Introduction
Fabry disease (FD) is an X-linked genetic disease
resulting from the deficient enzyme activity of α-galactosidase A
[α-gal A; Online Mendelian Inheritance in Man of the galactisidase
(GLA) gene, 301500; Enzyme Commission number of α-gal A, 3.2.1.22],
which is encoded by the GLA gene (Xq22) (1). This enzymatic defect leads to the
accumulation of glycolipid, which consists primarily of
globotriaosylceramide (Gb3), in tissues, organs and biological
fluids (2). Over time, a gradual
deterioration in organ function occurs, resulting in corresponding
renal, cardiac and vascular complications (3). The early diagnosis and treatment of FD
is vital to prevent irreversible damage to vital organs; however,
FD is often misdiagnosed due to its heterogeneous clinical
manifestations and inefficient diagnostic methods, such as enzyme
assays (3–5).
In males, the diagnosis of FD is usually based on
the dramatically decreased activity of α-gal A (6). However, enzyme assays are usually
inconclusive at diagnosing FD in females, as α-gal A activity
usually ranges between relatively low to normal levels in females
with FD (7). The association between
Gb3 levels and α-gal A activity is weak and it has therefore been
suggested is that Gb3 is not ideal as a primary diagnostic marker
of FD (8–11). Although genotyping is a powerful
diagnostic tool, the rate of mutation detection remains at ~10%
(12,13); therefore it is difficult to identify
novel GLA variants, as FD lacks defining clinical characteristics
(14). To avoid the misdiagnosis of
individuals with an unknown genetic composition, non-invasive
methods, including imaging examinations, may be used. However,
these are often unable to exclude FD in uncertain cases (15,16).
These patients are classified as uncertain cases as they exhibit
uncharacteristic FD manifestations and lack of a family history of
the disease; such patients only exhibited solitary unexplained
cardiac hypertrophy or stroke. Biopsy results from organs affected
by FD, such as the heart or kidneys, reveal the presence of
characteristic lamellar inclusion bodies following electron
microscopy assessment, which also confirms FD diagnosis (17,18).
However, biopsies are invasive and an unfeasible method of
diagnosing all patients with FD. Therefore, a novel diagnostic
biomarker of FD is urgently required.
In 2008, globotriaosylsphingosine (lyso-Gb3) was
introduced as a promising novel biomarker of FD, which has better
diagnostic sensitivity than α-gal A (19). Lyso-Gb3 is a cationic amphiphilic
compound containing a polar sugar group and is hydrophilic. It is
also a Gb3 metabolite and contains a free sphingosine amino group.
As lyso-Gb3 is able to freely access cells, it is more easily
detectable than Gb3 (20). Certain
methods used to measure lyso-Gb3, such as high-performance liquid
chromatography (HPLC), o-phthaldialdehyde (OPA)-derivatization or
fluorescence detection (19–21), did not exhibit high sensitivity to
lyso-Gb3. Previous studies have suggested that liquid
chromatography-tandem mass spectrometry (LC-MS/MS) may be a more
suitable technique of measuring lyso-Gb3 (22–25).
Lyso-Gb3-related analogues have been identified as novel biomarkers
for FD (26–29). However, levels of these analogues are
much lower than lyso-Gb3 in human plasma and are undetectable in
some patients with FD and healthy subjects. Therefore, the current
study focused on measuring lyso-Gb3 levels in human plasma.
To the best of our knowledge, the current study is
the first to measure plasma levels of lyso-Gb3 in Chinese patients
with FD. A simplified LC-MS/MS assay was used to measure lyso-Gb3
levels. An internal standard (IS) used to analyze sphingolipid
metabolism is easy to obtain and used to quantify lyso-Gb3 levels.
In addition, the clinical significance of α-gal A activity and
lyso-Gb3 were determined and compared to determine which would more
suitable for use in patients with FD.
Patients and methods
Patients and samples
Preliminary diagnoses of FD were based on the
presence of a myeloid body in histological studies and/or decreased
α-gal A enzyme activity; patients with FD exhibit α-gal A enzyme
activity ≤37.0 nmol/ml/h/mg (30–32). FD
diagnosis was confirmed by genetic sequencing, as previously
described (32). Between January
2012 and December 2014, 38 patients with FD (17 females and 21
males, 1:1.24; age, 34.7±16.0 years) and 120 healthy volunteers (60
females and 60 males, 1:1; age, 40.8±10.9 years) were enrolled at
Ruijin Hospital, The Medicine School of Shanghai Jiao Tong
University (Shanghai, China). The present study was approved by the
Ethics Committee of Ruijin Hospital and all participants provided
their informed consent prior to participation in the current study.
Written informed consent was obtained from the parents or guardians
of children enrolled. All EDTA-anti-coagulated blood samples from
patients with FD were collected prior to enzyme replacement therapy
(ERT).
Demographic and clinical
variables
For all patients with FD, demographic data,
including age, sex, blood pressure and body mass index, as well as
their clinical symptoms were recorded. Estimated glomerular
filtration rate (eGFR) was estimated using the abbreviated chronic
kidney disease epidemiology collaboration equation (33) in adults and the Schwartz formula
(34) in children. End-stage renal
disease was defined as an eGFR <15 ml/min or the requirement of
kidney transplant or dialysis. Abnormal brain magnetic resonance
imaging (MRI) results were indicated by the presence of white
matter lesions and lacunar infarctions. Left ventricular
hypertrophy was identified by echocardiography. Cornea verticillata
and high frequency sensorineural hearing loss were confirmed by the
slit-lamp examination and pure tone audiogram, respectively. The
presence of acroparesthesia was determining by assessing the
medical history of patients and identifying the presence of
neuropathic pain in the palms and soles. Physical examination and
history taking were used to detect angiokeratoma corporis
diffusum and abnormal sweating. Based on these data, the Mainz
severity score index (MSSI) was scored to evaluate overall disease
severity (35). Patients included in
the current study either exhibited classical FD phenotypes or
atypical FD phenotypes, which were defined by the diagnostic
criteria described in a previous study by our group (32).
Reagents
For LC-MS/MS, lyso-Gb3 was purchased as standard
(Matreya LLC, State College, PA, USA) whereas dimethyl psychosine
was selected as the IS (Avanti Polar Lipids, Alabastar, AL, USA).
Pure water (AppliChem GmbH, Darmstadt, Germany), acetonitrile and
methanol (Thermo Fisher Scientific, Inc., Waltham, MA, USA), formic
acid and ammonium formate (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and all other chemicals were HPLC grade. To detect the
α-gal A enzyme activity,
4-methylum-belliferyl-α-D-galactopyranoside (4MU-gal), 4MU and
N-acetyl-D-galactosamine (galNAc) were purchased from
Sigma-Aldrich; Merck KGaA.
Preparation of EDTA-plasma for
lyso-Gb3
A total of 3 ml whole blood was drawn from the elbow
vein of patients with FD and centrifuged at 1,500 × g for 10 min at
4°C. Plasma was separated and stored at −20°C until use. The neat
lyso-Gb3 standard was dissolved as recommended by the supplier and
diluted with methanol to a working solution concentration of 10
µg/ml. Aliquots of 100 µl standard were dried and stored at −20°C
for later use. For each analysis, a blank plasma pools from healthy
controls were used for preparing the calibration standards and
quality control (QC) samples. After adding appropriate volumes of
the working solution to blank plasma matrix, the final calibration
standards concentrations of lysoGb3 were 200, 100, 50, 25, 12.5,
6.25, 3.13, 1.56, 0.78 and 0 ng/ml. Final QC samples concentrations
of lysoGb3 were 4, 20 and 160 ng/ml. Glycolipids were extracted
from 50 ml plasma samples according to simultaneous protein
precipitation (36), which was
performed by adding a mixture of acetone and methanol (1 ml, 1:1)
to a plasma sample and centrifuging the mixture at 14,000 × g for
10 min at 4°C to remove the sediment. The supernatant was collected
and evaporated for LC-MS/MS analysis without the requirement of
further sample preparation, such as Solid-Phase Extraction. For
analysis, samples were re-dissolved in 100 µl pure methanol with
subsequent sonication at 40 kHz for 10 min, followed by vortexing
at 1,200 rpm for 3 min and centrifugation at 14,000 × g for 10 min
at 4°C. The supernatant was transferred into glass microvials and 5
µl was injected into the LC-MS/MS system.
LC-MS/MS quantification of
lyso-Gb3
Quantitative LC-MS/MS analysis was performed on an
AB SCIEX API 4000 triple quadrupole mass spectrometer (AB Sciex
LLC, Framingham, MA, USA) operating in positive mode. An XDB-C18
column (2.1×100 mm, 3.5 µm particles) was used in combination with
a VanGuard pre-column of the same material (Agilent Technologies,
Inc., Santa Clara, CA, USA). The mobile phases consisted of (A)
water and (B) acetonitrile/methyl alcohol (15/85=v/v). Each mobile
phase contained 0.1% formic acid and 2 mM ammonium formate. The two
mobile phases formed the following gradient: 0–0.25 (50% B) min,
0.25–2.0 min (50–99%, B), 2.0–4.5 min (99%, B), 4.5–4.75 min
(99–50% B) and 4.75–8.5 min (50%, B). The MS conditions were as
follows: ESI mode was positive, ion spray voltage was 5.5 KV,
nebulizer gas and auxiliary gas were 60 psi, curtain gas was 25
psi, the desolvation temperature was 600°C and the flow rate is 0.3
ml/min. The following multiple-reaction monitoring (MRM)
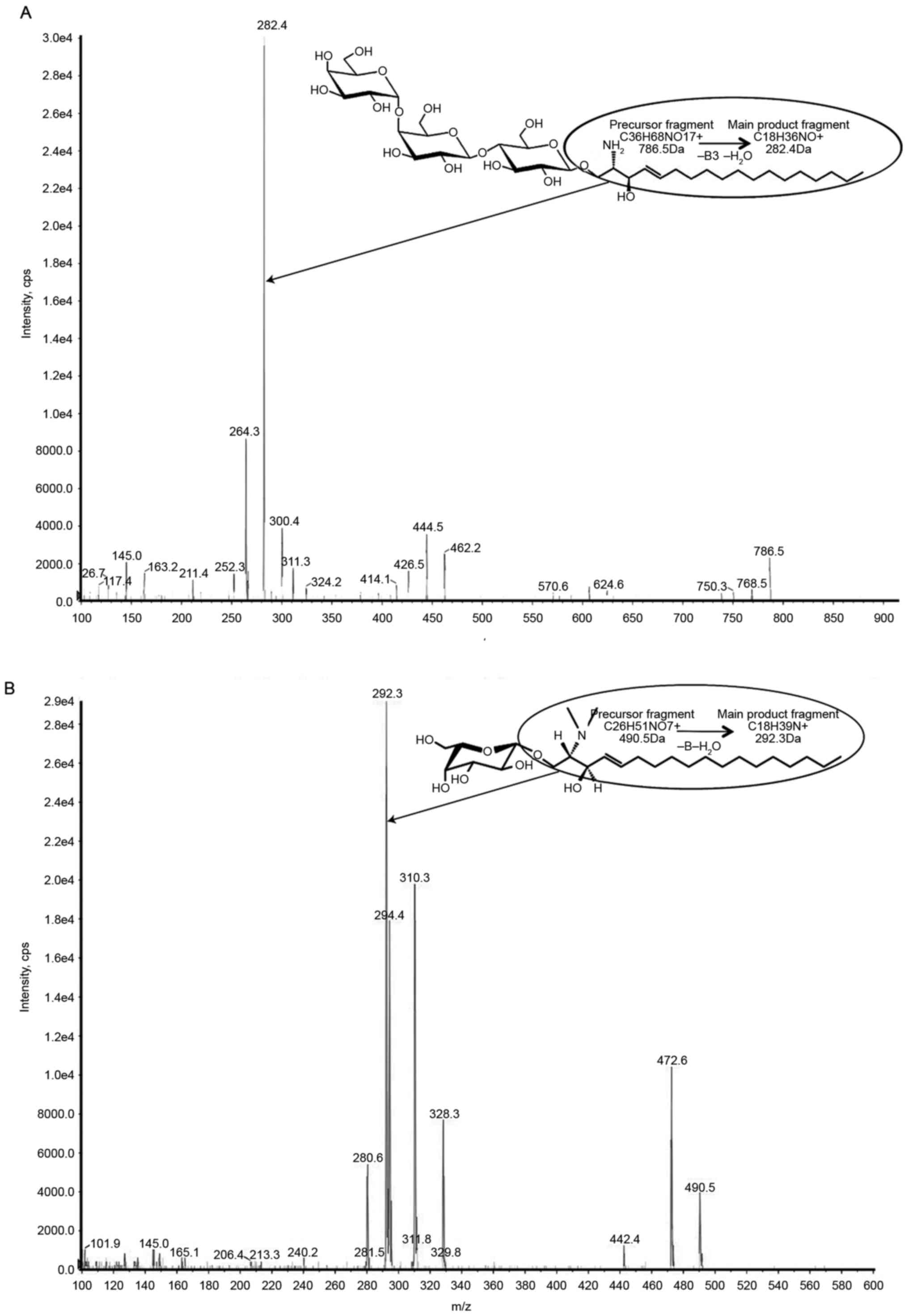
transitions were monitored, with a dwell time of 100 ms:
786.5>282.4 (lyso-Gb3) and 490.5>292.3 (IS). Collision energy
was 48.6 V and 38 V in the MRM traces of lyso-Gb3 and IS,
respectively (Fig. 1).
Measurement of α-gal A activity
α-gal A activity in leukocytes was detected using
the fluorimetric method, as described by Desnick et al
(31). Briefly, 4 ml whole blood was
added to 50-ml conical tubes. Leukocyte pellets were isolated and
resuspended in 100 µl sodium phosphate buffer. Following 10 cycles
of freezing/thawing, leukocyte lysates were centrifuged at 2,000 ×
g for 10 min at 4°C. Supernatants were stored at −20°C to allow
assaying at a later period. The mixture of 4MU-gal plus galNAc
solution was added to the leukocyte lysate and incubated at 37°C
for 30 min. Reactions were quenched using 0.1 M Na-Glycine buffer
(pH 10.7). Fluorescence readings were performed using a Turner
Fluorometer (Turner Designs, San Jose, CA, USA) using the following
wavelengths: Excitation at 360 nm and emission at 450 nm. Protein
values were determined using a standard BCA assay and were used to
calculate the specific enzyme activity of the lysates. Normal α-gal
A activity was defined as >37.0 nmol/ml/h/mg (32).
Statistical analysis
The distributions of quantitative variables were
assessed for normality. Results are presented as mean ± standard
deviation or median (range). All analyses were performed using IBM
SPSS Statistics, ver. 22 (IBM Corp, Armonk, NY, USA). A
Mann-Whitney U-test was used to assess differences between two
non-parametric variables, and a t-test was used to compare two
normally distributed variables. To analyze the relationship between
two variables, the correlation coefficient was calculated using
Spearman's correlation coefficient. A receiver operating
characteristic (ROC) curve was used to define the pathological
cutoff point of lyso-Gb3 and compare the diagnostic value between
it and enzyme activity. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic characteristics
The demographic variables of the 38 patients with FD
prior to ERT are presented in Table
I. Compared with female patients, males exhibited an earlier
age of onset (13 vs. 33 years). Cornea verticillata was the
presenting symptom most frequently reported by males and females
(70% of males and 50% of females reported experiencing this
symptom). Males with FD were more likely to report a clinical
event, including end-stage renal disease and life threatening
cardiovascular or cerebrovascular complications, compared with
females. Male patients exhibited significantly higher levels of
lyso-Gb3 accumulation and lower enzyme activity (each, P<0.001)
and also presented with significantly higher MSSI scores than
females (P<0.05).
 | Table I.Clinical baseline characteristics of
male and female patients with Fabry disease. |
Table I.
Clinical baseline characteristics of
male and female patients with Fabry disease.
| Parameter | Male (n=21) | Female (n=17) | P-value |
|---|
| Enzyme activity
(nmol/ml/h/mg) | 0.3 (0.0–23.2) | 64.4
(14.8–152.4) | <0.001 |
| Lyso-Gb3
(ng/ml) | 14.5
(0.9–113.4) | 2.8 (0.7–6.0) | <0.001 |
| BMI
(kg/m2) | 23.1±7.1 | 22.0±6.9 | 0.647 |
| Onset age
(year) | 13 (1–51) | 33
(5–54) | 0.114 |
| Age at diagnosis
(year) |
31.1±14.6 |
39.2±16.8 | 0.122 |
| SBP (mmHg) | 132.2±23.7 | 130.1±13.7 | 0.765 |
| DBP (mmHg) |
81.8±15.7 | 77.6±9.1 | 0.378 |
| PP (mmHg) |
50.4±15.6 |
52.5±11.2 | 0.674 |
| MAP (mmHg) |
98.6±17.3 | 95.1±9.5 | 0.461 |
| Angiokeratoma, %
(N) | 20.0 (4/20) | 7.1
(1/14) | 0.298 |
| Abnormal sweating,
% (N) | 35.0 (7/20) | 7.1
(1/14) | 0.059 |
| Cornea
verticillata, % (N) | 70.0
(14/20) | 50.0 (7/14) | 0.238 |
| Hearing loss, %
(N) | 37.5 (6/16) | 16.7 (2/12) | 0.227 |
| Acroparaesthesia, %
(N) | 50.0
(10/20) | 50.0 (7/14) | 0.999 |
| Brain MRI, %
(N) | 21.1 (4/19) | 38.5 (5/13) | 0.282 |
| LVH, % (N) | 21.1 (4/19) | 0.0
(0/14) | 0.067 |
| ESRD, % (N) | 14.3 (3/21) | 0.0
(0/17) | 0.104 |
| Classical
phenotype, % (N) | 47.6
(10/21) | 35.3 (6/17) | 0.521 |
| MSSI score |
19.6±10.0 |
7.6±5.1 | 0.003a |
Assay validation of the quantitation
of plasma lyso-Gb3
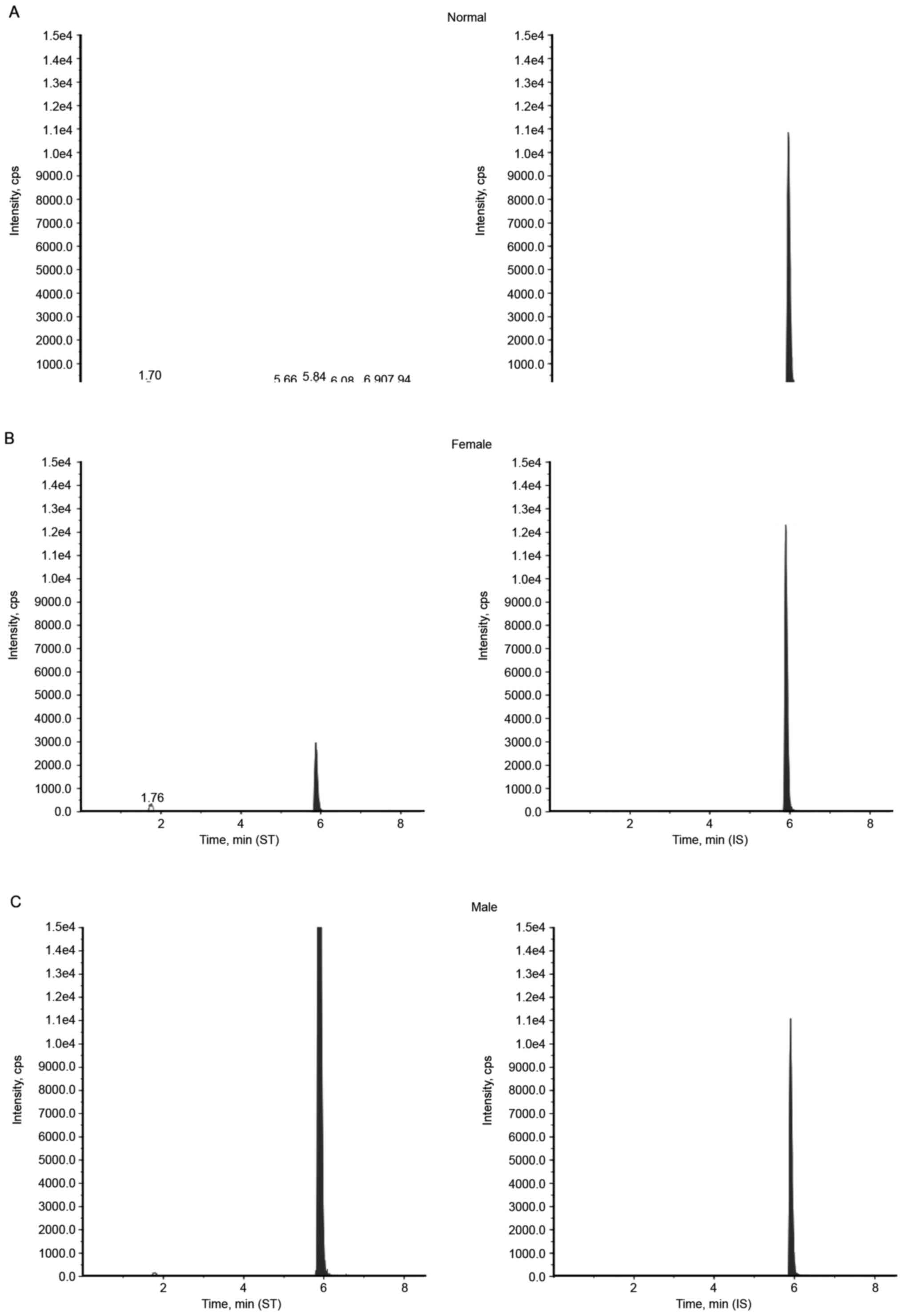
An individual from each group was selected as a
typical representative; the chromatography of LC-MS/MS results
revealed that a male patient displayed with a lyso-Gb3 level of
106.5, 6.04 ng/ml for a female patient and 0.61 ng/ml for a healthy
male control (Fig. 2). Diagnostic
assay validation was performed according to the criteria of the
Clinical and Laboratory Standards Institute (37,38).
Linearity was tested by a dilution series of blank plasma pools
analyzed in three separate runs. In this way, the limit of
detection representing the assay background (0.22±0.04 ng/ml) was
calculated from all subsequent sample dilutions that did not lead
to further signal reduction. Recoveries of 86.3–97.0% were measured
in QC samples prior to and following extraction (Table II). Intra-assay variation,
determined by analyzing a pooled sample in one batch, was 4.6–9.9%.
Inter-assay variation, determined by analyzing individually
prepared samples in different runs over different days, was
2.8–18.9%. Methanolic stock solutions of the standard and IS were
stable for ≥2 months at −20°C. Samples and controls were also
frozen at −20°C and did not exhibit a significant decrease
following 2 months storage. There were no noticeable influences on
quantitative results ≤3 freeze-thaw cycles. These results reveal
that using the LC-MS/MS method to quantify plasma lyso-Gb3 levels
is accurate, reproducible and highly sensitive.
 | Table II.Imprecision, inaccuracy and recovery
results for globotriaosylsphingosine quantification. |
Table II.
Imprecision, inaccuracy and recovery
results for globotriaosylsphingosine quantification.
| Quality control
samples | Low | Medium | High |
|---|
| Mean value ± SD,
g/l | 4.1±0.3 | 20.1±1.9 | 165.8±12.7 |
| CV% within-run
(batch) |
9.9 |
7.2 |
4.6 |
| CV% estimate of
between-day |
18.9 |
14.6 |
2.8 |
| CV% estimate of
between-run |
0a |
0a |
0a |
| CV% estimate of
within-run |
12.0 |
8.9 |
10.8 |
| Recovery %, mean ±
SD | 86.3±5.9 | 92.0±3.0 | 97.0±3.6 |
| Recovery max,
% | 93 | 95 | 101 |
| Recovery min,
% | 82 | 89 | 94 |
Diagnostic performance of plasma
lyso-Gb3 and α-gal A enzyme activity
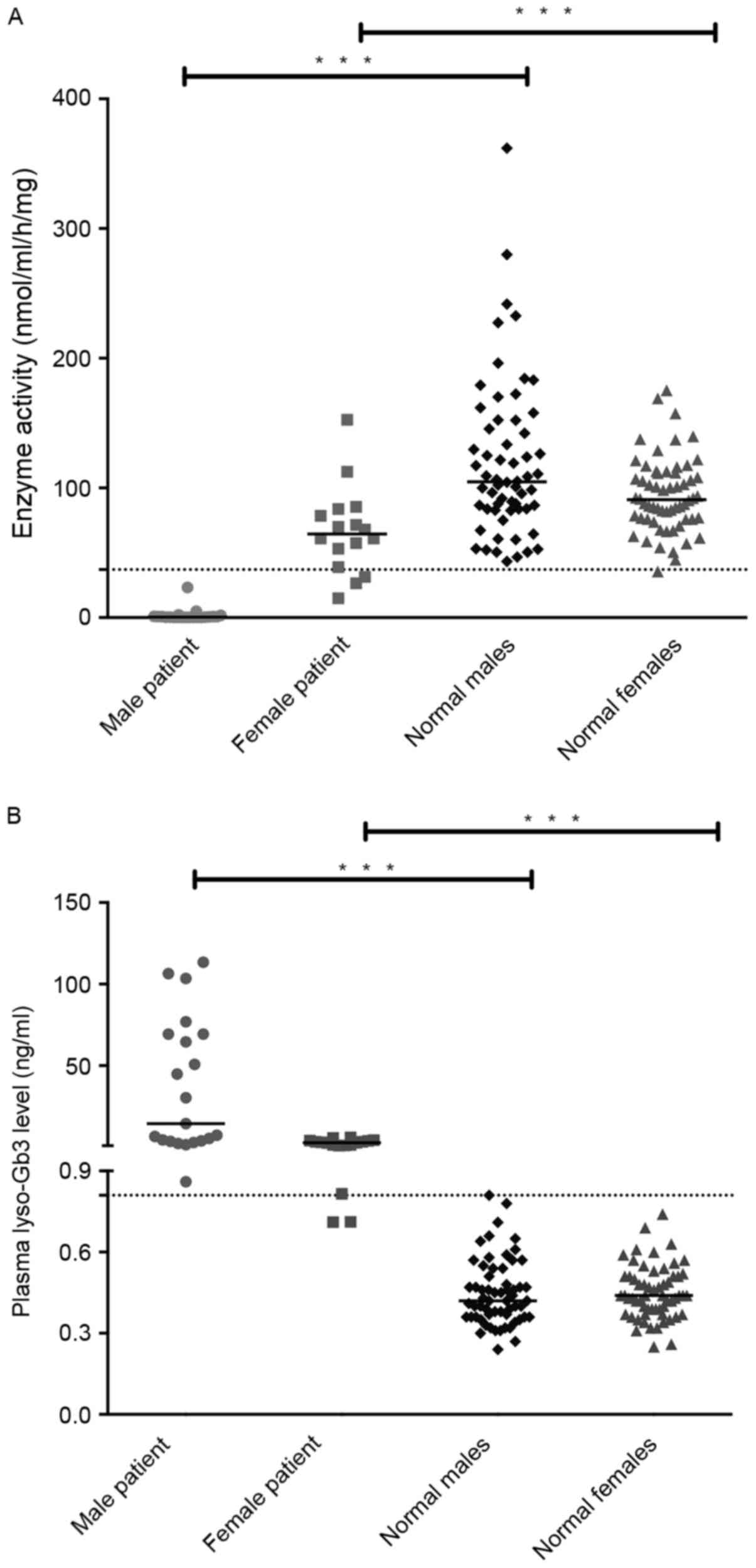
No significant difference in α-gal A enzyme activity
was observed between male and female controls (male, median 104.7
nmol/ml/h/mg; range, 43.2–361.8 nmol/ml/h/mg; female, median 90.8
nmol/ml/h/mg; range, 35.5–175.1 nmol/ml/h/mg; Fig. 3A). By contrast, enzyme activities in
leukocytes of male patients with FD (median, 0.3 nmol/ml/h/mg;
range, 0.0–23.2 nmol/ml/h/mg) were significantly lower than that of
female patients with FD (median, 64.4 nmol/ml/h/mg; range
14.8–152.4 nmol/ml/h/mg) (P<0.001; Table I). The significant differences were
also observed between male patients and male healthy controls, and
between female patients and female controls.
To verify the results of this assay and determine
the normal range of lyso-Gb3, 120 healthy controls were included in
the present study. No significant differences in lyso-Gb3 levels
were observed between healthy males and females. Only trace amounts
of it were present in healthy females (median, 0.44 ng/ml; range,
0.25–0.74 ng/ml) and healthy males (median, 0.42 ng/ml; range,
0.24–0.81 ng/ml). By contrast, median concentrations in male
patients were significantly higher than that in female patients
(14.50 vs. 2.79 ng/ml; P<0.001; Table
I), as expected. Although male and female patients had
significantly higher plasma lyso-Gb3 levels than healthy groups
(both P<0.001), there was a slight overlap between female
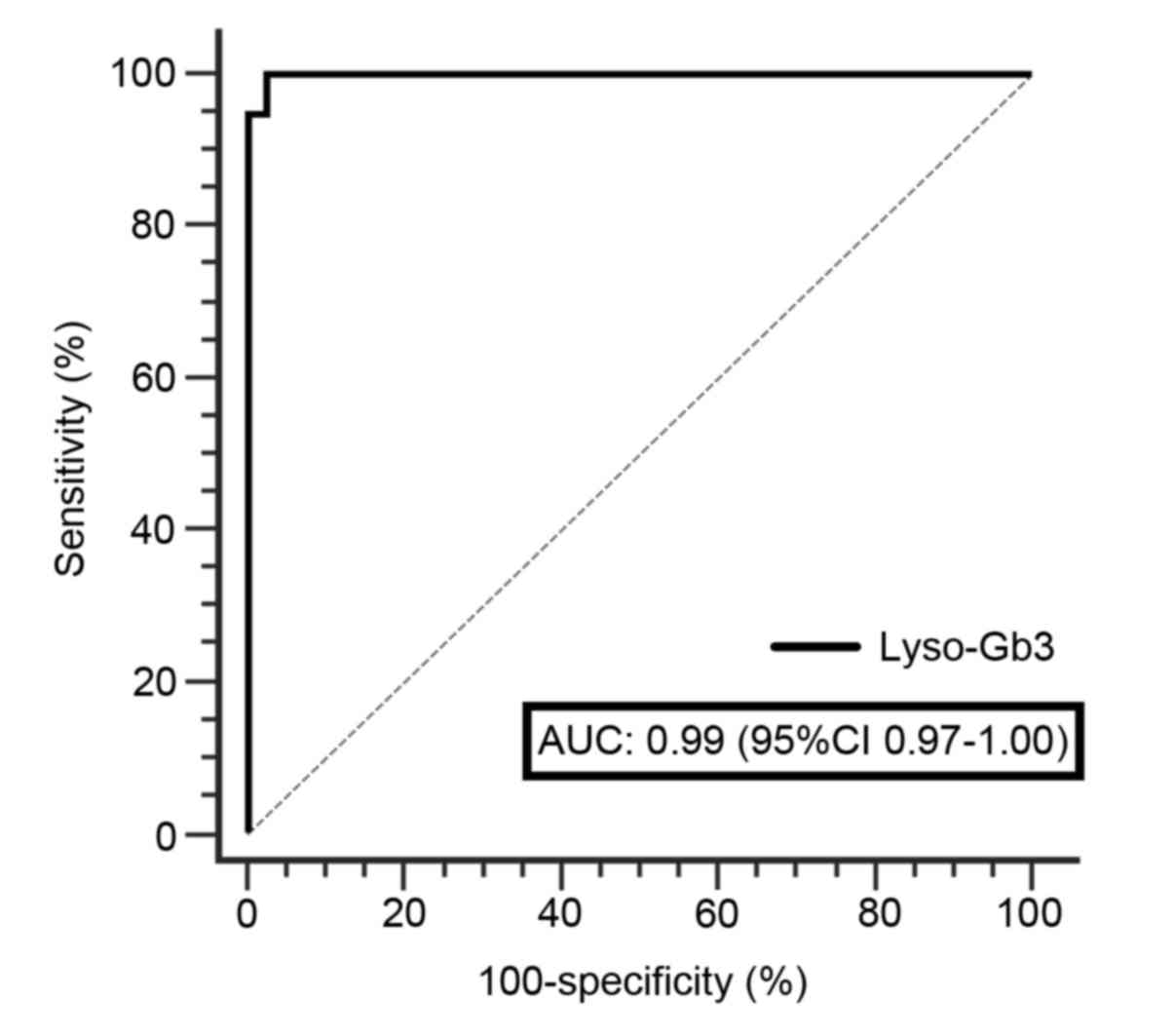
patients with FD and healthy individuals (Fig. 3B). The pathological threshold for
lyso-Gb3 was set to 0.81 ng/ml with 100% specificity and 94.74%
sensitivity, since all samples from healthy controls were clearly
below this cut-off value analyzed using a ROC curve (Fig. 4).
Comparison of clinical value between
two diagnostic biomarkers
To determine the usefulness of plasma lyso-Gb3
measurement in confirming FD diagnosis, enzyme activity was
measured in all plasma specimens. Linear correlation analysis of
all patients revealed a weak-negative correlation between lyso-Gb3
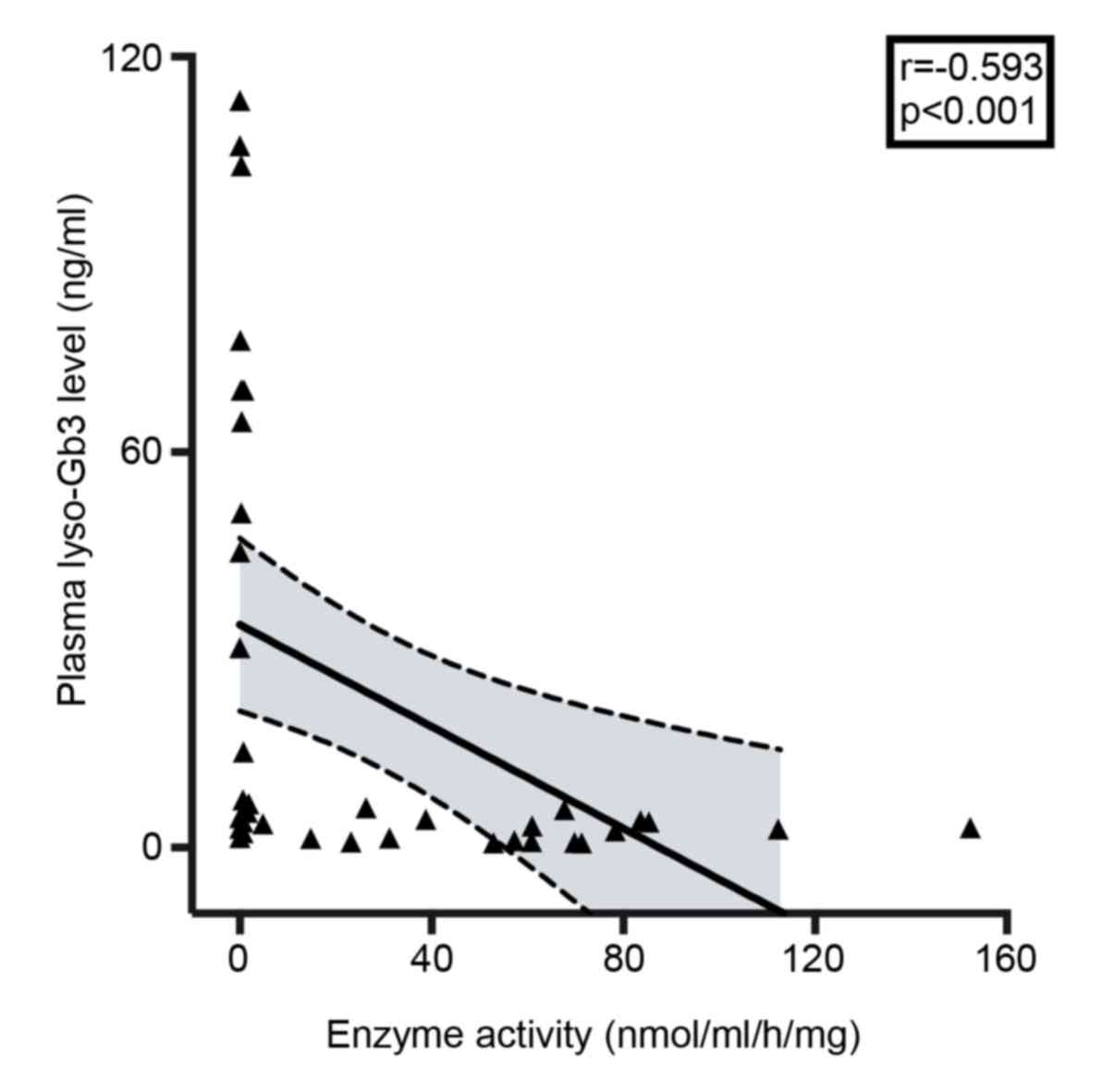
levels and enzyme activity (r=−0.593; P<0.001; Fig. 5). In males, FD diagnosis could be
confirmed by measuring plasma lyso-Gb3 concentration or enzyme
activity, as males with FD exhibit markedly higher plasma lyso-Gb3
concentrations and markedly decreased enzyme activity (25). The results of the current study
indicated that the sensitivities of two diagnostic indicators were
100% in males. However, the sensitivity of enzyme activity was
markedly lower than plasma lyso-Gb3 (23.5 vs. 82.4%) in females
with FD (Table III).
 | Table III.Diagnostic sensitivity of LysoGb3 and
enzyme activity. |
Table III.
Diagnostic sensitivity of LysoGb3 and
enzyme activity.
|
| Patients, N
(%) |
|
|---|
|
|
|
|
|---|
| Diagnostic
biomarkers | Male (N=21)
(%) | Female (N=17)
(%) | P-value |
|---|
| LysoGb3 |
|
| 0.193 |
|
>0.81 ng/ml | 100 | 82.4 |
|
| ≤0.81
ng/ml | 0 | 17.6 |
|
| Enzyme
activity |
|
| <0.001 |
| >37
nmol/ml/h/mg | 0 | 76.5 |
|
| ≤37
nmol/ml/h/mg | 100 | 23.5 |
|
Due to incomplete results from examinations, 15
patients lacking MSSI scores were excluded. In male patients,
plasma lyso-Gb3 and enzyme activity were correlated with MSSI
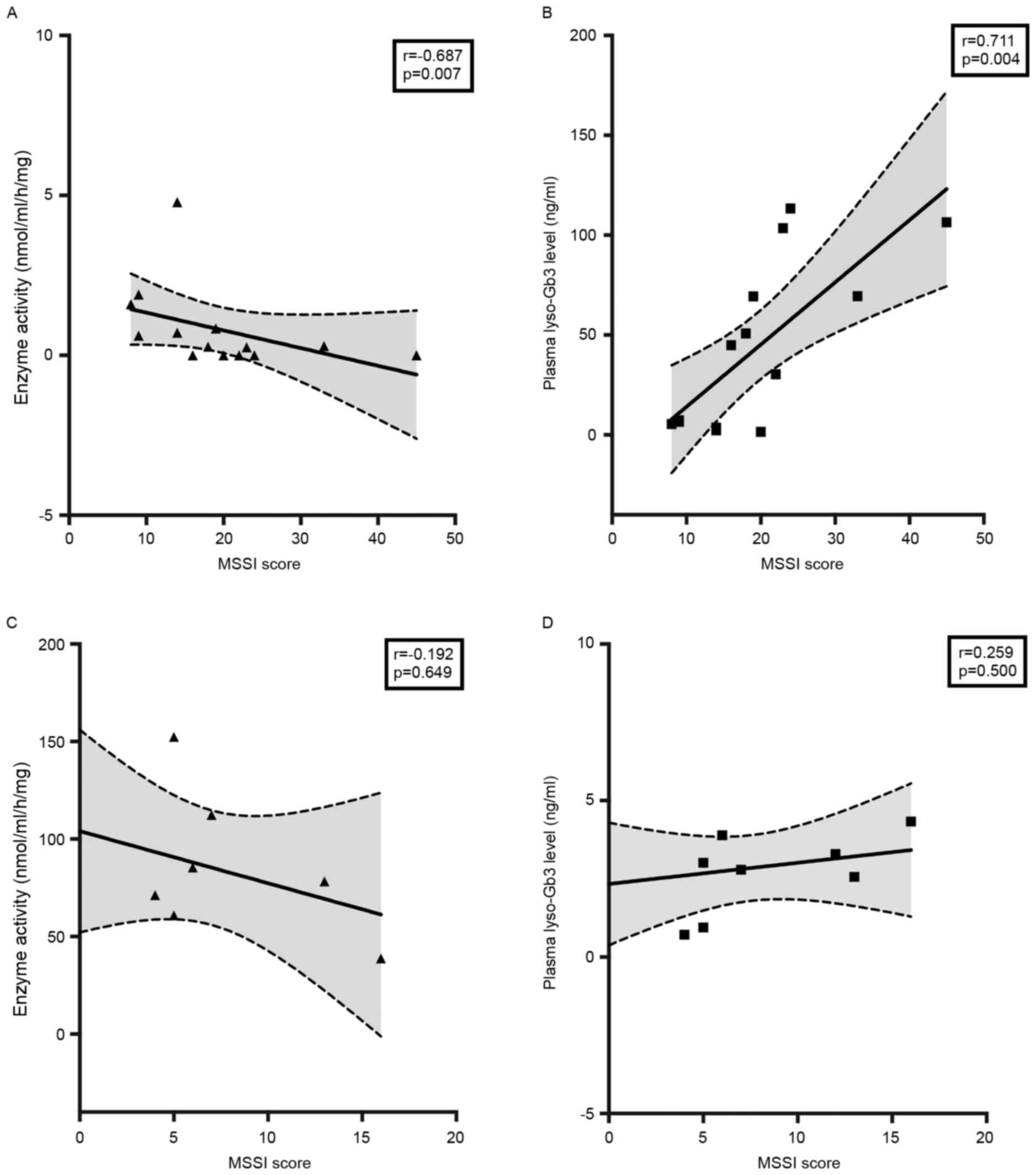
(P<0.01; Fig. 6A and B). Lyso-Gb3
was observed to be more strongly correlated with MSSI than enzyme
activity (r=0.711 vs. r=−0.687; Fig.
6). However, the analysis of correlation between lyso-Gb3 or
enzyme activity and MSSI showed no significant difference in female
patients (Fig. 6C and D).
Discussion
To the best of our knowledge, the current study is
the first to measure plasma lyso-Gb3 levels in Chinese patients
with FD. A robust LC-MS/MS assay with a short instrument run-time
and easy sample handling was developed and used. Instead of
choosing the 95th percentile calculation of the normal range (0.66
ng/ml) as the diagnostic value, a cut-off value of 0.81 ng/ml was
determined by ROC curve analysis. This value, with 94.74%
sensitivity and 100% specificity, was close to 0.9 ng/ml, the
pathological threshold set by Lukas et al (25). In the current study, lyso-Gb3 levels
in the majority of patients with FD were above the pathological
level of 0.81 ng/ml. In fact, only 17.65% heterozygote females
(3/17) exhibited weakly detectable lyso-Gb3 signals below the
cut-off limit. The diagnostic sensitivity of enzyme activity was
the same as lyso-Gb3 levels in male patients. However, in female
patients, it was remarkably lower than plasma lyso-Gb3 levels.
These results indicate that lyso-Gb3 levels are more reliable than
enzyme activity at diagnosing patients with FD, particularly in
women.
Recently awareness of FD in China has improved; but
the incidence of patients receiving delayed diagnoses and
misdiagnoses remains high, particularly in women (39). The results of the current study
indicate that the incidence of the delayed diagnosis in Chinese
patients with FD was 71.1% (27/38) and the mean delay in diagnosis
was 9.61 years (range, 0–47 years). Consequently, the incidence of
FD in China is generally underestimated, despite the existence of a
large population base (32).
Performing a biopsy of affected organs and/or tissues is an
effective method of diagnosing FD; however, this is not possible
for all patients, for example, in individuals presenting with an
isolated stroke (40). Although
measuring enzyme activity is the main method of diagnosing FD in
males, some female patients exhibit enzyme activity within normal
ranges (7). Furthermore, genetic
sequencing could not detect all mutations that lead to the
development of FD because the patients frequently lacked a family
history of the disease. The percentage of undetected mutation was
~10% (14).
Due to these weaknesses, a novel sensitive
diagnostic biomarker for FD is urgently required. In the current
study, male patients with FD exhibit markedly higher plasma
lyso-Gb3 concentrations and markedly decreased enzyme activity,
these results are similar to pervious studies (21,25). The
plasma lyso-Gb3 level was confirmed to be a reliable diagnostic
indicator of FD in other ethnic groups (21), however it has not been validated in
the Chinese population. To the best of our knowledge, this current
study is the first time it has been indicated that determining
lyso-Gb3 levels and leukocyte enzyme activity are useful biomarkers
when diagnosing Chinese male patients with FD. However, the plasma
lyso-Gb3 level assay was not 100% effective, as some female
patients with FD exhibit nearly normal levels of lyso-Gb3.
The results of a previous study indicated that there
is an association between disease severity and the α-gal A activity
in female patients with FD (32).
The observed residual enzyme activity in plasma or blood cells from
male patients is a poor predictor of clinical course (32); therefore better clinical indicators
are required for male patients. To confirm the clinical application
of lyso-Gb3 in the current study, the correlation between MSSI and
enzyme activity, as well as between MSSI and lyso-Gb3, were
evaluated. The MSSI score was confirmed to be a useful, specific
measure for objectively assessing the severity of FD. Due to
financial constraints, some patients refused a full set of
examinations at diagnosis. The MSSI score could not be assessed in
the patients who did not have ultrasonic cardiogram or brain MRI
examinations. Due to a lack of comprehensive examination data, 15
patients (7 males and 8 females) without MSSI scores were excluded
prior to performing the further analyses of MSSI.
In male patients, lyso-Gb3 levels were more strongly
correlated with MSSI (r=0.711; P=0.004) than enzyme activity
(r=−0.687; P=0.007). However, in female patients, lyso-Gb3 and
enzyme activity were not correlated with MSSI (Fig. 6C and D). The results were slightly
different from those of previous studies (16,18),
which identified a strong correlation between lyso-Gb3 and MSSI in
females. No correlation was noted between the lyso-Gb3 level and
MSSI score in male patients (19,21).
This may be due to the fact that the majority of male patients in
the aforementioned studies exhibit extremely high lyso-Gb3 levels.
Smid et al (41) found the
lyso-Gb3 level to be markedly higher in classical FD than atypical
FD, especially in male patients. The male patients included in the
current study consisted of 10 patients with classical phenotypes
and 11 with atypical phenotypes (Table
I). As the male patients in the current study did not uniformly
exhibit high levels of lyso-Gb3, the correlation analysis between
MSSI and lyso-Gb3 concentration is more likely to have statistical
significance. Furthermore, the very small number of female patients
(n=9) included in the MSSI analysis of the current study may have
affected the results.
In conclusion, the results of the current study
indicated that plasma lyso-Gb3 levels may be a novel diagnostic
biomarker for patients with FD. It is particularly helpful at
diagnosing females exhibiting near-normal levels of enzyme activity
with FD. Furthermore, the strong correlation between lyso-Gb3 and
MSSI in male patient means that lyso-Gb3 levels are more useful
than enzyme levels at assessing disease severity in male patients.
However, no correlation was identified between lyso-Gb3 and MSSI in
female patients. To some extent, the lower number of female
patients included in the current study may have resulted in a data
bias, which may have affected statistical significance. Therefore,
future studies involving a larger cohort of patients with FD are
required, to confirm that these results are accurate.
Acknowledgements
We thank Genzyme Corporation (Cambridge, MA, USA)
for their support to this study.
Funding
This study was supported by grants from the National
Basic Research Program of China (973 program) (Grant no.
2012CB517604), the Key Program of Shanghai Science and Technology
Commission (Grant no. 08dz1900502), and the National Natural
Science Foundation of China (Grant no. 30871001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NC conceived and designed the study. YO, XP, ZW, HR,
YX, LY and NC collected the clinical and pathological data patients
with Fabry disease. YO and BC performed the analysis of plasma
lyso-Gb3. LN and XY performed the enzyme activity assay. XP and XY
performed the DNA/RNA sequencing. YO and BC analyzed the data. YO,
BC, XP and NC wrote and revised the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Ruijin Hospital and all participants provided their
informed consent prior to participation in the current study.
Written informed consent was obtained from the parents or guardians
of children enrolled.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Germain DP: Fabry disease. Orphanet J Rare
Dis. 5:302010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Askari H, Kaneski CR, Semino-Mora C, Desai
P, Ang A, Kleiner DE, Perlee LT, Quezado M, Spollen LE, Wustman BA
and Schiffmann R: Cellular and tissue localization of
globotriaosylceramide in Fabry disease. Virchows Arch. 451:823–834.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Desnick RJ, Brady R, Barranger J, Collins
AJ, Germain DP, Goldman M, Grabowski G, Packman S and Wilcox WR:
Fabry disease, an under-recognized multisystemic disorder: Expert
recommendations for diagnosis, management, and enzyme replacement
therapy. Ann Intern Med. 138:338–346. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elleder M, Bradová V, Smíd F, Budĕsínský
M, Harzer K, Kustermann-Kuhn B, Ledvinová J, Bĕlohlávek, Král V and
Dorazilová V: Cardiocyte storage and hypertrophy as a sole
manifestation of Fabry's disease. Report on a case simulating
hypertrophic non-obstructive cardiomyopathy. Virchows Arch A Pathol
Anat Histopathol. 417:449–455. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakao S, Kodama C, Takenaka T, Tanaka A,
Yasumoto Y, Yoshida A, Kanzaki T, Enriquez AL, Eng CM, Tanaka H, et
al: Fabry disease: Detection of undiagnosed hemodialysis patients
and identification of a ‘renal variant’ phenotype. Kidney Int.
64:801–807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brady RO, Gal AE, Bradley RM, Martensson
E, Warshaw AL and Laster L: Enzymatic defect in Fabry's disease.
Ceramidetrihexosidase deficiency. N Engl J Med. 276:1163–1167.
1967. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Linthorst GE, Vedder AC, Aerts JM and
Hollak CE: Screening for Fabry disease using whole blood spots
fails to identify one-third of female carriers. Clin Chim Acta.
353:201–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kitagawa T, Ishige N, Suzuki K, Owada M,
Ohashi T, Kobayashi M, Eto Y, Tanaka A, Mills K, Winchester B and
Keutzer J: Non-invasive screening method for Fabry disease by
measuring globotriaosylceramide in whole urine samples using tandem
mass spectrometry. Mol Genet Metab. 85:196–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whitfield PD, Calvin J, Hogg S, O'Driscoll
E, Halsall D, Burling K, Maguire G, Wright N, Cox TM, Meikle PJ and
Deegan PB: Monitoring enzyme replacement therapy in Fabry
disease-role of urine globotriaosylceramide. J Inherit Metab Dis.
28:21–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rozenfeld PA, De Francesco NP, Borrajo GJ,
Ceci R and Fossati CA: An easy and sensitive method for
determination of globotriaosylceramide (Gb3) from urinary sediment:
Utility for Fabry disease diagnosis and treatment monitoring. Clin
Chim Acta. 403:194–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Young E, Mills K, Morris P, Vellodi A, Lee
P, Waldek S and Winchester B: Is globotriaosylceramide a useful
biomarker in Fabry disease? Acta Paediatr Suppl. 94:51–54;
discussion 37–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blaydon D, Hill J and Winchester B: Fabry
disease: 20 novel GLA mutations in 35 families. Hum Mutat.
18:4592001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schäfer E, Baron K, Widmer U, Deegan P,
Neumann HP, Sunder-Plassmann G, Johansson JO, Whybra C, Ries M,
Pastores GM, et al: Thirty-four novel mutations of the GLA gene in
121 patients with Fabry disease. Hum Mutat. 25:4122005. View Article : Google Scholar
|
|
14
|
van der Tol L, Smid BE, Poorthuis BJ,
Biegstraaten M, Deprez RH, Linthorst GE and Hollak CE: A systematic
review on screening for Fabry disease: Prevalence of individuals
with genetic variants of unknown significance. J Med Genet. 51:1–9.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fazekas F, Enzinger C, Schmidt R, Grittner
U, Giese AK, Hennerici MG, Huber R, Jungehulsing GJ, Kaps M,
Kessler C, et al: Brain magnetic resonance imaging findings fail to
suspect Fabry disease in young patients with an acute
cerebrovascular event. Stroke. 46:1548–1553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van der Tol L, Cassiman D, Houge G,
Janssen MC, Lachmann RH, Linthorst GE, Ramaswami U, Sommer C,
Tøndel C, West ML, et al: Uncertain diagnosis of fabry disease in
patients with neuropathic pain, angiokeratoma or cornea
verticillata: Consensus on the approach to diagnosis and follow-up.
JIMD Rep. 17:83–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Tol L, Svarstad E, Ortiz A, Tøndel
C, Oliveira JP, Vogt L, Waldek S, Hughes DA, Lachmann RH, Terryn W,
et al: Chronic kidney disease and an uncertain diagnosis of Fabry
disease: Approach to a correct diagnosis. Mol Genet Metab.
114:242–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smid BE, van der Tol L, Cecchi F, Elliott
PM, Hughes DA, Linthorst GE, Timmermans J, Weidemann F, West ML,
Biegstraaten M, et al: Uncertain diagnosis of Fabry disease:
Consensus recommendation on diagnosis in adults with left
ventricular hypertrophy and genetic variants of unknown
significance. Int J Cardiol. 177:400–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aerts JM, Groener JE, Kuiper S,
Donker-Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian
M, Wijburg FA, Linthorst GE, et al: Elevated
globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl
Acad Sci USA. 105:pp. 2812–2817. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Togawa T, Kodama T, Suzuki T, Sugawara K,
Tsukimura T, Ohashi T, Ishige N, Suzuki K, Kitagawa T and Sakuraba
H: Plasma globotriaosylsphingosine as a biomarker of Fabry disease.
Mol Genet Metab. 100:257–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rombach SM, Dekker N, Bouwman MG,
Linthorst GE, Zwinderman AH, Wijburg FA, Kuiper S, Vd Bergh Weerman
MA, Groener JE, Poorthuis BJ, et al: Plasma
globotriaosylsphingosine: Diagnostic value and relation to clinical
manifestations of Fabry disease. Biochim Biophys Acta.
1802:741–748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krüger R, Tholey A, Jakoby T, Vogelsberger
R, Mönnikes R, Rossmann H, Beck M and Lackner KJ: Quantification of
the Fabry marker lysoGb3 in human plasma by tandem mass
spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci
883–884. 1–135. 2012.
|
|
23
|
Gold H, Mirzaian M, Dekker N, Joao Ferraz
M, Lugtenburg J, Codée JD, van der Marel GA, Overkleeft HS,
Linthorst GE, Groener JE, et al: Quantification of
globotriaosylsphingosine in plasma and urine of fabry patients by
stable isotope ultraperformance liquid chromatography-tandem mass
spectrometry. Clin Chem. 59:547–556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boutin M, Gagnon R, Lavoie P and
Auray-Blais C: LC-MS/MS analysis of plasma lyso-Gb3 in Fabry
disease. Clin Chim Acta. 414:273–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lukas J, Giese AK, Markoff A, Grittner U,
Kolodny E, Mascher H, Lackner KJ, Meyer W, Wree P, Saviouk V and
Rolfs A: Functional characterisation of alpha-galactosidase a
mutations as a basis for a new classification system in fabry
disease. PLoS Genet. 9:e10036322013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lavoie P, Boutin M and Auray-Blais C:
Multiplex analysis of novel urinary lyso-Gb3-related biomarkers for
Fabry disease by tandem mass spectrometry. Anal Chem. 85:1743–1752.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boutin M and Auray-Blais C: Multiplex
tandem mass spectrometry analysis of novel plasma
lyso-Gb3-related analogues in Fabry disease. Anal Chem.
86:3476–3483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Auray-Blais C, Boutin M, Gagnon R, Dupont
FO, Lavoie P and Clarke JT: Urinary
globotriaosylsphingosine-related biomarkers for Fabry disease
targeted by metabolomics. Anal Chem. 84:2745–2753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dupont FO, Gagnon R, Boutin M and
Auray-Blais C: A metabolomic study reveals novel plasma lyso-Gb3
analogs as Fabry disease biomarkers. Curr Med Chem. 20:280–288.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sessa A, Toson A, Nebuloni M, Pallotti F,
Giordano F, Battini G, Maglio A, Meroni M, Calconi G, Bertolone G
and Gatti P: Renal ultrastructural findings in Anderson-Fabry
disease. J Nephrol. 15:109–112. 2002.PubMed/NCBI
|
|
31
|
Desnick RJ, Allen KY, Desnick SJ, Raman
MK, Bernlohr RW and Krivit W: Fabry's disease: Enzymatic diagnosis
of hemizygotes and heterozygotes. Alpha-galactosidase activities in
plasma, serum, urine, and leukocytes. J Lab Clin Med. 81:157–171.
1973.PubMed/NCBI
|
|
32
|
Pan X, Ouyang Y, Wang Z, Ren H, Shen P,
Wang W, Xu Y, Ni L, Yu X, Chen X, et al: Genotype: A crucial but
not unique factor affecting the clinical phenotypes in fabry
disease. PLoS One. 11:e01613302016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwartz GJ, Haycock GB, Edelmann CM Jr
and Spitzer A: A simple estimate of glomerular filtration rate in
children derived from body length and plasma creatinine.
Pediatrics. 58:259–263. 1976.PubMed/NCBI
|
|
35
|
Whybra C, Kampmann C, Krummenauer F, Ries
M, Mengel E, Miebach E, Baehner F, Kim K, Bajbouj M, Schwarting A,
et al: The mainz severity score index: A new instrument for
quantifying the Anderson-Fabry disease phenotype, and the response
of patients to enzyme replacement therapy. Clin Genet. 65:299–307.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bligh EG and Dyer WJ: A rapid method of
total lipid extraction and purification. Can J Biochem Physiol.
37:911–917. 1959. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
NCCLS, . Evaluation of Precision
Performance of Quantitative Measurement Methods; Approved
Guideline-Second Edition. NCCLS document EP5-A2. ISBN:
1-56238-542-9NCCLS; Wayne, PA: 2004
|
|
38
|
NCCLS, . Evaluation of the Linearity of
Quantitative Measurement Procedures: A Statistical Approach;
Approved Guideline. NCCLS document EP6-A. ISBN: 1-56238-498-8NCCLS;
Wayne, PA: 2003
|
|
39
|
Jamboti J and Forrest CH: Fabry disease;
early diagnosis improves prognosis but diagnosis is often delayed.
J Nephropathol. 6:130–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kes VB, Cesarik M, Zavoreo I, Butković SS,
Kes P, Bašić-Jukić N, Rački S, Jakić M, Delić-Brkljačić D, Jukić Z,
et al: Guidelines for diagnosis, therapy and follow up of
Anderson-Fabry disease. Acta Med Croatica. 68:223–232.
2014.PubMed/NCBI
|
|
41
|
Smid BE, van der Tol L, Biegstraaten M,
Linthorst GE, Hollak CE and Poorthuis BJ: Plasma
globotriaosylsphingosine in relation to phenotypes of Fabry
disease. J Med Genet. 52:262–268. 2015. View Article : Google Scholar : PubMed/NCBI
|