Introduction
Breast cancer has a high incidence and poses a
notable threat to human health, particularly in females (1). The worldwide recurrence and metastasis
rate in patients with early breast cancer is estimated to be ~30%
(1). Breast cancer metastasizes to
the lungs, bone, liver and brain (2). Metastasis is a complex process that
relies to a great extent on the interaction between primary tumors
and the tumor-associated host stromal tissue (2). Surgical procedures, radiation-,
endocrine- and/or chemotherapy are required for the treatment of
the disease (3).
A frequently used chemotherapeutic drug in breast
cancer is doxorubicin, which inhibits the replication of DNA by
intercalating into and inhibiting the enzyme topoisomerase II. In
doing so, the cell cycle stops and the cancerous cells undergo
apoptosis (4). However, some types
of breast cancer are known to develop resistance to anticancer
agents such as doxorubicin (5). An
increased risk of breast cancer development has been associated
with cigarette smoking in epidemiological and clinical studies in
which smokers and non-smokers were compared (5–7). Of the
4,000 components in cigarette smoke, the most important and
effective is known to be nicotine (2) for breast cancer.
Long-term smoking of tobacco clinically contributes
to the development of cardiovascular disease and also to the
development of cancer (8). While
smoking, 80–90% of inhaled nicotine (~1.0 mg per cigarette) is
systemically absorbed (9). Nicotine
is thought to have a role in the formation of various types of
cancer and has been demonstrated to increase angiogenesis in
previous proliferation model studies (10–15).
Cancer stem cells (CSCs) have become an important
topic in oncology research. Malignant cancer stem cells were first
identified and isolated from solid breast cancer tumor (16). Human cell surface antigen, cluster of
differentiation (CD24), is a marker for normal mammary stem cells;
however, when combined with other markers, CD24− breast
cancer cells have the greatest tumor-initiating potential and may
be successfully identified in stem cell populations (17).
Breast cancer stem cells are different from other
breast cancer cells with regards to volume, size, membrane
components, antigen expression, proliferation and metastasis
(18). In the cancer stem cell
model, a small group of tumor cells are responsible for cancer
formation, progression and recurrence (11). A study by Honeth et al
determined that the CD24 and CD44 cell surface proteins are
putative markers for cancer stem cell populations in breast cancer
(18).
In a previous study, breast CSCs were enriched in
the minority fraction of CD44+CD24low lineage
cells, with as few as 100 CD44+CD24low cells
able to initiate tumor growth in immunocompromised mice (19). A study by Al Hajj et al was
the first to isolate CSCs from human breast cancer. They
prospectively identified and isolated the tumorigenic cells as
CD44(+) CD24 (−/low) lineage (−) in 8/9 patients.
CSCs are hypothesized to be a subset of tumor cells
with stem cell-like features that have the ability to self-renew
and differentiate, which causes a heterogeneous tumor cell
population (20). This variability
led to diverse results in clinical studies (21). Previous studies reported that
CD44+CD24− breast CSCs enhance breast tumor
cells because of their angiogenic potential (20–25).
The effects of daily exposure to chemical agents may
be determined at a cellular level with advanced imaging techniques,
including positron-emission tomography (26). These analyses of cancer cells and
biotechnological advances are opening new horizons of molecular
oncology and also provide major advances in our understanding of
oncology.
Breast cancer is managed through surgical treatment,
radiation therapy, endocrine therapy and/or chemotherapy (21). Resistance to chemotherapy is a
challenge for the successful cure of several types of cancer;
breast cancer has evolved resistance to a number of anticancer
agents, such as doxorubicin (23,24).
There is limited experimental data that supports direct links
between breast cancer and exposure to nicotine. The present
findings demonstrated the harmful effects of nicotine following
metastasis of cancer due to the chemoresistance produced through
uninterrupted smoking, which may impact the effectiveness of
treatment.
Materials and methods
Cell culture and treatment
The human breast cancer cell line MCF-7 (American
Type Culture Collection, Manassas, VA, USA) used in the present
study was grown in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; PAA Laboratories; GE
Healthcare, Chicago, IL, USA), 2 mM glutamine, 10 U/l penicillin
and 100 µg/ml streptomycin (all Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). The cells were cultured in a humidified
incubator with a 37°C in an atmosphere containing 5%
CO2.
For nicotine treatment, cells were washed twice with
phosphate-buffered saline (PBS), dissociated with 0.25% trypsin
(Gibco; Thermo Fisher Scientific, Inc.) and seeded near confluence
(2×105 cells/well) in 6-well plates. Cells were cultured
in complete medium at 37°C in a 5% CO2 incubator for 24
h prior to nicotine treatment (Sigma-Aldrich) at concentrations of
0.01, 0.05, 0.1, 1 and 10 µM at the same time to the wells.
Cell diameter
MCF-7 cells were analyzed with an Vi-Cell XR cell
viability analyzer (Beckman Coulter, Inc., Brea, CA, USA). MCF-7
cell diameters were measured 24 and 48 h after treatment with
nicotine using the Vi-Cell XR cell viability analyzer.
Scanning electron microscopy
(SEM)
MCF-7 cells were plated on 6-well plates
(7×103 cell/well). The cells were cultured in DMEM
(Clonetics; Lonza Group, Ltd., Basel, Switzerland), which consisted
of 5% FBS, 0,1% penicillin strep. MCF-7 breast cancer cells were
centrifuged at 1600 × g. Cells were collected and prepared in
accordance with the method of Groebel et al (27) and analyzed with an FEI Quanta 450
FEG-EDS scanning electron microscope (Thermo Fisher Scientific,
Inc.).
Transmission electron microscopy
(TEM)
MCF-7 breast cancer cells were centrifuged at 1600 ×
g, 5 min at room temperature after treatment trypsin-EDTA. Cells
were collected and prepared in accordance with the method of
Groebel et al (27) and
analyzed using a Philips CM100 transmission electron microscope
(Philips Medical Systems, Inc., Bothell, WA, USA).
CSC analysis by flow cytometry
Allophycocyanin (APC)-conjugated mouse anti-human
CD44 monoclonal antibody (cat. no. BD 559942) and
phycoerythrin/cyanine 7 (PE/Cy7)-conjugated mouse anti-human CD24
monoclonal antibody (cat. no. BD 561646) were purchased from BD
Pharmingen (BD Biosciences, Franklin Lakes, NJ, USA). CD24 and CD44
expression was analyzed in cells derived from monolayer cultures
following dissociation in trypsin-EDTA at 37°C. At least
1×105 cells were pelleted by centrifugation at 500 × g
for 5 min at 4°C. Cells were washed in PBS, resuspended with
anti-CD24-PE/Cy7 (1:20 dilution) and anti-CD44-APC (1:20 dilution);
samples were incubated for 30 min at 4°C in the dark. The labeled
cells were washed using PBS and analyzed using a BD FACSAria II
flow cytometer and software DiVa (BD Biosciences). The negative
fraction was determined using appropriate isotype controls.
Actin immunostaining
MCF-7 breast cancer cells were plated onto
poly-D-lysine-coated 6-well glass chamber slides (7,000 cells/well)
and incubated at 37°C in a 5% CO2 incubator for
immunostaining. After 24 h incubation, cells were treated with
0.01, 0.05, 0.1, 1 or 10 µM nicotine for the indicated time points
(24 and 48 h). Immunofluorescence microscopy was used to determine
F-actin organization in MCF-7 breast cancer cells. The cells were
fixed in 3.5% paraformaldehyde in PBS for 10 min at room
temperature. Cells were permeabilized and blocked in PBS containing
0.1% Triton X-100 and 5% FBS for 30 min at room temperature. Actin
filaments were visualized using rhodamine-phalloidin label, and
nuclei were stained with DAPI (Invitrogen; Thermo Fisher
Scientific, Inc.). All images were obtained using an Olympus BX51
Microscope equipped with a DP72 camera, controlled by Olympus
DP2-TWAIN software (ver7.2; Olympus Corp., Tokyo, Japan) (28).
Doxorubicin resistance
A final doxorubicin (Sigma-Aldrich; Merck KGaA)
concentration of 2.5 µg/ml was used in the present study. Cells
were divided into a total of six groups (doxorubicin concentrations
were 0.5, 1, 2, 4 or 8 µM) and in the control group MCF-7 cells
received no treatment with doxorubicin. MCF-7 breast cancer cells
were shaken every 15–20 min during their incubation with
doxorubicin at 37°C for 1 h. The cell viability test was performed
using trypan blue (29).
Statistical analysis
Statistical analysis was performed and data were
presented as the mean ± standard deviation, For comparisons of two
normally distributed groups, a Student t-test was used. All
statistical analysis was performed using SPSS software for Windows
(version 21; IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of nicotine on actin
cytoskeleton in MCF-7 breast cancer cells
Lamellipodia formation on filamentous actin was
investigated using fluorescence microscopy, in order to evaluate
whether nicotine increases the metastatic potential in breast
cancer (Fig. 1). MCF-7 cells
appeared to have a diamond or polygonal shape. The F-actin
filaments of the MCF-7 breast cancer cells treated with nicotine
were altered to a rounded form with a smaller diameter. This effect
was more pronounced as the concentration and duration of nicotine
treatment increased.
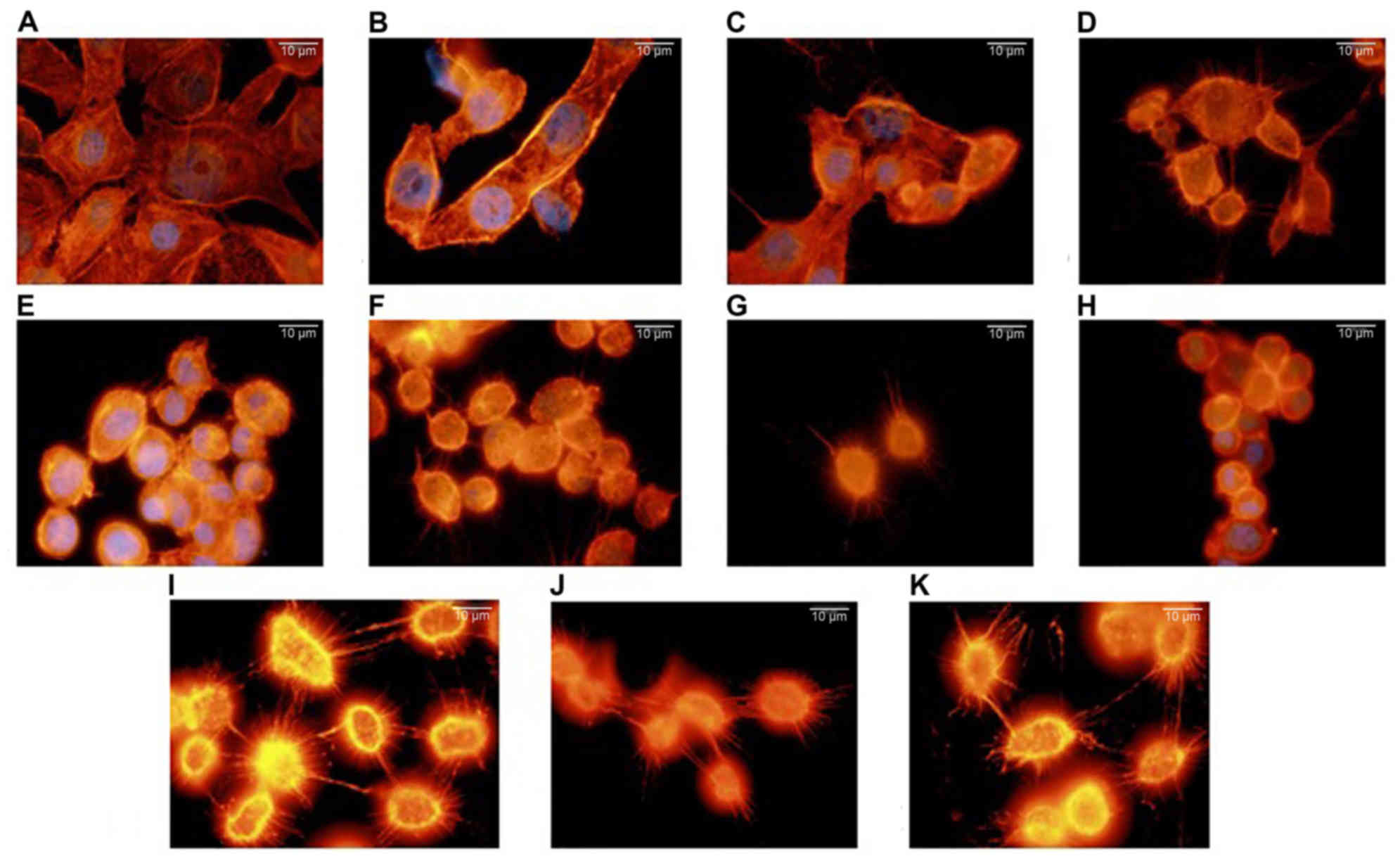 | Figure 1.Effect of nicotine treatment on the
actin cytoskeleton. MCF-7 cells were incubated in the presence of
nicotine: (A) 0 µM (control) (24 h), (B) 0.01 µM (24 h), (C) 0.05
µM (24 h), (D) 0.1 µM (24 h), (E) 1 µM (24 h), (F) 10 µM (24 h),
(G) 0.01 µM (48 h), (H) 0.05 µM (48 h), (I) 0.1 µM (48 h), (J) 1 µM
(48 h) or (K) 10 µM (48 h). Actin filaments were visualized using
rhodamine-phalloidin and nuclei were stained with DAPI for all
conditions. Scale bar=10 µm (magnification, ×100). |
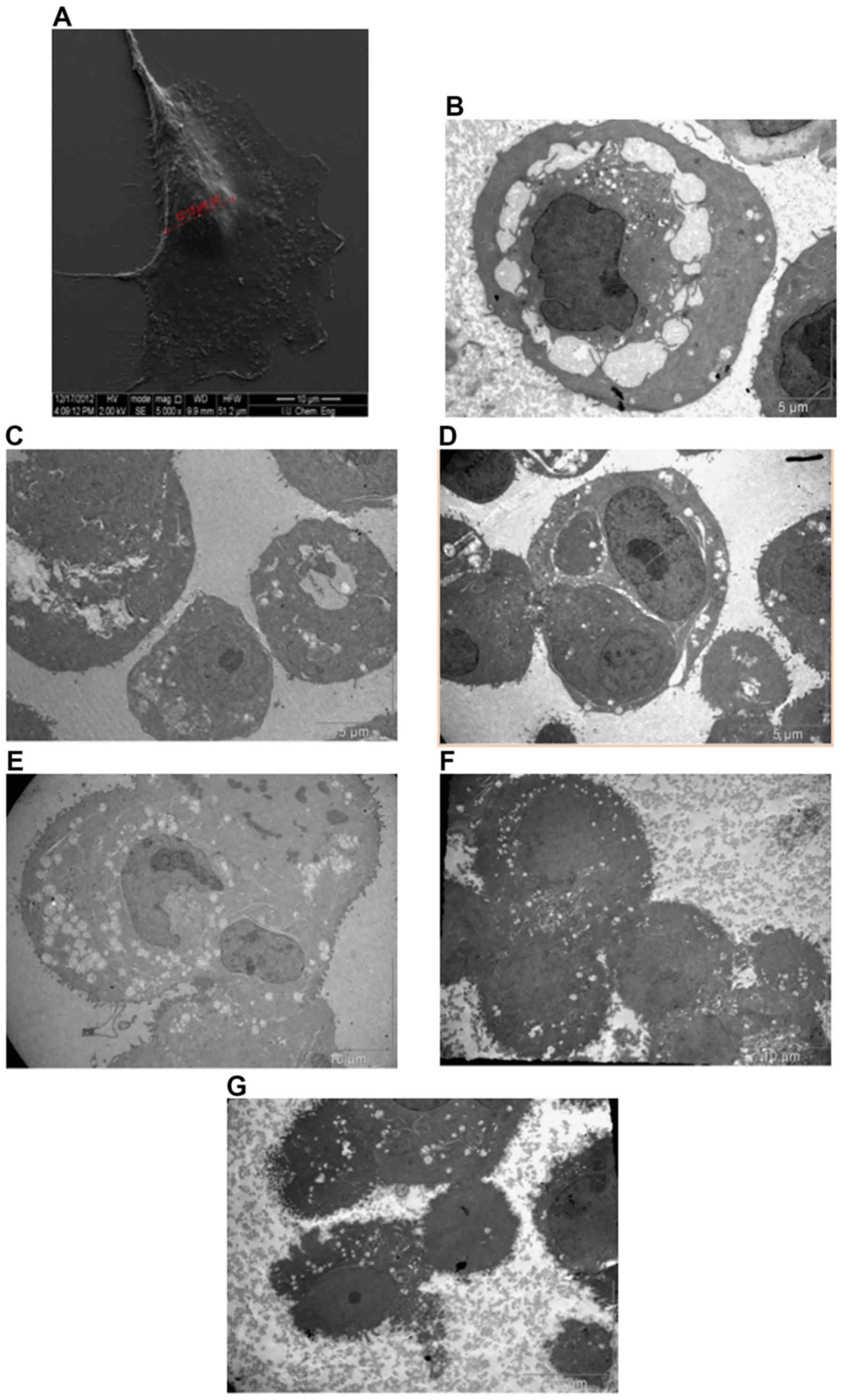
Effect of nicotine on cellular surface
morphology and ultrastructure
The surfaces of the nicotine treated MCF-7 cells
(grown in DMEM with 5% FBS) as viewed under SEM were almost
uniformly covered with an increased dense network of microvilli
(Fig. 2A), whereas nicotine-treated
cells typically exhibited an increase in the density and length of
surface microvilli (Fig. 2). The SEM
results supported our hypothesis in which an elevated number of
filopodia were identified with a higher extensively in the
nicotine-treated groups.
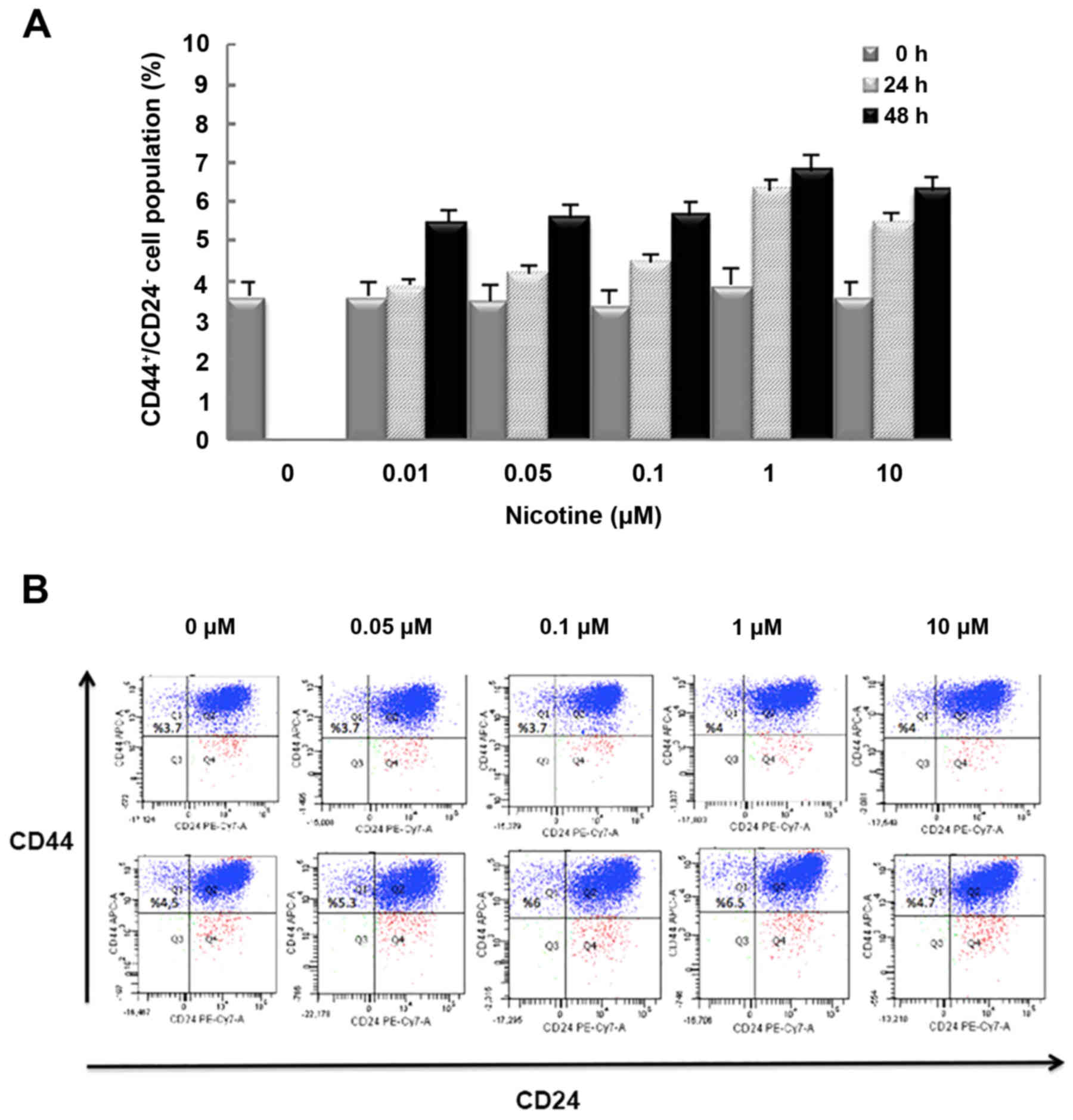
Nicotine increases the
CD44+CD24− cell population in MCF-7
cells
To investigate whether nicotine affects the size of
a CSC population, MCF-7 human breast cancer cells were
characterized using flow cytometry for surface expression of CD44
and CD24, which is used for the identification of CSCs. As
demonstrated in Fig. 3, stimulation
with 0.01, 0.05, 0.1, 1 or 10 µM nicotine increased the ratio of
CSCs. Treatment with 1 µM nicotine after 24 h, increased
CD44+CD24− cells (~1.6-fold), suggesting that
the effect of nicotine is due to the proliferation of
CD24− cells. As demonstrated in Fig. 3, the effect of nicotine was observed
in a dose-dependent manner until 1 µM nicotine treatment, after
which the cell population was decreased at 10 µM, which indicated
that maximal effects were achieved at a concentration of 1 µM. To
confirm whether nicotine increases the CSC population, expression
patterns of CD24 and CD44 in MCF-7 cells were analyzed by flow
cytometry were determined. The results suggested that nicotine
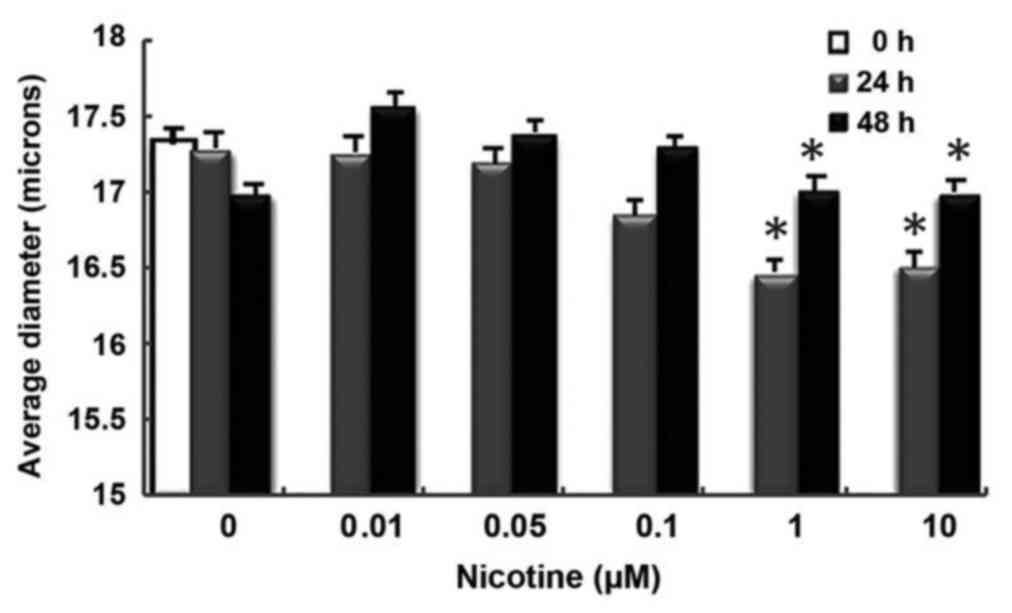
increased the proportion of CSCs in the cell population (Fig. 3B). Smaller changes between 24 and 48
h in cell diameter measurements were observed in nicotine-treated
groups compared with controls (Fig.
4).
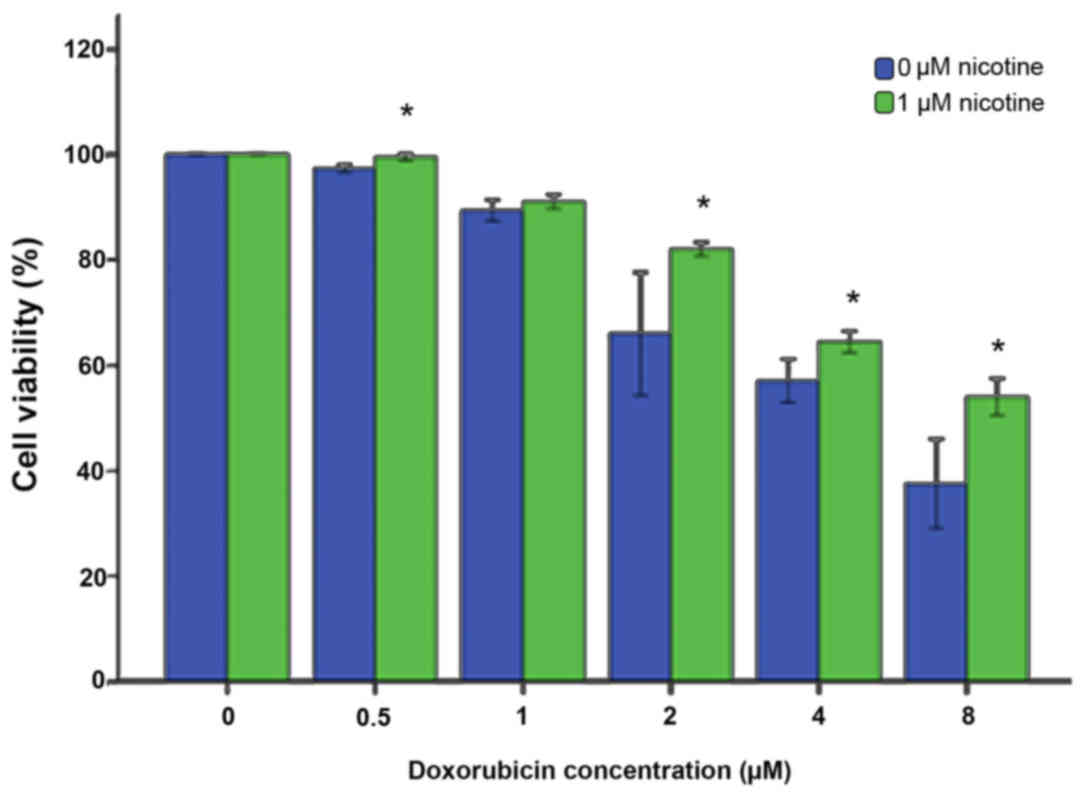
Nicotine treatment promotes cell
viability during doxorubicin exposure
It would be beneficial to determine the specific
compounds within cigarettes that may be responsible for promoting
drug resistance because cigarettes contain a mixture of compounds.
Therefore, the present study aimed to determine whether nicotine, a
specific component of cigarettes, may be responsible for
chemotherapy drug resistance. Trypan blue cell counting was
performed to compare survival curves between control cells and 1 µM
nicotine-treated cells in the presence of increasing doxorubicin
concentrations (Fig. 5. The results
demonstrated that nicotine significantly increased MCF-7 cell
viability during exposure to 0.5, 2, 4 or 8 µM doxorubicin
(P<0.01).
Discussion
Nicotine and its derivatives are well documented for
their role in addiction and wide range of effects in cancer.
Nicotine stimulates the cellular and molecular pathways in
carcinogenesis throughout the gastrointestinal tract (28). Furthermore, nicotine affects the
function of numerous systems in the human body (28). Nicotine is an established cause of
numerous cancer types (10–13); however its role in breast cancer
etiology is unclear. In the present study, the potential effects of
nicotine in human breast cancer cells were evaluated.
In vivo studies have provided evidence of
direct actions of nicotine, known as the dependency-inducing
ingredient of cigarettes, regarding tumor development by increasing
cell proliferation and interfering with apoptosis (13–15). It
is known that, aside from tumor development, nicotine also causes
changes in the human immune system (30–33).
Heeschen et al (13) has
demonstrated proliferation, membrane transport, metabolic and
hormonal changes in nicotine induced cells.
Nicotine in plasma was previously reported to be
between 10 nM and 10 µM in smokers (33). Therefore, in the present study, these
nicotine concentrations were used in accordance with previous
reports. Nicotine is taken into the body by chewing nicotine gum,
by sniffing tobacco or sucking it into the mouth through the use of
cigarettes. Plasma concentrations of nicotine rise slowly, reach a
peak level after 30 min and do not fall during the following 2 h
(34). In nicotine gums, nicotine is
secreted as the gum is chewed, therefore a complete extraction of
nicotine from the gum does not occur (2 mg gum provides 53%
secretion, while 4 mg gum ensures 72% secretion). In this
circumstance, a significant amount of nicotine is swallowed through
the mouth (34,35).
A previous study indicated that it took 15–30 min
for 4-mg gum to raise the plasma nicotine by an average of 9–11
ng/ml compared with an average increase of 8–27 ng/ml within 2 min
of completing each cigarette (36).
Furthermore, nicotine is believed to be responsible for the
occurrence and development of several cancer types, including
breast cancer (37). Previous study
models have also demonstrated that nicotine increases angiogenesis
and proliferation (38). In the
present study, when the results of the 24- and 48-h analyses of
MCF-7 cells on an XR viability analyzer were evaluated between
themselves, a nicotine dose-dependent increase in cell diameter was
observed some changes in cell diameter measurements in the cells
treated with nicotine compared with the controls. Cells were
suspended with trypsin/EDTA during the sphere shape conducted using
the cell XR viability analyzer.
CSCs, or tumor-initiating cells, have a major role
in the onset of cancer and they have a resistance against
chemotherapy and radiotherapy (39,40). In
the present study, the presence of nicotine, these cells increase
in number and invade, induced by changes in the dynamic values of
other cells, thus providing mobility. This suggests that treatment
would be hindered by nicotine intake, thereby deteriorating the
treatment process. There is an increasing amount of evidence that
suggests that breast cancer stem cells have a central role in the
development of cancer (38). A
clinical study has indicated that breast cancer metastases occur in
the lungs of smokers (40). The
findings of the present study suggested that in the presence of
nicotine, the number of CSCs is associated with an increase in the
CD24− cell population, which is active in metastasis.
Like alcohol, nicotine is a facilitating risk factor in the
metastasis process of breast cancer (38).
An investigation of the microcirculation of mammals
has indicated that nicotine results in cell proliferation, membrane
transport, metabolic and hormonal changes (41). The results of the present study are
consistent with these suggestions. Gradually increasing dosages of
nicotine, which were within the limits of nicotine in the blood
plasma of smokers, stimulated the number of MCF-7 cancer stem
cells. It was identified that 1 µM nicotine increased the number of
CD44+CD24− cells among the MCF-7 cells. The
decrease in viability and ratio of CD44+CD24−
cells following treatment with 10 µM nicotine may be explained by a
possible toxic effect.
Stimulation with nicotine may be the result of the
increase in the percentage of CD24− MCF-7 cells, as
identified by flow cytometry. Furthermore, the effects of nicotine
on a CSC population were investigated with flow cytometry based on
the CD44+CD24− characteristics, and it was
found that it also stimulates CSC proliferation. This finding
suggested a direct relation of nicotine with disease development
and progression in patients with breast cancer who smoke.
Cell morphology findings of the present study
demonstrated that nicotine increased cell invasion, although the
results with electron microscopy and florescent microscopy
indicated the orientation of cells but did not provide quantitative
information about the invasive ability of breast cancer cells
without characteristics of CSCs. Flow cytometry results suggested
that nicotine had a proliferation-inducing effect on the
CD44+CD24− cell population. This difference
was not observed in all cells in the study areas but it was
identified in higher numbers in the nicotine groups. This may be
explained by the heterogeneous quality of the population. Previous
research has demonstrated the effects of nicotine on cell
morphology, motility and the cytoskeleton, which leads to
degradation of the actin cytoskeleton and finally to cell
destruction (42,43).
In the present study, re-characterization of
F-actin, a basic cortical skeletal component of in vitro
cancer models, was also identified to be among the potential
effects of nicotine on breast cancer cells. Nicotine-administered
MCF-7 breast cancer cells exhibited changes in cell structure,
including becoming rounder in form with decreased diameters, which
was in direct proportion with the increase of nicotine
concentrations. Based on these results, it is hypothesized that
nicotine also has a role in the regulation of cellular cascades. It
is possible that sensors of cytosolic mechanisms send signals to
cell membrane proteins and to the nucleus, initiating mechanical
events such as clustering and interactions between the cortical
cell and skeletal structure.
In the present study, actin filaments were located
at the region underneath the nuclear membrane. Actin filaments were
also observed to increase around the inner part of the cell
membrane. Thus, the accumulation of F-actin was evaluated, which
was most likely related to the cellular motility. Thus, nicotine
has an effect on cell motility and associates with the metastatic
process.
In the present study, as a result of administration
of varying concentrations of nicotine to MCF-7 breast cancer cells,
morphological changes occurred in the structure of the cytoskeleton
and, at the same time, cells experienced stress depending on the
concentration of nicotine. A previous study demonstrated that one
of the most important obstacles in chemotherapy is the resistance
that cancer cells develop against apoptosis (44). One of the current approaches in this
context is to use various drugs along with anticancer medications
in order to ensure sensitivity of cancer cells for chemotherapy and
to break the resistance (45). Sak
(44) identified that this
resistance is developed in cancer patients who do not cease smoking
and continue their exposure to nicotine. The results of a
doxorubicin experiment, which was performed because
nicotine-administered MCF-7 cells had an increased number of stem
cells and these cells were resistant to chemotherapy, it was
demonstrated that MCF-7 breast cancer cells developed resistance
against this chemotherapeutic agent (44).
To evaluate whether nicotine increases metastatic
potential in breast cancer, lamellipodia formation on F-actin was
investigated using TEM and fluorescence microscopy. In light of
these parameters, the present findings regarding mobilization were
confirmed by SEM and fluorescence microscopy. Although these
results are considered to be associated with metastasis, they may
provide a basis for further studies on cancer development.
It is necessary to determine molecular and genetic
targets that would be helpful in destroying CSCs without damaging
normal cells, and to detect genetic and epigenetic events that
trigger cancer. To the best of our knowledge, no previous studies
have been conducted on the effect of nicotine on drug resistance.
Inhibition of signal pathways that are involved in the resistance
mechanism of CSCs would create novel treatment possibilities and
therefore further studies on signal pathways are required. In order
for gene sequence analyses and molecular markers that inhibit or
control the interaction of cells with nicotine to be realized in
the near future, studies addressing the genetic and molecular basis
of nicotine effects are required.
Resistance against doxorubicin may be explained by
an increased number of CSCs due to the presence of nicotine.
However, some transcriptional factors that may modulate resistance
to doxorubicin may be responsible, which was based on the present
result that no correlation was identified between the resistance
against doxorubicin and the elevation of the stem cell-induced
cancer.
In the present study, it was identified that the
population of CSCs increased in the presence of nicotine in MCF-7
cells. Nicotine was also indicated to increase the proliferation of
MCF-7 breast cancer cells and actin filaments. The present results
have aided in the understanding of drug resistance in breast cancer
cells.
In conclusion, it is believed that nicotine, which
is effective in the occurrence of breast cancer, is also one of the
factors that has a role in the development and metastasis of this
cancer. The results of the present study suggested that smoking and
other forms of long-term nicotine intake, such as through nicotine
gums, electronic cigarettes and nicotine pastilles, which are used
as aids for smoking cessation during the cancer process, also
contribute to the mobilization of cancer cells and affect the
progression and relapse of the disease.
Acknowledgements
The present study was supported by the Scientific
Research Projects Coordination Unit of Istanbul University (project
no. 25945).
References
|
1
|
Gonzalez-Angulo AM, Morales-Vasquez F and
Hortobagyi GN: Overview of resistance to systemic therapy in
patients with breast cancer. Adv Exp Med Biol. 608:1–22. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Laurentiis A, Pardo OE, Palamidessi A,
Jackson SP, Schoenwaelder SM, Reichmann E, Scita G and Arcaro A:
The catalytic class I(A) PI3K isoforms play divergent roles in
breast cancer cell migration. Cell Signal. 23:529–541. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bailly C: topoisomerase I poisons and
suppressors as anticancer drugs. Curr Med Chem. 7:39–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Housman G, Byler S, Heerboth S, Lapinska
K, Longacre M, Snyder N and Sarkar S: drug resistance in cancer: An
overview. Cancers (Basel). 6:1769–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Samet JM, Avila-Tang E, Boffetta P, Hannan
LM, Olivo-Marston S, Thun MJ and Rudin CH: Lung cancer in never
smokers: Clinical epidemiology and environmental risk factors. Clin
Cancer Res. 15:5626–5645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cancer among adults from exposure to
secondhand smoke, The health consequences of involuntary exposure
to tobacco smoke: A Report of the Surgeon General. Atlanta (GA):
Centers for Disease Control and Prevention (US); 2006
|
|
8
|
Benowitz NL: Biomarkers of Cigarette
SmokingMonograph 7: The FTC Cigarette Test Method for Detemining
Tar, Nicotine, and Carbon Monoxide Yields of U.S. Cigarettes.
National Cancer Institute; Bethesda, MD: pp. 93–111. 1988
|
|
9
|
Centers for Disease Control and Prevention
(US); National Center for Chronic Disease Prevention and Health
Promotion (US); Office on Smoking and Health (US): Nicotine
Addiction: Past and PresentHow Tobacco Smoke Causes Disease: The
Biology and Behavioral Basis for Smoking-Attributable Disease: A
Report of the Surgeon General. Centers for Disease Control and
Prevention (US); Atlanta, GA: 2010
|
|
10
|
Dani JA and De Biasi M: Cellular
mechanisms of nicotine addiction. Pharmacol Biochem Behav.
70:439–446. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clark CA, McEachern MD, Shah SH, Rong Y,
Rong X, Smelley CL, Caldito GC, Abreo FW and Nathan CO: Curcumin
inhibits carcinogen and nicotine-induced mammalian target of
rapamycin pathway activation in head and neck squamous cell
carcinoma. Cancer Prev Res (Phila). 3:1586–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaal C and Chellappan SP:
Nicotine-mediated cell proliferation and tumor progression in
smoking-related cancers. Mol Cancer Res. 12:14–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heeschen C, Jang JJ, Weis M, Pathak A,
Kaji S, Hu RS, Tsao PS, Johnson FL and Cooke JP: Nicotine
stimulates angiogenesis and promotes tumor growth and
atherosclerosis. Nat Med. 7:833–839. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petros WP, Younis IR, Ford JN and Weed SA:
Effects of tobacco smoking and nicotine on cancer treatment.
Pharmacotherapy. 32:920–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh S, Pillai S and Chellappan S:
Nicotinic acetylcholine receptor signaling in tumor growth and
metastasis. J Oncol. 2011:4567432011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suraneni MV and Badeaux MD:
Tumor-Initiating cells, cancer metastasis and therapeutic
implications, madame curie bioscience databaseMadame Curie
Bioscience Database [Internet]. Landes Bioscience; Austin, TX:
2013
|
|
17
|
Davis R, Rizwani W, Banerjee S, Kovacs M,
Haura E, Coppola D and Chellappan S: Nicotine promotes tumor growth
and metastasis in mouse models of lung cancer. PLoS One.
4:e75242009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Honeth G, Bendahl PO, Ringner M, Saal LH,
Gruvberger-Saal SK, Lövgren K, Grabau D, Fernö M, Borg A and
Hegardt C: The CD44+/CD24− phenotype is
enriched in basal-like breast tumors. Breast Cancer Res.
10:R532008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lorico A and Rappa G: Phenotypic
heterogeneity of breast cancer stem cells. J Oncol.
2011:1350392011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J and Song E: let-7 Regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Laurentiis M, Cianniello D, Caputo R,
Stanzione B, Arpino G, Cinieri S, Lorusso V and De Placido S:
Treatment of triple negative breast cancer (TNBC): Current options
and future perspectives. Cancer Treat Rev. 36 Suppl 3:S80–S86.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muñoz-Pinedo C, El Mjiyad N and Ricci JE:
Cancer metabolism: Current perspectives and future directions. Cell
Death Dis. 3:e2482012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rivera E and Gomez H: Chemotherapy
resistance in metastatic breast cancer: The evolving role of
ixabepilone. Breast Cancer Res. 12 Suppl 2:S22010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SY, Lee HE, Li H, Shipitsin M, Gelman
R and Polyak K: Heterogeneity for stemcell-related markers
according to tumor subtype and histologic stage in breast cancer.
Clin Cancer Res. 16:876–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hussain T and Nguyen QT: Molecular imaging
for cancer diagnosis and surgery. Adv Drug Deliv Rev. 66:90–100.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Groebel K, Hoelzle K, Wittenbrink MM,
Ziegler U and Hoelzle LE: Mycoplasma suis invades porcine
erythrocytes. Infect Immun. 77:576–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varol B, Bektaş M, Nurten R and Bermek E:
The cytotoxic effect of diphtheria toxin on the actin cytoskeleton.
Cell Mol Biol Lett. 17:49–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitchell RR, Szabo E, Benoit YD, Case DT,
Mechael R, Alamilla J, Lee HJ, Fiebig-Comyn A, Gillespie DC and
Bhatia M: Activation of neural cell fate programs toward direct
conversion of adult human fibroblasts into tri-potent neural
progenitors using OCT-4. Stem Cells Dev. 23:1937–1946. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jensen K, Afroze S, Munshi MK, Guerrier M
and Glaser SS: Mechanisms for nicotine in the development and
progression of gastrointestinal cancers. Transl Gastrointest
Cancer. 1:81–87. 2012.PubMed/NCBI
|
|
31
|
Karamboulas C and Ailles L: Developmental
signaling pathways in cancer stem cells of solid tumors. Biochim
Biophys Acta. 1830:2481–2495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pereira ML, Carvalho JC, Peres F,
Gutierres M and Fernandes MH: Behaviour of human osteoblastic cells
cultured on plasma-sprayed titanium implants in the presence of
nicotine. Clin Oral Implants Res. 19:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Berger MR and Zeller WJ: Interaction of
nicotine with anticancer treatment. Klin Wochenschr. 66 Suppl
11:S127–S133. 1988.
|
|
34
|
Benowitz NL, Hukkanen J and Jacob P III:
Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp
Pharmacol. 29–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hukkanen J, Jacob P III and Benowitz NL:
Metabolism and disposition kinetics of nicotine. Pharmacol Rev.
57:79–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Russell MA, Feyerabend C and Cole PV:
Plasma nicotine levels after cigarette smoking and chewing nicotine
gum. Br Med J. 1:1043–1046. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sobus SL and Warren GW: The biologic
effects of cigarette smoke on cancer cells. Cancer. 120:3617–2627.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Warren GW and Singh AK: Nicotine and lung
cancer. J Carcinog. 12:12013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei W and Lewis MT: Identifying and
targeting tumor-initiating cells in the treatment of breast cancer.
Endocr Relat Cancer. 22:R135–R155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Murin S and Inciardi J: Cigarette smoking
and the risk of pulmonary metastasis from breast cancer. Chest.
119:1635–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pomorski P, Wasik A, Kołodziejczyk J,
GrêBecka L and Kłopocka W: Nicotine affects behaviour, morphology
and cortical cytoskeleton of amoeba proteus. Acta Protozool.
43:193–198. 2004.
|
|
42
|
Krause RM, Buisson B, Bertrand S,
Corringer PJ, Galzi JL, Changeux JP and Bertrand D: Ivermectin: A
possible allosteric effector of the alpha 7 neuronal nicotinic
acetylcholine receptor. Mol Pharmacol. 53:283–294. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sak K: Chemotherapy and dietary
phytochemical agents. Chemother Res Pract.
2012:2825702012.PubMed/NCBI
|
|
45
|
You HB and Park K: Targeted drug delivery
to tumors: Myths, reality and possibility. J Control Release.
153:198–205. 2011. View Article : Google Scholar : PubMed/NCBI
|