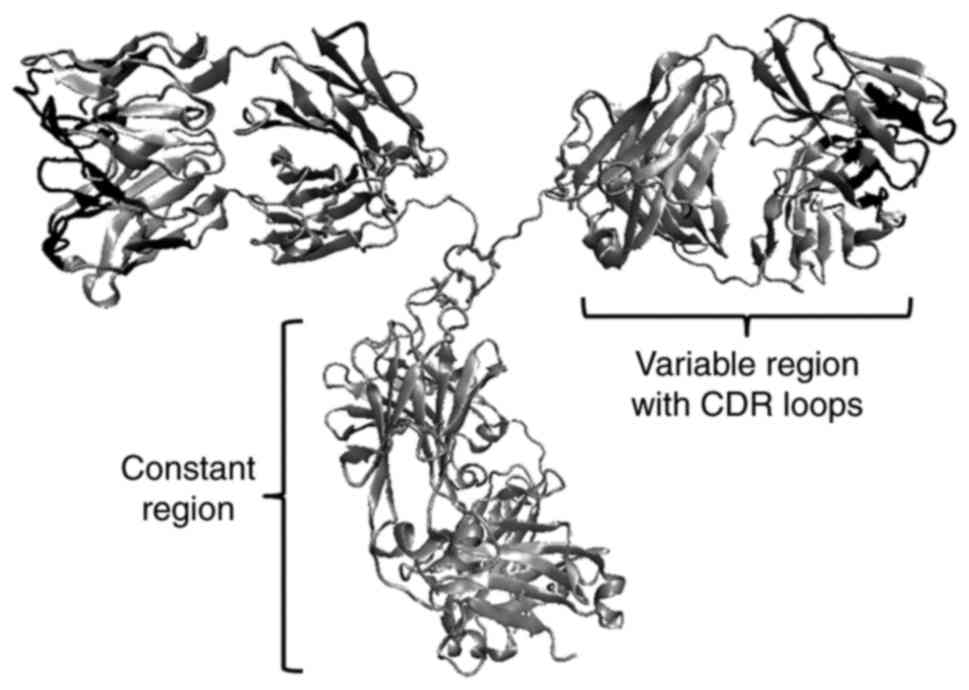
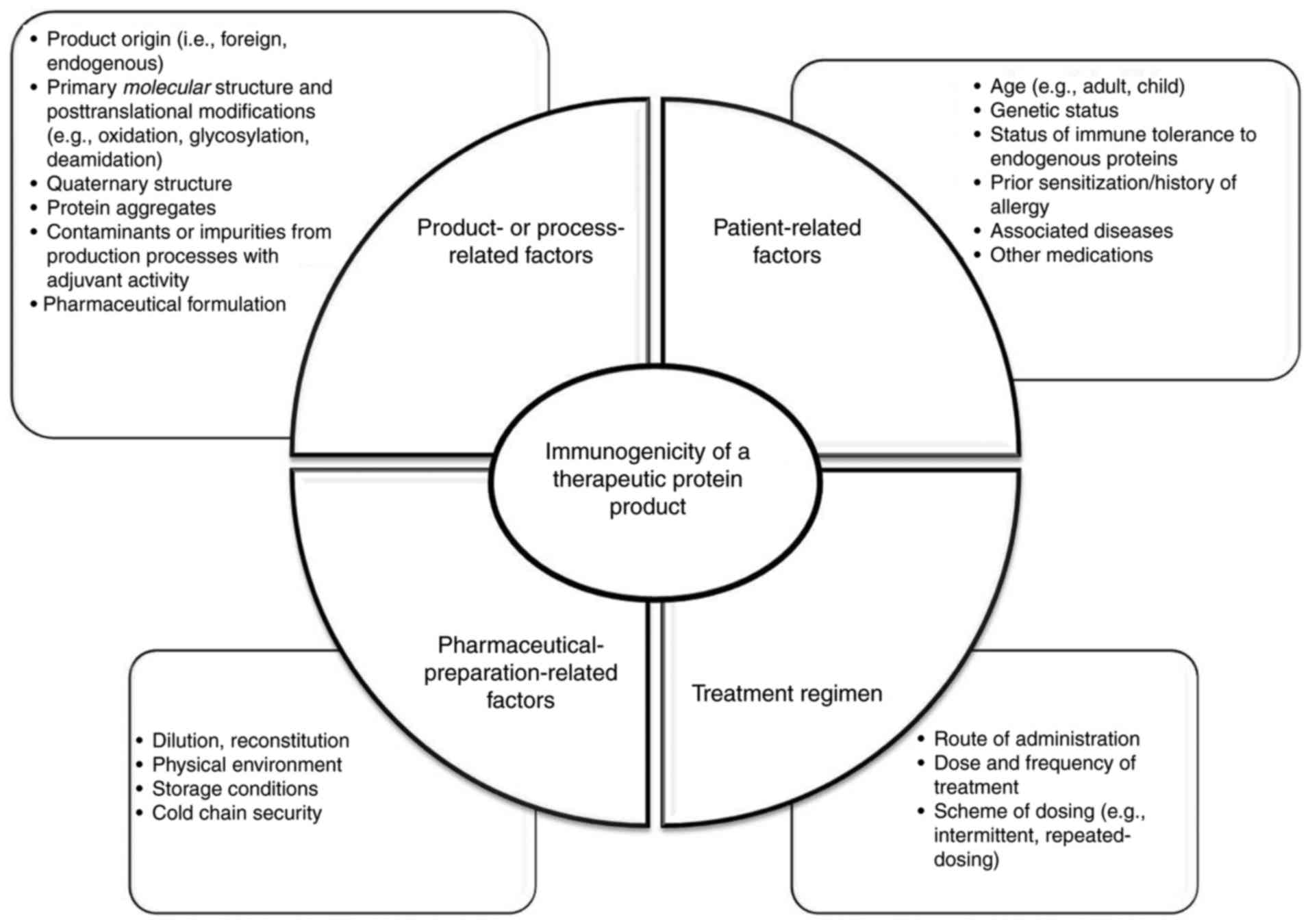
|
1
|
Das G and Rees A: Use of monoclonal
antibodies for proprotein convertase subtilisin kexin type 9
inhibition: Issues with efficacy, tolerability and safety. Curr
Opin Lipidol. 25:96–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elvin JG, Couston RG and van der Walle CF:
Therapeutic antibodies: Market considerations, disease targets and
bioprocessing. Int J Pharm. 440:83–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shukla D, Schneider CP and Trout BL:
Molecular level insight into intra-solvent interaction effects on
protein stability and aggregation. Adv Drug Deliv Rev.
63:1074–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W: Instability, stabilization, and
formulation of liquid protein pharmaceuticals. Int J Pharm.
185:129–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berrill A, Biddlecombe J and Bracewell D:
Product quality during manufacture and supplyPeptide and protein
delivery. Chris Van Der W: Academic Press; Boston, MA: pp. 313–339.
2011, View Article : Google Scholar
|
|
6
|
Kamerzell TJ, Esfandiary R, Joshi SB,
Middaugh CR and Volkin DB: Protein-excipient interactions:
Mechanisms and biophysical characterization applied to protein
formulation development. Adv Drug Deliv Rev. 63:1118–1159. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bee JS, Nelson SA, Freund E, Carpenter JF
and Randolph TW: Precipitation of a monoclonal antibody by soluble
tungsten. J Pharm Sci. 98:3290–3301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davies MJ: The oxidative environment and
protein damage. Biochim Biophys Acta. 1703:93–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Margiloff L, Chaplia L, Chow A, Singhal PC
and Mattana J: Metal-catalyzed oxidation of immunoglobulin G
impairs Fc receptor-mediated binding to macrophages. Free Radic
Biol Med. 25:780–785. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu D, Ren D, Huang H, Dankberg J,
Rosenfeld R, Cocco MJ, Li L, Brems DN and Remmele RL Jr: Structure
and stability changes of human IgG1 Fc as a consequence of
methionine oxidation. Biochemistry. 47:5088–5100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mian IS, Bradwell AR and Olson AJ:
Structure, function and properties of antibody binding sites. J Mol
Biol. 217:133–151. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dimitrov JD, Ivanovska ND,
Lacroix-Desmazes S, Doltchinkova VR, Kaveri SV and Vassilev TL:
Ferrous ions and reactive oxygen species increase antigen-binding
and anti-inflammatory activities of immunoglobulin G. J Biol Chem.
281:439–446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omersel J, Jurgec I, Cucnik S, Kveder T,
Rozman B, Sodin-Semrl S and Bozic B: Autoimmune and proinflammatory
activity of oxidized immunoglobulins. Autoimmun Rev. 7:523–529.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daugherty AL and Mrsny RJ: Formulation and
delivery issues for monoclonal antibody therapeutics. Adv Drug
Deliv Rev. 58:686–706. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang L, Lu J, Wroblewski VJ, Beals JM and
Riggin RM: In vivo deamidation characterization of monoclonal
antibody by LC/MS/MS. Anal Chem. 77:1432–1439. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vermeer AW and Norde W: The thermal
stability of immunoglobulin: Unfolding and aggregation of a
multi-domain protein. Biophys J. 78:394–404. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Gaza-Bulseco G and Sun J:
Characterization of the stability of a fully human monoclonal IgG
after prolonged incubation at elevated temperature. J Chromatogr B
Analyt Technol Biomed Life Sci. 837:35–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye H: Simultaneous determination of
protein aggregation, degradation, and absolute molecular weight by
size exclusion chromatography-multiangle laser light scattering.
Anal Biochem. 356:76–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Segura A, Herrera M, González E, Vargas M,
Solano G, Gutiérrez JM and León G: Stability of equine IgG
antivenoms obtained by caprylic acid precipitation: Towards a
liquid formulation stable at tropical room temperature. Toxicon.
53:609–615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arakawa T, Prestrelski SJ, Kenney WC and
Carpenter JF: Factors affecting short-term and long-term
stabilities of proteins. Adv Drug Deliv Rev. 46:307–326. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Nema S and Teagarden D: Protein
aggregation-pathways and influencing factors. Int J Pharm.
390:89–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hawe A, Kasper JC, Friess W and Jiskoot W:
Structural properties of monoclonal antibody aggregates induced by
freeze-thawing and thermal stress. Eur J Pharm Sci. 38:79–87. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W: Lyophilization and development of
solid protein pharmaceuticals. Int J Pharm. 203:1–60. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang BS, Kendrick BS and Carpenter JF:
Surface-induced denaturation of proteins during freezing and its
inhibition by surfactants. J Pharm Sci. 85:1325–1330. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cleland JL and Langer R: Formulation and
Delivery of Proteins and Peptides: Design and Development
Strategies. (ACS Symposium Series 567). American Chemical Society;
Washington, DC: 1994, View Article : Google Scholar
|
|
26
|
Hermeling S, Crommelin DJ, Schellekens H
and Jiskoot W: Structure-immunogenicity relationships of
therapeutic proteins. Pharm Res. 21:897–903. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosenberg AS: Effects of protein
aggregates: An immunologic perspective. AAPS J. 8:E501–E507. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Groot AS and Scott DW: Immunogenicity
of protein therapeutics. Trends Immunol. 28:482–490. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keiserman M, Codreanu C, Handa R,
Xibillé-Friedmann D, Mysler E, Briceño F and Akar S: The effect of
antidrug antibodies on the sustainable efficacy of biologic
therapies in rheumatoid arthritis: Practical consequences. Expert
Rev Clin Immunol. 10:1049–1057. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schellekens H and Jiskoot W:
Eprex-associated pure red cell aplasia and leachates. Nat
Biotechnol. 24:613–614. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suntharalingam G, Perry MR, Ward S, Brett
SJ, Castello-Cortes A, Brunner MD and Panoskaltsis N: Cytokine
storm in a phase 1 trial of the anti-CD28 monoclonal antibody
TGN1412. N Engl J Med. 355:1018–1028. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang WY and Foote J: Immunogenicity of
engineered antibodies. Methods. 36:3–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joubert MK, Hokom M, Eakin C, Zhou L,
Deshpande M, Baker MP, Goletz TJ, Kerwin BA, Chirmule N, Narhi LO
and Jawa V: Highly aggregated antibody therapeutics can enhance the
in vitro innate and late-stage T-cell immune responses. J Biol
Chem. 287:25266–25279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brinks V, Jiskoot W and Schellekens H:
Immunogenicity of therapeutic proteins: The use of animal models.
Pharm Res. 28:2379–2385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swanson SJ and Bussiere J: Immunogenicity
assessment in non-clinical studies. Curr Opin Microbiol.
15:337–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Breckenridge A: Report of the working
party on the addition of drugs to intravenous infusion fluids
(HC(76)9). Department of Health and Social Security; London:
1976
|
|
37
|
Langford S, Fradgley S, Evans M and Blanks
C: Assessing the risk of handling monoclonal antibodies. Hospital
Pharm. 15:60–64. 2008.
|
|
38
|
Odou P: Medical devices for safe handling
of cytotoxic drugs. Eur J Oncol Pharm. 4:17–19. 2010.
|
|
39
|
Paul M, Vieillard V, Jaccoulet E and
Astier A: Long-term stability of diluted solutions of the
monoclonal antibody rituximab. Int J Pharm. 436:282–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grundstein A, Meentemeyer V and Dowd J:
Maximum vehicle cabin temperatures under different meteorological
conditions. Int J Biometeorol. 53:255–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laguerre O and Flick D: Temperature
prediction in domestic refrigerators: Deterministic and stochastic
approaches. Int J Refrigeration. 33:41–51. 2010. View Article : Google Scholar
|
|
42
|
James SJ, Evans J and James C: A review of
the performance of domestic refrigerators. J Food Eng. 87:2–10.
2008. View Article : Google Scholar
|