Introduction
Diabetic retinopathy is one of the common
complications in patients with advanced diabetes, which is the main
cause for patient's hypopsia and even blindness (1). With the change in eating habits and
lifestyle of the Chinese people, the incidence rate of diabetes is
rising significantly. Some studies indicate that the prevalence of
type 2 diabetes among people aged over 60 years is close to 10%
(2). In addition, the probability of
developing diabetic retinopathy is nearly 30% among patients with
diabetes for more than 20 years (3).
To date, there is no very effective method for treating the
disease.
Oxidative stress and signaling pathways activated by
stress are involved in the occurrence and development of diabetic
retinopathy. Studies have shown that (3) increase in oxidative stress can be
observed both in the retina of diabetic individuals and in the
retinal endothelial cells and pericytes cultured in high glucose.
Oxidative stress can damage the retinal endothelial cells and
pericytes, resulting in pathological changes of the retina.
Probucol has strong anti-oxidative stress and hypolipidemic
effects, clinical application of probucol in the treatment of
diabetic nephropathy and prevention and treatment of
atherosclerosis associated with type 2 diabetes has achieved
preferable results (4,5). However, there is scarce literature on
pobucol for the treatment of diabetic retinopathy.
The primary purpose of this study was to investigate
the effect of probucol in the treatment of diabetic retinopathy and
analyze its impact on its hemodynamics, rheology and blood
lipid.
Patients and methods
Patient data
A total of 80 patients with diabetic retinopathy who
were treated in the Ninth People's Hospital of Chongqing
(Chongqing, China) from January 2015 to August 2016 were selected,
and all the patients were confirmed by glucose tolerance test,
glucose determination and ophthalmoscopy. Before enrollment, signed
informed consent of the patients was obtained and the research was
approved by the Ethics Committee of The Ninth People's Hospital of
Chongqing. Signed written informed consents were obtained from the
patients and/or guardians. Patients with one of the following
events were excluded: complicated with serious cardiac and
pulmonary dysfunction, associated with type 1 diabetes, complicated
with secondary hypertension, associated with chronic obstructive
pulmonary disease, complicated with heart failure, associated with
severe hepatic or renal dysfunction, complicated with coagulation
disorders, associated with nephrotic syndrome, use of lipid
regulating drugs within 3 months before enrollment, use of drugs
affecting the hemodynamics within 3 months before enrollment and
refusal to sign for enrollment. All the patients were divided into
two groups by random number table, with 40 patients in each group.
In observation group, there were 28 men and 12 women aged 40–80
years, with an average age of 58.2±3.1 years; the duration of
diabetes was 10–35 years, with an average duration of 19.2±2.1
years; the duration of complicated retinopathy was 1–6 years, with
an average duration of 3.1±0.2 years; there were 23 cases of
microangioma, 7 cases of hemorrhage spot, 5 cases of yellow and
white exudate and 5 cases of macular edema. In control group, there
were 29 men and 11 women aged 40–80 years, with an average age of
58.3±3.0 years; the duration of diabetes was 10–35 years, with an
average duration of 19.3±2.0 years; the duration of complicated
retinopathy was 1–6 years, with an average duration of 3.0±0.2
years; the number of cases of microangioma, hemorrhage spot, yellow
and white exudate as well as macular edema was 24, 6, 6 and 4,
respectively. The differences in gender, age, duration of diabetes,
duration of complicated retinopathy and clinical manifestations of
fundus lesions between the two groups were not statistically
significant (P>0.05) (Table
I).
 | Table I.Demographic data for the patients in
the two groups. |
Table I.
Demographic data for the patients in
the two groups.
| Group | N | Male/female | Age (years) | Duration of diabetes
(years) | With
microangioma | With hemorrhage
spot | With yellow and white
exudate | With macular
edema |
|---|
| Observation | 40 | 28/12 | 58.2±3.1 | 19.2±2.1 | 23 | 7 | 5 | 5 |
| Control | 40 | 29/11 | 58.3±3.0 |
| 24 | 6 | 6 | 4 |
Therapeutics for two groups
Dietary control, exercise therapy and oral
hypoglycemic drugs were applied to all the enrolled patients to
treat diabetes. Whether subcutaneous injection of insulin was
performed or not was determined by the change of blood glucose. It
was generally recommended that fasting blood glucose be controlled
within 7.0 mmol/l and 2 h postprandial blood glucose within 11.1
mmol/l and glycosylated hemoglobin be regulated <7.0%.
Meanwhile, health education about diabetes in patients was
strengthened. Control group was given metformin and acarbose to
control the blood glucose, and observation group was treated with
probucol (0.375 g twice a day) (Shanghai First Pharma Ltd.,
Shanghai, China) on basis of blood glucose control.
Observation indicators
Outpatient follow-up was performed to all the
patients for 6 months. Among the blood rheology, the changes in
blood viscosity and erythrocyte aggregation indexes at different
time points before and after intervention in the two groups were
compared. Mean blood flow velocities in renal artery, renal artery
pulse indexes and renal artery resistance indexes at different time
points were recorded. Changes in blood lipid of the two groups
before and after intervention were compared, and the rate of
complications during the treatment was calculated.
Detection methods
The measurement of whole blood viscosity (at high
and low shear rates) and plasma viscosity were performed using
LG-R-80F series automated hematology analyzer manufactured by
Beijing Steellex Scientific Instrument Co. (Beijing, China), and
SYSMEX CA-7000 automatic blood biochemical analyzer was used to
measure the erythrocyte aggregation index. Color Doppler ultrasound
(Bioson Corp., Beijing, China) was utilized to detect renal blood
flow. The patients were in the left lateral position and right
lateral position during the examination. The flow spectrain
bilateral main renal arteries, segmental renal arteries and middle
sections of renal interlobar arteries were recorded, respectively,
to calculate the mean blood flow velocities as well as the pulse
indexes and resistance indexes. The values of the above mentioned
examinations were based on the mean values of the main renal
arteries, segmental renal arteries and renal interlobar arteries,
so that the mean of the measured values in the bilateral main renal
arteries was taken as the enrollment data. As for indexes related
to blood lipid, total cholesterol (TC), triglyceride (TG), low
density lipoprotein cholesterol (LDL-C) and high-density
lipoprotein cholesterol (HDL-C) were detected using Aeroset series
automatic biochemistry analyzers (Abbott, Abbott Park, IL, USA).
Samples collection method was that the venous blood was taken when
the patients were enrolled and fasting venous blood was taken in
the morning 6 months after intervention.
Statistical analysis
SPSS 21.0 (IBM Corp., New York, NY, USA) was used
for statistical processing. The measurement data are presented as
mean ± standard deviation, and the enumeration data were calculated
at %; t-test was used for mean comparison between groups, analysis
of variance of repeated measures was performed for mean comparison
within the group, and χ2 test was used for rate
comparison between groups. P<0.05 suggested that the difference
was statistically significant and comparable.
Results
Comparisons of plasma viscosity
changes in blood rheology in two groups before and after
intervention
Before intervention, the differences in whole blood
viscosity at high shear rate, whole blood viscosity at low shear
rate and plasma viscosity between the two groups were not
statistically significant (P>0.05). After intervention, the
whole blood viscosity at high and low shear rates and plasma
viscosity in observation group were lower than those before
intervention (P<0.05), and those indexes were lower than those
in control group after intervention (P<0.05) (Table II).
 | Table II.Comparisons of plasma viscosity
changes in blood rheology in two groups before and after
intervention (mPa/s, mean ± SD). |
Table II.
Comparisons of plasma viscosity
changes in blood rheology in two groups before and after
intervention (mPa/s, mean ± SD).
| Group |
| Whole blood viscosity
at high shear rate | Whole blood viscosity
at low shear rate | Plasma viscosity |
|---|
| Observation | Before
intervention | 5.99±0.09 | 12.03±0.08 | 1.85±0.03 |
|
| After
intervention |
5.56±0.03a,b |
10.82±0.06a,b |
1.61±0.01a,b |
| Control | Before
intervention | 5.98±0.10 | 12.04±0.08 | 1.86±0.03 |
|
| After
intervention | 5.73±0.06 | 11.12±0.05 | 1.75±0.02 |
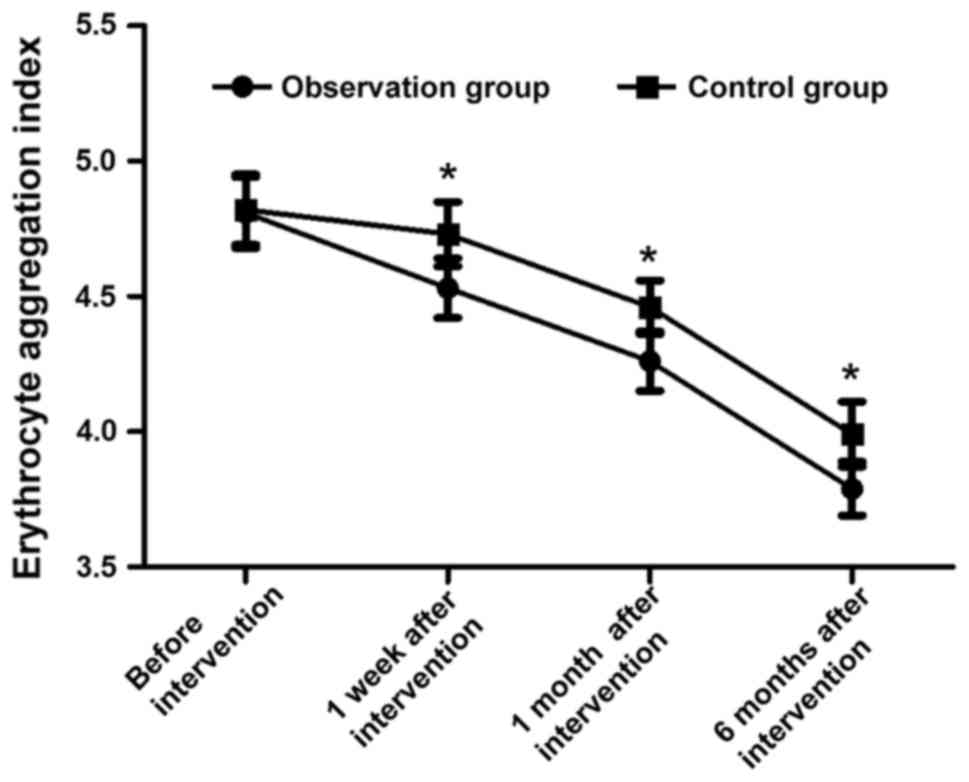
Comparisons of erythrocyte aggregation
indexes at different time points during intervention in two
groups
Before intervention, the erythrocyte aggregation
indexes in the two groups were 4.81±0.13 and 4.82±0.13,
respectively, and the difference was not statistically significant
(t=0.344, P>0.05). The erythrocyte aggregation indexes in
observation groups were 4.53±0.11, 4.26±0.11 and 3.79±0.10 at 1
week, 1 month and 6 months after intervention, respectively, which
were obviously decreased compared with those in control group
[4.73±0.12, 4.46±0.10 and 3.99±0.12, respectively; (t=7.770,
t=19.995 and t=8.098, respectively, P<0.05)] (Fig. 1).
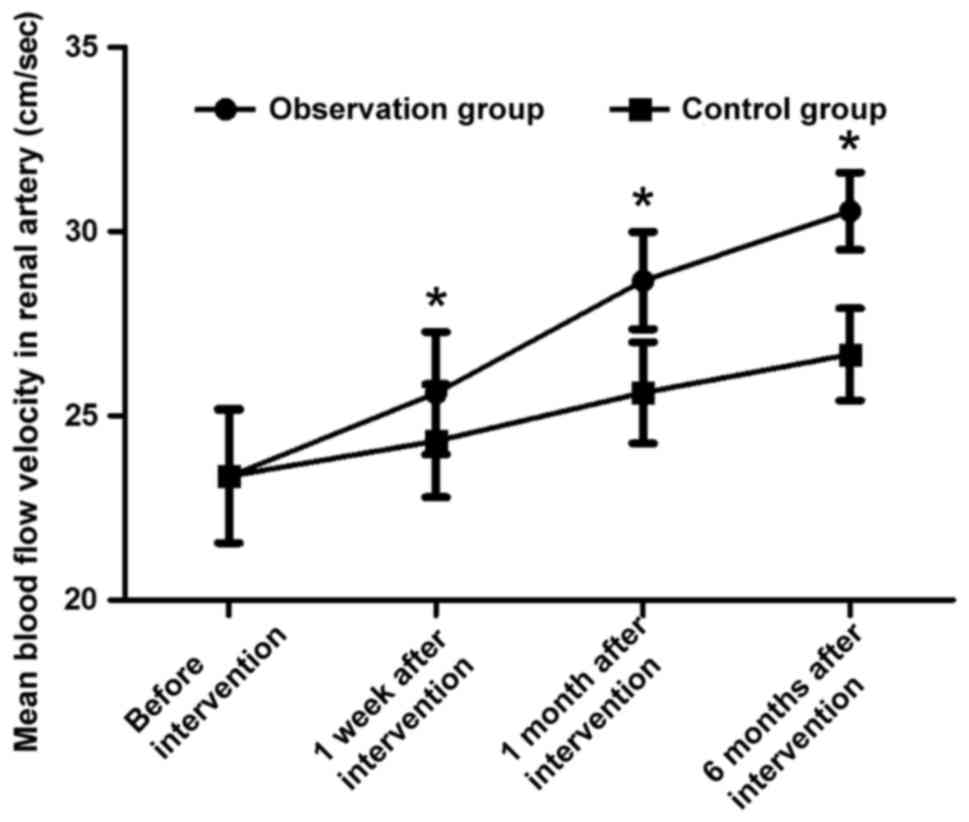
Comparisons of mean blood flow
velocities in renal artery at different time points during
intervention in two groups
Before intervention, the mean blood flow velocities
in renal artery in the two groups were 23.36±1.81 cm/sec and
23.37±1.82 cm/sec, respectively, and the difference was not
statistically significant (t=0.025, P>0.05). The mean blood flow
velocities in renal artery in observation group were 25.62±1.66
cm/sec, 28.67±1.32 cm/sec and 30.56±1.05 cm/sec at 1 week, 1 month
and 6 months after intervention, respectively, which were
remarkably higher than those in control group [24.32±1.53 cm/sec,
25.63±1.38 cm/sec and 26.67±1.25 cm/sec, respectively; (t=3.642,
t=10.068 and t=15.071, respectively, P<0.05)] (Fig. 2).
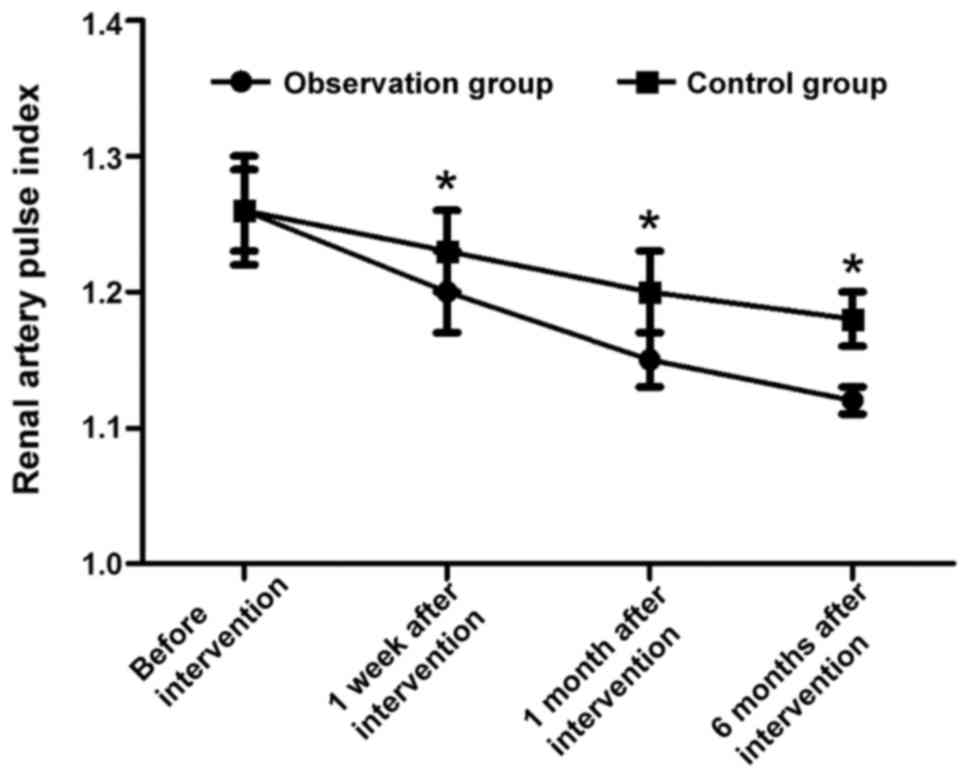
Comparisons of renal artery pulse
indexes at different time points during intervention in two
groups
Before intervention, the renal artery pulse indexes
in the two groups were 1.26±0.04 and 1.26±0.03, respectively, and
the difference was not statistically significant (t=0.000,
P>0.05). The renal artery pulse indexes in observation group
were 1.20±0.03, 1.15±0.02 and 1.12±0.01 at 1 week, 1 month and 6
months after intervention, respectively, which were notably lower
than those in control group [1.23±0.03, 1.20±0.03 and 1.18±0.02,
respectively; (t=4.472, t=8.771 and t=16.971, respectively,
P<0.05)] (Fig. 3).
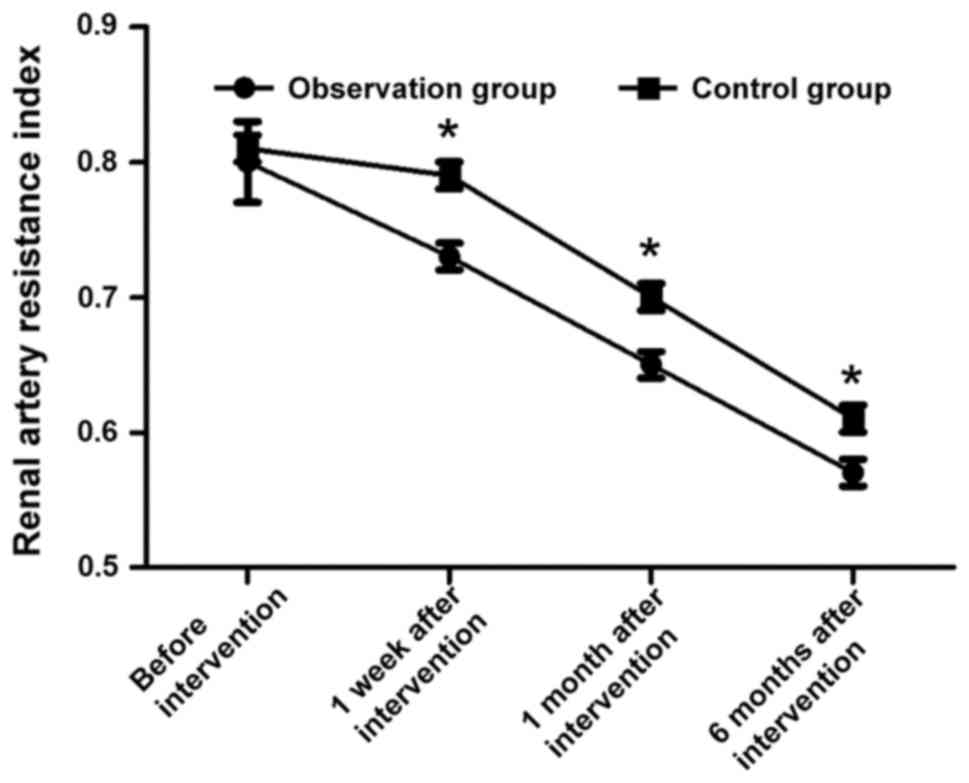
Comparisons of renal artery resistance
indexes at different time points during intervention in two
groups
Before intervention, the renal artery resistance
indexes in the two groups were 0.80±0.03 and 0.81±0.03,
respectively, and the difference was not statistically significant
(t=1.491, P>0.05). The renal artery resistance indexes in
observation group were 0.73±0.01, 0.65±0.01 and 0.57±0.01 at 1
week, 1 month and 6 months after intervention, respectively, which
were much lower than those in control group [0.79±0.01, 0.70±0.01
and 0.61±0.01, respectively; (t=26.833, t=22.361 and t=17.889,
respectively, P<0.05)] (Fig.
4).
Comparisons of changes in blood lipid
in the two groups before and after intervention
By comparing the levels of TC, TG, LDL-C and HDL-C
before intervention, the differences between the two groups were
not statistically significant (P>0.05). In observation group,
the levels of TC, TG and LDL-C after intervention were decreased
compared with those before intervention (P<0.05), while the
level of HDL-C was increased (P<0.05). After intervention, the
levels of TC, TG and LDL-C in observation group were lower than
those in control group (P<0.05), while the HDL-C level was
higher (P<0.05) (Table
III).
 | Table III.Comparisons of changes in blood lipid
in the two groups before and after intervention (mmol/l, mean ±
SD). |
Table III.
Comparisons of changes in blood lipid
in the two groups before and after intervention (mmol/l, mean ±
SD).
| Group |
| TC | TG | LDL-C | HDL-C |
|---|
| Observation | Before
intervention | 6.13±0.02 | 2.35±0.02 | 3.5±0.03 | 1.32±0.02 |
|
| After
intervention |
4.61±0.01a,b |
1.45±0.01a,b | 2.3±0.02a,b |
1.49±0.03a,b |
| Control | Before
intervention | 6.15±0.03 | 2.36±0.03 | 3.6±0.03 | 1.33±0.02 |
|
| After
intervention | 5.51±0.01 | 1.85±0.01 | 2.7±0.02 | 1.39±0.02 |
Comparisons of complications during
treatment in the two groups
During the treatment, the total incidence of
phlebitis, chills, fever, rash and maculopapule in observation
group was obviously lower than that in control group (P<0.05)
(Table IV).
 | Table IV.Comparisons of complications during
treatment in the two groups (n, %). |
Table IV.
Comparisons of complications during
treatment in the two groups (n, %).
| Items | Phlebitis | Chills and fever | Rash and
maculopapule | Total incidence |
|---|
| Observation | 0 | 1 | 1 | 2 (5.0%) |
| Control | 2 | 3 | 5 | 10 (25.0%) |
| χ2
value | – | 4.804 |
|
|
| P-value | – | 0.028 |
|
|
Discussion
Diabetic retinopathy, one of the most common
microvascular diseases of advanced diabetes, is a specific ocular
fundus vascular lesion and a serious complication of diabetes
(6). Its diagnosis and typing is
based on whether there is retinal neovascularization. Patients
without retinal neovascularization are integrated into simple-type
or background-type diabetic retinopathy (7), while patients with retinal
neovascularization are integrated into proliferative diabetic
retinopathy (8). With regard to
treatment of this disease, patients with early lesions generally do
not need specific treatment. Enhanced clinical observation and
follow-up is sufficient, of which observation of changes in
clinical ocular symptoms and control of blood glucose should be
conducted (9) to prevent and
decrease further development of ocular diseases. When complicated
ocular fundus hemorrhage and neovascularization occur, fluoresce in
angiography should be performed in time for diagnosis confirmation
so as to exclude non-perfused regions in ocular fundus (10). Alternatively, panretinal photo
coagulation or even vitrectomy can be implemented in the early
stage to avoid irreversible changes of vision acuity in patients
(11). So far, however, there is no
particularly efficient agent in drug therapies, especially the
medical treatment for patients in early and middle stage, in
clinical practices (12).
In this study, observation group was given probucol
therapy on the basis of conventional glycemic control. By comparing
the changes in the blood rheology in the two groups before and
after intervention, it was found that the whole blood viscosities
at high and low shear rates as well as plasma viscosity in
observation group were lower than those before probucol
intervention and those in control group. Moreover, by comparing the
erythrocyte aggregation indexes at different time points during the
intervention in the two groups, it was discovered that the
erythrocyte aggregation indexes in observation group were obviously
increased compared with those in control group at 1 week, 1 month
and 6 months after intervention. It suggests that application of
probucol in the treatment of diabetic nephropathy can remarkably
improve the rheological indexes of the patients, reduce blood
viscosity and raise the blood flow velocity in renal artery.
Moreover, in terms of the mean blood flow velocities in renal
artery, renal artery pulse indexes and resistance indexes at
different time points during the intervention, it was found that
the mean blood flow velocities in renal artery in observation group
were remarkably higher than those in control group at 1 week, 1
month and 6 months after intervention, while the renal artery
resistance indexes and pulse indexes in observation group were
lower than those in control group in the same period. It indicates
that use of probucol therapy for treatment of diabetic retinopathy
can ameliorate the hemodynamic indexes of the patients, improve
blood flow in renal arteries and decrease vascular resistance. By
comparing the blood lipid changes before and after intervention in
the two groups, it showed that in the observation group, the levels
of TC, TG and LDL-C after intervention were decreased compared with
those before intervention (P<0.05), while the HDL-C level was
increased. After intervention, the levels of TC, TG and LDL-C in
observation group were lower than those in control group
(P<0.05), while the HDL-C level was higher. It suggests that for
patients with diabetic retinopathy, probucol therapy can better
regulate the metabolism of blood lipid and lower the blood lipid
level. Finally, through comparison of the complications during the
treatment in the two groups, it was found that the total incidence
of phlebitis, chills, fever, rash and maculopapule in observation
group was obviously lower than that in control group during the
treatment. It proves that probucol therapy has high safety.
Probucol can effectively decrease blood lipid and
ameliorate the blood rheology and hemodynamics (13). On treating diabetic retinopathy, it
can improve the blood flow in the retinal vessels and reduce
formation of thrombus by means of inhibiting platelet aggregation
and thromboxane release produced by it in the body (14). Moreover, the drug can adjust the
prostaglandin level in the body and reduce retinal
vasoconstriction. Furthermore, it can lower the fibrinogen level in
plasma (15), improve coagulation
function of the body, regulate fibrinolytic function and decrease
the blood viscosity (16). Probucol
can also reduce endothelin release in the body, thus increasing the
level of nitric oxide in the blood vessels (17), promoting vasodilatation and
ameliorating rheological indexes (18). In respect of blood lipid regulation,
probucol can effectively alleviate endothelial injury and reduce
platelet aggregation, which can not only have an anti-coagulating
effect (19), but also lower the
levels of TC, TG and LDL-C in the body, thus having a hypolipidemic
effect (20).
However, there are still some limitations of our
study. The sample size of cases and controls is small due to the
limitation of time and funds, and we will increase the sample size
for further study later. The relationship between various indices
of diabetic retinopathy and outpatient follow-up of the impact of
probucolin was not investigated according to the severity of the
disease because the sample size was not large, and we will further
study the related issues in subsequent studies. In conclusion,
application of probucol in treatment of diabetic retinopathy can
significantly improve the hemodynamic and rheological indexes and
lower blood lipid in the body.
Acknowledgements
We would like to thank Dr Wen Wei and Dr Jie Zhang
for their assistance.
Funding
The present study was supported by the Chongqing
Municipal Health Bureau (no. 2016MSXM145).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HL performed the study and wrote the manuscript. MC
analysed the data and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Ninth People's Hospital of Chongqing (Chongqing, China). Signed
written informed consents were obtained from the patients and/or
guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fei YX, Wang SQ, Yang LJ, Qiu YY, Li YZ,
Liu WY, Xi T, Fang WR and Li YM: Salvia miltiorrhiza Bunge
(Danshen) extract attenuates permanent cerebral ischemia through
inhibiting platelet activation in rats. J Ethnopharmacol.
207:57–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bi X, Zhang K, He L, Gao B, Gu Q, Li X,
Chen J and Wang J: Synthesis and biological evaluation of
tanshinone IIA derivatives as novel endothelial protective agents.
Future Med Chem. 9:1073–1085. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sha J, Sui B, Su X, Meng Q and Zhang C:
Alteration of oxidative stress and inflammatory cytokines induces
apoptosis in diabetic nephropathy. Mol Med Rep. 16:7715–7723. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murata Y, Sugi T, Weiss LM and Kato K:
Identification of compounds that suppress Toxoplasma
gondiitachyzoites and bradyzoites. PLoS One. 12:e01782032017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Endo K, Miyashita Y, Sasaki H, Ohira M,
Saiki A, Koide N, Otsuka M, Oyama T, Takeyoshi M, Ito Y, et al:
Probucol delays progression of diabetic nephropathy. Diabetes Res
Clin Pract. 71:156–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ojha S, Balaji V, Sadek B and Rajesh M:
Beneficial effects of phytochemicals in diabetic retinopathy:
Experimental and clinical evidence. Eur Rev Med Pharmacol Sci.
21:2769–2783. 2017.PubMed/NCBI
|
|
7
|
Qin YL, Chen L, He W, Su M, Jin Q, Fang Z,
Ouyang PK and Guo K: Continuous synthesis and anti-myocardial
injury of tanshinone IIA derivatives. J Asian Nat Prod Res. 8:1–9.
2017.
|
|
8
|
Yao NW, Lu Y, Shi LQ, Xu F and Cai XH:
Neuroprotective effect of combining tanshinone IIA with low-dose
methylprednisolone following acute spinal cord injury in rats. Exp
Ther Med. 13:2193–2202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen PX, Zhang YL, Xu JW, Yu MH, Huang JH,
Zhao L and Zhou WL: Sodium tanshinone IIA sulfonate stimulated
Cl-secretion in mouse trachea. PLoS One. 12:e01782262017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Li Z, Li X, Liu B and Liu Z:
Mechanisms of Tanshinone IIA inhibits malignant melanoma
development through blocking autophagy signal transduction in A375
cell. BMC Cancer. 17:3572017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen F, Li H, Zhu G, Chen X and Tang Z:
Sodium tanshinone IIA sulfonate improves inflammation, aortic
endothelial cell apoptosis, disseminated intravascular coagulation
and multiple organ damage in a rat heat stroke model. Mol Med Rep.
16:87–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang LM, Wang LX, Wang ZY, Sun LF, Pan XD
and Pan GQ: Tanshinone IIA ameliorates lead (Pb)-induced cognitive
deficits and oxidative stress in a rat pup model. Bratisl Lek
Listy. 118:196–201. 2017.PubMed/NCBI
|
|
13
|
Parthasarathy S, Young SG, Witztum JL,
Pittman RC and Steinberg D: Probucol inhibits oxidative
modification of low density lipoprotein. J Clin Invest. 77:641–644.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Endo K, Miyashita Y, Sasaki H, Ebisuno M,
Ohira M, Saiki A, Koide N, Oyama T, Takeyoshi M and Shirai K:
Probucol and atorvastatin decrease urinary
8-hydroxy-2′-deoxyguanosine in patients with diabetes and
hypercholesterolemia. J Atheroscler Thromb. 13:68–75. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma J, Zhao S, Gao G, Chang H, Ma P and Jin
B: Probucol protects against asymmetric dimethylarginine-induced
apoptosis in the cultured human brain microvascular endothelial
cells. J Mol Neurosci. 57:546–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mooranian A, Negrulj R, Arfuso F and
Al-Salami H: Multicompartmental, multilayered probucol
microcapsules for diabetes mellitus: Formulation characterization
and effects on production of insulin and inflammation in a
pancreatic β-cell line. Artif Cells Nanomed Biotechnol.
44:1642–1653. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong JK, Guo ZG, Li C, Wang ZK, Lai WY
and Tu Y: Probucol alleviates atherosclerosis and improves high
density lipoprotein function. Lipids Health Dis. 10:2102011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Santos DB, Peres KC, Ribeiro RP, Colle D,
dos Santos AA, Moreira EL, Souza DO, Figueiredo CP and Farina M:
Probucol, a lipid-lowering drug, prevents cognitive and hippocampal
synaptic impairments induced by amyloid β peptide in mice. Exp
Neurol. 233:767–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braun A, Zhang S, Miettinen HE, Ebrahim S,
Holm TM, Vasile E, Post MJ, Yoerger DM, Picard MH, Krieger JL, et
al: Probucol prevents early coronary heart disease and death in the
high-density lipoprotein receptor SR-BI/apolipoprotein E double
knockout mouse. Proc Natl Acad Sci USA. 100:7283–7288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yakushiji E, Ayaori M, Nishida T, Shiotani
K, Takiguchi S, Nakaya K, Uto-Kondo H, Ogura M, Sasaki M, Yogo M,
et al: Probucol-Oxidized products, spiroquinone and diphenoquinone,
promote reverse cholesterol transport in mice. Arterioscler Thromb
Vasc Biol. 36:591–597. 2016. View Article : Google Scholar : PubMed/NCBI
|