds were
used to remove the non-migrated cells from the upper compartment
and the migrated cells were then attached to the filter on the
other side, fixed using formaldehyde (3.5%) for 30 min and stained
with crystal violet. Every five fields were calculated and
quantified by recording absorbance at 570 nm for each elution of
crystal violet using acetic acid (5%). Data were represented as the
mean of triplicate measurements.
Western blot analysis
Initially, the A427 pretreated cells were washed
three times with ice-cold PBS solution. Cells were then lysed using
radioimmunoprecipitation lysis buffer containing Tris-HCl (10 mM,
pH 7.4), EDTA (1 mM), 1% sodium deoxycholate, sodium chloride (100
mM) and NP-40 along with 1X Roche protease inhibitor composed of
aprotinin and leupeptin (both 5 mg/ml). Centrifugation was
performed at 12,000 × g for 10 min at 4°C to obtain the cell
lysates and protein concentrations were estimated using Bio-Rad
Protein Assay reagent (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Proteins were subsequently separated by SDS-PAGE and
electrophoretically transferred onto polyvinylidene difluoride
membranes followed by blocking with bovine serum albumin (1%) for
~3 h at room temperature. Membranes were subsequently incubated at
4°C overnight with primary antibodies against E-Cadherin, Snail and
β-actin at dilution ratios of 1:1,000, 1:1,000 and 1:1,500,
respectively. Blots obtained after washing were treated with
infra-red (IRDye 800 CW Goat anti-Human IgG)-associated secondary
antibodies (cat. no. 926-32232; LI-COR Biotechnology, Lincoln, NE,
USA) at a dilution ratio of 1:10,000 for ~2 h at room temperature.
The resultant blots were scanned using a LAS 4000 Image Quant gel
documentation system (GE Healthcare, Chicago, IL, USA).
Statistical analysis
One-way analysis of variance (ANOVA) was used for
all statistical calculations. Tukey's honest significant difference
was used as a post hoc test method following ANOVA. P<0.05 was
considered to indicate a statistically significant difference. All
data were expressed as the mean ± standard deviation of triplicate
measurements.
Results
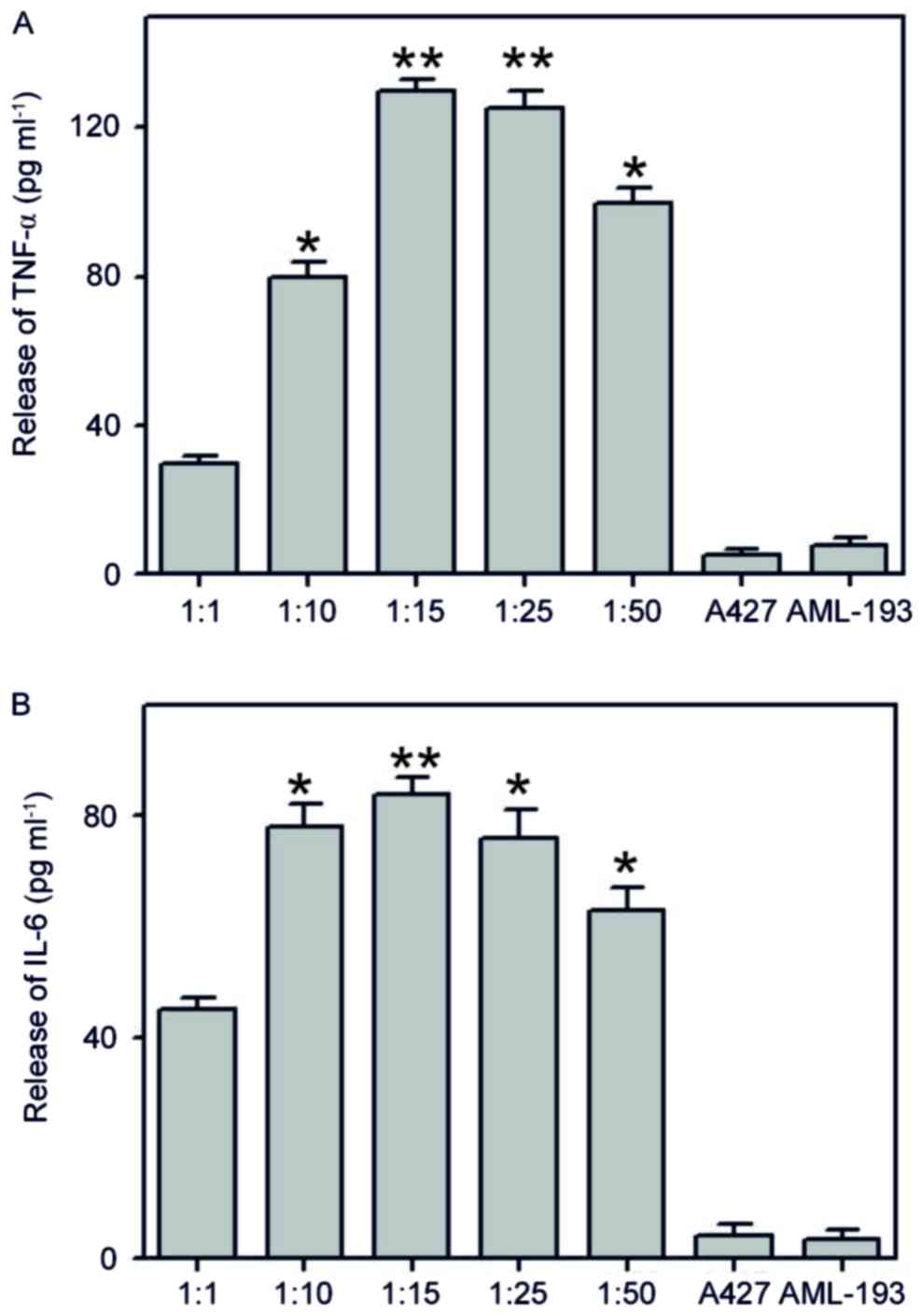
Determination of pro-inflammatory
coculture requirements
To assess the potential of the pro-inflammatory
co-culture method in simulating the original cancer
micro-environment background, co-culture with different ratios
(1:1, 1:10, 1:15, 1:25 and 1:50) of A427 human lung cancer cell
line with AML-193 human leukemic monocytic cell lines were
performed for ~6 h. Once the cocultured conditioned medium was
attained, we estimated the levels of cytokines (TNF-α and IL-6)
using enzyme-associated immunoassays. Results of coculture
indicated the formation of a significant amount of TNF-α and IL-6
and the high response was monitored at a 1:15 cocultured ratio
(Fig. 1). These types of cancer and
myeloid cells (A427/AML-193) are generally identified only in solid
tumors but not in liquid medium (31). In addition, generation of cytokines
(TNF-α and IL-6) was not observed when using either A427 or AML-193
cultures alone. These results indicate the possible liberation of a
stimulus from A427 cells that induces pro-inflammatory
characteristics in AML-193 cells.
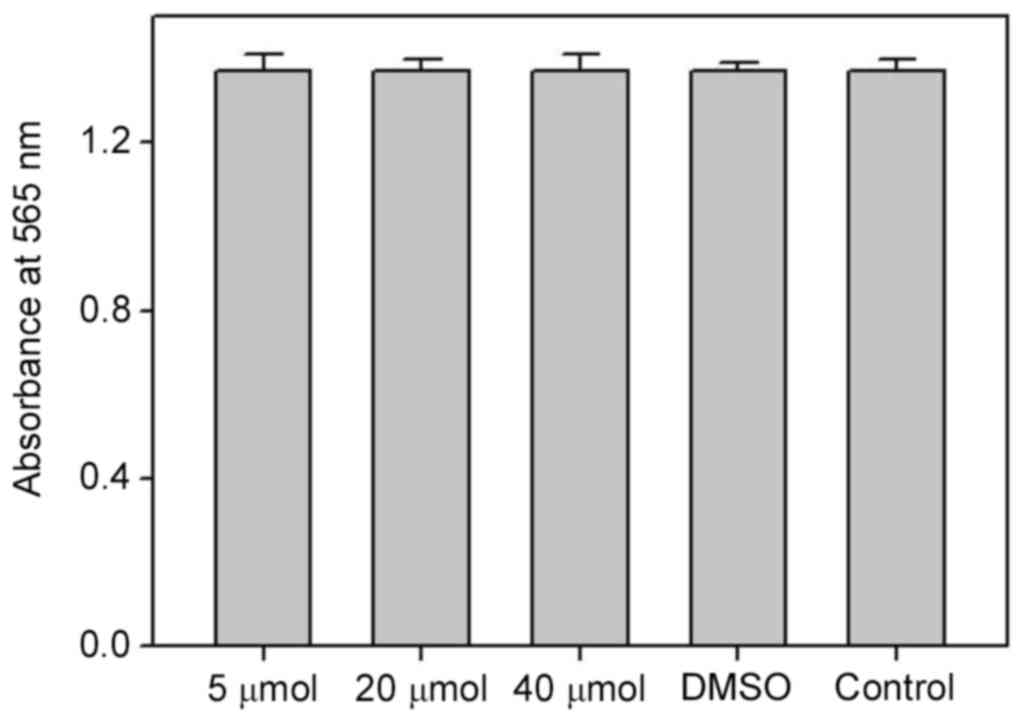
Assessment of sub-lethal dosages of
biochanin A
In order to evaluate the role of biochanin A on cell
viability and to examine the operative concentration that is able
to produce negligible cytotoxicity to A427 cells, an MTT assay was
conducted with sub-toxic dosages of biochanin A (5–40 µM). Results
of MTT demonstrated good cell viability, and thus no cytotoxicity,
at all the measured dosages of biochanin A (Fig. 2).
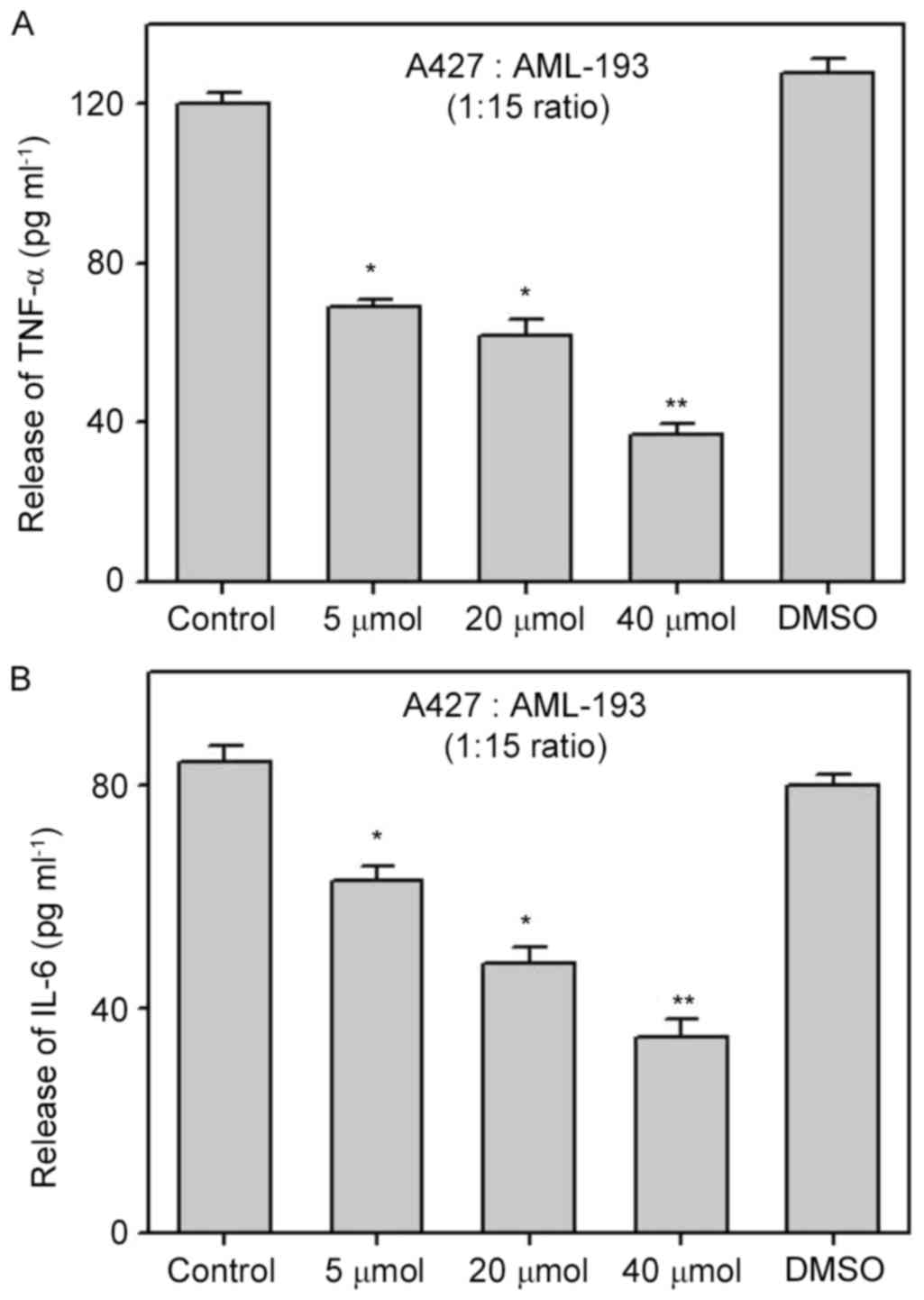
Biochanin A inhibits the release of
cancer cell generated pro-inflammatory markers from monocytic
cells
In order to elucidate the effects of biochanin A on
the pro-inflammatory response via a cocultured method, we performed
pretreatment of A427 cells with biochanin A at sub-lethal
concentrations of 5–40 µM for ~24 h prior to coculture with AML-193
cells. Cytokine levels (TNF-α and IL-6) were quantified from the
supernatant solutions of cell culture. Results demonstrated the
significant suppression of TNF-α and IL-6 liberation from AML-193
cells when compared to that of non-treated (control) or dimethyl
sulfoxide treated cocultures (Fig.
3). It is known that pro-inflammatory cytokines have a crucial
role in cancer metastasis (12) and
the use of biochanin A results in the inhibition of the
pro-inflammatory response from A427 cells, which might alter the
cancer cell micro-environment.
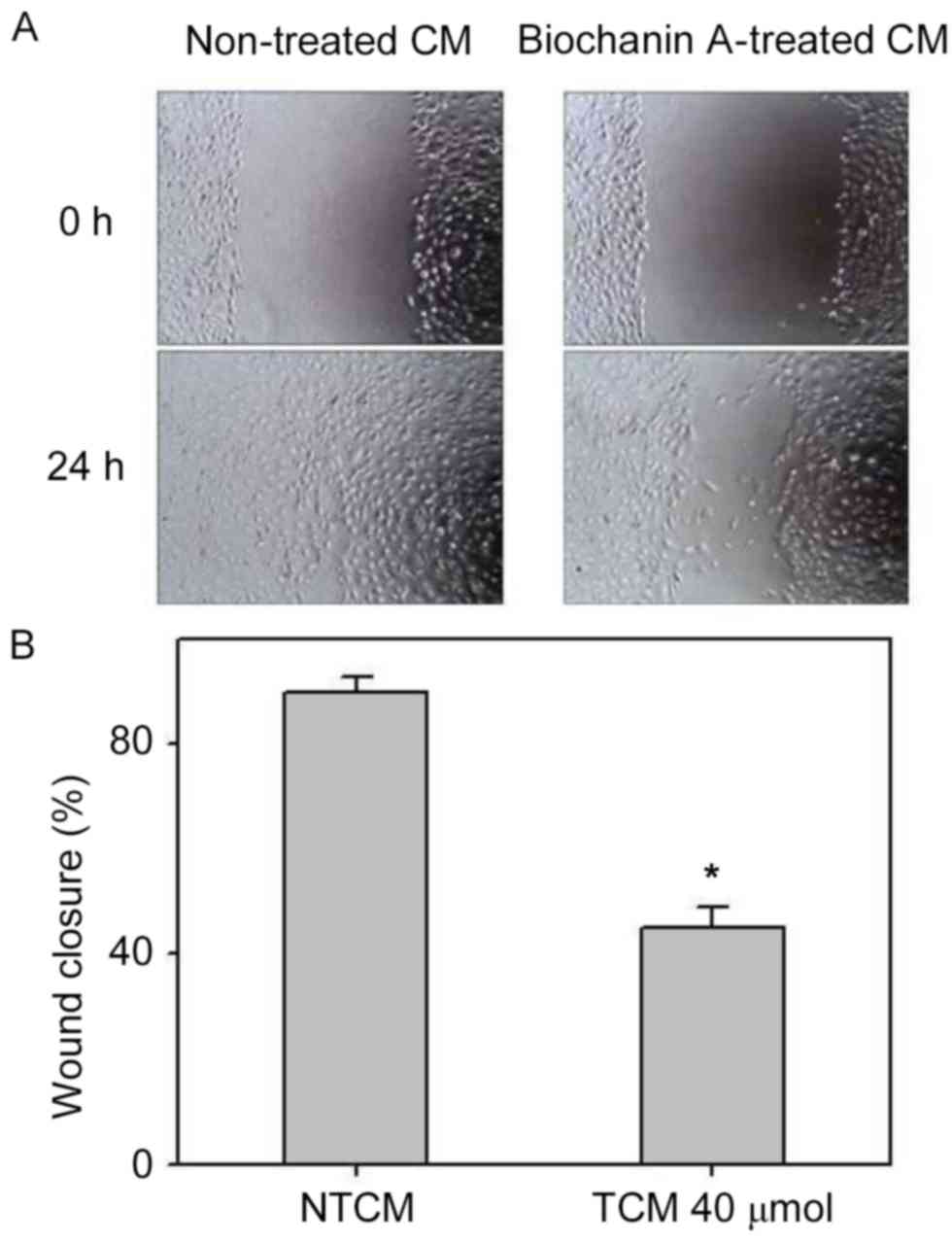
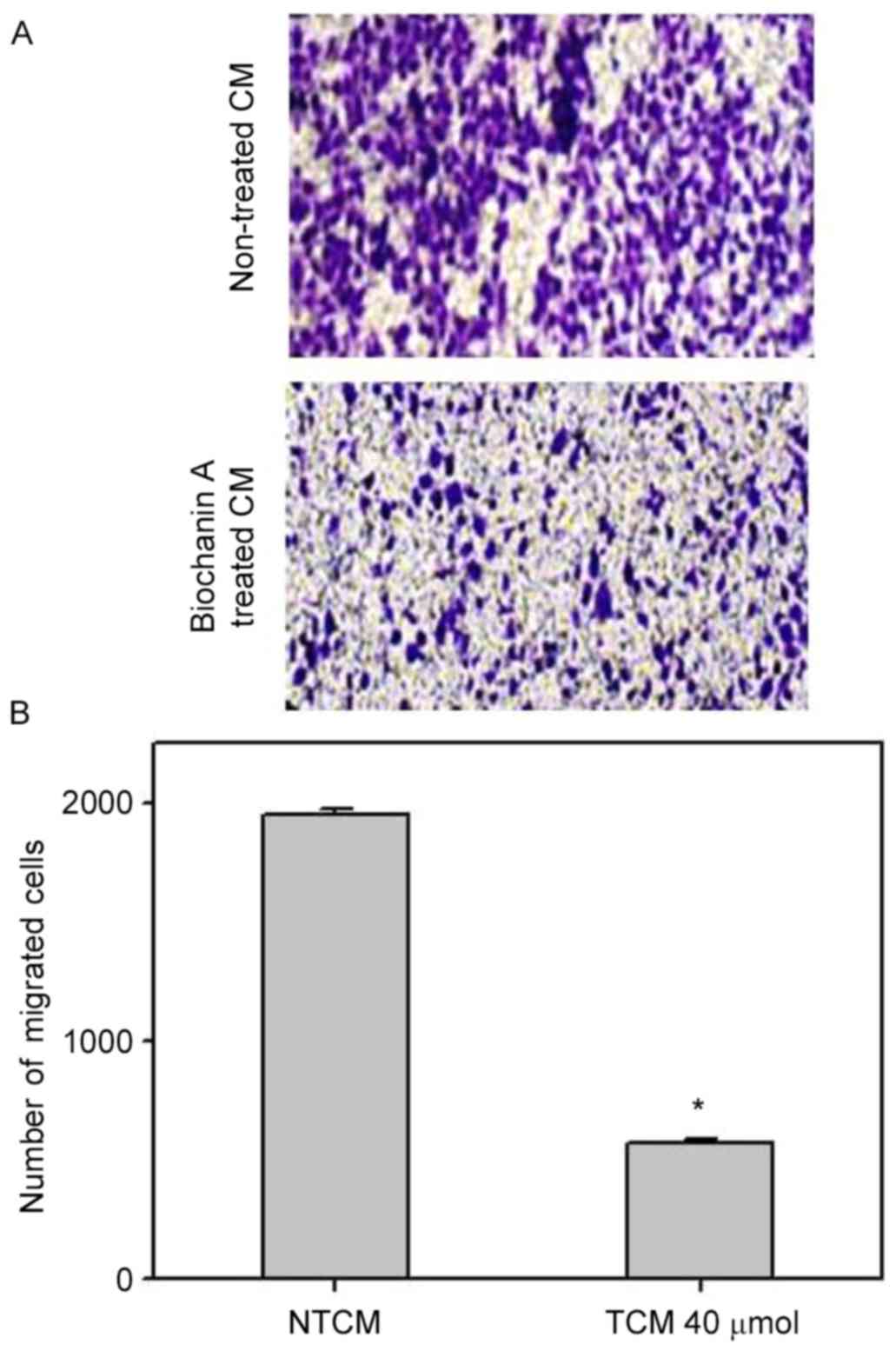
Effect of biochanin A on the
inhibition of migration and invasion in A427 cells
Pro-inflammatory cytokines, such as TNF-α and IL-6,
occurring in the cancer microenvironment governs the migration and
invasive characteristics to the tumor cells (32). Since the results demonstrated that
A427 induced AML-193 cells to produce TNF-α and IL-6 cytokines, we
examined whether biochanin A was able to alter the metastatic
properties of A427 cells. Migration and invasion studies were
conducted with conditioned medium from biochanin A added cocultures
of A427 and AML-193 cells. A427 cell lines were cultured to minimal
confluency, wounded and further cultured in biochanin A-treated
(TCM) or non-treated (NTCM) cocultured conditioned medium,
respectively. The data revealed that biochanin A markedly reduced
the transition of A427 cells as it reduced the wound healing
process (Fig. 4). In addition to
these results, A427 cells were cultured in NTCM or TCM (40 µM
biochanin A) cocultured conditioned medium to further explore the
effects using different in vitro cell line models. Data
indicated that biochanin A strongly repressed the
coculture-stimulated invasion of A427 cells (Fig. 5); a 19-fold reduction in the number
of invasive cells was noted for TCM, as compared with that of
NTCM.
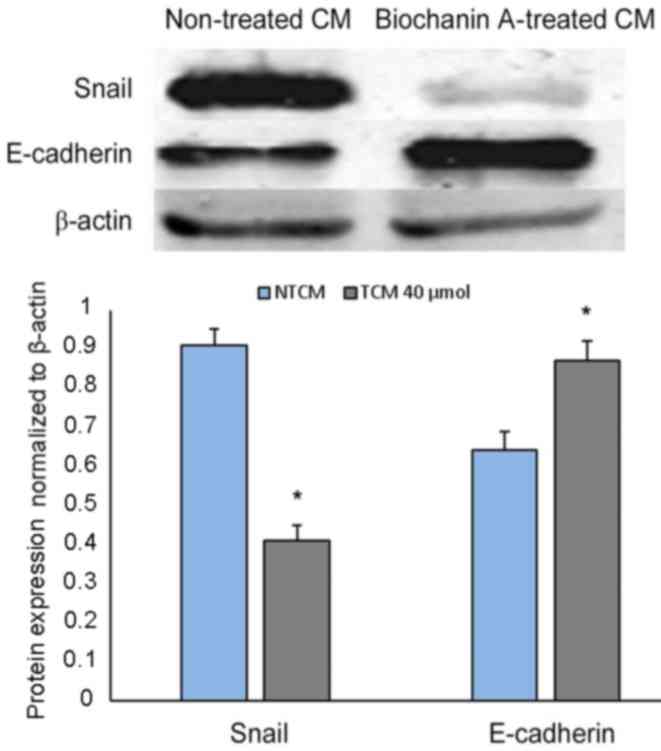
Effect of biochanin A on E-cadherin
and Snail expression via EMT
Strong correlations have been identified between EMT
and pro-inflammatory cancer cell microenvironments (33,34),
which helps to activate cancer metastasis. It has previously been
demonstrated that biochanin A is able to reduce pro-inflammatory
activities and thereby hinder cancer metastasis (35); however, whether biochanin A can alter
epithelial-mesenchymal transition still remains to be established.
To analyze this, A427 cell lines were developed in non-treated or
biochanin A-treated cocultured medium. A significant reduction in
Snail expression and a simultaneous depression in E-cadherin was
determined from western blot analysis data (Fig. 6), which is indicative of EMT as these
are EMT bio-markers (32).
Discussion
There are numerous reports of successful induction
of apoptosis in cancer cells using natural extracts derived from
herbs (36,37). Many of chemopreventive substances are
currently used, but these kill both the normal and cancer cells
with side effects. However, the usage of isolated natural compounds
that are specific to cancer cells may decrease the side effects and
indicate their use as successful therapeutic agents. It has been
demonstrated that the inflammatory micro-environment has a crucial
function in the metastatic development of several cancers (38). Numerous experimental studies and
clinical trials, have clearly demonstrated that existence of
tumor-infiltrating immune cells, particularly macrophages, which
may promote cancer growth (39,40).
Certainly, tumor cells recruit macrophages and discharge elements
that interact with cells in the cancer cavity to produce an
inflammatory micro-environment. A number of studies have
demonstrated the utility of anti-inflammatory substances in
preventing tumors through anti-cancer treatments (40,41).
The present study investigated the use of biochanin
A as an anti-inflammatory drug to control the swelling stimulated
by metastatic growth. We investigated, for the first time, whether
the preparation of lung cancer cell lines with biochanin A prevents
the potential of these tumor cells to induce leukemic monocytes
apart from hindering coculture triggered liberation of TNF-α and
IL-6 cytokines at sub-lethal dosages in a concentration-dependent
fashion. The findings demonstrated the hindering potential of
biochanin A on the liberation of pro-inflammatory inducement from
A427 lung cancer cell lines, which relates to the stimulation and
consequent liberation of cytokines from AML-193 monocytes. Under
the cancer micro-environment, it has been observed that cytokine
release and other pro-inflammatory features result in the
activation of cancer cells to perform EMT, thus advancing their
migration and invasion (42,43).
TNF-α has been suspected as driving functions that
characterize the invasive nature of cancer by stimulating Snail
sustainment, which is a prime transcription element that
coordinated the cellular and molecular functions associated with
EMT (42). In our case, we observed
that A427 lung cancer cell lines cocultured with AML-193 leukemic
monocytes resulted in the liberation of pro-inflammatory cytokines
(TNF-α and IL-6) in the respective cultured medium. Conditioned
medium accumulated from these AML-193 cells emphasized the
transition potential of A427 cell lines, as determined by wound
healing and migration assays. This was further demonstrated by the
elevation of Snail expression with a simultaneous reduction in
E-cadherin expression. However, biochanin A pre-treatment of A427
cells reduced activation and release of cytokines from AML-193
cells, as was mirrored by trace amounts of TNF-α and IL-6 in the
cocultured condition medium. This was indicated by the reduced
migration ability of A427 cells that received treatment with
biochanin A, and altered E-cadherin and Snail protein
expression.
In conclusion, data acquired from various
experiments revealed that biochanin A hindered pro-inflammatory
effects triggered from leukemic AML-193 monocytes in A427 human
lung cancer cell lines. This characteristic of biochanin A was
induced by the blockage of an inducing stimulus from A427 cells by
a molecular mechanism that is still to be elucidated. Therefore,
biochanin A was shown to possess strong inhibiting effects against
cancer-evoked inflammation.
Acknowledgements
The authors would like to thank Hubei University of
Medicine for their technical assistance.
References
|
1
|
Xu J, Liu X, Zhou S and Wei M: Combination
of immunotherapy with anaerobic bacteria for immune gene therapy of
solid tumours. Gene Ther Mol Biol. 13:36–52. 2009.
|
|
2
|
Bender E: Epidemiology: The dominant
malignancy. Nature. 513:S2–S3. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shibuya K, Mathers CD, Boschi-Pinto C,
Lopez AD and Murray CJ: Global and regional estimates of cancer
mortality and incidence by site: II. Results for the global burden
of disease. BMC Cancer. 2:372002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al: Global surveillance of cancer survival 1995–2009: Analysis
of individual data for 25,676,887 patients from 279
population-based registries in 67 countries (CONCORD-2). Lancet.
385:977–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Denlinger CE, Ikonomidis JS, Reed CE and
Spinale FG: Epithelial to mesenchymal transition: The doorway to
metastasis in human lung cancers. J Thorac Cardiovasc Surg.
140:505–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Batlle E, Sancho E, Franci C, Dominguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cano A, Perez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Graham RE, Gandhi TK, Borus J, Seger AC,
Burdick E, Bates DW, Phillips RS and Weingart SN: Risk of
concurrent use of prescription drugs with herbal and dietary
supplements in ambulatory careAdvances in Patient Safety: New
Directions and Alternative Approaches. 4. Henriksen K, Battles JB,
Keyes MA and Grady ML: Agency for Healthcare Research and Quality
(US); Rockville, MD: pp. 1–22. 2008, View Article : Google Scholar
|
|
14
|
Peng CC, Glassman PA, Trilli LE,
Hayes-Hunter J and Good CB: Incidence and severity of potential
drug-dietary supplement interactions in primary care patients: An
exploratory study of 2 outpatient practices. Arch Intern Med.
164:630–636. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rhee SM, Garg VK and Hershey CO: Use of
complementary and alternative medicines by ambulatory patients.
Arch Intern Med. 164:1004–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dergal JM, Gold JL, Laxer DA, Lee MS,
Binns MA, Lanctôt KL, Freedman M and Rochon PA: Potential
interactions between herbal medicines and conventional drug
therapies used by older adults attending a memory clinic. Drugs
Aging. 19:879–886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Breinholt V, Hossaini A, Svendsen GW,
Brouwer C and Nielsen E: Estrogenic activity of flavonoids in mice.
The importance of estrogen receptor distribution, metabolism and
bioavailability. Food Chem Toxicol. 38:555–564. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang QY, Meng QH, Zhang ZT, Tian ZJ and
Liu H: Synthesis solubility lipids-lowering and liver-protection
activities of sulfonated formononetin. Yao Xue Xue Bao. 44:386–389.
2009.(In Chinese). PubMed/NCBI
|
|
19
|
Kole L, Giri B, Manna SK, Pal B and Ghosh
S: Biochanin-A, an isoflavon, showed antiproliferative and
anti-inflammatory activities through the inhibition of iNOS
expression, p38-MAPK and ATF-2 phosphorylation and blocking NF κB
nuclear translocation. Eur J Pharmacol. 653:8–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michaelis M, Sithisarn P and Cinatl J Jr:
Effects of flavonoid-induced oxidative stress on anti-H5N1
influenza a virus activity exerted by baicalein and biochanin A.
BMC Res Notes. 7:3842014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu L, Lin B, Lin Z, Lin YP, Lin M and
Yang X: Biochanin A ameliorates the cytokine secretion profile of
lipopolysaccharide-stimulated macrophages by a PPARγ-dependent
pathway. Mol Med Rep. 5:217–222. 2012.PubMed/NCBI
|
|
22
|
Chen HQ, Jin ZY and Li GH: Biochanin A
protects dopaminergic neurons against lipopolysaccharide-induced
damage through inhibition of microglia activation and
proinflammatory factors generation. Neurosci Lett. 417:112–117.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biradar SM, Joshi H and Chheda TK:
Biochanin-A ameliorates behavioural and neurochemical derangements
in cognitive-deficit mice for the betterment of Alzheimer's
disease. Hum Exp Toxicol. 33:369–382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mishra P, Kale RK and Kar A:
Chemoprevention of mammary tumorigenesis and chemomodulation of the
antioxidative enzymes and peroxidative damage in prepubertal
Sprague Dawley rats by Biochanin A. Mol Cell Biochem. 312:1–9.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S and Morris ME: Effect of the
flavonoids biochanin A and silymarin on the P-glycoprotein-mediated
transport of digoxin and vinblastine in human intestinal Caco-2
cells. Pharm Res. 20:1184–1191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harini R, Ezhumalai M and Pugalendi KV:
Antihyperglycemic effect of biochanin A, a soy isoflavone, on
streptozotocin-diabetic rats. Eur J Pharmacol. 676:89–94. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ko WC, Lin LH, Shen HY, Lai CY, Chen CM
and Shih CH: Biochanin A, a phytoestrogenic isoflavone with
selective inhibition of phosphodiesterase 4, suppresses
ovalbumininduced airway hyperresponsiveness. Evid Based Complement
Alternat Med. 2011:6350582011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan JW, Tham CL, Israf DA, Lee SH and Kim
MK: Neuroprotective effects of biochanin A against
glutamate-induced cytotoxicity in PC12 cells via apoptosis
inhibition. Neurochem Res. 38:512–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Breikaa RM, Algandaby MM, El-Demerdash E
and Abdel-Naim AB: Multimechanistic antifibrotic effect of
biochanin a in rats: Implications of proinflammatory and
profibrogenic mediators. PLoS One. 8:e692762013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rah B, Amin H, Yousuf K, Khan S, Jamwal G,
Mukherjee D and Goswami A: A novel MMP-2 inhibitor
3-azidowithaferin A (3-azidoWA) abrogates cancer cell invasion and
angiogenesis by modulating extracellular par-4. PLoS One.
7:e440392012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiménez-Orozco FA, López-González JS,
Nieto-Rodriguez A, Velasco-Velázquez MA, Molina-Guarneros JA,
Mendoza-Patiño N, García-Mondragón MJ, Elizalde-Galvan P,
León-Cedeño F and Mandoki JJ: Decrease of cyclin D1 in the human
lung adenocarcinoma cell line A-427 by 7-hydroxycoumarin. Lung
Cancer. 34:185–894. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of Snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
DeNardo DG, Andreu P and Coussens LM:
Interactions between lymphocytes and myeloid cells regulate
pro-versus anti-tumor immunity. Cancer Metastasis Rev. 29:309–316.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Breikaa RM, Algandaby MM, El-Demerdash E
and Abdel-Naim AB: Multimechanistic antifibrotic effect of
biochanin A in rats: Implications of proinflammatory and
profibrogenic mediators. PLoS One. 16:e692762013. View Article : Google Scholar
|
|
36
|
Banjerdpongchai R, Khawon P and Pompimon
W: Phytochemicals from Goniothalamus griffithii Induce Human Cancer
Cell Apoptosis. Asian Pac J Cancer Prev. 17:3281–3287.
2016.PubMed/NCBI
|
|
37
|
Shyur LF, Chen CH, Lo CP, Wang SY, Kang
PL, Sun SJ, Chang CA, Tzeng CM and Yang NS: Induction of apoptosis
in MCF-7 human breast cancer cells by phytochemicals from
Anoectochilus formosanus. J Biomed Sci. 11:928–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Torisu H, Ono M, Kiryu H, Furue M, Ohmoto
Y, Nakayama J, Nishioka Y, Sone S and Kuwano M: Macrophage
infiltration correlates with tumor stage and angiogenesis in human
malignant melanoma: Possible involvement of TNFa and IL-1alpha. Int
J Cancer. 85:182–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rayburn ER, Scharri SJ and Zhang R:
Anti-inflammatory agents for cancer therapy. Mol Cell Pharmacol.
1:29–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ricchi P, Zarrilli R, Di Palma A and
Acquaviva AM: Anti-inflammatory drugs in colorectal cancer: From
prevention to therapy. Br J Cancer. 88:803–807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of Snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|