Introduction
Activity-based rehabilitation is a promising
strategy for improving functional recovery after spinal cord injury
(SCI) by enhancing the growth of descending and ascending fiber
tracts around or through the lesion, restoring the levels of
neurotrophins (1,2), and preventing secondary damage
following spinal cord injury (3).
For decades, activity-based rehabilitation strategies aimed at
improving locomotor functions have been extensively investigated in
humans and in animal models with partial or complete SCI.
Parameters, such as type of training (4–6), amount
of training (7) and speed (8), training program duration (9,10), and
combinations of rehabilitation methods (11–13) have
been assessed to evaluate the outcome of different activity-based
rehabilitation strategies, which have identified the potential of
specific training paradigms to enhance locomotor function after
SCI.
Walking recovery is the goal of patients who
experience SCI (14). Marked
restoration of stepping in the paralyzed hindlimbs in spinalized
animals has been reported following treadmill training (15–17).
Therefore, retraining for walking after SCI has largely focused on
mass practice with repetitive stepping on a treadmill or over
ground (18). Body-weight-supported
treadmill training (BWSTT) is a type of step training therapy that
incorporates treadmill training with body weight support. BWSTT has
demonstrated improvements in walking in animal models with SCI
(19), particularly in rats. Due to
the positive outcomes (100% in rats and cats for treadmill training
and 75% for BWSTT in rats and 100% in cats) (19), especially restoration of walking,
treadmill training, and BWSTT have been frequently applied in
animal models with SCI.
Treadmill training and BWSTT are generally
introduced in the early phase after SCI, which suggests that the
timing of training may be important, since most neurological
recovery occurs within the first month after injury. A long delay
(20) between injury and the
beginning of training appears to reduce the beneficial effects of
training regimens on the regrowth of nerve fibers and the
plasticity of the spinal cord (19,20).
Furthermore, an early initiation of the exercise plan can attenuate
muscle atrophy and bone loss (21).
However, the primary limitation of step treadmill training is that
it requires a certain residual function, which is typically absent
during the early phase of SCI. Therefore, assistance, either by a
robotic device or manually, is initially required. Studies have
shown that robotic devices can facilitate training by providing
precise control over limb weight bearing and improve locomotor
functions (22) and cardiovascular
fitness during exercises (23).
However, to date, few animal or human studies have investigated the
effects of assistive training stepping patterns. Previous studies
using robotic arms as assistive tools did not report the details of
the assistive training patterns or methods (17). Several studies used manually recorded
assistive training patterns (16,24). Lee
et al first reported the use of manual assistance (MA) for
training patterns by electric robotic arms, to provide assistance
to the hindlimbs of spinally contused rats during BWSTT, and
demonstrated that assist-as-need (AAN) step training resulted in
better hindlimbs stepping performance than full assist (FA) step
training (24). However, the primary
deficit of MA is that it is considerably different from the normal
gait pattern of the animals and these differences may limit the
generation of independent stepping or coordination of the limbs. In
theory, the aim of gait training is to assist patients to recover
normal step function; therefore, it should typically mimic the
normal step pattern. To overcome this limitation, a novel algorithm
has been implemented, which can synchronize the robotic device with
the actual motion of the individual in real-time (25) or trajectory tracking (26), establishing subject-specific
trajectories (26). Recently, a new
training pattern has been developed for hemiparetic patients that
records the movement of the unimpaired contralateral limb to train
the impaired limb (27). However,
the effectiveness of robotic step training relative to manually
assisted step training is unclear (28). It is important to compare these two
assistance training patterns in animal models as the first step to
identify the best training pattern to enhance the rehabilitation
effect of BWSTT following SCI.
Early initiation of step training and effective
assistance are critical factors that influence the outcomes of
BWSTT after SCI. Robotic devices have the advantage of providing
repetitive, systematic, prolonged gait training sessions (29) and reducing the labor of therapists.
Therefore, the establishment of an effective robotic assistance
pattern of treadmill training is vital for early initiation of
training. The purpose of this study was to examine and identify the
effectiveness of different assistance patterns for spinal cord
injury in a rat model.
Materials and methods
Animals
Procedures involving animals and their care were
conducted in accordance with the institutional guidelines of the
local Ethics Committee for Animal Research at Dalian University,
and complied with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals. Forty adult male
Sprague-Dawley rats weighing 280±20 g were obtained from the
Experimental Animal Center of Dalian Medical University (Dalian,
China). The rats were raised in separated cages under controlled
temperature (22–24°C) and humidity (40–50%) with a standardized
12-h light-and-dark cycle and free access to food and tap
water.
Eight of the 40 rats were used for normal step
pattern recording and electrophysiological baseline testing. The
remaining 32 rats were randomly assigned to the following 4 groups
(8 rats/group): The sham group (including rats who underwent
laminectomy with no injury to the spinal cord and without BWSTT),
the sedentary group (SCI but without treadmill training), the
normal rat stepping pattern assistance (NRSPA) group (BWSTT for 3
weeks beginning at 3 days after SCI with NRSPA), and the MA group
(BWSTT for 3 weeks beginning 3 days after SCI with MA).
The study has been approved by the Ethics Committee
of Affiliated Zhongshan Hospital of Dalian University
(2017082).
Surgery and spinal cord contusion
injury
Rats were anesthetized with 7% chloral hydrate (350
mg/kg) intraperitoneally. The rats were placed in the supine
position on a surgical table, and the spinous process of the T10
thoracic vertebra was located by palpating the ribs. The dorsal
skin was shaved and disinfected with iodine at the incision site. A
20-mm midline incision was made in middle of the thoracic region of
the dorsum with a fine scalpel and the overlying fascia and muscles
were retracted to expose the dorsal surface of the T10 vertebrae.
The spinous process and the dorsal parts of vertebral plate of T10
were resected with bone rongeurs until the dorsal epidural surface
of the spinal cord was exposed. Twenty-four of the 32 rats received
a severe mid-thoracic contusion of the spinal cord, while in the
remaining 8 rats, the T10 lamina was opened, exposing the spinal
cord, without causing spinal cord contusion (the sham group).
Spinal cord contusion injury was made by dropping a 10-g rod from a
distance of 25 mm to directly impact the dorsal surface of the
exposed spinal cord. The rod was removed immediately following the
injury. Indicators of successful contusion include the red and
swollen appearance of the local spinal cord, fluttering of both
hindlimbs immediately after the impact, and bilateral hindlimbs
paralysis when the rats regained consciousness. The surrounding
tissues were then closed layer by layer with surgical sutures.
After the surgery, the rats were immediately placed on warm water
pads until they regained consciousness. Penicillin (160 mg/kg) was
administered intraperitoneally at the end of the surgery for 3
consecutive days to prevent infection. Manual bladder expression
was performed twice daily until the rats recovered spontaneous
micturition.
Training procedures after SCI
Establishment of stepping play-back pattern for
assistance during training. The Rodent Robotic Motor Performance
System (RRMPS) (Robomedica, Inc., Mission Viejo, CA, USA) was used
to train and test treadmill stepping in the rats. RRMPS is the
updated version of Rodent Robot 3000 (Robomedica, Inc.) (24). RRMPS has a motorized-variable-speed
treadmill, two robotic arms, and a body-weight-support arm. The
rats are secured to the weight-supporting arm of the robotic device
with a vest. The ankles of the rats were attached to the distal
ends of the robotic arms with rubber loops that wrapped around the
ankle. RRMPS can record stepping patterns and playback the
recording stepping patterns.
First, the stepping patterns of the intact rats were
recorded. Eight rats [Basso, Beattie, and Bresnahan (BBB)
score=21]were trained to adapt to RRMPS for 4 walking sessions per
day, lasting approximately 15 min/session, separated by a 15-min
resting period in the, with food rewards after each training
session. After 1 month of training, the rats could successfully
step on the treadmill. Repetitive stepping recordings were
performed using RRMPS at a treadmill speed of 7 cm·sec−1
and 80% weight support with both forelimbs and hindlimbs touching
the surface of the treadmill at the same time to maintain the
normal quadrupedal locomotion pattern of the rats. The rats were
observed and recorded until the most stable stepping pattern was
visualized on the computer. The recorded stepping patterns were
analyzed and edited using the analysis software provided by RRMPS.
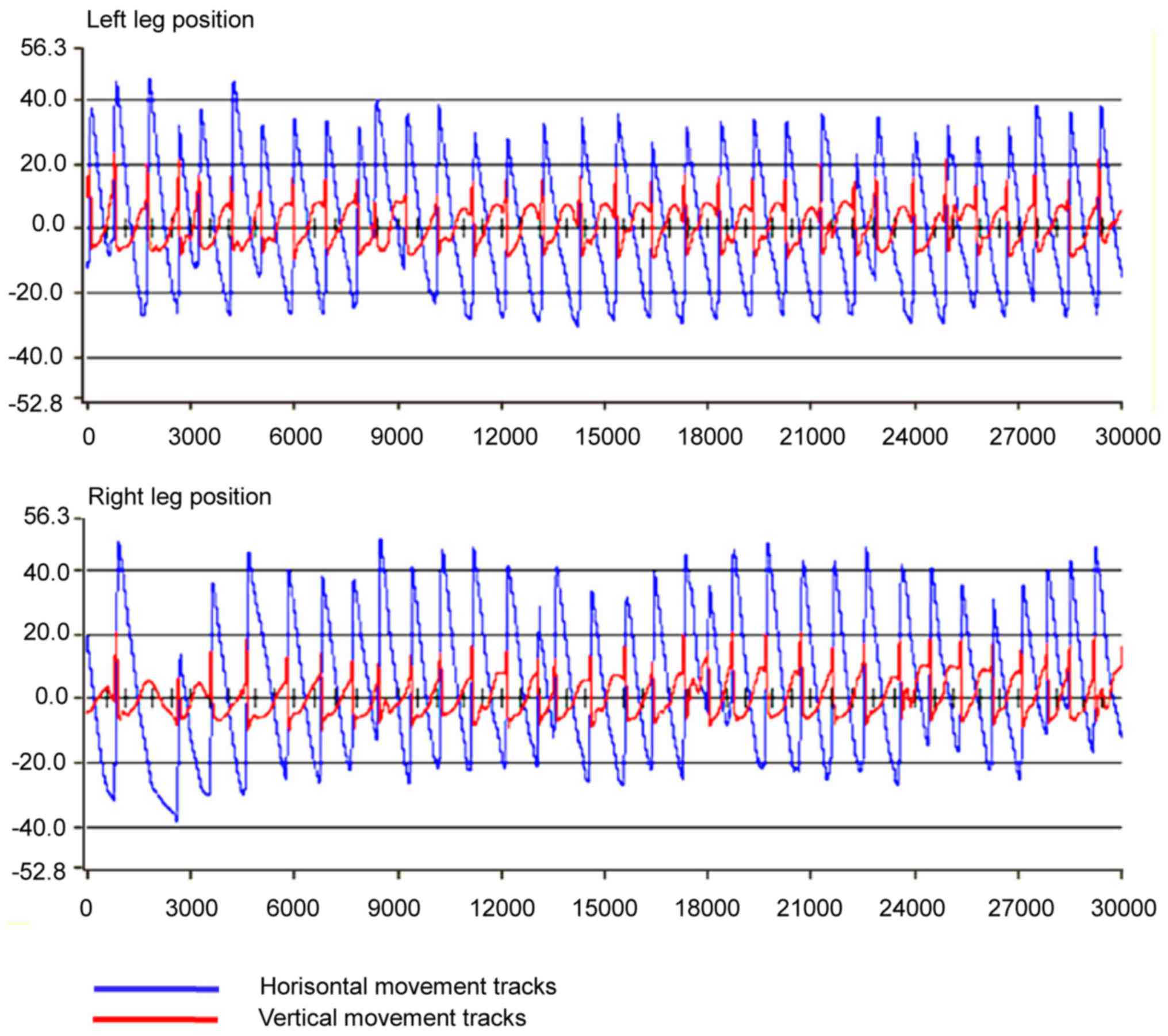
A 30-sec consecutive hindlimbs step playback-pattern of a normal
rat was determined (Fig. 1).
To test the reliability and constancy of the
recorded step pattern, 6 primary parameters related to step
characters were analyzed: Step length (horizontal movements of the
toe between two successive paw contacts of the same limb); step
height (vertical movements of the toe between two successive paw
contacts of the same limb); step cycle duration (the time between
two successive contact points of the same paw on the treadmill);
stance duration (the time when the foot is on the treadmill during
1 step, i.e., from the end of 1 step to the beginning of the next
step of the same foot); swing duration (the time during which the
foot is lifted during 1 step); and stance duration/swing duration
(the ratio of stance time and swing time in 1 step). The t-test was
used to determine significant differences between right and left
limbs in this step pattern based on the six parameters, and no
significant differences (P>0.05) were found. The coefficients of
variance (CV) of the six parameters of the same limb were all under
15%, indicating the consistency and stability of the step pattern
(Table I).
 | Table I.Descriptive statistical data of
30-sec normal rat step mode recorded by the rodent robotic motor
performance system (mean ± SD). |
Table I.
Descriptive statistical data of
30-sec normal rat step mode recorded by the rodent robotic motor
performance system (mean ± SD).
| Data
classification |
| Left hindlimb | Right hindlimb |
|---|
| Step length
(mm) | mean ± SD | 63.51±4.79 | 63.80±7.13 |
|
| CV% | 7.54 | 11.26 |
| Step height
(mm) | mean ± SD | 23.36±3.17 | 23.17±3.03 |
|
| CV% | 13.58 | 13.09 |
| One step cycle
(sec) | mean ± SD | 1.45±0.23 | 1.46±0.22 |
|
| CV% | 12.33 | 12.89 |
| Stance duration of
one step (sec) | mean ± SD | 1.02±0.21 | 1.01±0.19 |
|
| CV% | 12.89 | 12.20 |
| Swing duration of
one step (sec) | mean ± SD | 0.43±0.03 | 0.44±0.02 |
|
| CV% | 14.30 | 13.64 |
| Stance/Swing in one
step cycle | mean ± SD | 2.37±1.04 | 2.23±1.01 |
|
| CV% | 14.10 | 14.54 |
Training protocol
Because the injury did not affect the forelimbs of
the SCI rats, forelimb stepping following the speed of the
treadmill can contribute to body support and interappendicular
coordination. Quadrupedal stepping is the normal stepping pattern
rather than bipedal stepping (30).
Hence the quadrupedal stepping protocol was adopted in this
study.
One week prior to surgery, all the rats were
familiarized with RRMPS (including the vest, the robotic arms, the
body support system and the speed of the treadmill). The rats in
the NRSPA and MA groups started BWSTT 3 days after the surgery.
BWSTT of the SCI rats was performed for 15 min twice per day with a
15-min break between sessions. The total duration of training was 3
weeks with 5 days of training per week. The speed of the treadmill
was maintained constant (7 cm·sec−1) and 80% of the body
weight was supported. The recorded normal rat stepping pattern
(NRSP) performed in a repetitive loop was implemented on the
hindlimbs of the rats from the NRSPA group by the robotic arms
controlled by the RRMPS software (version 1.0; Robomedica Inc.). MA
by an experienced trainer was implemented as required to the
hindlimbs of the rats from MA group.
Locomotor function assessment and
analysis step detection
Locomotor function assessment and analysis step
detection was performed for all the groups before BWSTT (3 days
post-surgery) and at the termination of BWSTT. BBB scores were
assessed 4 times for all groups: 3 days post-surgery and at the end
of every week BWSTT.
Step detection and parameters analysis
by RRMPS
No robotic assistance was provided to the ankles
during the tests. The stepping patterns of the rats were
consecutively recorded for 30 sec with a treadmill speed of 7
cm·sec−1 and 80% weight support. The robotic arms of
RRMPS recorded the kinematic features of stepping, including step
length and height, swing duration, and successful step numbers,
which can quantitatively reflect the subtle locomotor function
recovery of the SCI rats.
A successful step cycle was identified by RRMPS
analysis software (Robomedica, Inc.), which was defined as a
minimum 10 mm in step length and 3 mm in step height (24). The data for each stepping kinematic
feature of the rats were averaged and stored on a computer for
further analysis.
BBB score
The BBB score consists of an ordinal scale from 0
(flaccid paralysis) to 21 points (normal gait) (31). Higher scores are associated with
better locomotor function. The BBB score was evaluated by two
experienced investigators who were double blinded. During the
evaluation, the rats were allowed to move voluntarily in a square
plastic box (100×100×4 cm) without cover for 4 min, and the
movements of hindlimb were observed; BBB scores were averaged for
each group.
Neurophysiological assessment
Transcranial electrical motor-evoked potentials
(tceMEPs) were obtained using the EMG/EP System NDI-094 (Haishen
Medical Electronic Instrument Co., Ltd., Shanghai, China). Typical
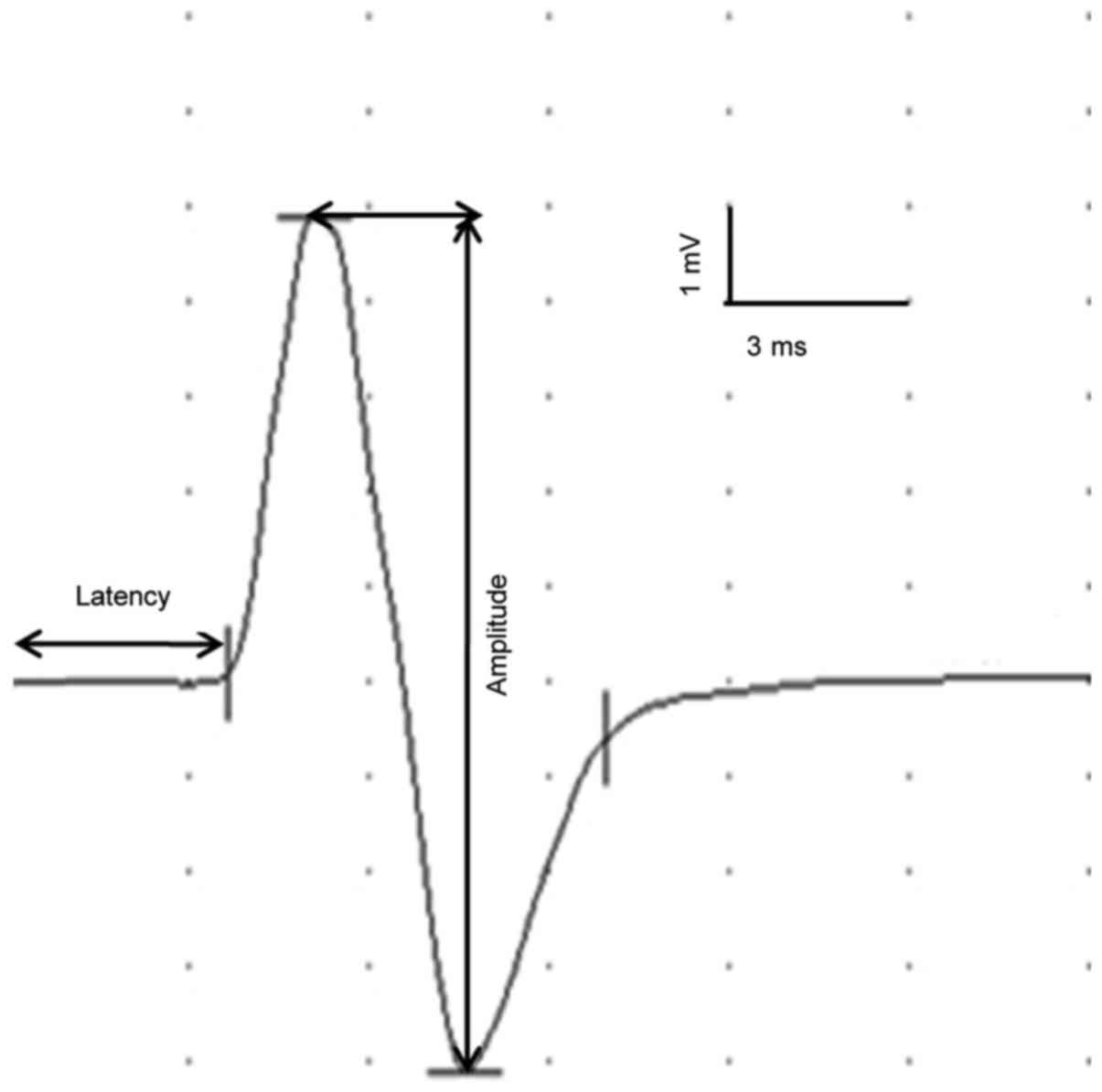
waveforms (Fig. 2) and parameters of
tceMEPs were recorded in intact rats as the baseline. The tceMEPs
for all experimental groups were recorded twice when the BWSTT
training was initiated (3 days post-surgery) and terminated (3
weeks after the initiation of BWSTT).
For tceMEPs, a dosage of 7% chloral hydrate (350
mg/kg) was administered to the rats intraperitoneally to induce
anesthesia. The head region of the rats was shaved and disinfected
with 75% ethyl alcohol. The stimulating needle was positioned
beneath the scalp, 2 mm anterior to the coronal suture and 2 mm
lateral to the sagittal suture. The reference electrode was placed
0.5 cm posterior to the recording electrode. The ground electrode
slice was positioned on the skin of left forelimbs. An
intramuscular mono-polar recording needle was inserted into the
belly of the gastrocnemius muscle in the right hindlimb. The
reference electrode was inserted into the distal tendon (32).
Monopolar electrical stimulation was used to induce
transcranial electrical stimulation, with a current intensity of
15–20 mA, a pulse width of 0.2 msec, frequency of 2 Hz. A total of
50 superimposed traces were recorded, with a scanning speed of 3
msec/D and sensitivity of 1 mV/D. Stimulation intensity was
increased gradually, until the movement of both forelimbs developed
and a clear MEPs pattern was detected. The latency and amplitude of
tceMEPs and the tceMEPs waveforms were recorded and stored on the
computer for analysis.
Tissue preparation and
immunohistochemistry
Immediately following the last locomotor function
and electrophysiological assessments, the rats were perfused
transcardially with 4% paraformaldehyde in 0.1 mol/l phosphate
buffer saline (PBS, pH 7.4) at 4°C for 20–30 min after deep
anesthesia with 7% chloral hydrate (350 mg/kg). The spinal cords
were harvested and post-fixed in the same fixation fluid for 48 h
(33). The spinal cords were cut
into 1.0-cm long pieces along the rostrocaudal axis with the lesion
area at the center. Paraffin specimens were prepared by a paraffin
embedder (EG1150H) after dehydration using semi-enclosed bench top
tissue processor (TP1020) and cooled on the cold plate (EG1150C;
all from Leica Microsystems GmbH, Wetzlar, Germany) at a constant
temperature of 5°C for 30 min. Twenty-five adjacent serial 6-µm
thick cross-sectional paraffin sections were obtained at the
epicenter of the lesion area for each spinal cord sample using
manual rotary microtome (RM2235; Leica), and every 5th section was
selected for immunohistochemistry. The procedure for
immunocytochemistry has been described previously (34) and in this study, we chose fluorescent
staining instead of DAB. The primary antibody was neurofilament 200
(NF200) antibody (BM0100) diluted 1:200 and the secondary antibody
was anti-GAPDH rabbit monoclonal antibody (M00227) (both from
BosterBio, Pleasanton, CA, USA) diluted 1:1,000. Digital
photomicrographs of 5 visual areas in the dorsal horn in a section
were taken using a fluorescent microscope (Ci-1; Nikon, Tokyo,
Japan) with a 20X objective lens under a constant exposure
condition for all the sections. Image-Pro Plus 6.0 software (Media
Cybernetics, Silver Springs, MD, USA) was used to analyze the area,
integrated density (ID), and area fraction (AF) of
NF200+ expression of each image after normalization of
the background intensity. The mean density (MD) of NF200-labeled
axons for each sample was calculated for further statistical
analysis.
Statistical analysis
All data were analyzed using SPSS 19.0 (SPSS, Inc.,
Chicago, IL, USA). Mean and standard deviation (SD) were used to
describe the sample parameters. Factorial design ANOVA was used to
determine the source of variation in step analysis. Multigroup
comparisons of the means were carried out by one-way analysis of
variance (ANOVA) with post hoc contrasts by least significant
difference (LSD). P<0.05 was considered to indicate a
statistically significant difference.
Results
Rats in the NRSPA group achieved
better stepping quality than those in the MA group
Before the initiation of BWSTT, the hindlimbs of the
sham rats could step, although the number of steps was less than
that of the intact rats, due to pain and weakness after
laminectomy. The step cycle of the sham rats was consistent with
that of the intact rats. The hindlimbs of all SCI rats could not
perform stepping.
After 3 weeks of BWSTT, the rats in the NRSPA and MA
groups gained greater locomotor recovery than the sedentary group
in terms of longer horizontal movement (step length), higher
vertical movement (step height), longer swing duration, and greater
number of steps in the 30-sec testing, although these results were
significantly poorer than those recorded for the rats in the sham
group. Compared to the MA group, the hindlimbs of the rats in the
NRSPA group performed better stepping (Fig. 3). Factorial design ANOVA for stepping
analysis showed that the source of the variation was different
according to the different groups (P<0.001) but not according to
the different hindlimbs (P>0.05). Therefore, in all rats, the
right and left hindlimbs could perform consistently, and the
differences in stepping quality improvement were due to the
differences between the groups in step training schemes and
assistive modes.
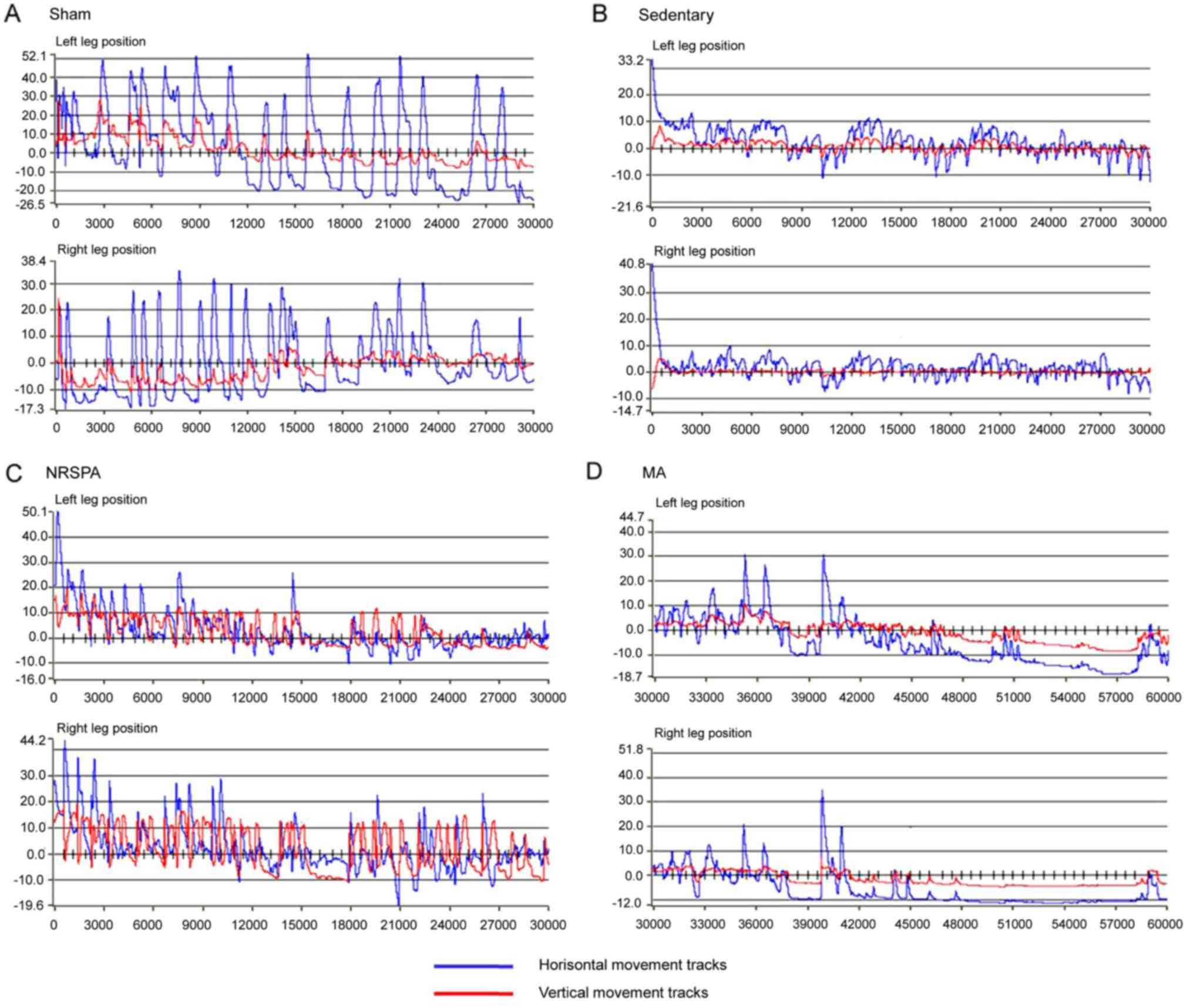 | Figure 3.Representative images of paw
positions of the right and left limb during 30-sec testing for each
experimental group after 3 weeks of BWSTT. (A-D) represent the
trajectories of the right and left paws' movements for sham,
sedentary, NRSPA and MA groups, respectively. For detailed
explanations see Fig. 1. According
to the amplitudes of the blue lines (horizontal movements) and red
lines (vertical movements), the trajectories of the two BWSTT
groups (NRSPA and MA) are obviously lower than that of the sham
group and greater than that of the sedentary group. Furthermore, by
comparison of C and D, the movements were greater in the NRSPA
group than in the MA group, particularly in relation to the red
lines (vertical movements). BWSTT, body-weight-supported treadmill
training; NRSPA, normal rat stepping pattern assistance; MA, manual
assistance. |
Step length (horizontal movement)
Intact horizontal movements (left, 50.33±6.59 mm;
right, 51.3±5.37 mm) were generated by the hindlimbs of the sham
rats (Figs. 3A and 4A), which are consistent with those of the
intact rats. The hindlimbs of sedentary rats performed occasional
step-like movements (left, 5.87±2.04 mm; right, 7.25±2.98 mm) that
could not be recognized as real stepping by RRMPS (Figs. 3B and 4A). The stepping patterns in the NRSPA and
MA group were similar to those of the sham rats, although the
amplitudes were smaller. This finding indicated that the rats
benefited from BWSTT for improvement of the horizontal movement of
the hindlimbs. The rats in the NRSPA group achieved a significantly
longer step length (left, 25.08±3.06 mm; right, 25.23±3.91 mm;
P<0.05) (Figs. 3C and 4A) than those in the MA group (left,
18.54±2.84 mm; right, 17.86±4.03 mm) (Figs. 3D and 4A).
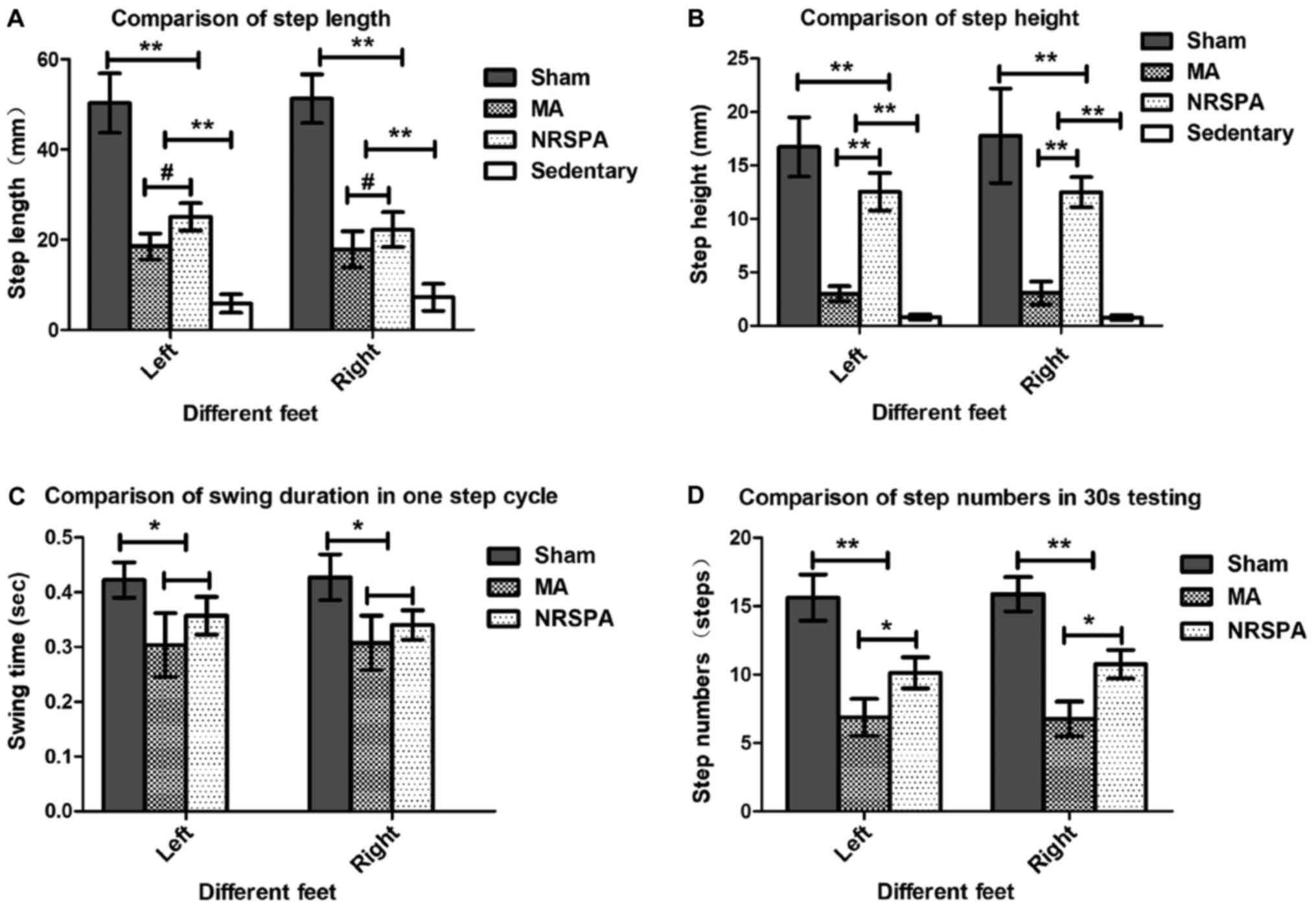 | Figure 4.Comparisons of the step parameters
analysis between different groups during 30-sec testing after 3
weeks of BWSTT. The charts represent the outcomes of step
parameters statistical analysis by comparison between all the
experimental groups for step length (A), step height (B), swing
duration in 1 step cycle (C), and step numbers (D) in the 30-sec
testing. The sedentary group was not included in the analysis of
swing duration and step number as they did not meet the criterion
for a step. One-way ANOVA and LSD were used for statistical
analysis and P<0.05 was statistically significant.
#P<0.05, *P<0.01, **P<0.001. From the above
parts, the sham group had the best outcomes for all the parameters
of step analysis compared to the NRSPA, MA and sedentary groups
(P<0.001). On comparison between the NRSPA and MA group, (A)
shows significant differences in step length (P<0.05); (B) shows
significant differences in step height (P<0.001); (C) shows no
significant difference in swing duration (P>0.05); and (D) shows
significant difference in step number (P<0.01). BWSTT,
body-weight-supported treadmill training; NRSPA, normal rat
stepping pattern assistance. |
Step height (vertical movement)
The hindlimbs of the sham rats performed normal
vertical movement (left, 16.75±2.77 mm; right, 17.78±2.42 mm)
(Figs. 3A and 4B) and the stepping was coordinated as the
step height correlated with the step length. The sedentary rats
performed minimal vertical movements (left, 0.81±0.23 mm; right,
0.77±0.23 mm), which were not recognized as step movements by the
analysis software. The improvement in vertical movements among the
NRSPA rats (left, 12.55±1.75 mm; right, 12.52±1.41 mm) (Fig. 3C) was significantly different
(P<0.001) (Fig. 4B) than that
recorded among the MA rats (left, 3.00±0.69 mm; right, 3.07±1.07
mm) (Figs. 3D and 4B). The vertical movements of the hindlimbs
in the NRSPA group markedly improved and were repeated
consistently, following the rhythm of movements of the forelimbs to
some extent. However, the rats in the MA group did not perform
obvious vertical movements, which were disproportionate to the
amplitude of horizontal movements (Fig.
3D).
Swing duration
Among the sedentary rats, no steps were recognized
by the analysis software; therefore, the comparison of the swing
duration only included the sham group, NRSPA group, and MA group.
After 3 weeks of BWSTT, the increase in the swing duration in the
NRSPA group (left, 0.35±0.04 sec; right, 0.35±0.03 sec) was greater
than that in the MA group (left, 0.30±0.06 sec; right, 0.31±0.05
sec), although the difference was not statistically significant
(P>0.05). In both groups, swing duration was significantly
shorter (P<0.01) than that in the sham rats (left, 0.42±0.03
sec; right, 0.43±0.04 sec) (Fig.
4C).
Step number
The hindlimbs of the sedentary rats generated some
step-like movements but did not meet the criteria for complete
steps. Following 3 weeks of BWSTT, the average number of complete
steps in the 30-sec testing performed by the NRSPA group (left,
10.13±1.13; right, 10.75±1.04) and the MA group (left, 6.88±1.36;
right, 6.75±1.28) was significantly less than that of the sham rats
(left, 15.63±1.69; right, 15.88±1.25) (P<0.001). The number of
complete steps in the 30-second testing performed by the NRSPA
group was significantly greater than that of the MA group
(P<0.01) (Fig. 4D).
Comparison of BBB scores between the
groups provided consistent evidence of step detection by RRMPS
All the rats presented normal locomotion before
surgery. Three days after SCI, all the rats with spinal contusion
had the lowest scores (0) and the rats in the sham group had the
highest scores (20.50±0.76). At the end of first week of training,
the BBB scores increased slightly across all SCI groups, and the
BWSTT groups (NRSPA group and MA group) had higher scores than the
sedentary group (BBB score, 1.5±0.53) (P<0.01). No significant
difference was found between the NRSPA group (BBB score, 3.5±1.07)
and the MA group (BBB score, 3.38±1.19) (P>0.05) (Fig. 5A and B). After 2 weeks of training,
the BBB scores of the NRSPA group (BBB score, 6.35±0.74) increased
significantly compared to the scores recorded during the first
week, although no obvious improvement was observed in the MA group
(BBB score, 4.63±0.52); the difference between the two BWSTT groups
was statistically significant (P<0.001) (Fig. 5A and C). Assessment was repeated at
the end of the third week of BWSTT and the BBB scores of the NRSPA
group, the MA group, and the sedentary group were significantly
lower than those of the sham group which exhibited normal
continuous plantar stepping (P<0.001). The sedentary rats did
not regain further functional activity and their hindlimbs
exhibited minimal joint movement (BBB score, 3.13±0.45). The BBB
scores of the MA group (BBB score, 5.88±0.83) were significantly
lower than those of the NRSPA group (BBB score, 9.13±2.59)
(P<0.001) (Fig. 5A and D). After
3 weeks of training, the rats in the NRSPA group showed occasional
plantar stepping without weight support and coordinated forelimb
gait. The rats in the MA group showed coordinated and extensive
movements of the hip-joint and knee-joint and slight movement of
the ankle-joint of the hindlimb, but no plantar stepping and weight
support.
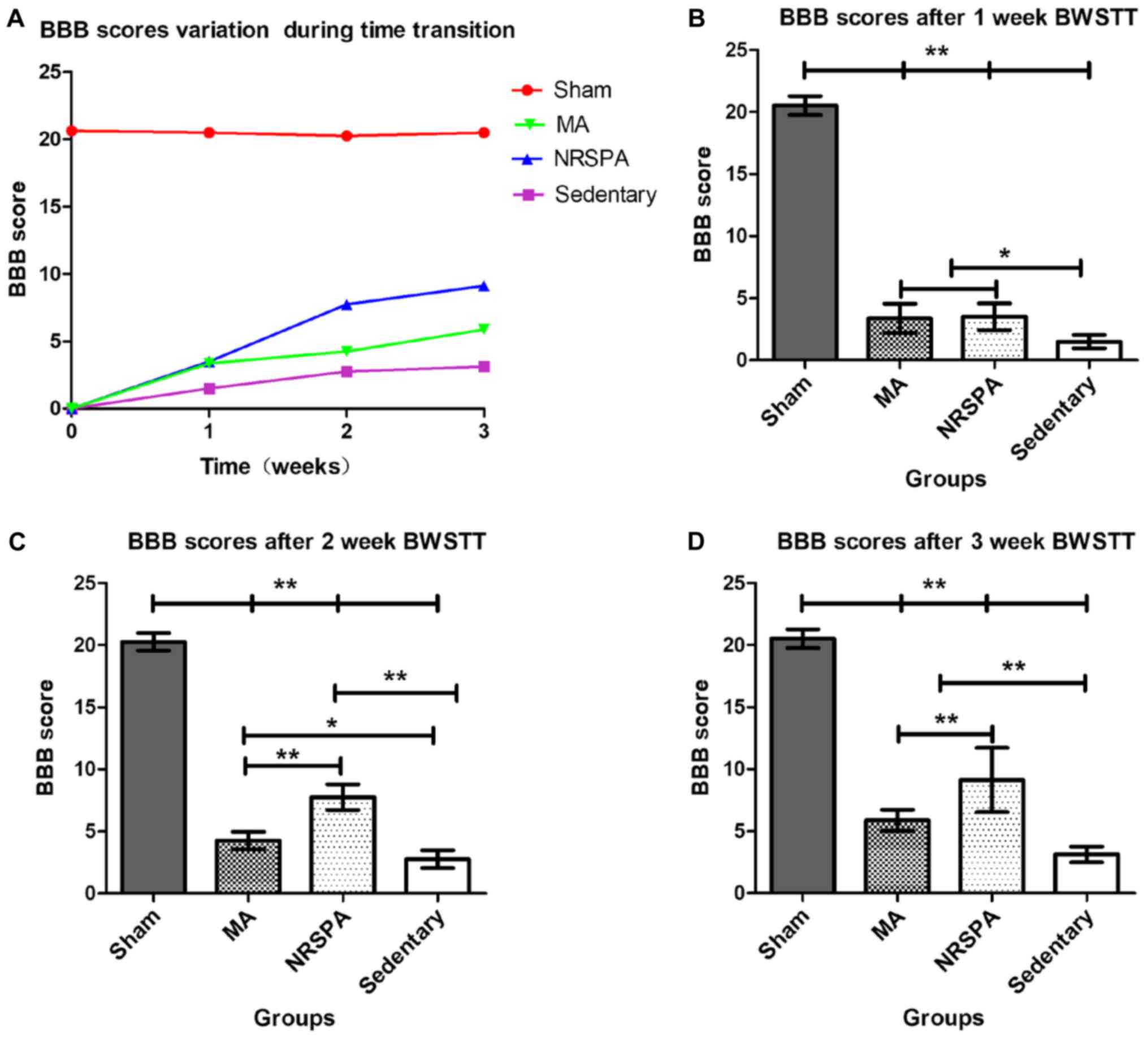 | Figure 5.Comparison of BBB scores for all
experimental groups following time transition. As shown in (A), BBB
scores show a variation trend from the beginning to the end of the
BWSTT. BBB scores of all the SCI groups showed a growth trend over
time and the BBB score of the sham group remained stable. The BBB
score of the NRSPA group increased faster than that of the MA group
from the second week of BWSTT, although the BBB scores of the two
groups were similar at the end of 1-week BWSTT. (B-D) show the
comparison of BBB scores between the different groups at the end of
1, 2, and 3 weeks of BWSTT, respectively. One-way ANOVA and LSD
were used for statistical analysis and P<0.05 was statistically
significant. *P<0.01 and **P<0.001. On comparison between the
NRSPA and MA group, (B) shows no significant difference in BBB
scores after the first week, while (C and D) show significant
differences in BBB scores in the second and third week,
respectively (P<0.001). BBB, Basso, Beattie, and Bresnahan;
BWSTT, body-weight-supported treadmill training; SCI, spinal cord
injury; NRSPA, normal rat stepping pattern assistance. |
Electrophysiological assessments
demonstrated uneven improvement between the groups
The waveforms of tceMEPs were not detectable in all
SCI groups (NRSPA, MA, sedentary) 3 days after surgery (Fig. 6A). No significant changes in latency
and amplitude were found in the sham group compared to the baseline
of intact rats throughout the whole procedure. After 3 weeks of
BWSTT, the waveforms of tceMEPs were measured in the different SCI
groups. The latency and amplitude were restored slightly in the
sedentary rats, which were significantly different to the sham rats
(P<0.001) and the two BWSTT groups (P<0.01) (Fig. 6B and C). The two BWSTT groups with
different assistive modes presented shorter latency and taller
amplitude compared to the sedentary group (Fig. 6A), which indicated that the
electrical signal conduction function of the spinal cord was
improved by BWSTT. Furthermore, the rats in the NRSPA group
exhibited better conduction parameters than the rats in MA group
(P<0.05) (Fig. 6B and C).
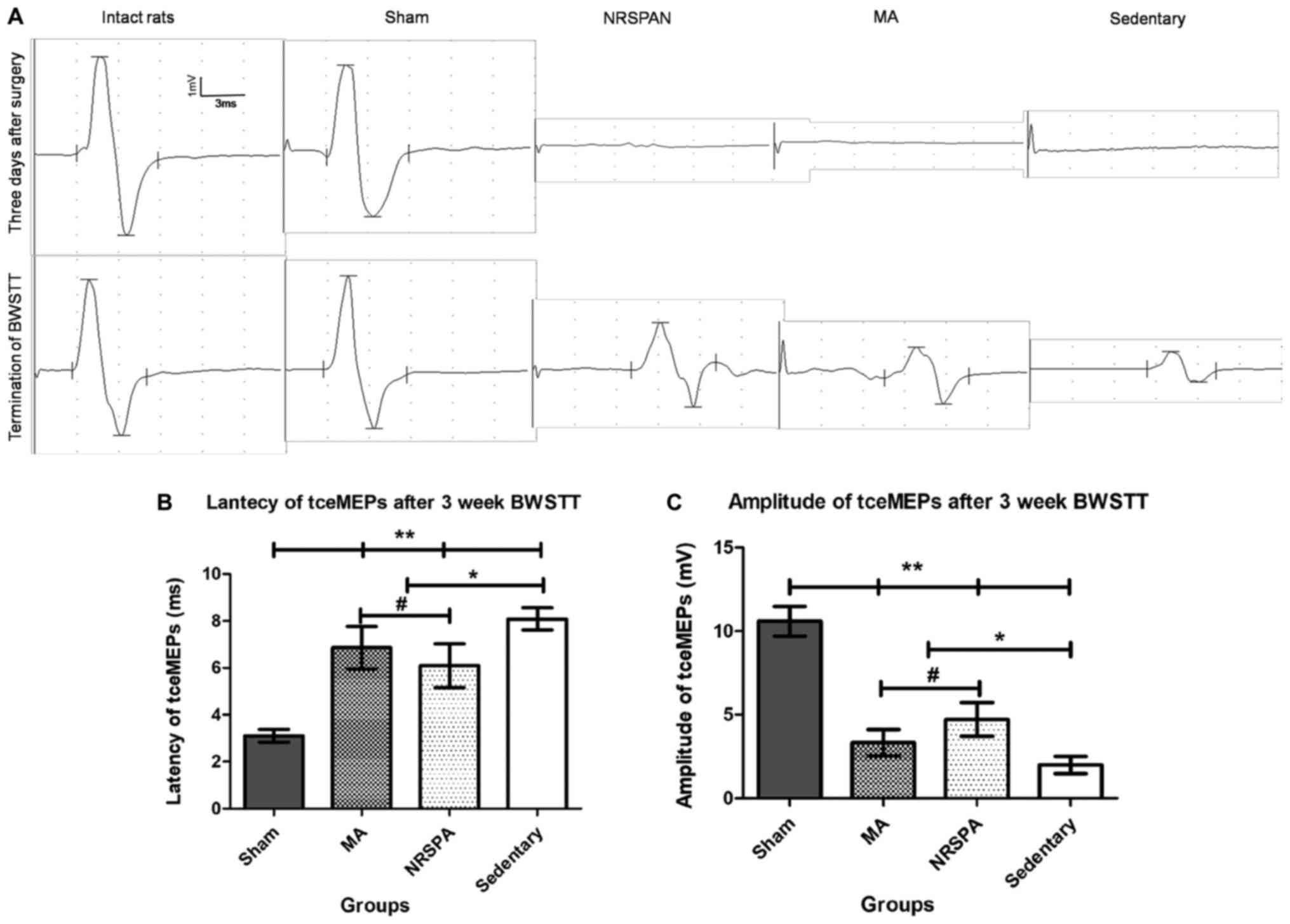 | Figure 6.The representative tceMEPs waveforms
at the beginning and the termination of BWSTT and the tceMEPs
parameters analysis. (A) The variation trends of tceMEPs waveforms
in all experimental groups by comparing the tceMEPs waveforms
obtained at 3 days after surgery and the termination of the 3 weeks
of BWSTT. The sham group had exactly the same waveform as the
intact rats. The tceMEPs waveforms could not be detected in the
three SCI groups (NRSPA, MA and sedentary) 3 days after surgery.
Following a 3-week BWSTT, the latencies and amplitudes of tceMEPs
waveform in NRSPA group gained the greatest increase in contrast to
those of the MA and sedentary groups. (B and C) The results of
statistical analysis for latency and amplitude of tceMEPs,
respectively. One-way ANOVA and LSD were used for statistical
analysis and P<0.05 was statistically significant.
#P<0.05, *P<0.01, **P<0.001. (B) represents
significant differences in the latency of tceMEPs between the NRSPA
group and MA group (P<0.05). (C) The significant differences in
the amplitude of tceMEPs between the two BWSTT groups (NRSPA and MA
group) (P<0.05). tceMEPs, transcranial electrical motor-evoked
potentials; BWSTT, body-weight-supported treadmill training; SCI,
spinal cord injury; NRSPA, normal rat stepping pattern assistance;
MA, manual assistance. |
NF200 protein expression in spinal
cord lesion area
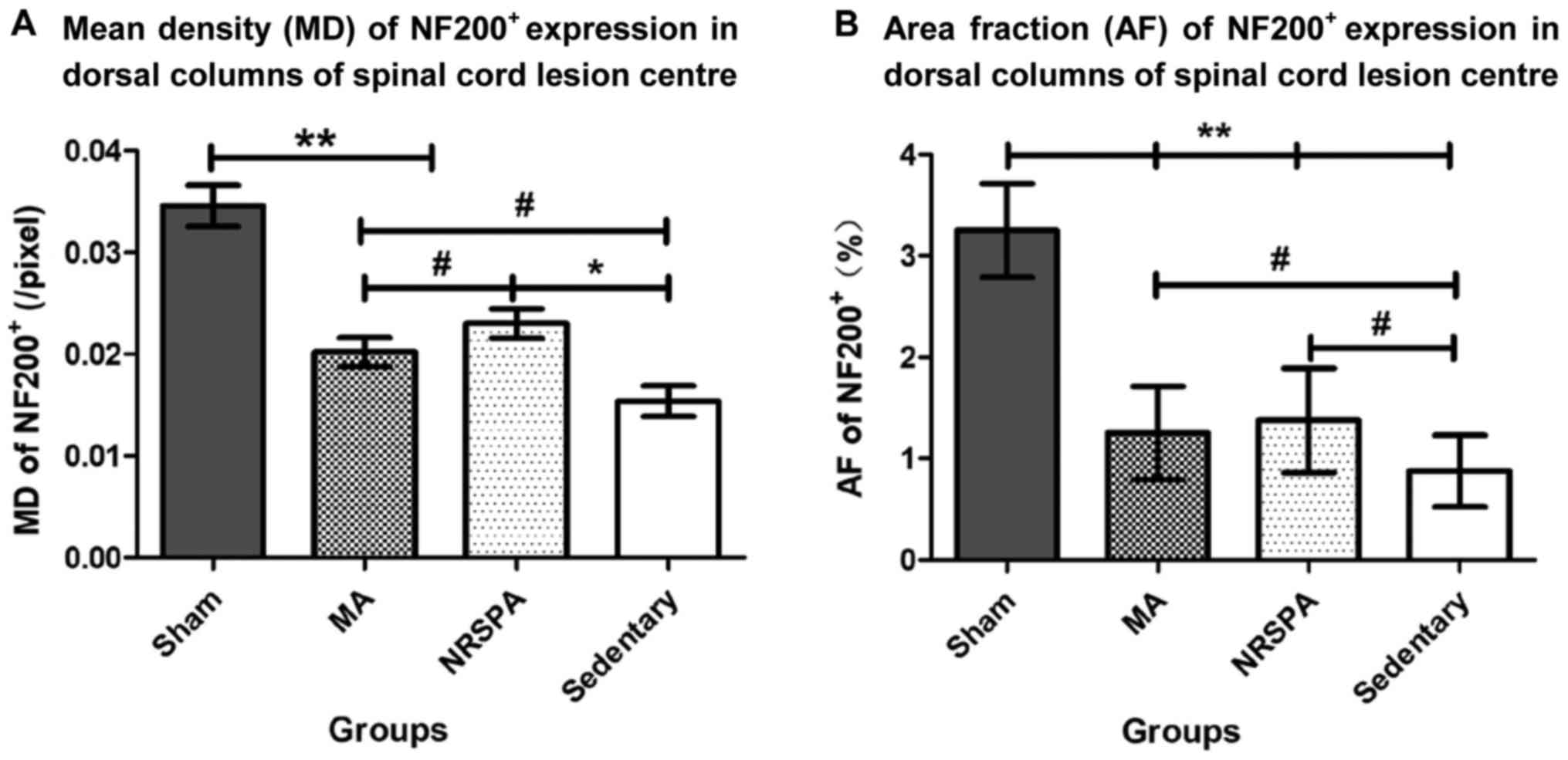
After the 3-week experimental period, the mean
density (MD) and positive area fraction (AF) of NF200+
expression in the dorsal horn of the spinal cord lesion center were
analyzed and compared between groups. The AF and MD values of the
sham group were significantly higher than those in all the other
SCI groups (P<0.001) (Figs. 7 and
8). The AF of NF200+
expression in the two BWSTT groups (NRSPA and MA) increased
significantly compared to the sedentary group (P<0.05), although
expression remained significantly below the normal level
(P<0.001) (Fig. 8B). There was no
significant difference in the AF values between the NRSPA group and
MA group (P>0.05) (Fig. 8B). A
significant difference in MD values was found between the sedentary
group and the two BWSTT groups (MA group, P<0.05; NRSPA group,
P<0.01). The MD values in the MA group were significantly lower
than those in the NRSPA group (P<0.05) (Fig. 8A).
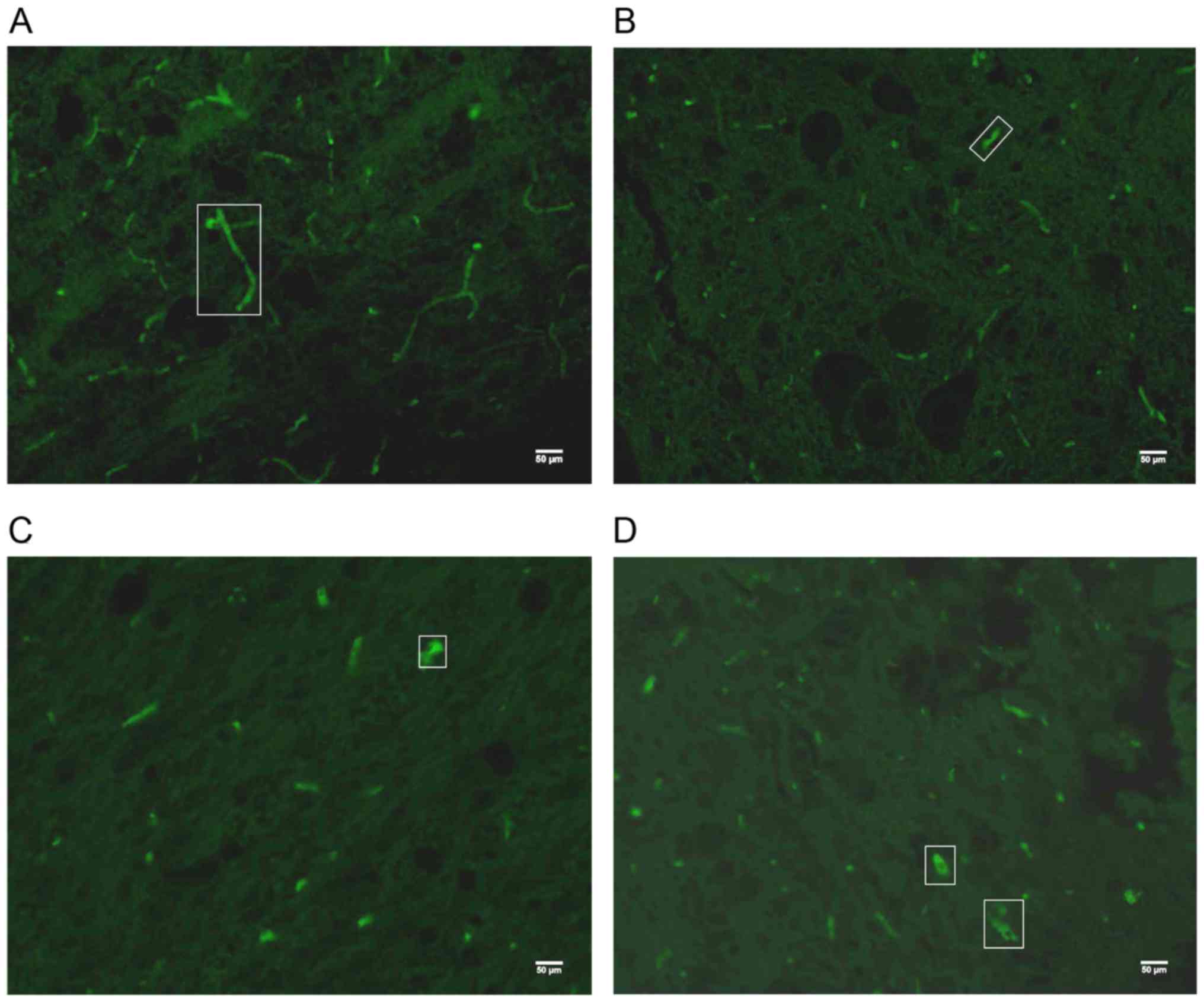 | Figure 7.The representative images of
NF200+ expression under a 20X objective lens in the
dorsal horn of the spinal cord lesion epicenter after 3 weeks of
BWSTT. (A-D) NF200+ expression for the sham, sedentary,
NRSPA, and MA groups, respectively, and the green fluorescence in
the white boxes is typical NF200+ expression. (A) The
extensive axonal connection is visible in the sham group, while in
(B) the NF200+ expression is scattered, short, and thin
in the sedentary group. Scattered, short and dotted
NF200+ expression with limited strong positive
expression can been seen in the NRSPA (C) and MA groups (D); the
differences between the two groups are not obvious, as shown in the
image. NF200, neurofilament 200; BWSTT, body-weight-supported
treadmill training; NRSPA, normal rat stepping pattern assistance;
MA, manual assistance. |
Discussion
In this study, we compared two assistive training
patterns for the hindlimbs during BWSTT to assess the improvement
of the locomotor function, nerve conduction function, and nerve
regeneration in the spinal cord based on a rat model of acute SCI.
We demonstrated that the rats in the NRSPA group achieved better
recovery in terms of stepping function, neural conduction of the
spinal cord, and axon regeneration than the rats in the MA group.
These results showed that NRSPA for the hindlimbs was more
effective than MA during BWSTT for the improvement of axon
regeneration and neural conduction in the early phase of acute
SCI.
It is recognized that some degree of locomotor
function of the hindlimbs can recover spontaneously in rats with
incomplete SCI. This is consistent with the findings of this study
that the BBB scores and kinematic parameters of the hindlimbs in
the sedentary group increased gradually without any exercise
training. However, recovery was limited for the outcomes of step
analysis as the hindlimb movements of sedentary rats did not meet
the stepping criteria for height and length of hindlimb movement.
The hindlimbs of the rats in the two BWSTT groups achieved
considerable improvement in locomotor function after 3 weeks of
step training, indicating that BWSTT with assistance to the
paralyzed limbs is an effective method for SCI rehabilitation in
the early phase of acute SCI.
With advances in technology, rehabilitation
interventions based on the use of robotics have been widely
implemented, demonstrating trends for strong growth over the past
two decades (35). Multiple studies
have proved the effectiveness of robotic assistance to improve
motor function (23,36,37) and
it is widely recognized that robotics are efficient substitutes of
physiotherapists (36). It is not
clear from the available research on humans which are the most
effective (38) although animal
studies are scarce. Theoretically, robotic assistance under
computer control, should be more stable while MA is more variable.
Natural walking is characterized by variability and inconsistency
in step length, step height, step speed, and center of mass dur to
variable environmental features; therefore, the training is varied
and fits the features of natural walking. Thus, it is increasingly
recognized that movement variability is an essential requirement
for skilled, adaptable movements (11,39). It
is increasingly being recognized that the practice occurring to
achieve these skilled movements must allow for variability
(40). Therefore, unlike MA, the
lack of variability of robotic assistance has been a criticism of
robotic assistance. However, from another perspective, the spinal
cord is able to sustain several forms of learning and memory,
including limb-position training and several forms of adaptive
motor plasticity, which can generate profound effects on locomotor
behavior. Therefore, the lack of variability and the precise
repetition of training cycles may be advantages of robotic
assistance because the precise movements with high number of
repetitions are recognized as beneficial to learning (35). In this study, we used the BWSTT
platform to compare the effects of different assistance training
patterns for BWSTT. One of these is assistance by robotic arms
following a trajectory of normal rat hindlimb stepping (NRSPA)
which has been shown to be consistent (Table I and Fig.
1). The other is assistance by the hands of an experienced
trainer (MA) who can assist the hindlimbs of the SCI rats as evenly
as possible. From the outcomes of the BBB score over 3 weeks of
training, the rats in the NRSPA group achieved greater improvements
than those in the MA group at the end of the second and third week
of training. Although no differences were found between the BBB
scores of the NRSPA group and those of the MA group after the first
week of training, the average BBB score in the NRSPA group was
higher than that of in the MA group. The results of step detection
and analysis also supported the above outcomes, the hindlimbs of
rats in the NRSPA group gained longer horizontal movement (step
length), higher vertical movement (step height), longer swing
duration and more step numbers (Fig.
4). Based on the above locomotor function evaluation results,
the NRSPA achieved consistent and stable characteristics more
effectively than the MA. However, locomotor function recovery in
the early stages of SCI is the most effective time for step
learning rather than accommodating skilled movements.
Generally, locomotion in mammals, including humans,
is based on the activity of neuronal circuits within the spinal
cord. It has been proved that incomplete lesions to the spinal cord
are accompanied by axonal sprouting in the vicinity of the lesion
within weeks of SCI (41). Post-SCI
axonal sprouting can form new intraspinal neuronal circuits that
allow descending pathways to bypass the site of the lesion. Recent
studies have shown that exercise training can enhance axon
sprouting following SCI (42) and
axon regrowth remains the major prerequisite for plasticity,
regeneration, circuit formation, and eventually functional recovery
(43). Recent studies have suggested
a plastic behavior of the spinal neuronal circuits (44–46) and
alteration of the step characteristics (47) were observed following SCI after
locomotor training, that is, the relearning of the spinal cord.
However, this relearning process of the spinal cord is affected by
the pattern of stimuli during training, and noncontingent stimuli
can prevent future attempts at learning through a central
sensitization mechanism (48).
Therefore, theoretically, the activity-dependent plasticity within
the spinal cord should be carefully modified to promote adaptive
spinal training. Stimulation delivered in a limb position-dependent
manner or at a fixed interval are able to induce adaptive
plasticity that promotes spinal cord learning (30). Based on the plasticity mechanism of
spinal neuronal circuits, we hypothesized that NRSPA may be a more
effective stimulus than MA because NRSPA was congruent with the
normal stepping pattern. To prove our hypothesis, we used the
tceMEPs assessment which was an objective assessment of electrical
conduction through the associated neural pathways (49–51) and
immunohistochemistry analysis for neurofilament protein NF200
(NF200) expression, which was the main component of the neuronal
and axonal cytoskeleton. By tceMEPs assessment, the waveforms of
tceMEPs have not be detected 3 days after surgery in all the SCI
rats for the acute injury at the T10 level. After 3 weeks of BWSTT,
the tceMEPs waveforms in the NRSPA group demonstrated greater
improvement compared to the MA group for shorter latencies and
greater amplitudes (Fig. 6),
indicating greater neural conduction in the spinal cord than the
later. The quantitative analysis for NF200+ expression
by immunohistochemistry in the dorsal horn of the spinal cord
lesion epicenter was performed to validate the tceMEPs set-up. Our
results indicated that the positive area fraction (AF) and mean
density (MD) of the NF200+ expression in the two BWSTT
groups (NRSPA and MA) increased significantly compared to those of
the sedentary group (P<0.05) (Figs.
7 and 8). Importantly, the MD
values of the NF200-labeled axons in the MA group were
significantly lower than those in the NRSP group, although there
was no statistically significant difference in AF values between
the two BWSTT groups. Therefore, on the basis of the results of the
tceMEPs assessment and NF200 immunoreactivity, we conclude that the
rats undergoing BWSTT and receiving NRSPA can achieve greater nerve
regeneration and neural conduction in the spinal cord than those
undergoing BWSTT and receiving MA.
In conclusion, NRSPA for paralytic hindlimbs was
more effective than MA in promoting the stepping learning process
during BWSTT in the early phase of acute SCI. This finding is
demonstrated by improved locomotor function evaluation, neural
conduction, and nerve regeneration of the spinal cord lesion area.
These results indicate that the establishment of an accurate
assistive pattern of training to correct the subtle mistakes during
stepping learning from the beginning is critical. However, stepping
is a complex movement involving controlling the central nervous
system and the functioning of a group of skeletal muscles, joints,
and limb coordination, and the different training duration and
training intensity may influence the results to some extent. All
the above parts were not evaluated in this study. Therefore,
further studies are required to validate the different effects of
NRSPA and MA from a more comprehensive perspective.
We examined the effects of NRSPA for
robotic-assisted treadmill training on locomotor recovery in rats
with SCI. Spinally contused rats receiving NRSPA during BWSTT
performed better hindlimb stepping and had greater tceMEPs recovery
and NF200+ expression in the spinal cord lesion area
than rats receiving MA. This new assistive pattern of training for
BWSTT is potentially a better platform support for animal
experiments.
Acknowledgements
We are thankful for the support of all staff from
the Experimental Center of the School of Nursing, Dalian University
in China. This study was funded by the Education Department of
Liaoning Province (grant no. L2014489) and the Science and
Technology Department of Liaoning Province (grant no.
201602022).
Glossary
Abbreviations
Abbreviations:
|
SCI
|
spinal cord injury
|
|
NRSPA
|
normal rat stepping pattern
assistance
|
|
MA
|
manual assistance
|
|
tceMEPs
|
transcranial electrical motor-evoked
potentials
|
|
NF200
|
neurofilament 200
|
|
BWSTT
|
body weight-supported treadmill
training
|
|
RRMPS
|
rodent robotic motor performance
system
|
|
MD
|
mean density
|
|
AF
|
area fraction
|
References
|
1
|
Macias M, Dwornik A, Ziemlinska E, Fehr S,
Schachner M, Czarkowska-Bauch J and Skup M: Locomotor exercise
alters expression of pro-brain-derived neurotrophic factor,
brain-derived neurotrophic factor and its receptor TrkB in the
spinal cord of adult rats. Eur J Neurosci. 25:2425–2444. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Macias M, Nowicka D, Czupryn A, Sulejczak
D, Skup M, Skangiel-Kramska J and Czarkowska-Bauch J:
Exercise-induced motor improvement after complete spinal cord
transection and its relation to expression of brain-derived
neurotrophic factor and presynaptic markers. BMC Neurosci.
10:1442009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rangasamy SB: Locomotor recovery after
spinal cord hemisection/contusion injures in bonnet monkeys:
Footprint testing-a minireview. Synapse. 67:427–453. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith RR, Brown EH, Shum-Siu A, Whelan A,
Burke DA, Benton RL and Magnuson DS: Swim training initiated
acutely after spinal cord injury is ineffective and induces
extravasation in and around the epicenter. J Neurotrauma.
26:1017–1027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Onifer SM, Zhang O, Whitnel-Smith LK, Raza
K, O'Dell CR, Lyttle TS, Rabchevsky AG, Kitzman PH and Burke DA:
Horizontal ladder task-specific re-training in adult rats with
contusive thoracic spinal cord injury. Restor Neurol Neurosci.
29:275–286. 2011.PubMed/NCBI
|
|
6
|
Côté MP, Azzam GA, Lemay MA, Zhukareva V
and Houlé JD: Activity-dependent increase in neurotrophic factors
is associated with an enhanced modulation of spinal reflexes after
spinal cord injury. J Neurotrauma. 28:299–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Leon RD, See PA and Chow CH:
Differential effects of low versus high amounts of weight supported
treadmill training in spinally transected rats. J Neurotrauma.
28:1021–1033. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe S, Oya Y, Iwata J and Someya F:
Influences of changes in the level of support and walking speed on
the H Reflex of the soleus muscle and circulatory dynamics on body
weight-supported treadmill training: investigation in healthy
adults. J Phys Ther Sci. 26:1345–1350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SW, Kim YS, Jun TW, Seo JH, Kim K,
Shin MS and Kim CJ: The impact of duration of one bout treadmill
exercise on cell proliferation and central fatigue in rats. J Exerc
Rehabil. 9:463–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim YP, Kim HB, Jang MH, Lim BV, Kim YJ,
Kim H, Kim SS, Kim EH and Kim CJ: Magnitude- and time-dependence of
the effect of treadmill exercise on cell proliferation in the
dentate gyrus of rats. Int J Sports Med. 24:114–117. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah PK, Gerasimenko Y, Shyu A, Lavrov I,
Zhong H, Roy RR and Edgerton VR: Variability in step training
enhances locomotor recovery after a spinal cord injury. Eur J
Neurosci. 36:2054–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Navarrete-Opazo A, Alcayaga JJ, Sepúlveda
O and Varas G: Intermittent hypoxia and locomotor training enhances
dynamic but not standing balance in patients with incomplete spinal
cord injury. Arch Phys Med Rehabil. 98:415–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamgar P, Agarwal A, Chao T, Askari S, Tan
M, Honor R and Won DS: Step trajectory analysis of spinal cord
injured rats trained with neuromuscular electrical stimulation
coordinated with robotic treadmill training. Conf Proc IEEE Eng Med
Biol Soc. 2012:pp. 1864–1867. 2012; PubMed/NCBI
|
|
14
|
Scivoletto G, Tamburella F, Laurenza L,
Torre M and Molinari M: Who is going to walk? A review of the
factors influencing walking recovery after spinal cord injury.
Front Hum Neurosci. 8:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martinez M, Delivet-Mongrain H and
Rossignol S: Treadmill training promotes spinal changes leading to
locomotor recovery after partial spinal cord injury in cats. J
Neurophysiol. 109:2909–2922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Battistuzzo CR, Rank MM, Flynn JR, Morgan
DL, Callister R, Callister RJ and Galea MP: Effects of treadmill
training on hindlimb muscles of spinal cord-injured mice. Muscle
Nerve. 55:232–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heng C and de Leon RD: Treadmill training
enhances the recovery of normal stepping patterns in spinal cord
contused rats. Exp Neurol. 216:139–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang JF, Musselman KE, Livingstone D,
Brunton K, Hendricks G, Hill D and Gorassini M: Repetitive mass
practice or focused precise practice for retraining walking after
incomplete spinal cord injury? A pilot randomized clinical trial.
Neurorehabil Neural Repair. 28:314–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Battistuzzo CR, Callister RJ, Callister R
and Galea MP: A systematic review of exercise training to promote
locomotor recovery in animal models of spinal cord injury. J
Neurotrauma. 29:1600–1613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Norrie BA, Nevett-Duchcherer JM and
Gorassini MA: Reduced functional recovery by delaying motor
training after spinal cord injury. J Neurophysiol. 94:255–264.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Panisset MG, Galea MP and El-Ansary D:
Does early exercise attenuate muscle atrophy or bone loss after
spinal cord injury? Spinal Cord. 54:84–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hornby TG, Zemon DH and Campbell D:
Robotic-assisted, body-weight-supported treadmill training in
individuals following motor incomplete spinal cord injury. Phys
Ther. 85:52–66. 2005.PubMed/NCBI
|
|
23
|
Gorman PH, Scott W, York H, Theyagaraj M,
Price-Miller N, McQuaid J, Eyvazzadeh M, Ivey FM and Macko RF:
Robotically assisted treadmill exercise training for improving peak
fitness in chronic motor incomplete spinal cord injury: A
randomized controlled trial. J Spinal Cord Med. 39:32–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee C, Won D, Cantoria MJ, Hamlin M and de
Leon RD: Robotic assistance that encourages the generation of
stepping rather than fully assisting movements is best for learning
to step in spinally contused rats. J Neurophysiol. 105:2764–2771.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aoyagi D, Ichinose WE, Harkema SJ,
Reinkensmeyer DJ and Bobrow JE: A robot and control algorithm that
can synchronously assist in naturalistic motion during
body-weight-supported gait training following neurologic injury.
IEEE Trans Neural Syst Rehabil Eng. 15:387–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Emken JL, Harkema SJ, Beres-Jones JA,
Ferreira CK and Reinkensmeyer DJ: Feasibility of manual
teach-and-replay and continuous impedance shaping for robotic
locomotor training following spinal cord injury. IEEE Trans Biomed
Eng. 55:322–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vallery H, van Asseldonk EH, Buss M and
van der Kooij H: Reference trajectory generation for rehabilitation
robots: Complementary limb motion estimation. IEEE Trans Neural
Syst Rehabil Eng. 17:23–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hornby TG, Campbell DD, Kahn JH, Demott T,
Moore JL and Roth HR: Enhanced gait-related improvements after
therapist-versus robotic-assisted locomotor training in subjects
with chronic stroke: A randomized controlled study. Stroke.
39:1786–1792. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hussain S, Xie SQ and Liu G: Robot
assisted treadmill training: mechanisms and training strategies.
Med Eng Phys. 33:527–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shah PK, Garcia-Alias G, Choe J, Gad P,
Gerasimenko Y, Tillakaratne N, Zhong H, Roy RR and Edgerton VR: Use
of quadrupedal step training to re-engage spinal interneuronal
networks and improve locomotor function after spinal cord injury.
Brain. 136:3362–3377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D and Zhang J: Electrophysiological
functional recovery in a rat model of spinal cord hemisection
injury following bone marrow-derived mesenchymal stem cell
transplantation under hypothermia. Neural Regen Res. 7:749–755.
2012.PubMed/NCBI
|
|
33
|
Li WT, Zhang XY, Xue H, Ni CP, Wang EG and
An LB: Comparison of three different time points of starting
treadmill training in spinal cord injured rats. Dev Neurorehabil.
16:382–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen G, Zhang Z, Wang S and Lv D: Combined
treatment with FK506 and nerve growth factor for spinal cord injury
in rats. Exp Ther Med. 6:868–872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Esquenazi A and Packel A: Robotic-assisted
gait training and restoration. Am J Phys Med Rehabil. 91(11 Suppl
3): S217–S231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shin JC, Kim JY, Park HK and Kim NY:
Effect of robotic-assisted gait training in patients with
incomplete spinal cord injury. Ann Rehabil Med. 38:719–725. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schwartz I and Meiner Z: Robotic-assisted
gait training in neurological patients: Who may benefit. Ann Biomed
Eng. 43:1260–1269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Srivastava S, Kao PC, Reisman DS, Scholz
JP, Agrawal SK and Higginson JS: Robotic assist-as-needed as an
alternative to therapist-assisted gait rehabilitation. Int J Phys
Med Rehabil. 4:3702016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harbourne RT and Stergiou N: Movement
variability and the use of nonlinear tools: Principles to guide
physical therapist practice. Phys Ther. 89:267–282. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fetters L: Perspective on variability in
the development of human action. Phys Ther. 90:1860–1867. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bareyre FM, Kerschensteiner M, Raineteau
O, Mettenleiter TC, Weinmann O and Schwab ME: The injured spinal
cord spontaneously forms a new intraspinal circuit in adult rats.
Nat Neurosci. 7:269–277. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Houle JD and Côté MP: Axon regeneration
and exercise-dependent plasticity after spinal cord injury. Ann N Y
Acad Sci. 1279:154–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Filous AR and Schwab JM: Determinants of
axon growth, plasticity and regeneration in the context of spinal
cord injury. Am J Pathol. 188:53–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gossard JP, Delivet-Mongrain H, Martinez
M, Kundu A, Escalona M and Rossignol S: Plastic changes in lumbar
locomotor networks after a partial spinal cord injury in cats. J
Neurosci. 35:9446–9455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Martinez M, Delivet-Mongrain H, Leblond H
and Rossignol S: Incomplete spinal cord injury promotes durable
functional changes within the spinal locomotor circuitry. J
Neurophysiol. 108:124–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Adkins DL, Boychuk J, Remple MS and Kleim
JA: Motor training induces experience-specific patterns of
plasticity across motor cortex and spinal cord. J Appl Physiol
(1985). 101:1776–1782. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rossignol S and Frigon A: Recovery of
locomotion after spinal cord injury: Some facts and mechanisms.
Annu Rev Neurosci. 34:413–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu GT, Ferguson AR, Crown ED, Bopp AC,
Miranda RC and Grau JW: Instrumental learning within the rat spinal
cord: Localization of the essential neural circuit. Behav Neurosci.
119:538–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Morris SH, Howard JJ, Rasmusson DD and
El-Hawary R: Validity of transcranial motor evoked potentials as
early indicators of neural compromise in rat model of spinal cord
compression. Spine (Phila Pa 1976). 40:E492–E497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alam M, Garcia-Alias G, Shah PK,
Gerasimenko Y, Zhong H, Roy RR and Edgerton VR: Evaluation of
optimal electrode configurations for epidural spinal cord
stimulation in cervical spinal cord injured rats. J Neurosci
Methods. 247:50–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Petersen JA, Spiess M, Curt A, Dietz V and
Schubert M; EM-SCI Study Group, : Spinal cord injury: One-year
evolution of motor-evoked potentials and recovery of leg motor
function in 255 patients. Neurorehabil Neural Repair. 26:939–948.
2012. View Article : Google Scholar : PubMed/NCBI
|