Introduction
Bone tissue engineering has been widely applied for
repairing and regeneration of bone defects caused by traumatic
injury, congenital malformation or surgery for bone cancer
(1–3). Existing treatment methods include
autogenous, allogenic and synthetic bone grafts (4–6). Among
them, autograft is a generally preferred choice of bone grafting
material (7,8). However, the application of autograft is
limited by the inadequate supply for autograft tissues. Though some
reports demonstrated that allografts were effective in vertical
ridge augmentation of the atrophic posterior mandible Laino
(9), problems like disease transfer
and histo-incompatibilities which are very likely to occur in the
case of allografts (10,11). Due to the aforementioned limitations,
engineered biomaterials combined with growth factors have emerged
as an alternative choice in bone repair and regeneration (12). A number of different growth factors,
including bone morphogenetic proteins (BMPs), transforming growth
factor-β (TGF-β), vascular endothelial growth factor (VEGF),
fibroblast growth factor (FGF) and insulin growth factor (IGF) have
been shown to stimulate bone growth, collagen synthesis and
fracture repair both in vitro and in vivo (13–16).
In particular, BMPs are osteoinductive proteins
originally identified in demineralized bone and are known to
facilitate bone healing without bone tissue transferring (17). Among this group of proteins, it is
well known that BMP-2 can promote the healing process of segmental
bone defects and the osteogenesis ability of bone marrow stromal
cells (BMSCs) (18,19). However, the circulation half-life of
BMP-2 is rather short. BMP-2 is easily to be inactivated due to
dilution or interaction with enzymes in blood if applied alone by
intravenous injection (20,21). Another drawback is that the
intravenous injection of BMP-2 alone may produce burst effect,
which may lead to soft tissue hematoma and bone absorption
phenomenon (22). Therefore, it is
necessary to develop appropriate delivery systems for BMP-2 to
extend its blood circulation time, achieve sustained local lease,
and at the same time to avoid the adverse effects such as the burst
release (23).
In searching for such delivery system, it is noticed
that chitosan (CS), a cationic polysaccharide, has been widely used
as a drug carrier due to its good biocompatibility and
biodegradability and CS-based microsphere have shown some distinct
advantages in delivery of various bio-active species (24). It has been demonstrated that CS-based
microspheres as a drug delivery system (DDS) is able to reduce side
effects, improve drug stability and enhance therapeutic efficacy of
drugs and proteins (25,26).
In our previous study, we successfully prepared
rhBMP-2 loaded CS microsphere. However, the entrapment efficiency
and drug loading ratio (mass ratio of protein to carrier of the
microsphere) were quite low, presumably because of the low binding
affinity of CS towards BMP-2. On the other hand, it was reported
that dextran sulfate (DS) sodium, a sulfated anionic
polysaccharide, showed fairly strong affinity towards proteins,
which have heparin or heparan sulfate glycosaminoglycans, such as
rhBMP-2 (27,28). Furthermore, it was reported that CS
and DS could form stable polyelectrolyte complexes (PECs) which
could efficiently encapsulate and stabilize therapeutic proteins
(29,30). It has also been demonstrated that
heparin sulfate glucose amino glucan could be utilized to adjust
the biological activity of exogenous growth factors (31). According to the above, we
hypothesized that, by introducing DS as the BMP-2 binding component
in to our CS-microsphere delivery vehicles, the encapsulation
efficiency would be greatly enhanced. And more importantly, the
polyelectrolyte complexes formed by CS and DS might significantly
increase the stability of the resulting protein-loaded delivery
assembly.
Herein, we reported the preparation of CS/DS based
microspheres as an efficient delivery vehicle for recombinant human
bone morphogenetic protein-2 (rhBMP-2). Our preliminary study
revealed that our CS/DS based microsphere has high entrapment
efficiency (85.6±3%) and high drug loading ratio (47.245±3.321
µg/mg) for rhBMP-2, as well as sustained release kinetics and good
osteogenic activity. This study may provide new orientation for
bone tissue engineering for repairing and regeneration of bone
defects in clinic.
Materials and methods
Preparation of DS/CS blank
microsphere
A certain amount of CS, molecular weight ranging
from 50,000-190,000, off 85% of the acetic acid, Shanghai Aladdin
Bio-Chem Technology Co., Ltd., (Shanghai, China) was dissolved in
0.175% (V/V) acetic acid to prepare 1 mg/ml CS solution which was
then filtered successively through 0.45 µm-diameter membrane and
0.22 µm-diameter membrane. DS soluble to double-distilled water was
weighed and prepared into 1 mg/ml DS solution which was then
filtered successively through 0.45 µm-diameter membrane and 0.22
µm-diameter membranes. 0.2 ml DS (molecular weight 500,000,
Shanghai Aladdin Bio-Chem Technology Co., Ltd.) was taken and
stirred at a speed of 1,500 rpm/min, following by addition of
further proportion of CS with 0.1 ml ZnSO4, which were
stirred continuously for 30 min. Thereafter, the microsphere was
moved to a centrifuge tube which was supplemented with 5% mannitol
and then centrifuged for 15 min at 4°C, 15,000 rpm/min. The
supernatant was discarded and the microsphere was lyophilized for
use after centrifugation.
Preparation of CS/DS/rhBMP-2
microsphere
After preparation of CS and DS solutions as
described above, 0.2 ml DS and 0.04 ml rhBMP-2 were added and
stirred at a speed of 1,500 rpm for 20 min. Then, 0.12 ml CS
solution was added following with addition of 0.1 m
ZnSO4 5 min later, which were stirred continuously for
30 min. The microsphere was moved to a centrifuge tube which was
supplemented with 5% mannitol and then centrifuged for 15 min at
4°C, 15,000 rpm/min. The supernatant was discarded and the
microsphere were lyophilized for use after centrifugation for three
times.
Characterization of CS/DS/rhBMP-2
microsphere
The average particle size of CS/DS/rhBMP-2
nanoparticles, particle size distribution, and the dispersion of
potential value were evaluated. The DS and CS solutions were added
with a pipette according to a certain mass ratio (10:1~10:11), and
were stirred to form a uniform state at room temperature to obtain
the DS/CS complex solution. Analyzing grain diameter of DS/CS blank
microsphere was conducted by Zeta potential to determine the best
quality ratio. According to above experiment, the DS:CS ratio was
set to 10:6, and the ratio of DS:CS:rhBMP-2 was set to 10:6:1,
10:6:1.2, 10:6:1.5, 10:6:2, 10:6:2.5, 10:6:3 to find the optimized
mass ratio.
The microsphere morphology of the CS/DS/rhBMP-2
microsphere was observed by a scanning electron microscope (S-4800;
Hitachi, Ltd., Tokyo, Japan) and a three-dimensional morphology was
obtained using an atomic force microscope (S-5500; Hitachi, Ltd.).
Briefly, the CS/DS/rhBMP-2 microsphere solution was dropped on a
uniformly thin layer on a silicon wafer after diluted, and then was
observed after freeze-drying. Then, a high-resolution Zeta
potential and particle size analyzer (Brookhaven American Company)
was used to determine the average particle size, size distribution,
and the potential of the dispersion of potential value.
Determination of the entrapment
efficiency and drug loading ratio
The supernatant liquid was collected after
centrifugation. The drug loading rate and entrapment efficiency of
rhBMP-2 CS nanoparticles were calcula`ted by detecting the amount
of rhBMP-2 in the supernatant with Enzyme-linked immunosorbent
assay (ELISA) kit (RayBiotech, Norcross, GA, USA). The absorbance
[optical density (OD)] of the supernatant was determined at value
A450 according to ELISA kit instructions and the standard curve was
drawn. All experiments were performed in triplicate. Calculation
formulas for entrapment efficiency and drug loading ratio were as
follows:
Drug loading=(Total quality of
rhBMP–2)–(rhBMP–2inupernatants)Total quality of
microspheres×100%
Entrapment efficiency=(Total quality of
rhBMP–2)–(rhBMP–2insupernatants)Total quality of
rhBMP–2×100%
In vitro sustained release
profile
50 mg CS/DS/rhBMP-2 microsphere was added with 2 ml
phosphate buffer solution with pH 7.4, following with ultrasonic
vibration at room temperature for 10 min. The solution was
centrifuged for 15 min at the speed of 15,000 rpm/min. 100 ml
supernatant was taken at 6, 12, 18, 24, 48, and 72 h every 3 days
and was ultrasonically oscillated with 100 ml PBS. The content of
rhBMP-2 in the supernatant was measured by ELISA kit as described
above. All experiments were performed in triplicate.
Biocompatibility assessment
Extracts preparation
1 g CS/DS/rhBMP-2 composite microsphere and 1 g
CS/DS sample blank microsphere were added into two flasks
respectively, following by addition of 40 ml RPMI1640 medium ml
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to each
flask. The flasks were placed in a cell incubator (37°C, 5%
CO2) (V/V) and were cultured for 24 h, sterilized by
microporous membrane (0.22 µm), and stored at 4°C.
Cell culture
The L929 cells (provided by General Hospital of
Guangzhou Military Command, department of Experimental Medicine)
were thawed 1~2 min at 37°C water bath, and then 15 ml L929 cells
liquid were sterilized 30 min using the UV ultra-bacteria station.
Cells were then centrifuged for 5 min, 1,000 rpm/min. The
supernatant was discarded, and cells were re-suspended with the
H-DMEM medium (Gibco; Thermo Fisher Scientific, Inc.) containing
10% fetal bovine serum (FBS) (V/V) (Gibco; Thermo Fisher
Scientific, Inc.), and cultured at the condition of 37°C, 5%
CO2. Cells in the logarithmic phase were trypsinized and
formulated into cell suspension of concentration of
1×104/ml. Then cells were seeded in 96-well culture
plate with 100 µl per hole and cultured under 37°C, 5%
CO2.
MTT assay
Samples were divided into CS/DS/rhBMP-2 composite
microsphere (200 ng/ml) group, rhBMP-2 (200 ng/ml group, CS/DS
blank microsphere (200 ng/ml) group and control group; each group
contained three parallel samples. 10 µl MTT (5 mg/ml) was added for
each group at 1, 3, 5, 7 days. The relative growth rate (RGR of
each group of cells was detected at the OD of 490 after cultured
for 4 h, namely (experimental group OD/negative control OD
×100%.
Cytotoxicity evaluation
Cytotoxicity was evaluated according to the United
States Pharmacopoeia cytotoxic grading standards (32), 0 level: RGR≥100; 1 level: 75~99; 2
level: 50~74; 3 level: 25–49; 4 level: 2~14; 5 level: 0.
Differentiation and proliferation studies of
BMSCs-C57 cells
BMSCs-C57 subculture experiments
Cells were divided into four groups: CS/DS/rhBMP-2
microsphere group, added with CS/DS/rhBMP-2 composite microsphere
(concentration of the drug in accordance with the conversion rate,
rhBMP-2 concentration of 200 µg/l); rhBMP-2 group, added with
rhBMP-2 (200 µg/l); CS/DS group, added with CS/DS microsphere;
blank control group. Cultured the BMSCs-C57 of third generation was
cultured, and the growth of cells was observed every day. Cells
were cultured after digestion with 0.25% trypsin when convergence
rate>80%.
Proliferation assessment of
BMSCs-C57cells
Proliferation of BMSCs-C57 cells was determined
using MTT method. Cell culture and MTT procedure were described as
above. Cell proliferation in each group was determined at 2, 4 and
6 days.
Differentiation analysis of BMSCs-C57
cells
The differentiation analysis of BMSCs-C57 cells was
conducted by detecting the alkaline phosphatase (ALP) activity of
different groups of cells when cultured at 1, 3, 5, 7, 9, 11 and 14
days using an Alkaline Phosphatase Detection Kit (Nanjing built
Biotechnology Co., Ltd., China). The growth of calcium nodules in
each group was observed by alizarin red staining.
Statistical analysis
Results were expressed as mean ± standard deviation
(SD) from three or more separate experiments. All the data were
analyzed by SPSS v19.0 statistic package software. The statistical
differences were analyzed by using the ANOVA test. Pairwise
comparisons in two groups were analyzed by the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization and optimization of
microspheres
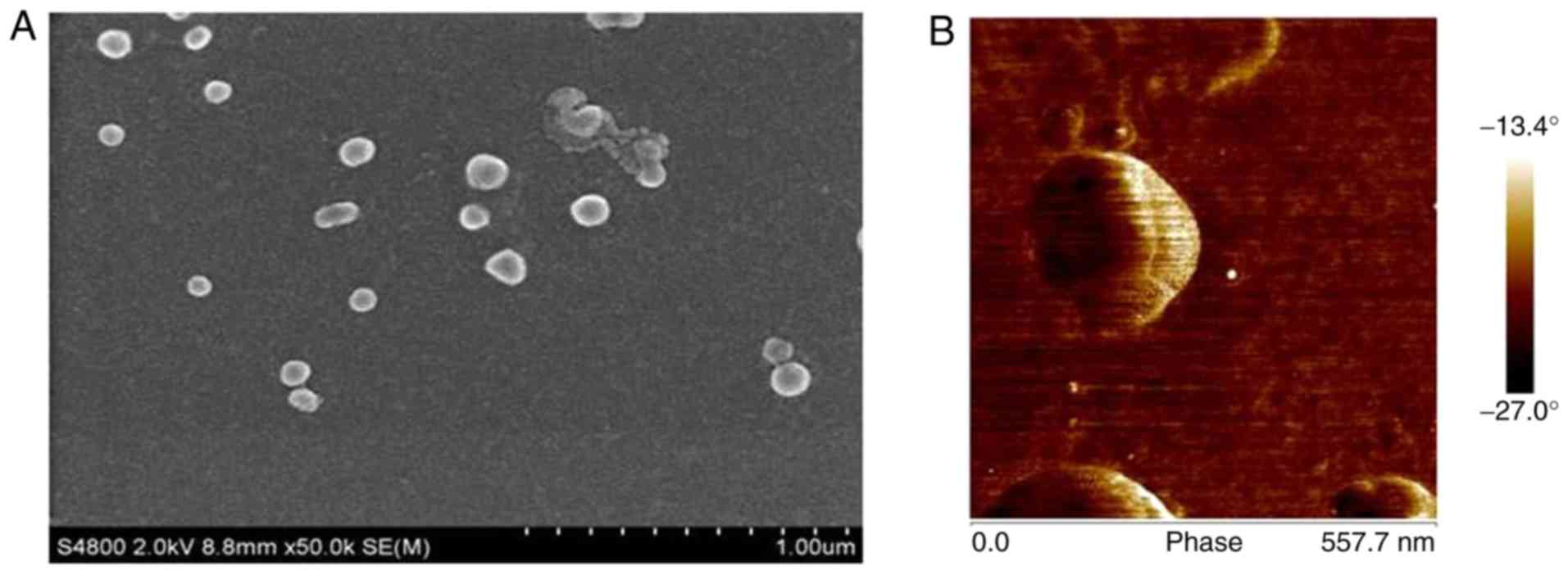
Representative SEM images of CS/DS/rhBMP-2 are shown
in Fig. 1. It could be observed that
prepared CS/DS/rhBMP-2 nano-composites with smooth surfaces were
spherical and evenly disperses without obvious agglomeration, which
suggested the successful formation of the microspheres. As shown in
Table I, the diameter of the
CS/DS/rhBMP-2 microsphere could be adjusted between 200 nm ~900 nm.
When the mass ratio of DS and CS was 10:1, the grain diameter was
larger than 1 µm. The diameter of CS/DS microsphere was 928.2±8.8
nm when mass ratio of DS:CS was 10:2. It was found that the
increase of CS/DS mass ratio would lead to decrease of the diameter
of the resulting microsphere. When the mass ratio of DS/CS reached
10:6, the particle diameter of the microsphere was around 217 nm
and the polydispersity was the narrowest (0.221±0.008). The
particle diameter of CS/DS microsphere increased when more CS was
added. When mass ratio of DS:CS was over 10:10, the diameter of
CS/DS microsphere was larger than 1 µm.
 | Table I.Grain diameter, dispersion and Zeta
potential values of DS/CS microsphere under different mass ratios
of DS:CS. |
Table I.
Grain diameter, dispersion and Zeta
potential values of DS/CS microsphere under different mass ratios
of DS:CS.
| R(DS:CS) | Particle (nm) | Polydispersity | Zeta potential
(mV) |
|---|
| 10:2 | 928.2±8.8 | 0.205±0.012 | −23.82±0.68 |
| 10:3 | 725.1±12.8 | 0.212±0.017 | −16.24±3.25 |
| 10:5 | 212.3±11.5 | 0.214±0.004 | −16.61±1.57 |
| 10:6 | 217.2±15.7 | 0.221±0.008 | −16.50±0.76 |
| 10:9 | 930.7±14.5 | 0.061±0.072 | −11.35±5.60 |
Table II shows the
average particle size, degree of dispersion and Zeta potential
values of CS/DS/rhBMP-2 microspheres with different DS: CS: rhBMP-2
mass ratios. It was found that when the mass ratio of DS: CS:
rhBMP-2 was 10: 6: 1.2, the average particle size of CS/DS/rhBMP-2
microsphere is about 248.1 nm, polydispersity was about 0.188, Zeta
potential value was about −9.55 mV. Under this mass ratio,
CS/DS/rhBMP-2 microspheres had the optimized stability, good
dispersion and the appropriate diameter for delivery purpose, which
were used for our further studies.
 | Table II.Grain diameter, dispersion and Zeta
potential values of CS/DS/rhBMP-2 microsphere under different mass
ratios of DS:CS:rhBMP-2. |
Table II.
Grain diameter, dispersion and Zeta
potential values of CS/DS/rhBMP-2 microsphere under different mass
ratios of DS:CS:rhBMP-2.
|
R(DS:CS:rhBMP-2) | Particle (nm) | Polydispersity | Zeta Potential
(mV) |
|---|
| 10:6:1 | 247.9±10.8 | 0.121±0.032 | −15.61±1.75 |
| 10:6:1.2 | 248.1±2.7 | 0.188±0.012 | −9.55±1.54 |
| 10:6:1.5 | 264.6±2.4 | 0.122±0.048 | −9.88±2.69 |
| 10:6:2 | 259.3±3.1 | 0.182±0.023 | −8.40±1.81 |
| 10:6:2.5 | 252.5±3.2 | 0.167±0.002 | −7.96±4.22 |
| 10:6:3 | 266.2±2.4 | 0.185±0.017 | −17.80±0.49 |
Drug loading ratio, entrapment
efficiency and release Kinetics of rhBMP-2
CS/DS/microspheres showed the highest entrapment
efficiency of 85.6±3%, and the highest the drug loading ratio
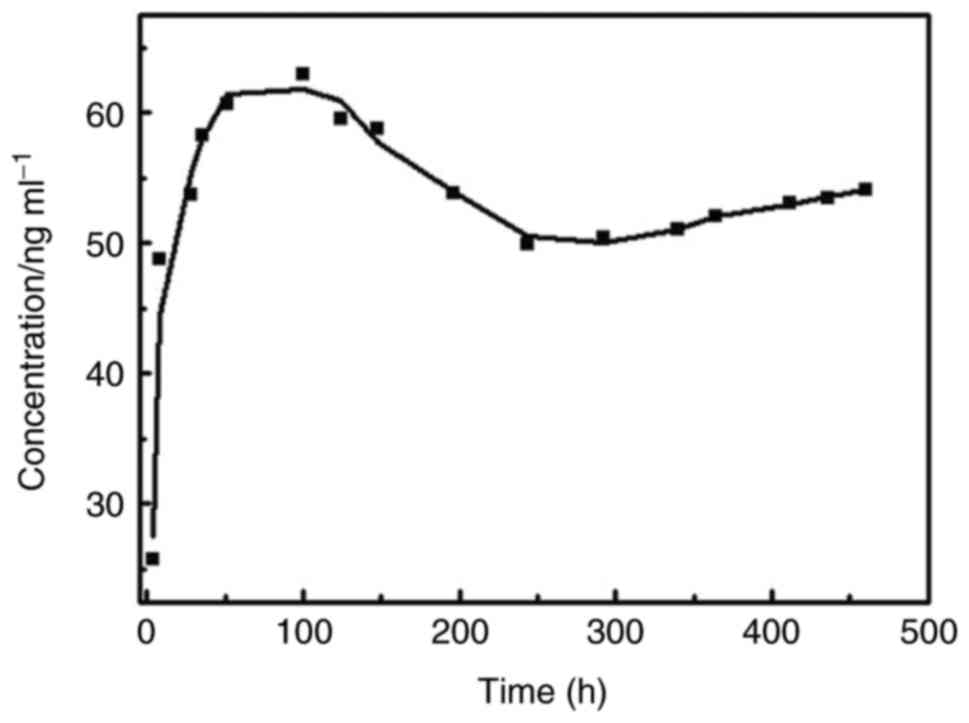
(rhBMP-2 to CS/DS/microspheres of 47.245±3.321 µg/mg). It was
observed that the microsphere release started to undergo a sudden
release period in vitro 2 h after the experiment, and the
release concentration reached a peak at day 4, followed by a slow
decline. The release cycle lasted about 20 days, which was well
consistent with a biphasic release kinetic model (Fig. 2).
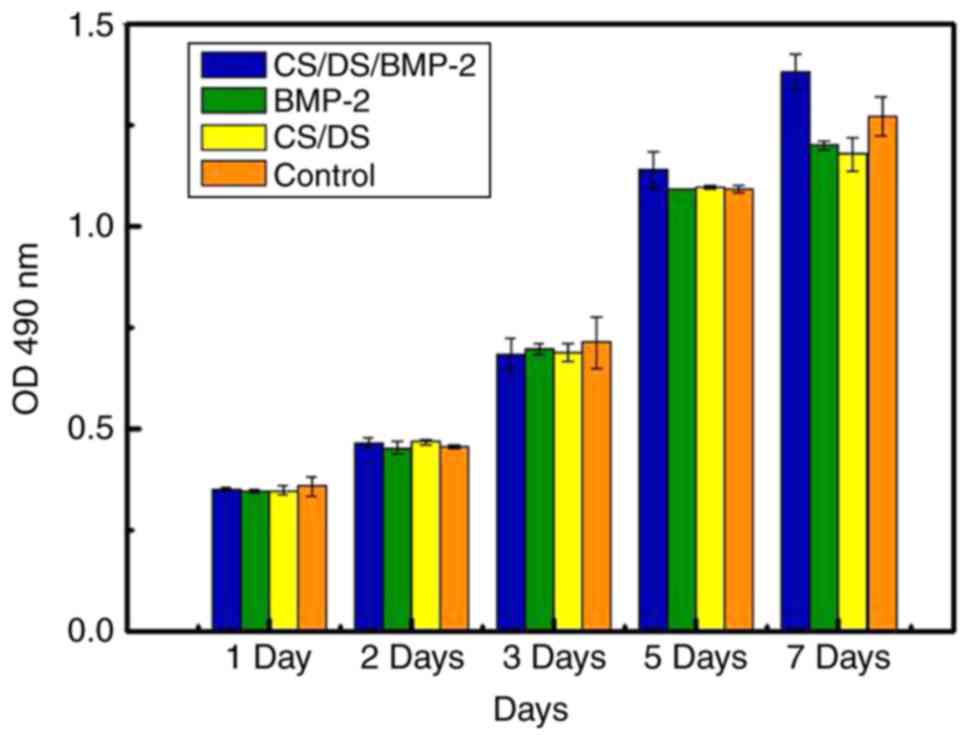
CS/DS/ and CS/DS/rhBMP-2 microspheres
had no effect on BMSCs-C57 cells proliferation
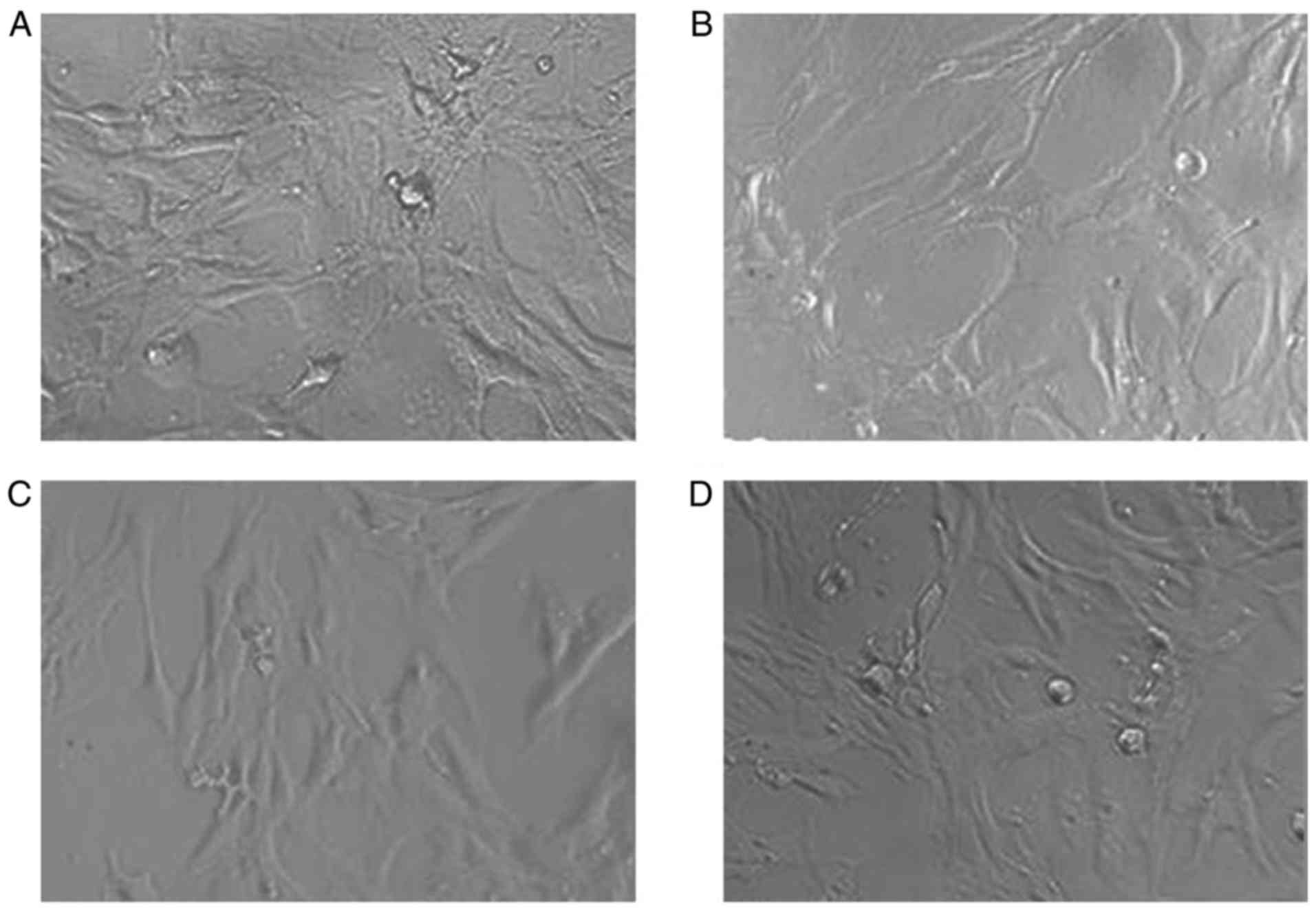
MTT assay was conducted to measure the effect of
different kinds of microspheres on BMSCs-C57 cells. Results showed
that in all cell groups, the cell morphology were normal, fusiform
or polygonal, cell bodies were plump and no significant difference
were noticed among the groups (Fig.
3), suggesting that microspheres didn't show significant effect
on cells. After cultured 2, 4, 6 days, results of OD values of all
BMSCs-C57 cells also showed no significant difference (P>0.05,
Table III).
 | Table III.Optical density values of BMSC-C57 at
different time points of the different groups co-cultured with bone
marrow mesenchymal stem cells-C57. |
Table III.
Optical density values of BMSC-C57 at
different time points of the different groups co-cultured with bone
marrow mesenchymal stem cells-C57.
|
| Culture time
(days) |
|---|
|
|
|
|---|
| Group | 2 | 4 | 6 |
|---|
| CS/DS/rhBMP-2
microsphere | 0.606±0.054 | 1.266±0.124 | 2.752±0.254 |
| rhBMP-2 |
0.632±0.076a |
1.256±0.142a |
1.985±0.171a |
| CS/DS |
0.574±0.027a |
1.196±0.054a |
1.984±0.100a |
| Blank control |
0.611±0.063a |
1.105±0.112a |
2.325±0.245a |
Effect of CS/DS/and CS/DS/rhBMP-2
microspheres on differentiation of BMSCs-C57 cells
To investigate the effects of different microspheres
on differentiation of BMSCs-C57 cells, the ALP assay of BMSCs-C57
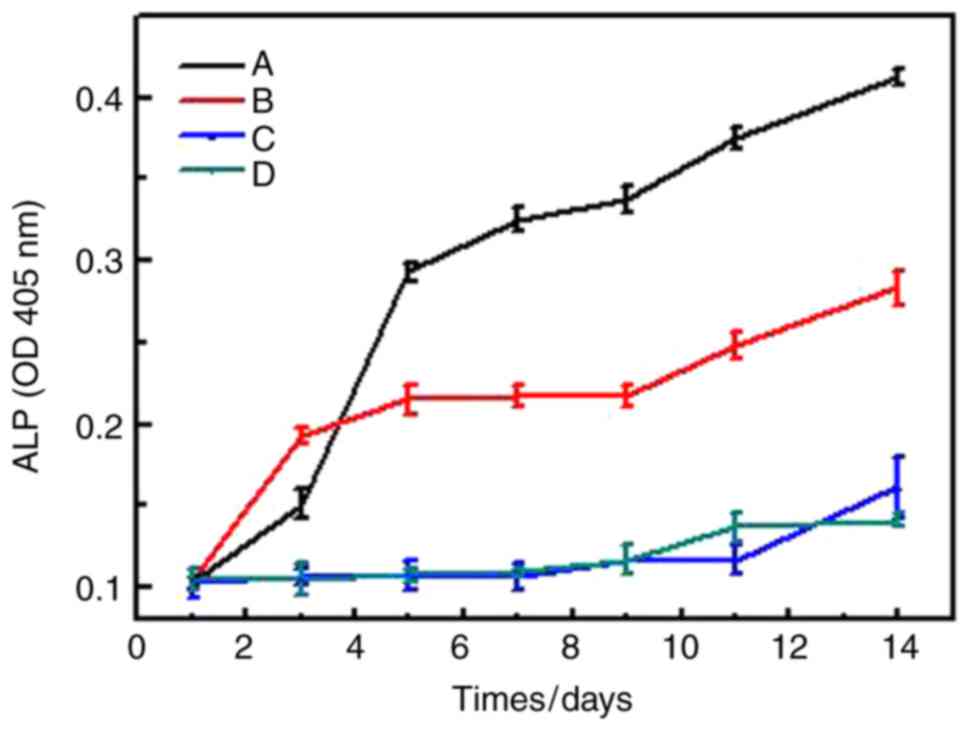
cells and alizarin red staining were performed. Results showed that
in CS/DS/rhBMP-2 microsphere group, ALP activity was significantly
lower than rhBMP-2 group, (P<0.05, Table IV and Fig. 4), but when cultured 5 days, the ALP
activity of CS/DS/rhBMP-2 microsphere group was significantly
higher than other 3 groups (P<0.05, Table IV and Fig. 4); and the ALP activity of BMSCs-C57
in rhBMP-2 group was significantly higher than CS/DS group and
control group, (P<0.05, Table IV
and Fig. 4). These results suggested
that CS/DS/rhBMP-2 microsphere had stronger ability to induce ALP
activity in a long term period. Results of alizarin red staining
also showed that after osteogenic culturing for 14 days, clear
crystal formation in BMSCs-C57 cells could be found in
CS/DS/rhBMP-2 microsphere group and rhBMP-2 group. Cells showed
multilayered structure and granular materials could be seen clearly
on the cell surface. However there was no appreciable formation of
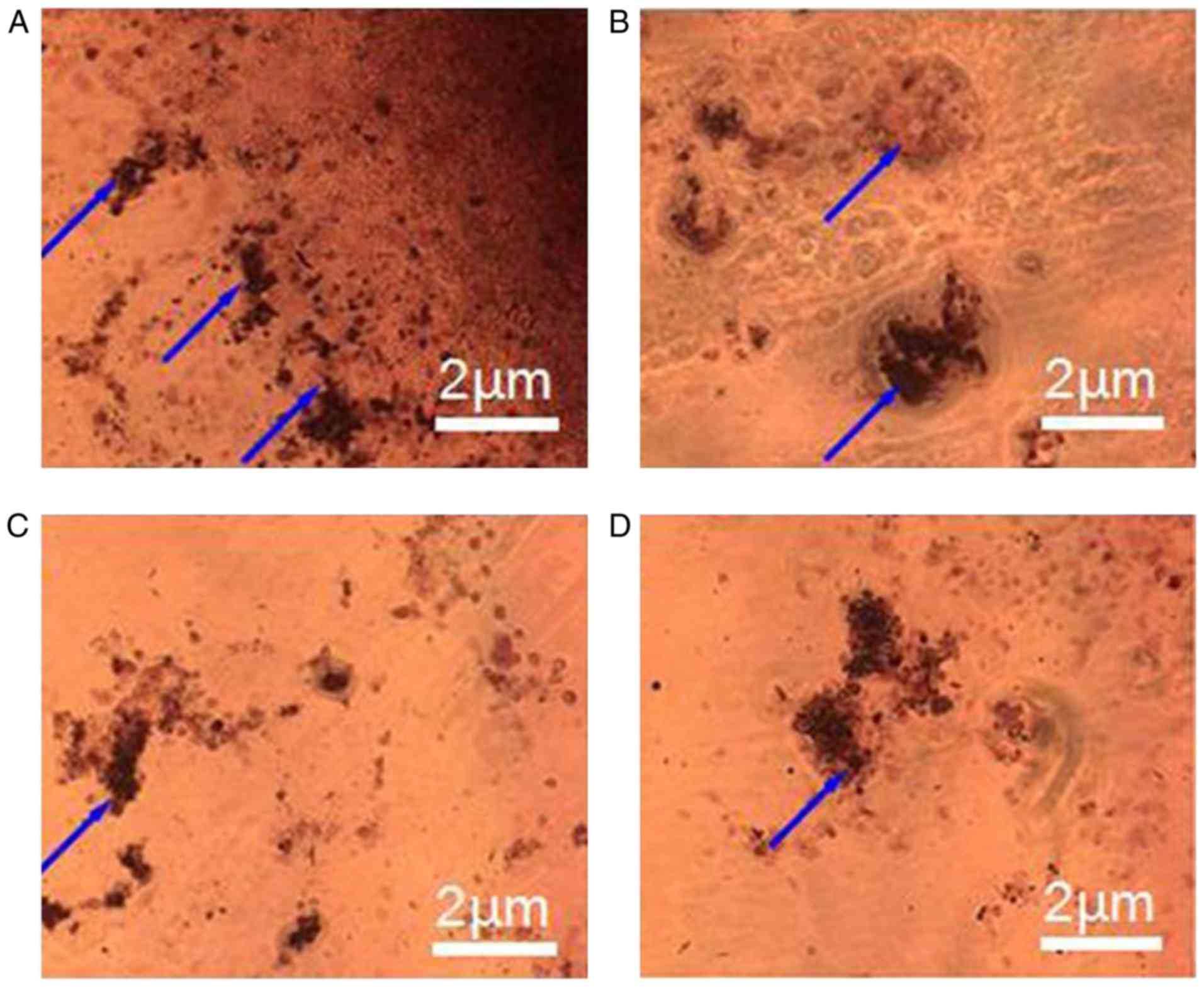
calcium nodules in CS/DS group and the control group (Fig. 5), which further demonstrated the
differentiation induction ability of CS/DS/rhBMP-2 microsphere.
 | Table IV.Alkaline phosphatase activity of bone
marrow mesenchymal stem cells-C57 in each group. |
Table IV.
Alkaline phosphatase activity of bone
marrow mesenchymal stem cells-C57 in each group.
|
| Training time
(days) |
|---|
|
|
|
|---|
| Group | 1 | 3 | 5 | 7 | 9 | 11 | 14 |
|---|
| CS/DS/rhBMP-2
microsphere | 0.103±0.003 | 0.151±0.009 | 0.293±0.006 | 0.325±0.007 | 0.337±0.008 | 0.375±0.007 | 0.412±0.005 |
| rhBMP-2 | 0.105±0.007 |
0.193±0.005a |
0.215±0.009a |
0.217±0.007a |
0.217±0.007a |
0.248±0.008a |
0.283±0.010a |
| CS/DS | 0.103±0.009 |
0.107±0.005a,b |
0.107±0.009a,b |
0.107±0.008a,b |
0.117±0.009a,b |
0.117±0.009a,b |
0.161±0.019a,b |
| Blank control | 0.105±0.007 |
0.105±0.009a,b |
0.108±0.004a,b |
0.109±0.003a,b |
0.117±0.009a,b |
0.137±0.009a,b |
0.141±0.004a,b |
| F-value | 0.525 | 11.029 | 14.422 | 21.819 | 22.577 | 23.894 | 23.076 |
| P-value | 0.618 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
The biocompatibility of CS/DS/rhBMP-2
microsphere
At last we determined The biocompatibility of
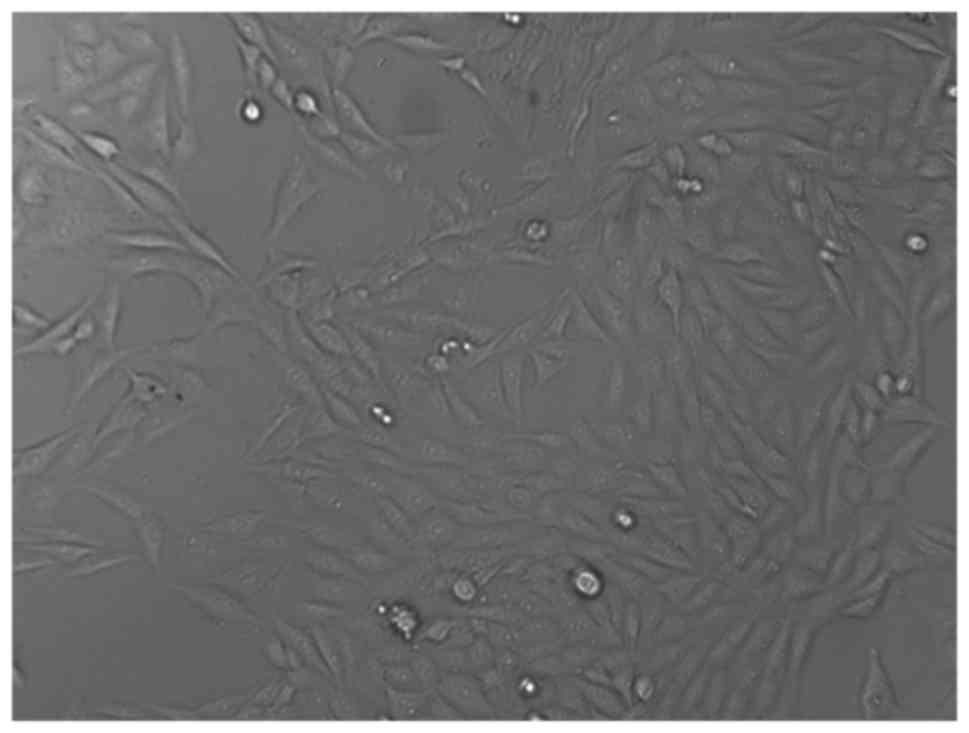
CS/DS/rhBMP-2 microsphere using toxicity test. Morphology study
showed that the sub-cultured L929 cells were fully extended,
spindle or polygonal and the nuclei were clear to be seen and
appeared circular or oval (Fig. 6).
The results of the cytotoxicity evaluation carried on L929 cells
were shown in Table V and Fig. 7. After incubation for 7 days, there
was no significant difference in OD values for CS/DS/rhBMP-2
microsphere group and other groups (P>0.05. And the toxicity
grading was between level 0 and 1. These results indicated that
CS/DS/rhBMP-2 microsphere and CS/DS blank microsphere (200 ng/ml)
were not toxic to L929 cells in the concentrations studied.
 | Table V.Optical density values, relative
growth ratio and toxicity grading of each group following
incubation with L929 cells. |
Table V.
Optical density values, relative
growth ratio and toxicity grading of each group following
incubation with L929 cells.
| Group | Optical
density | Relative growth
ratio (%) | Toxicity
grading |
|---|
| CS/DS/rhBMP-2
microsphere | 0.468±0.0123 | 102.41 | 0 |
| rhBMP-2 | 0.454±0.0161 | 99.34 | 1 |
| CS/DS | 0.470±0.00723 | 102.85 | 0 |
| Blank control | 0.457±0.00265 | 100.00 | 0 |
Discussion
Microspheres are known as carriers to significantly
enhance the efficacy of loaded therapeutics (33). In this study, we employ an organic
solvent-free method to fabricate CS/DS polyelectrolyte complex
based microspheres that can efficiently entrap and stabilize
rhBMP-2 protein. BMP-2 was first bound to anionic DS, DS sodium,
with its heparin binding domain, then forms polyelectrolyte complex
with a cationic polymer, CS, to form the protein-loaded CS/DS
microspheres (28). The morphology
and size of resultant CS/DS/rhBMP-2 microsphere were characterized
and results showed that the protein-loaded CS/DS microspheres had
smooth surfaces and evenly dispersed without obvious agglomeration
in solution, suggesting successfully construction of the delivery
system. The diameter of the CS/DS/rhBMP-2 microsphere can be
adjusted between 200 to 900 nm the optimized mass ratio of
DS:CS:rhBMP-2 was found to be 10: 6: 1.2. At this mass ratio, the
average particle size of CS/DS/rhBMP-2 microsphere was about 248.1
nm, the polydispersity was around 0.188 and Zeta potential value
was −9.55 mV.
Then, the entrapment efficiency, drug loading rate,
and in vitro release profile of the CS/DS/rhBMP-2
microspheres were assessed by an enzyme-linked immunosorbent assay
(ELISA). The entrapment efficiency and protein to carrier loading
ratio were found to be 85.6% and 47.245 ug/mg, respectively, which
were higher than that of CS/BMP-2 microsphere. It confirmed our
hypothesis that introduction of DS as the BMP-2 binding component
to our CS based delivery vehicle could greatly enhance the
encapsulation efficiency and protein to carrier loading ratio. The
results of in vitro release studies suggested that burst
effect of loaded microsphere was avoided. In fact, around 20% of
entrapped protein was released in the first two hour and at day 4,
the release reached a peak, followed by a slow decline. The release
lasted about 20 days, which is in consistent with a biphasic
release kinetic model. It was likely that the observed release in
first 4 days could be attributed to the relatively weak binding
force between CS and rhBMP-2 in the microspheres. After that,
rhBMP-2 that was strongly bounded with DS was gradually released.
Next, the cell cytotoxicity of the CS/DS/rhBMP-2 microsphere to the
mouse fibroblasts L929 cell was assessed by MTT assay. The data
showed that the RGR value of CS/DS/rhBMP-2 microsphere is 102.41%,
the cytotoxicity level is 0. The OD values of CS/DS/rhBMP-2
microsphere group and DS/CS blank microsphere group co-cultured
with L929 were not statistically significant, P>0.05, and the
toxicity grading was found to be between 0 and 1. These results
indicated that CS/DS/rhBMP-2 microsphere had good
biocompatibility.
The osteogenic effect of CS/DS/rhBMP-2 microsphere
on bone marrow stromal cells (BMSCs-C57) was investigated. BMSCs
are widely used as seed cells for tissue engineering. Also, they
possess multiple differentiation potential and can be induced to
differentiate into a variety of cell types (34,35). It
is known that BMP-2 can induce BMSC's differentiation, stimulate
ALP activities and matrix calcification (36). In CS/DS/rhBMP-2 group, rhBMP-2 group,
CS/DS group and negative control group, the form of cells in each
group looked fusiform or polygonal, cell body appeared plump, and
the cell nucleus were found to be large and clear, no significant
differences among the four groups were observed. In addition, there
were no significant differences (P>0.05) in the OD values of
each group. These results suggested that rhBMP-2 loaded CS/DS
microsphere, CS/DS microsphere, and free rhBMP-2, do not have
significantly effect of promoting the proliferation of BMSCs-C57.
ALP, as a biological mineralization marker, ALP assay is usually
used to verify cell osteogenic transformation and matrix
calcification osteoinductive (37).
RhBMP-2 loaded CS/DS microspheres were found to induce increase of
ALP level in BMSCs-C57, which indicated that they had significantly
ability of promoting differentiation of BMSCs, which might be due
to controlled release of rhBMP-2 from the microspheres. In
contrary, rhBMP-2, blank CS/DS microsphere and the control group
did not show appreciable differentiation of BMSCs. Herford et
al (38), demonstrated that the
addition of rhBMP2 into the between of the bone fragment induced a
rapid increase in hard and soft tissue healing, which is in
consistent with our study. Calcium nodule formation were checked
with an optical microscope by Alizarin Red staining protocol, it
was found that, no calcium nodules after incubation for 7 days in
any groups. After 14 days, the calcium nodule growth in the group
of rhBMP-2 loaded CS/DS microsphere could be clearly observed but
not in the rhBMP-2 group, CS/DS blank microsphere group or control
group, which suggested that rhBMP-2 loaded CS/DS microsphere were
able to promoted calcium nodule formation in BMSC cells. All these
results verified our assumption that the polyelectrolyte complexes
formed between CS and DS indeed significantly increase the
osteogenic activity of the encapsulated BMP-2 protein.
The present study also has some limitations. First,
we only used ALP activity and the growth condition of calcium
nodules to demonstrate the effects of rhBMP-2 CS nanoparticles on
cell differentiation and didn't investigate alteration of
differentiation related proteins. Secondly, it is unclear that
which signaling pathways are involved in the progress for the
regulation of rhBMP-2 CS nanoparticles on the cell differentiation.
At last the potential clinical use for rhBMP-2 CS nanoparticles is
still far away and needs further studies to improve the
process.
In summary, DS-CS microspheres were prepared by an
ionic cross-linking method. The resultant microsphere could
efficiently entrap rhBMP-2 and formed stable rhBMP-2 loaded CS/DS
microspheres. By varying mass ratio of DS:CS:rhBMP-2, stable
microspheres with suitable particle size could be fabricated.
Sustained release of rhBMP-2 from the CS/DS microspheres was
observed. RhBMP-2 loaded CS/DS microspheres showed good
bio-compatibility and could increase the osteogenic activity of the
encapsulated BMP-2. Our results suggested that microspheres from
CS/DS based polyelectrolyte complexes might have great potential as
carriers of therapeutic proteins in bone tissue engineering.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (51303031; Guangdong Natural
Science Foundation of China (S2012010009743; Applied basic research
project in Guangdong province (2013J4100120).
References
|
1
|
Lalwani G, Henslee AM, Farshid B, Lin L,
Kasper FK, Qin YX, Mikos AG and Sitharaman B: Two-dimensional
nanostructure-reinforced biodegradable polymeric nanocomposites for
bone tissue engineering. Biomacromolecules. 14:900–909. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia Z, Yu X, Jiang X, Brody HD, Rowe DW
and Wei M: Fabrication and characterization of biomimetic
collagen-apatite scaffolds with tunable structures for bone tissue
engineering. Acta Biomater. 9:7308–7319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrauskaite O, Gomes Pde S, Fernandes MH,
Juodzbalys G, Stumbras A, Maminskas J, Liesiene J and Cicciù M:
Biomimetic mineralization on a macroporous cellulose-based matrix
for bone regeneration. Biomed Res Int. 2013:4527502013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Visser R, Rico-Llanos GA, Pulkkinen H and
Becerra J: Peptides for bone tissue engineering. J Control Release.
244:122–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rogers GF and Greene AK: Autogenous bone
graft: Basic science and clinical implications. J Craniofac Surg.
23:323–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandez-Bances I, Perez-Basterrechea M,
Perez-Lopez S, Nuñez Batalla D, Fernandez Rodriguez MA,
Alvarez-Viejo M, Ferrero-Gutierrez A, Menendez Menendez Y,
Garcia-Gala JM, Escudero D, et al: Repair of long-bone
pseudoarthrosis with autologous bone marrow mononuclear cells
combined with allogenic bone graft. Cytotherapy. 15:571–577. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng Y, Wang S, Jin D, Sheng J, Chen S,
Cheng X and Zhang C: Free vascularised fibular grafting with
OsteoSet®2 demineralised bone matrix versus autograft
for large osteonecrotic lesions of the femoral head. Int Orthop.
35:475–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Genuario JW, Faucett SC, Boublik M and
Schlegel TF: A cost-effectiveness analysis comparing 3 anterior
cruciate ligament graft types: Bone-patellar tendon-bone autograft,
hamstring autograft, and allograft. Am J Sports Med. 40:307–314.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laino L, Iezzi G, Piattelli A, Lo Muzio L
and Cicciù M: Vertical ridge augmentation of the atrophic posterior
mandible with sandwich technique: Bone block from the chin area
versus corticocancellous bone block allograft-clinical and
histological prospective randomized controlled study. Biomed Res
Int. 2014:9821042014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petite H, Viateau V, Bensaïd W, Meunier A,
de Pollak C, Bourguignon M, Oudina K, Sedel L and Guillemin G:
Tissue-engineered bone regeneration. Nat Biotechnol. 18:959–963.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Widuchowski W, Widuchowska M, Koczy B,
Dragan S, Czamara A, Tomaszewski W and Widuchowski J: Femoral
press-fit fixation in ACL reconstruction using bone-patellar
tendon-bone autograft: Results at 15 years follow-up. BMC
Musculoskelet Disord. 13:1152012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herford AS, Tandon R, Stevens TW,
Stoffella E and Cicciu M: Immediate distraction osteogenesis: The
sandwich technique in combination with rhBMP-2 for anterior
maxillary and mandibular defects. J Craniofac Surg. 24:1383–1387.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lissenberg-Thunnissen SN, de Gorter DJ,
Sier CF and Schipper IB: Use and efficacy of bone morphogenetic
proteins in fracture healing. Int Orthop. 35:1271–1280. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Habibovic P and Barralet JE: Bioinorganics
and biomaterials: Bone repair. Acta Biomater. 7:3013–3026. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian H, Bi X, Li CS, Zhao KW, Brochmann
EJ, Montgomery SR, Aghdasi B, Chen D, Daubs MD, Wang JC and Murray
SS: Secreted phosphoprotein 24 kD (Spp24) and Spp14 affect TGF-β
induced bone formation differently. PLoS One. 8:e726452013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma AK, Bury MI, Fuller NJ, Rozkiewicz
DI, Hota PV, Kollhoff DM, Webber MJ, Tapaskar N, Meisner JW,
Lariviere PJ, et al: Growth factor release from a chemically
modified elastomeric poly(1,8-octanediol-co-citrate) thin film
promotes angiogenesis in vivo. J Biomed Mater Res A. 100:561–570.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oryan A, Alidadi S, Moshiri A and
Bigham-Sadegh A: Bone morphogenetic proteins: A powerful
osteoinductive compound with non-negligible side effects and
limitations. Biofactors. 40:459–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martínez A, Arana P, Fernández A, Olmo R,
Teijón C and Blanco MD: Synthesis and characterisation of
alginate/chitosan nanoparticles as tamoxifen controlled delivery
systems. J Microencapsul. 30:398–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cicciù M, Herford AS, Cicciù D, Tandon R
and Maiorana C: Recombinant human bone morphogenetic protein-2
promote and stabilize hard and soft tissue healing for large
mandibular new bone reconstruction defects. J Craniofac Surg.
25:860–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Retraction note: Drying of a plasmid
containing formulation: Chitosan as a protecting agent. Daru.
24:112016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jun SH, Lee EJ, Jang TS, Kim HE, Jang JH
and Koh YH: Bone morphogenic protein-2 (BMP-2) loaded hybrid
coating on porous hydroxyapatite scaffolds for bone tissue
engineering. J Mater Sci Mater Med. 24:773–782. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nitta SK and Numata K: Biopolymer-based
nanoparticles for drug/gene delivery and tissue engineering. Int J
Mol Sci. 14:1629–1654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheburu CN, Stoica B, Neamţu A and Vasile
C: Biocompatibility testing of chitosan hydrogels. Rev Med Chir Soc
Med Nat Iasi. 115:864–870. 2011.PubMed/NCBI
|
|
24
|
Honarkar H and Barikani M: Applications of
biopolymers I: Chitosan. Monatshefte für Chemie-Chem Month.
140:14032009. View Article : Google Scholar
|
|
25
|
Budiraharjo R, Neoh KG and Kang ET:
Enhancing bioactivity of chitosan film for osteogenesis and wound
healing by covalent immobilization of BMP-2 or FGF-2. J Biomater
Sci Polym Ed. 24:645–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Islam MA, Firdous J, Choi YJ, Yun CH and
Cho CS: Design and application of chitosan microspheres as oral and
nasal vaccine carriers: An updated review. Int J Nanomedicine.
7:6077–6093. 2012.PubMed/NCBI
|
|
27
|
Fan H, Li H, Zhang M and Middaugh CR:
Effects of solutes on empirical phase diagrams of human fibroblast
growth factor 1. J Pharm Sci. 96:1490–1503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
des Rieux A, Ucakar B, Mupendwa BP, Colau
D, Feron O, Carmeliet P and Préat V: 3D systems delivering VEGF to
promote angiogenesis for tissue engineering. J Control Release.
150:272–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sullad AG, Manjeshwer LS and Aminabhavi
TM: Novel semi-interpenetrating microspheres of
dextran-grafted-acrylamide and Poly(Vinyl Alcohol) for controlled
release of abacavir sulfate. I & Eng Chem Res.
50:pp11778–11784. 2011. View Article : Google Scholar
|
|
30
|
Gribova V, Crouzier T and Picart C: A
material's point of view on recent developments of polymeric
biomaterials: Control of mechanical and biochemical properties. J
Mater Chem. 21:14354–14366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hudalla GA and Murphy WL: Biomaterials
that regulate growth factor activity via bioinspired interactions.
Adv Funct Mater. 21:1754–1768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lewis RA: Hawley's Condensed Chemical
Dictionary. 16th. John Wiley & Sons; Hoboken, NJ: 2016
|
|
33
|
Palazzo B, Gallo A, Casillo A, Nitti P,
Ambrosio L and Piconi C: Fabrication, characterization and cell
cultures on a novel composite chitosan-nano-hydroxyapatite
scaffold. Int J Immunopathol Pharmacol. 24 1 Suppl 2:S73–S78. 2011.
View Article : Google Scholar
|
|
34
|
Raghunath J, Salacinski HJ, Sales KM,
Butler PE and Seifalian AM: Advancing cartilage tissue engineering:
The application of stem cell technology. Curr Opin Biotechnol.
16:503–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma HL, Chen TH, Low-Tone Ho L and Hung SC:
Neocartilage from human mesenchymal stem cells in alginate: Implied
timing of transplantation. J Biomed Mater Res A. 74:439–446. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Majumdar MK, Wang E and Morris EA: BMP-2
and BMP-9 promotes chondrogenic differentiation of human
multipotential mesenchymal cells and overcomes the inhibitory
effect of IL-1. J Cell Physiol. 189:275–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsiridis E, Upadhyay N and Giannoudis P:
Molecular aspects of fracture healing: Which are the important
molecules? Injury. 38 Suppl 1:S11–S25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Herford AS, Cicciù M, Eftimie LF, Miller
M, Signorino F, Famà F, Cervino G, Giudice GL, Bramanti E,
Lauritano F, et al: rhBMP-2 applied as support of distraction
osteogenesis: A split-mouth histological study over nonhuman
primates mandibles. Int J Clin Exp Med. 9:17187–17194. 2016.
|