Introduction
Preeclampsia is characterized by development of
hypertension and proteinuria after 20 weeks of gestation, and is
considered to be a multisystem disorder associated with pregnancy.
Worldwide, >70,000 maternal deaths per annum are associated with
hypertensive disorders arising during pregnancy, mainly
preeclampsia (1). Preeclampsia is
also associated with increased long-term cardiovascular mortality
for mother and infant (2).
In 1979, Crandon and Isherwood (3) first reported that patients who had
taken aspirin during pregnancy were less likely to suffer from
preeclampsia than those who had not. Over subsequent decades,
>50 trials and 27 meta-analyses have investigated the use of
low-dose aspirin for the prevention of preeclampsia. However, based
on the results provided by high-quality, multicenter randomized
controlled trials (RCTs) involving a large number of women and of
systematic reviews, the efficacy of aspirin in reducing
preeclampsia and associated outcomes remains controversial
(4–7). Of note, the effectiveness of low-dose
aspirin in preventing preeclampsia may be associated with the
time-point of treatment initiation. The World Health Organization
recommend that administration of low-dose aspirin (75 mg/day) for
the prevention of preeclampsia in high-risk females should start
during early pregnancy (8).
Recently, a multicenter, double-blinded,
placebo-controlled trial including 1,776 women with singleton
pregnancies who received low-dose aspirin or placebo from early
gestation until 36 weeks of gestation indicated that aspirin
decreases the incidence of preterm preeclampsia. However, no
significant differences were identified between groups regarding
the incidence of neonatal adverse outcomes or other adverse events
(7). Therefore, it is possible that
early use of aspirin may be more effective in preventing preterm
than term preeclampsia, or in preventing other adverse outcomes. It
is important to better understand the effects of aspirin associated
with this indication, as it is currently the best option for
improving outcomes for females at risk of preeclampsia and
associated adverse sequelae. The aim of the present study was to
evaluate the efficacy of low-dose aspirin administration to females
at risk of preeclampsia commenced at ≤16 weeks of gestation in
preventing preeclampsia, including preterm and term preeclampsia,
as well as the impact on associated maternal and neonatal adverse
events.
Materials and methods
Search strategy
In the present study, a systematic review and
meta-analysis of RCTs that evaluated the effect of aspirin intake
during pregnancy was performed. Relevant citations from January
1979 until October 2017 were extracted from the Embase, PubMed,
Cochrane Central Register of Controlled Trials and Web of Science
databases. A combination of keywords and MeSH terms was used for
the search: ‘aspirin’, ‘antiplatelet’, ‘acetylsalicylic acid’,
‘ASA’, ‘pregnancy-complication’, ‘pregnancy’, ‘eclampsia’,
‘hypertens*’, ‘blood press*’, ‘*eclamp*’, ‘PIH’ and ‘toxemia’. No
language restriction was imposed. A first reviewer sorted all
articles by citations and abstract for more detailed evaluation.
Two independent reviewers then selected relevant abstracts and
citations for complete evaluation of the studies. The quality of
this review was validated according to the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses statement (9).
Selection criteria
Only prospective, randomized controlled trials were
included. The included population was pregnant females at risk of
preeclampsia who were randomized into a low-dose aspirin and a
placebo or no treatment group, at ≤16 weeks of gestation. Low-dose
aspirin was defined as 50–150 mg daily. The following exclusion
criteria were applied: Trials with i) no control group; ii)
incomplete data or no data; and iii) repeated studies on the same
subjects. Each potentially eligible study was assessed
independently by at least two researchers and the risk of bias of
the studies was evaluated using the Cochrane Handbook for
Systematic Reviews of Interventions (10). Discrepancies were resolved by
discussion or by consultation with a third reviewer.
Outcomes
The primary outcome was preeclampsia (hypertension
with new-onset proteinuria at ≥20 weeks of gestation regardless of
delivery time) and its subcategories: Preterm preeclampsia
(delivered at <37 weeks) and term preeclampsia. Secondary
outcomes were other maternal adverse events, including gestational
hypertension, preterm birth (delivered at <34 weeks) and
postpartum hemorrhage, as well as neonatal adverse events,
including intrauterine growth retardation (IUGR), infant small for
gestational age (SGA), stillbirth or infant death, and newborn
weight.
Statistical analysis
The data were analyzed using RevMan 5.3 software
(The Cochrane Collaboration, London, UK). The significance
threshold for the chi-square test was set at α=0.1, and it was
deemed that heterogeneity existed when P<0.1. Heterogeneity
between studies was determined by calculating the Higgins
I2 value and considered high if it was ≥50%. The
individual risk ratio (RR) and 95% confidence intervals (CI) were
estimated using a fixed-effects model if no heterogeneity existed;
otherwise, the random-effects model was used. Publication bias was
tested by visual inspection of funnel plots generated using a
Begg's test.
Results
Study selection and evaluation
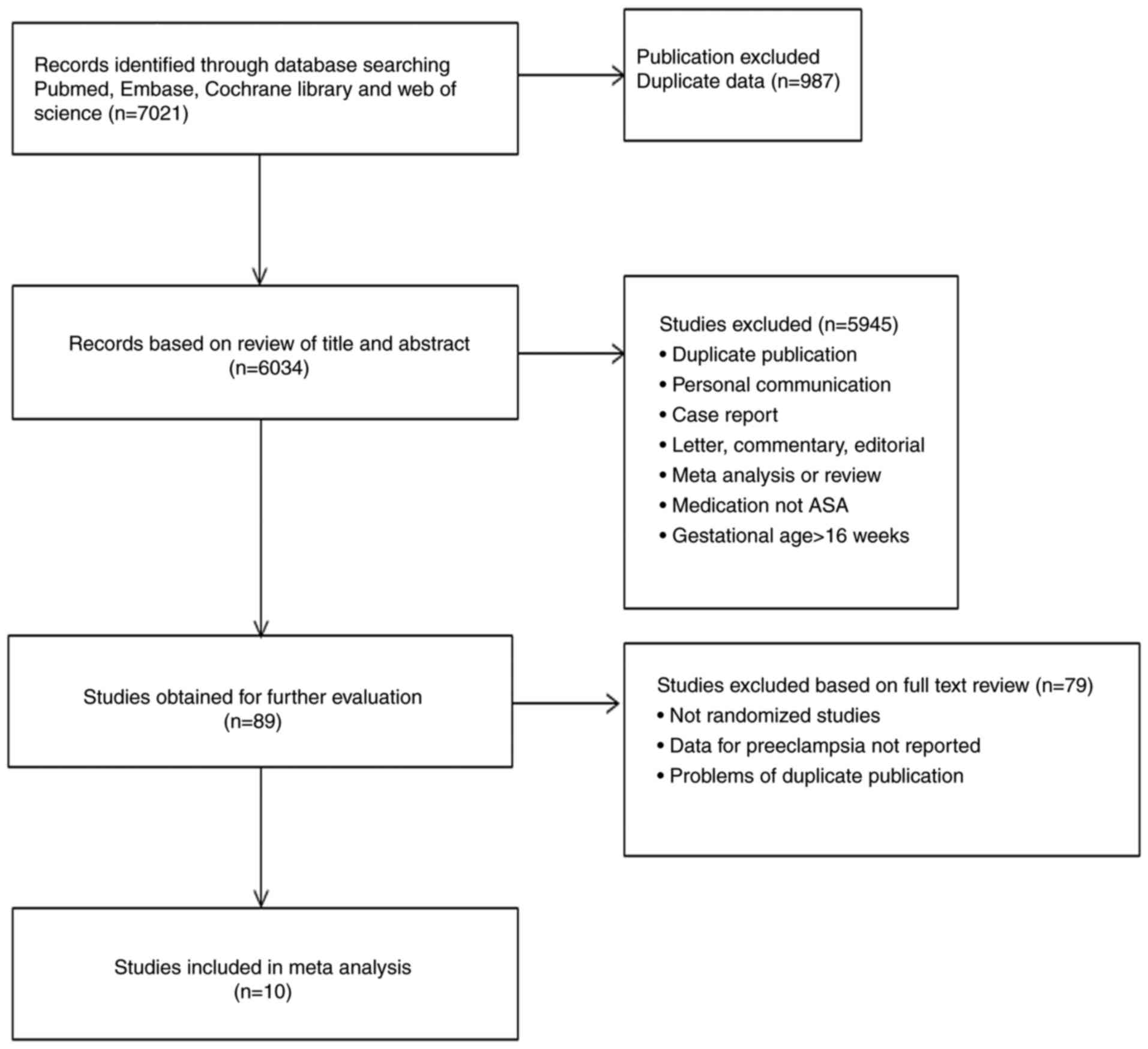
Fig. 1 displays the
flowchart of the study selection process. The initial 7,021
identified citations were reduced to 6,932 following review for
duplicated publications and of the title and abstract against the
inclusion criteria. The full text of the remaining 89 studies was
evaluated, resulting in the exclusion of a further 79 studies. The
remaining 10 RCTs comprising 3,168 participants were included,
including 1,581 patients treated with low-dose aspirin and 1,587
who received placebo or no treatment. The details of the regimens
are listed in Table I. No
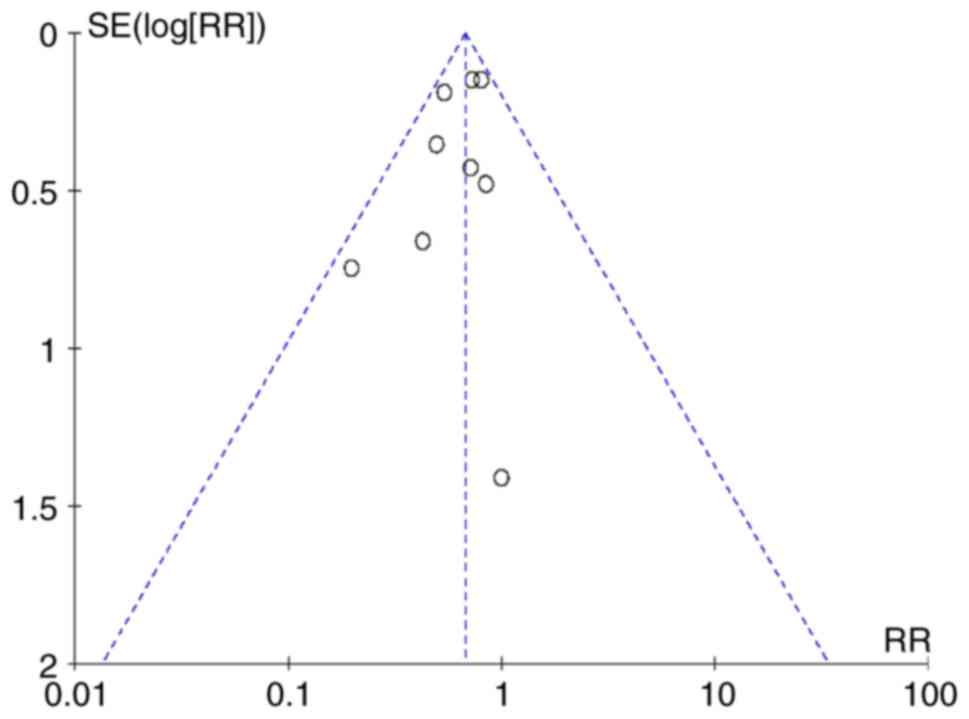
publication bias was identified by the funnel plot method on the
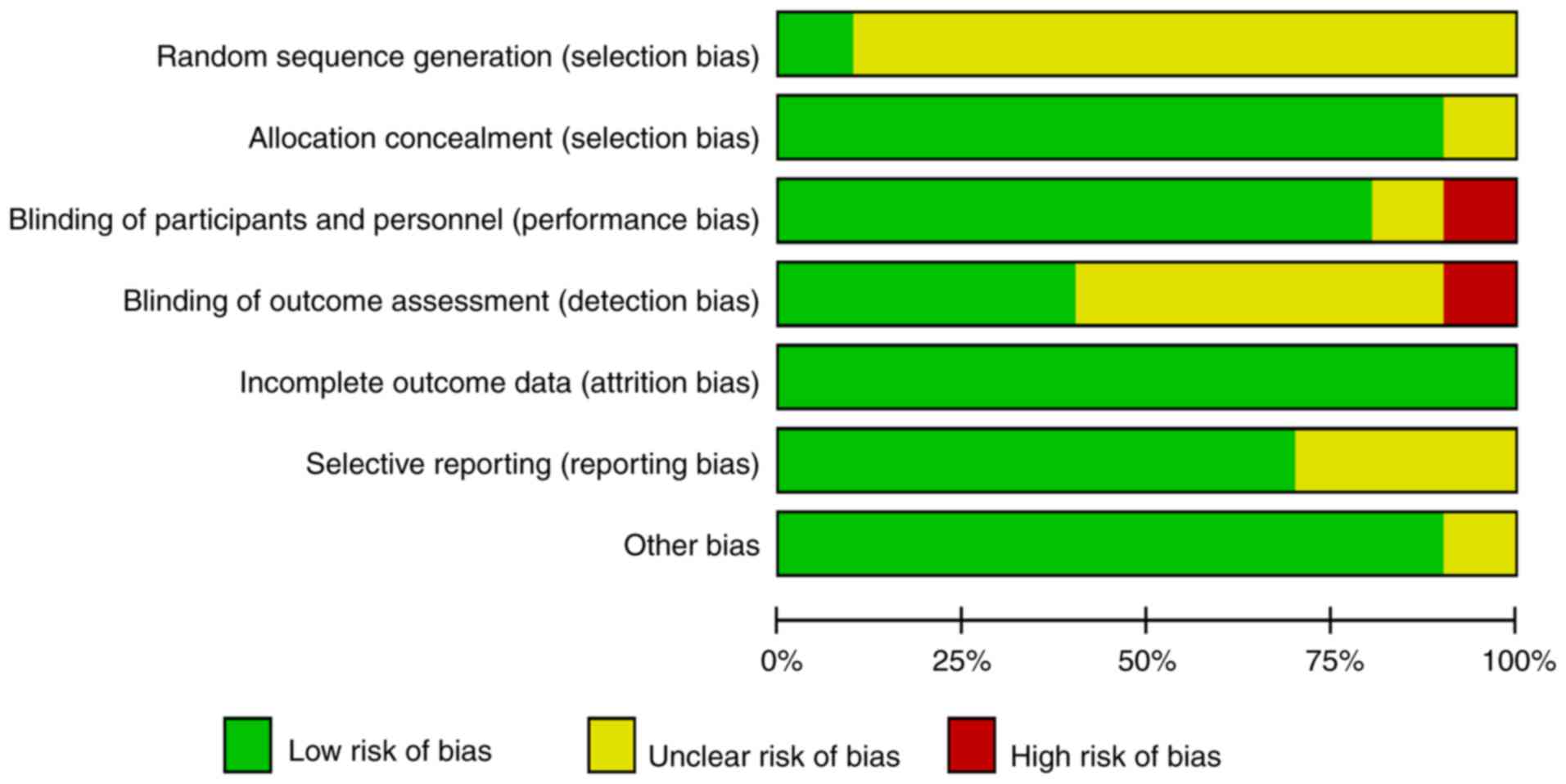
basis of data on the total rate of efficacy (Fig. 2). In addition the quality of RCTs was
assessed by The Cochrane Collaboration's tool for assessing risk of
bias (Fig. 3). The majority of
studies used the correct allocation concealment strategies,
reported incomplete outcome data and were double-blind. A moderate
number of studies were randomized incorrectly in sequence
generation and were not blinded to the outcome assessment.
 | Table I.Characteristics of randomized
controlled trials. |
Table I.
Characteristics of randomized
controlled trials.
| Study (year) | Gestation age
(weeks) | N | Inclusion
criteria | Intervention | Outcomes | (Refs.) |
|---|
| Ayala et al
(2013) | ≤16 | 350 | Pregnant women with
higher risk for gestational hypertension or preeclampsia | ASA 100 mg/d vs.
placebo | PE; preterm birth;
IUGR; stillbirth; newborn weight; Apgar score; gestational
hypertension; postpartum hemorrhage | (27) |
| Bakhti and
Vaiman (2011) | 8–10 | 164 | Women without
previous vasculo-renal pathology | ASA 100 mg/d vs. no
treatment | Preterm PE; PE; IUGR;
gestation hypertension; postpartum hemorrhage; stillbirth; preterm
birth; newborn weight | (28) |
| Benigni et al
(1989) | 12 | 33 | Women with
hypertension or previous obstetrical history: Fetal death, severe
IUGR, early onset of preeclampsia | ASA 60 mg/d vs.
placebo | PE; gestational
hypertension; preterm birth; IUGR; perinatal death; newborn
weight | (29) |
| Caritis et al
(1998) | 13–16 | 523 | Women with diabetes
mellitus, chronic hypertension or a history of PE | ASA 60 mg/d vs.
placebo | PE; IUGR; newborn
weight. | (30) |
| Chiaffarino et
al (2004) | <14 | 35 | Women with chronic
hypertension, history of severe pre-eclampsia or eclampsia or IUGR
or intrauterine fetal death | ASA 100 mg/d vs. no
treatment | PE; gestational
hypertension; abortion; birth weight | (31) |
| Ebrashy et al
(2005) | 14–16 | 139 | A high-risk factor
for preeclampsia or IUGR, including previous history of the
disease, essential hypertension, family history of or underlying
vascular disorder, maternal age <20 or >40 years, and
gestational diabetes mellitus | ASA 75 mg/d vs. no
treatment | Preterm PE; PE; IUGR;
preterm birth; apgar score; maternal hemorrhage; newborn
weight | (32) |
| Hermida et
al (1997) | 12–16 | 100 | Women with risk
factors of pre-eclampsia: Family or own history of gestational
hypertension or PE, chronic HT, cardiovascular or endocrine
problem, bleeding or endocrine disease | ASA 100 mg/d vs.
placebo | PE; gestational
hypertension; preterm birth; IUGR; perinatal death; birth
weight | (33) |
| Rolnik et al
(2017) | 11–14 | 1,620 | Women with high
risk high risk (>1 in 100) for preterm preeclampsia according to
the screening algorithm | ASA 150 mg/d vs.
placebo | Preterm PE; PE;
gestational hypertension; preterm birth; stillbirth; abruption;
SGA | (7) |
| Vainio et al
(2002) | 12–14 | 86 | Women considered to
be at high risk of preeclampsia or intrauterine growth retardation
were screened by transvaginal Doppler ultrasound | ASA 0.5 mg/kg/d vs.
placebo | Preterm PE; PE;
gestational hypertension; preterm birth; stillbirth; abruption;
SGA | (34) |
| Villa et al
(2013) | 12–13 |
| Women with risk
factors for pre-eclampsia or abnormal uterine artery Doppler
velocimetry | ASA 100 mg/d vs.
placebo | Preterm PE; PE;
gestational hypertension; newborn birthweight; Apgar score | (35) |
Low-dose aspirin commenced at ≤16
weeks of gestation reduces in the risk of preeclampsia
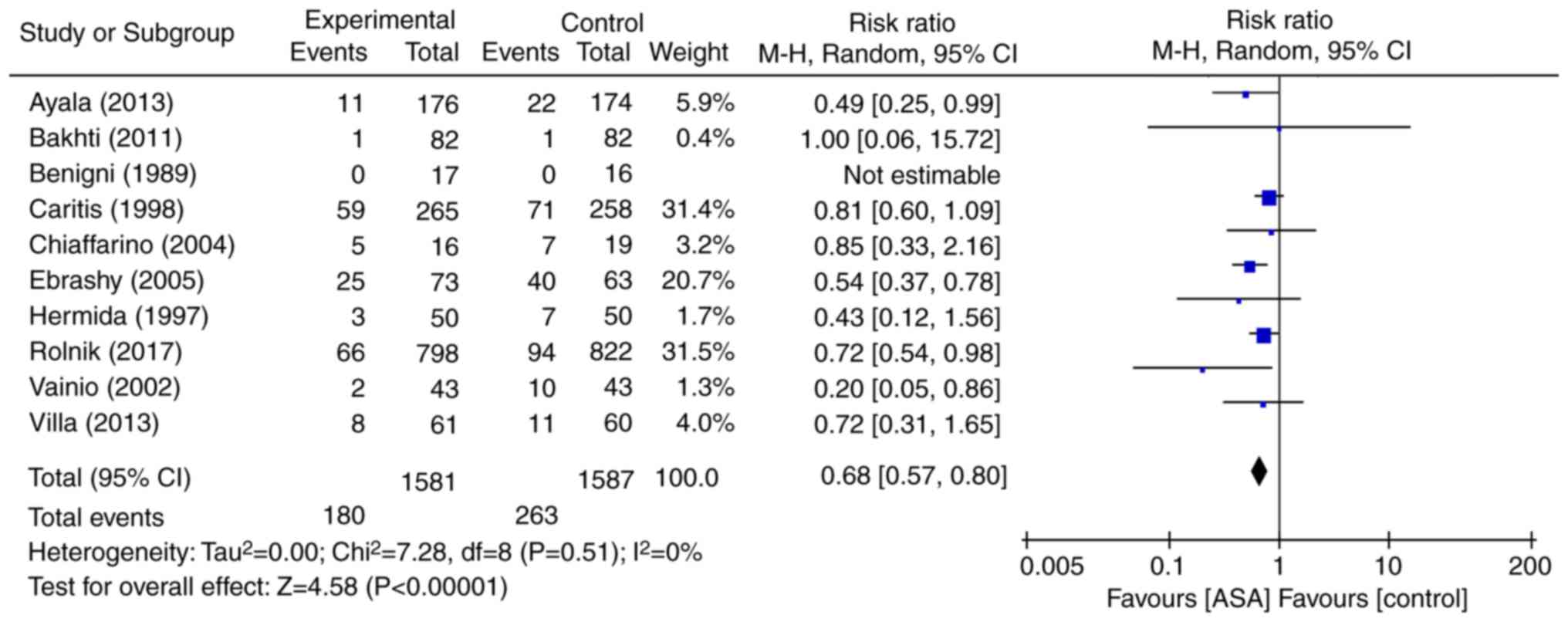
All 10 RCTs evaluated the effect of low-dose aspirin
for the prevention of preeclampsia irrespective of the time to
delivery (Fig. 4). As no
heterogeneity was identified (P=0.51, I2=0%), the
fixed-effects model was used for the meta-analysis. The results
indicated that, compared with placebo or no treatment, low-dose
aspirin was associated with a 33% reduction in the relative risk of
preeclampsia regardless of the time to delivery (RR=0.68, 95%
CI=0.57–0.80; P<0.0001). Next, the efficacy in the two subgroups
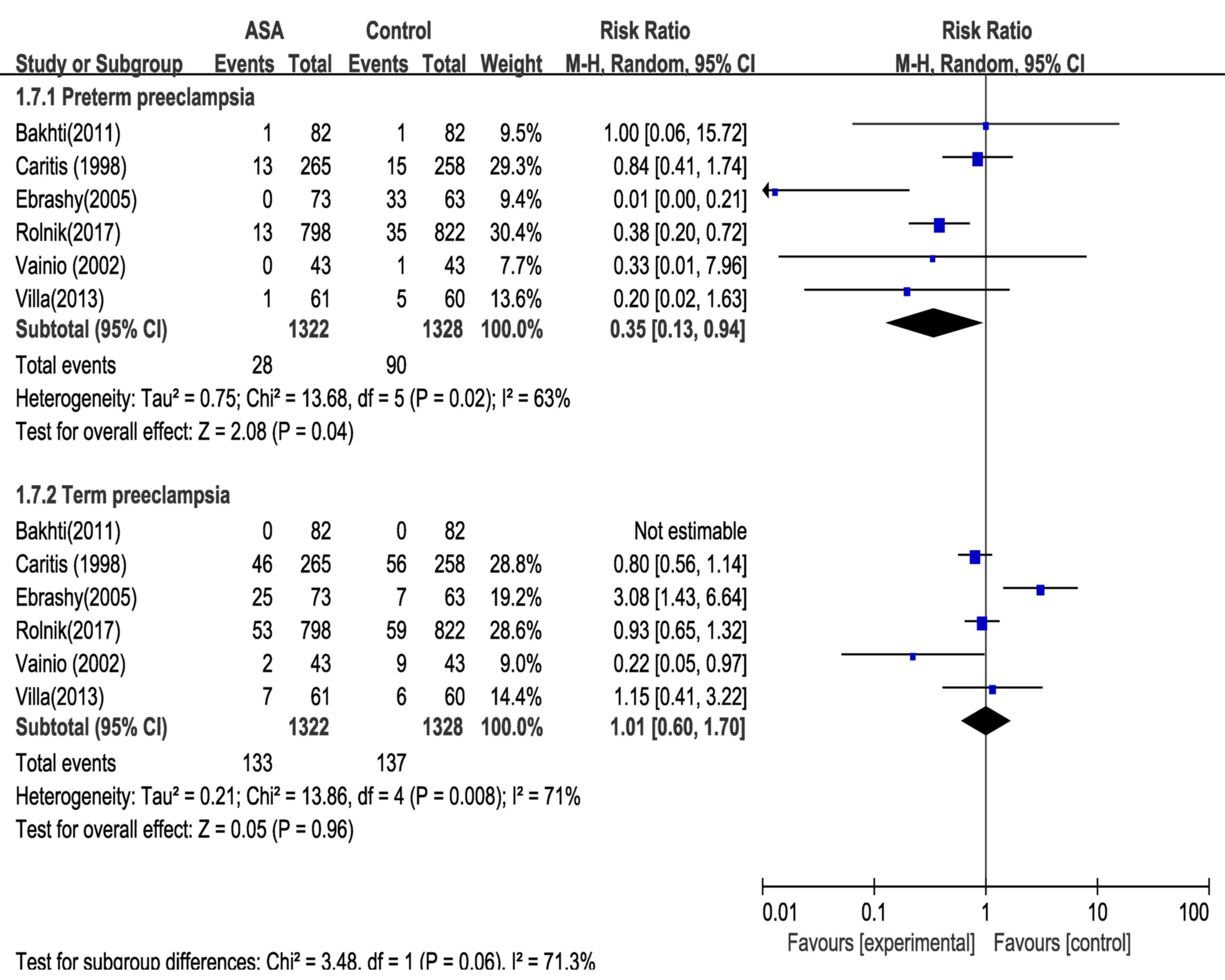
of preterm and term preeclampsia was evaluated (Fig. 5). Analysis of the data from 6 RCTs
indicated that low-dose aspirin, administered at ≤16 weeks of
gestation, was associated with a 65% reduction in the risk of
preterm preeclampsia. By contrast, no reduction in the relative
risk of term preeclampsia by administration of low-dose aspirin was
obtained (RR=1.01; 95% CI=0.60–1.70).
Low-dose aspirin commenced at ≤16
weeks of gestation reduces in the risk of gestational hypertension
and preterm birth
As no heterogeneity was identified (P=0.59,
I2=0%), the fixed-effects model was used for the
meta-analysis. The results for the other maternal adverse outcomes
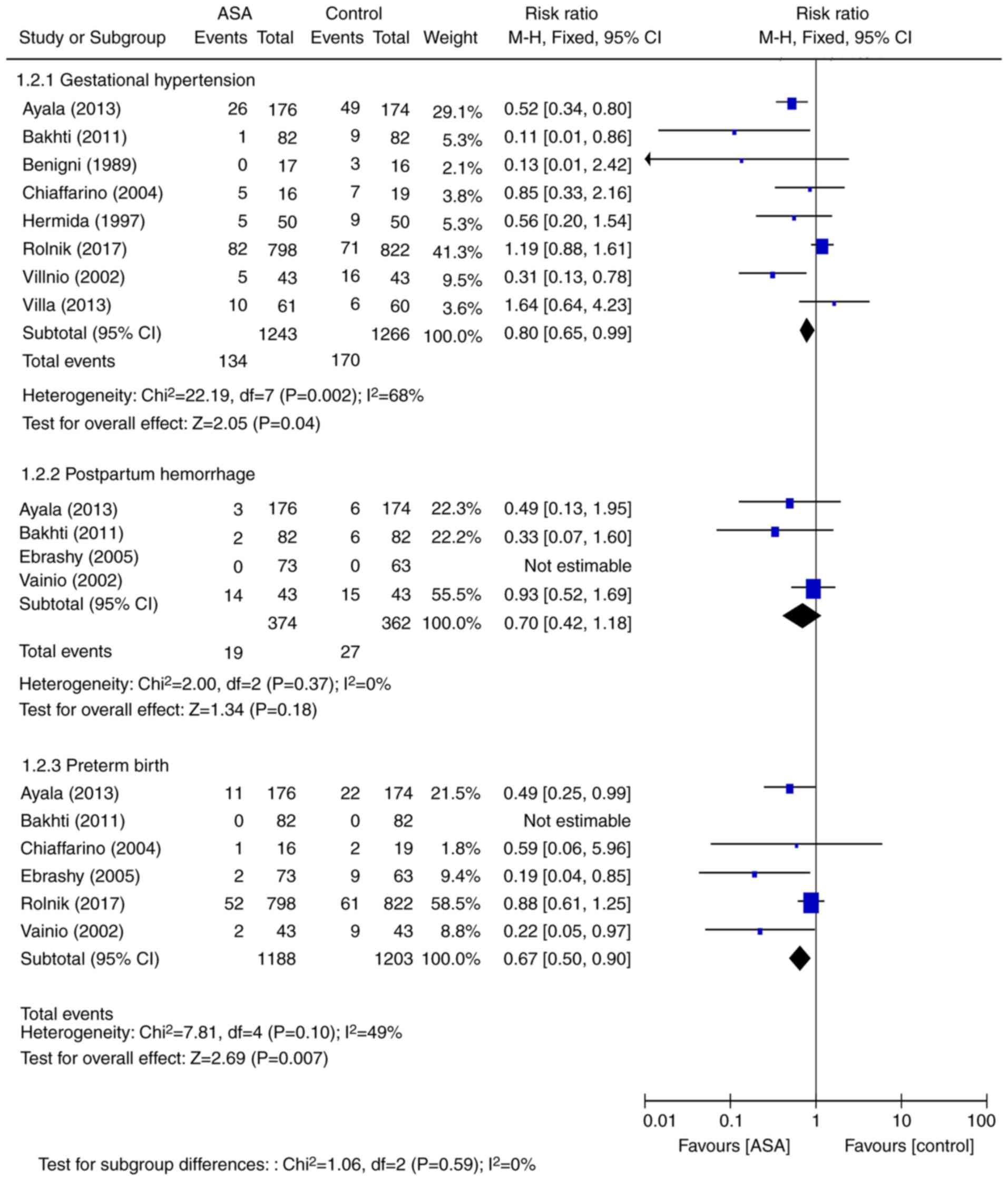
are presented in Fig. 6. A total of
9 RCTs comprising 2,508 cases reported on gestational hypertension,
with results indicating a 20% reduction in the RR of gestational
hypertension with aspirin (RR=0.80, 95% CI=0.65–0.99; P=0.0400).
Similarly, meta-analysis of the results from 6 RCTs comprising
2,391 cases indicated a 23% reduction in the RR for preterm birth
(RR=0.67, 95% CI=0.50–0.90; P=0.0070). The results obtained from 4
RCTs comprising 736 maternal patients suggested a 30% reduction in
the likelihood of postpartum hemorrhage (RR=0.70, 95% CI=0.42–1.18;
P=0.18), however this was not significant.
Low-dose aspirin commenced at ≤16
weeks of gestation reduces in the risk of neonatal adverse
outcomes
As no heterogeneity was identified (IUGR or SGA,
I2=0%; still birth or mortality, I2=0%;
newborn weight, I2=0%, respectively), the fixed-effects
model was used for the meta-analysis. Neonatal adverse outcomes are
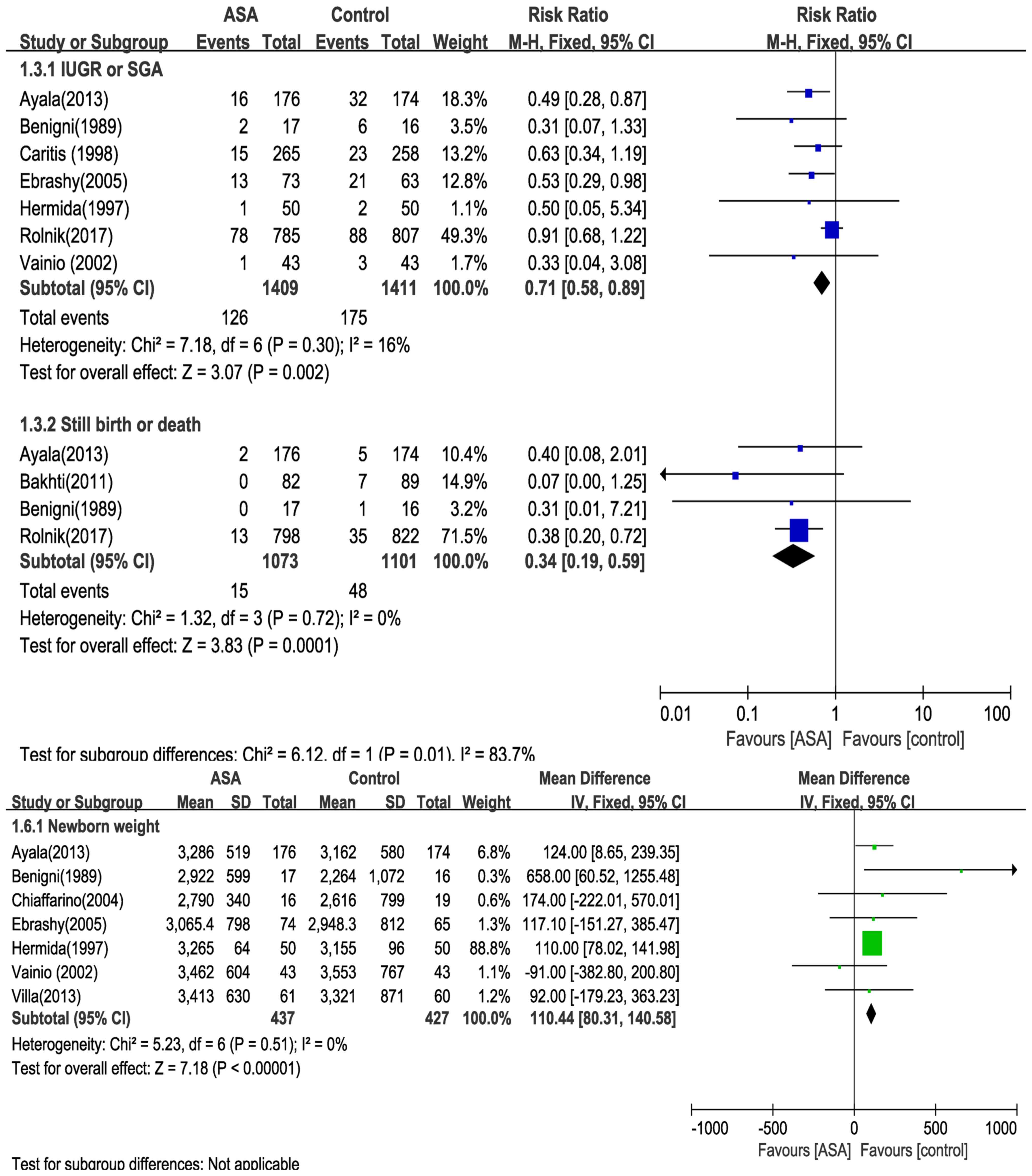
presented in Fig. 7. Meta-analysis
of the results from 7 RCTs comprising 2,820 maternal patients
suggested that aspirin reduced the risk of IUGR or SGA (RR=0.71;
95% CI=0.58–0.89; P=0.0040). Furthermore, analysis of the data of 4
RCTs involving 2,174 maternal patients suggested fewer still births
or deaths associated with aspirin intake (RR=0.34, 95%
CI=0.19–0.59; P=0.0001). In addition, data on 864 infants suggested
that aspirin intake during pregnancy was associated with an
increased newborn weight by 110.44 g (95% CI=80.31–140.58 g;
P<0.0001) in the setting of maternal risk of preeclampsia.
Overall, low-dose aspirin administration at ≤16
weeks of gestation to women at risk of preeclampsia was associated
with a reduction in maternal and neonatal adverse outcomes,
compared with placebo or no treatment. Aspirin administration was
also associated with an improvement of fetal growth.
Discussion
The present systematic review and meta-analysis was
restricted to RCTs that assessed outcomes of low-dose aspirin
administration commenced at ≤16 weeks of gestation in females at
risk of preeclampsia. The outcomes were preeclampsia (including two
subgroups of preterm and term preeclampsia), as well as maternal
adverse outcomes, including gestational hypertension, postpartum
hemorrhage and preterm birth, and neonatal adverse outcomes,
including IUGR or SGA, stillbirth or death, and newborn weight. The
inclusion criteria were met by 10 RCTs comprising a total of 3,168
female patients. The meta-analysis revealed a major beneficial
effect of low-dose aspirin, commenced at ≤16 weeks of gestation, on
the risk of preeclampsia regardless of the time to delivery
(RR=0.68; 95% CI=0.57–0.80). This appeared mainly due to a
reduction in preterm preeclampsia (RR=0.35; 95% CI=0.13–0.94), as
low-dose aspirin was not associated with any significant reduction
in the risk of term preeclampsia. Furthermore, maternal adverse
outcomes, including gestational hypertension and preterm birth, and
neonatal adverse outcomes, including IUGR or SGA, stillbirth or
infant death, and newborn weight, were improved by maternal aspirin
intake.
In recent decades, the ability of antiplatelet
agents to prevent or delay preeclampsia and its complications has
been widely tested in numerous studies. While various studies have
reported significant benefits (11–13),
others have not (14–16). In the present study, the efficacy of
low-dose aspirin therapy in maternal patients at risk of
preeclampsia commenced at ≤16 weeks of gestation was assessed
regarding the prevention of preeclampsia and the results of the
meta-analysis were similar to those observed in a previous
meta-analysis of individual patient data, which indicated a
moderate but consistent reduction in the RR for maternal and
neonatal adverse events (4). A
recent multicenter, double-blind, placebo-controlled trial of 1,776
women with singleton pregnancies at high risk for preterm
preeclampsia demonstrated that low dose aspirin significantly
reduced the incidence of this diagnosis compared with the placebo
(7). In the present study randomized
controlled trials were selected that met the indicated inclusion
criteria, including this recent study. The results of the present
study supported those of previous studies, demonstrating that the
use of aspirin commenced at ≤16 weeks of gestation may be
particularly effective in preventing preeclampsia.
Although the exact underlying cause of preeclampsia
remains to be fully elucidated, it is widely accepted that
abnormalities including angiogenesis, oxidative stress and
inflammation are involved. To date, numerous attempts at primary
and secondary prevention of preeclampsia using various supplements
and medications, including anti-hypertensives (17), calcium (18), or the antioxidants vitamins C and E
(19), have failed. Pilot studies
suggest a promising beneficial effect of pravastatin (20). However, its benefits (and safety in
pregnancy) require investigation in a large and well-designed RCT
with a sample size that is sufficiently large to achieve high
statistical power, prior to its implementation in routine clinical
practice.
Normal implantation and placentation are critical
for a successful pregnancy. It is thought that the first wave of
trophoblast invasion is already complete by around 10 weeks of
gestation and continues until the 20th week (21). It is also known that aspirin exerts
beneficial effects on endothelial function, as well as early
formation and development of the placenta (22,23).
Bujold et al (24) reported
that administration of low-dose aspirin commenced at ≤16 weeks of
gestation significantly decreases the risk of preeclampsia and
other adverse maternal and neonatal outcomes, whereas the effect is
not present with later commencement of aspirin. However, a recent
meta-analysis reported a consistent effect of low-dose aspirin on
preeclampsia and its complications regardless of whether it was
started prior to or after 16 weeks of gestation (25). These conflicting results may be due
to different inclusion criteria, and of note, the latter review
included participants who received one or more antiplatelet agents
(e.g., dipyridamole or low-molecular-weight heparin). Furthermore,
in the latter meta-analysis of individual participant data, studies
were selected where antiplatelet agent initiation was not
restricted to the first 16 weeks of pregnancy, thereby including
more studies.
The present result that low-dose aspirin reduced the
risk of maternal and neonatal adverse outcomes is compatible with
an earlier meta-analysis, which indicated that antiplatelet agents
achieved reductions in preterm birth, SGA and other adverse
maternal and fetal outcomes (4). The
major limitation of the present meta-analysis was the small number
of studies included. In particular, the presence of heterogeneity
for maternal and newborn adverse outcome suggests variance between
the included studies. The presence of heterogeneity
(I2=68%) for gestational hypertension and heterogeneity
(I2=83.7%) for subgroup analyses in IUGR or SGA and
still birth or death, suggested the presence of a variance between
the included studies. Females in the present small trial appeared
to have a larger than average reduction in the risk ratio for
adverse events. The recognition of bias from small trials is well
known and these outcomes should be interpreted with caution
(26).
In conclusion, the present meta-analysis indicated
that initiation of low-dose aspirin commenced at ≤16 weeks of
gestation resulted in a 33% decrease in the occurrence of
preeclampsia, mostly due to a 63% reduction of preterm
preeclampsia, although this change was not significant. However,
low-dose aspirin had no effect on the risk of term preeclampsia.
The present study also indicated that aspirin produced significant
reductions in maternal and neonatal adverse events. The most likely
explanation for these results is that early administration of
low-dose aspirin improves early formation and development of the
placenta. This should be discussed with women at risk of developing
preeclampsia to help them make informed choices regarding their
antenatal care. However, the potential benefit of low-dose aspirin
regarding the prevention of preeclampsia, as well as associated
maternal and neonatal adverse outcomes, remains controversial.
Additional clinical trials of higher quality and with a larger
sample size are necessary to further verify the effectiveness of
aspirin for this indication.
Acknowledgements
The authors thank Dr Zhi Wang and Dr Yifang Zhu in
the Department of Health and Human Services, Yiwu Maternity and
Children Health Care Hospital for their assistance.
Funding
No funding received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
FZ designed the study and checked the results. YC
and BZ performed the experiments and analyzed the data. The final
version of the manuscript was read and approved by all authors.
Ethical approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tooher J, Thornton C, Makris A, Ogle R,
Korda A, Horvath J and Hennessy A: Hypertension in pregnancy and
long-term cardiovascular mortality: A retrospective cohort study.
Am J Obstet Gynecol. 214:722.e1–6. 2016. View Article : Google Scholar
|
|
3
|
Crandon AJ and Isherwood DM: Effect of
aspirin on incidence of pre-eclampsia. Lancet. 1:13561979.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Askie LM, Duley L, Hendersonsmart DJ and
Stewart LA: PARIS Collaborative Group: Antiplatelet agents for
prevention of pre-eclampsia: A meta-analysis of individual patient
data. Lancet. 369:1791–1798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Groeneveld E, Lambers MJ, Lambalk CB,
Broeze KA, Haapsamo M, de Sutter P, Schoot BC, Schats R, Mol BW and
Hompes PG: Preconceptional low-dose aspirin for the prevention of
hypertensive pregnancy complications and preterm delivery after
IVF: A meta-analysis with individual patient data. Hum Reprod.
28:1480–1488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
CLASP: A randomized trial of low-dose
aspirin for the prevention and treatment of pre eclampsia among
9364 pregnant-women. CLASP (Collaborative Low-dose Aspirin Study in
Pregnancy) Collaborative Group. Lancet. 343:619–629. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rolnik DL, Wright D, Poon LC, O'Gorman N,
Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D,
Singh M, et al: Aspirin versus placebo in pregnancies at high risk
for preterm preeclampsia. N Engl J Med. 377:613–622. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
World Health Organization (WHO): WHO
recommendations for prevention and treatment of pre-eclampsia and
eclampsia. WHO; Geneva: 2011
|
|
9
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green S: Cochrane Handbook for Systematic
Reviews of Interventions: Cochrane Book Series. Wiley-Blackwell;
2008, View Article : Google Scholar
|
|
11
|
Roberge S, Nicolaides K, Demers S, Hyett
J, Chaillet N and Bujold E: The role of aspirin dose on the
prevention of preeclampsia and fetal growth restriction: Systematic
review and meta-analysis. Am J Obstet Gynecol. 216:110–120.e6.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberge S, Nicolaides KH, Demers S, Villa
P and Bujold E: Prevention of perinatal death and adverse perinatal
outcome using low-dose aspirin: A meta-analysis. Ultrasound Obstet
Gynecol. 41:491–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henderson JT, Whitlock EP, O'Conner E,
Senger CA, Thompson JH and Rowland MG: Low-dose aspirin for
prevention of morbidity and mortality from preeclampsia: A
systematic evidence review for the U.S. preventive services task
force. Ann Intern Med. 160:695–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roberge S, Sibai B, McCaw-Binns A and
Bujold E: Low-dose aspirin in early gestation for prevention of
preeclampsia and small-for-gestational-age neonates: Meta-analysis
of large randomized trials. Am J Perinatol. 33:781–785. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bergeron TS, Roberge S, Carpentier C,
Sibai B, Mccaw-Binns A and Bujold E: Prevention of preeclampsia
with aspirin in multiple gestations: A systematic review and
meta-analysis. Am J Perinatol. 33:605–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meher S and Alfirevic Z: Aspirin for
pre-eclampsia: Beware of subgroup meta-analysis. Ultrasound Obstet
Gynecol. 41:479–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abalos E, Duley L, Steyn DW and
Henderson-Smart DJ: Antihypertensive drug therapy for mild to
moderate hypertension during pregnancy. Cochrane Database Syst Rev:
Cd002252. 2007. View Article : Google Scholar
|
|
18
|
Levine RJ, Hauth JC, Curet LB, Sibai BM,
Catalano PM, Morris CD, DerSimonian R, Esterlitz JR, Raymond EG,
Bild DE, et al: Trial of calcium to prevent preeclampsia. N Engl J
Med. 337:69–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Conde-Agudelo A, Romero R, Kusanovic JP
and Hassan SS: Supplementation with vitamins C and E during
pregnancy for the prevention of preeclampsia and other adverse
maternal and perinatal outcomes: a systematic review and
metaanalysis. Am J Obstet Gynecol. 204:503.e1–12. 2011. View Article : Google Scholar
|
|
20
|
Costantine MM, Cleary K, Hebert MF, Ahmed
MS, Brown LM, Ren Z, Easterling TR, Haas DM, Haneline LS, Caritis
SN, et al: Safety and pharmacokinetics of pravastatin used for the
prevention of preeclampsia in high-risk pregnant women: a pilot
randomized controlled trial. Am J Obstet Gynecol.
214:720.e1–720.e17. 2016. View Article : Google Scholar
|
|
21
|
Knöfler M and Pollheimer J: IFPA award in
placentology lecture: Molecular regulation of human trophoblast
invasion. Placenta. 33 Suppl:S55–S62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quyyumi AA: Effects of aspirin on
endothelial dysfunction in atherosclerosis. Am J Cardiol.
82:31S–33S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tarim E, Bal N, Kilicdag E, Kayaselcuk F,
Bağiş T and Kuscu E: Effects of aspirin on placenta and perinatal
outcomes in patients with poor obstetric history. Arch Gynecol
Obstet. 274:209–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bujold E, Roberge S, Lacasse Y, Bureau M,
Audibert F, Marcoux S, Forest JC and Giguère Y: Prevention of
preeclampsia and intrauterine growth restriction with aspirin
started in early pregnancy: A meta-analysis. Obstet Gynecol.
116:402–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meher S, Duley L, Hunter K and Askie L:
Antiplatelet therapy before or after 16 weeks' gestation for
preventing preeclampsia: an individual participant data
meta-analysis. Am J Obstet Gynecol. 216:121–128.e2. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Borenstein M, Hedges LV, Higgins JPT and
Rothstein HR: Introduction to meta-analysis. John Wiley & Sons;
Hoboken, NJ: 2009, View Article : Google Scholar
|
|
27
|
Ayala DE, Ucieda R and Hermida RC:
Chronotherapy with low-dose aspirin for prevention of complications
in pregnancy. Chronobiol Int. 30:260–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bakhti A and Vaiman D: Prevention of
gravidic endothelial hypertension by aspirin treatment administered
from the 8th week of gestation. Hypertens Res.
34:1116–1120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Benigni A, Gregorini G, Frusca T,
Chiabrando C, Ballerini S, Valcamonico A, Orisio S, Piccinelli A,
Pinciroli V, Fanelli R, et al: Effect of low-dose aspirin on fetal
and maternal generation of thromboxane by platelets in women at
risk for pregnancy-induced hypertension. N Engl J Med. 321:357–362.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caritis S, Sibai B, Hauth J, Lindheimer
MD, Klebanoff M, Thom E, VanDorsten P, Landon M, Paul R, Miodovnik
M, et al: Low-dose aspirin to prevent preeclampsia in women at high
risk National Institute of Child Health and Human Development
Network of Maternal-Fetal Medicine Units. N Engl J Med.
338:701–705. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiaffarino F, Parazzini F, Paladini D,
Acaia B, Ossola W, Marozio L, Facchinetti F and Del Giudice A: A
small randomised trial of low-dose aspirin in women at high risk of
pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 112:142–144. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ebrashy A, Ibrahim M, Marzook A and Yousef
D: Usefulness of aspirin therapy in high-risk pregnant women with
abnormal uterine artery Doppler ultrasound at 14–16 weeks
pregnancy: Randomized controlled clinical trial. Croat Med J.
46:826–831. 2005.PubMed/NCBI
|
|
33
|
Hermida RC, Ayala DE, Iglesias M, Mojón A,
Silva I, Ucieda R and Fernández JR: Time-dependent effects of
low-dose aspirin administration on blood pressure in pregnant
women. Hypertension. 30:589–595. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vainio M, Kujansuu E, Iso-Mustajärvi M and
Mäenpää J: Low dose acetylsalicylic acid in prevention of
pregnancy-induced hypertension and intrauterine growth retardation
in women with bilateral uterine artery notches. BJOG. 109:161–167.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Villa PM, Kajantie E, Räikkönen K, Pesonen
AK, Hämäläinen E, Vainio M, Taipale P and Laivuori H: PREDO Study
group: Aspirin in the prevention of pre-eclampsia in high-risk
women: A randomised placebo-controlled PREDO Trial and a
meta-analysis of randomised trials. BJOG. 120:64–74. 2013.
View Article : Google Scholar : PubMed/NCBI
|