Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors and the leading cause of cancer-associated
mortality in humans (1). CRC is the
third most commonly diagnosed cancer in males and the second in
females, with an estimated 1.4 million cases and 693,900 deaths
occurring worldwide in 2012 (2). In
the USA, CRC is the third leading cause of cancer-associated
mortality (3), while tumor invasion
and metastasis are the leading causes of patient mortality
(4). Many CRCs are metastatic at the
time of diagnosis (5). Diabetes
mellitus (DM) is a metabolic disorder characterized by increased
blood glucose levels (6) and is
considered to be one of the most important health problems
worldwide (7). It has been
demonstrated that DM is associated with an elevated risk of CRC in
both men and women (8). A
meta-analysis of 8 studies identified a positive correlation
between type 2 (T2)DM with a 1.21-fold increased risk of CRC
(9). Patients with colorectal cancer
and DM have an increased risk of cancer-specific mortality and have
worse disease-free survival than those who do not have DM (10,11). DM
has also been reported to be a risk factor for CRC, although this
remains controversial (11–14).
Epithelial-mesenchymal transition (EMT) is the
morphological transformation of epithelial-like cancer cells to an
elongated mesenchymal phenotype (15). During EMT, cancer cells stop
expressing adhesion proteins, including epithelial (E)-cadherin and
claudin-1, and increase the expression of mesenchymal phenotype
markers, including vimentin, neural (N)-cadherin and Snail
(16). EMT serves an important role
in the invasion and metastasis of CRC (17) and is able to induce circulating tumor
cell properties in transformed colorectal epithelial cells
(18). Furthermore, EMT is highly
prognostic for colon cancer recurrence (19). High glucose (HG) induces EMT in
breast cancer cells (20) and human
peritoneal mesothelial cells (21);
however, this effect has not been studied in CRC.
The aim of the present study was to investigate the
association between HG and the migration, invasion and apoptosis of
colorectal cancer cells. The expression of EMT-associated proteins
was detected and the underlying mechanisms were investigated.
Materials and methods
Cell culture and transfection
The human CRC cell lines HCT-116 and HT-29 were
obtained from American Type Culture Collection (Manassas, VA, USA).
Both cell lines were cultured in Dulbecco's modified Eagle's medium
(DMEM; Genom Biotech Pvt., Ltd., Bhandup, Mumbai) containing 10%
fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA,
USA), 100 unit/ml penicillin, 100 µg/ml streptomycin with normal
glucose (NG; 5.5 mmol/l) or HG (30 mmol/l). Cultures were
maintained at 37°C in a humidified atmosphere containing 5%
CO2.
Human samples
A total of 6 CRCs with or without T2DM in this study
were histologically and clinically diagnosed at Ningbo Urology and
Nephrology Hospital between October 2015 to March 2016 and the
tissues were collected immediately following surgical resection for
diagnosis. The inclusion criteria was as follows: i) Patients had
to be diagnosed with CRC by preoperative pathological biopsy using
a colonoscope; ii) aged between 18 and 75 years; iii) exhibit no
distant metastasis; and iv) with or without diabetes. Patients were
excluded if they: i) Received radiotherapy and chemotherapy prior
to surgery; ii) exhibited acute infection; or iii) had a history of
abdominal surgery or other malignant tumors. The specimens were
then stored at −80°C. The present study was approved by Ningbo
Yinzhou Ethics Committee and signed informed consent was obtained
from the patients or their family. Patient data is summarized in
Table I.
 | Table I.Patient data. |
Table I.
Patient data.
| Patients | Number of
patients | Age | Sex ratio
(F:M) | Comorbidities |
|---|
| With diabetes | 3 | 56–64 | 2:1 | No
comorbidities |
| Without
diabetes | 3 | 60–65 | 2:1 | One with
hypertension |
Immunofluorescence
CRC tissues were fixed in 4% formaldehyde solution
for 2 h at 25°C and then sectioned into 5-µM-thick frozen sections.
The sections were washed in cold PBS 3 times and subsequently
blocked with 2% bovine serum albumin V at 25°C (BSA-V; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) for 1
h. Samples were incubated with primary antibodies against
E-cadherin (1:20; sc-8426; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) diluted with 1% BSA-V overnight at 4°C. Following 3 washes
with PBS, the sections were incubated with tetramethylrhodamine
conjugated goat anti-rabbit secondary antibody (1:1,000; sc-362281;
Santa Cruz Biotechnology, Inc.) diluted with 1% BSA-V in the dark
for 1 h at 25°C and washed in PBS again for 3 times. DAPI diluted
with PBS was used to stain the nuclei at 25°C. Images at a
magnification of ×40 were captured using an inverted fluorescence
microscope (Nikon Corp., Tokyo, Japan).
Western blotting
Tissues and cells were homogenized in a
radioimmunoprecipitation assay lysis buffer (Beijing Solarbio
Science & Technology Co., Ltd.). A BCA protein assay kit
(Cwbiotech, Beijing, China) was used to determine protein
concentrations. Proteins (20 µg) were separated by 10% SDS-PAGE and
electrotransferred to polyvinylidene fluoride membranes, which were
blocked in 5% nonfat milk for 1 h at 25°C and probed with primary
antibodies against E-cadherin (1:400; #AF0131; Affinity
Biosciences, Jiangsu, China), vimentin (1:600; #AF0292; Affinity
Biosciences), GAPDH (1:10,000; #T0004; Affinity Biosciences) and
high-mobility group A protein 2 (HMGA2; 1:500; 5269s; Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. The
membranes were subsequently incubated with goat anti-rabbit
antibody diluted with 0.3‰ TBST (1:1,000; #SC2004; Santa Cruz
Biotechnology, Inc.) or goat anti-mouse antibody diluted with 0.3‰
TBST (1:1,000; #SC2005; Santa Cruz Biotechnology, Inc.) for 1 h at
room temperature. The membranes were scanned with the Tanon 5200
automated image analysis system (Tanon, Shanghai, China) and the
ImageJ software (version 1.48U; National Institutes of Health,
Bethesda, MD, USA) was used to evaluate the band intensity.
Scratch assay
HCT-116 and HT-29 cells (3–5×105
cells/well) were seeded in 6-well plates and cultured in DMEM
containing NG or HG for 4 days at 37°C. Confluent cultures were
scratched with sterile 200 µl pipette tips and washed gently with
PBS to remove floating cells. Then the cells were cultured in DMEM
containing NG or HG and 5% FBS. Cells were viewed under an inverted
fluorescence microscope (magnification, ×40) and images were
captured after 0, 24, 48 and 72 h.
Transwell assays
HCT-116 and HT-29 cells were cultured in DMEM
containing NG or HG for 4 days and then serum-starved for 12 h. The
cells (1×105 cells/well) were seeded into Boyden
chambers (EMD Millipore, Billerica, MA, USA) with 8-µm pore size
filter membranes. The inserts were coated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) for invasion assays or not
coated for migration assays. The chambers were then placed in
24-well plates containing DMEM and 10% FBS at 37°C. After 72 h, the
non-invaded cells on the upper side of the filter were removed with
a cotton swab and cells attached to the underside of the membrane
were fixed in ethanol, stained with crystal violet and images were
counted using a microscope (CKX41; Olympus Corporation, Tokyo,
Japan; magnification, ×40).
HMGA2 knockdown
The RNA interference technique was used to
downregulate HMGA2 in HCT-116 and HT-29 cells (22). HMGA2 small interfering 400 ng
(si)RNAs (siHMGA2-1, 5′-GAAAGCAGAGACCAUUGGATT-3′; siHMGA2-2,
5′-GAAAGCAGAGACCAUUGGATT-3′; Shanghai Genechem Co., Ltd., Shanghai,
China) were synthesized and transfected into cells using RNAiMAX
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. Following 48 h
transfection, HMGA2 expression was confirmed by western blotting.
Western blotting was then performed as aforementioned.
MTT assay
HCT-116 and HT-29 proliferation was measured using
an MTT assay. Cells were incubated with 0.35 mg/ml MTT solution at
37 °C for 4 h. The medium was removed, 100 µl dimethylsulfoxide
(DMSO) was added and the mixture was vortexed at 112 × g for 10 min
at 25°C. The optical density was read at 490 nm and all experiments
were performed 3 times.
Ki-67 expression and apoptosis
analysis
Cells were seeded in 6-well plates and treated with
NG or HG, respectively, for 4 days. Cells were digested using 1 ml
trypsin (#C0201; Beyotime Institute of Biotechnology, Beijing,
China), washed twice with PBS and incubated in 100 µl fixation
buffer (Biolegend, Inc., San Diego, CA, USA) at room temperature
for 15 min. Cells were then washed with 100 µl permeabilisation
buffer (Biolegend, Inc.). Following centrifugation at 1,500 × g for
3 min at 25°C, the cells were resuspended in 100 µl
permeabilisation wash buffer containing Alexa Fluor 647 mouse
anti-Ki-67 antibody (1:100; 561126; BD Biosciences) and incubated
at room temperature in the dark for 30 min. A total of 400 µl
permeabilisation wash buffer was added to resuspend the cells for
flow cytometric analysis using a FACS flow cytometer (BD
Biosciences).
Cell apoptosis was assayed using the Annexin
V-phycoerythrin (PE) Apoptosis Detection kit (BD Biosciences).
Cells were washed twice with cold PBS and resuspended in Annexin V
Binding buffer at a concentration of 1.0×106 cells/ml.
Specifically, this suspension (100 µl) comprised 1 µl Annexin V-PE,
1 µl 7-aminoactinomycin D and 98 µl Binding buffer. The cells were
vortexed gently and incubated for 15 min at room temperature in the
dark. To each tube, 400 µl of Binding buffer added and cells were
analyzed using a FACS flow cytometer (BD Biosciences) and FlowJo
7.6 software (FlowJo LLC, Ashland, OR, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
One-way analysis of variance was used to test the Homogeneity of
variance, then a Mann-Whitney U was used to compare differences
between groups. All statistical analyses were performed using
GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) and
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
HG induces EMT in CRC tissues and
cells
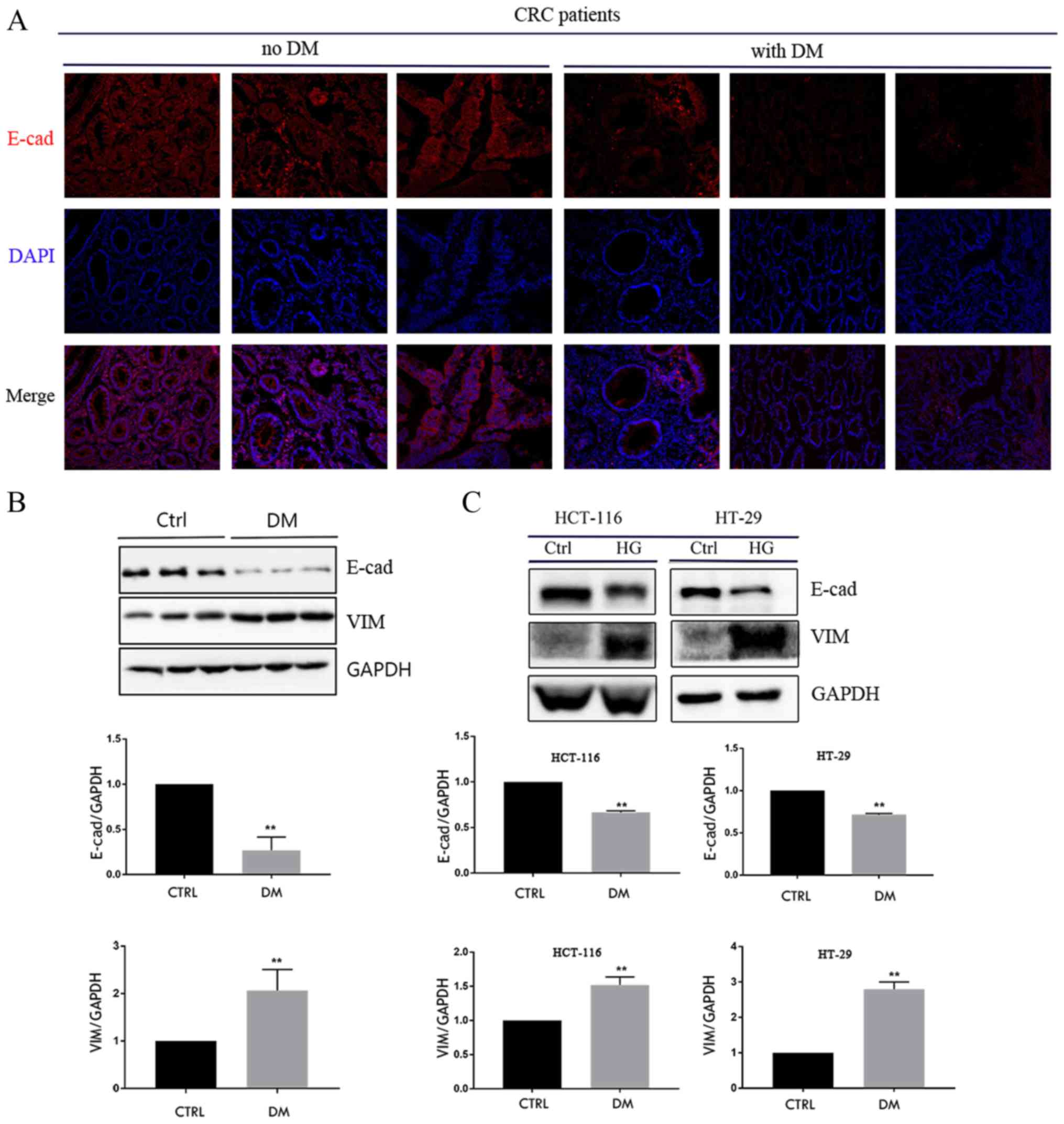
E-cadherin protein expression was measured in tumor
tissues from 3 patients with CRC and DM and 3 patients with CRC
without DM using immunofluorescence and western blotting. The area
of tumor cells with positive E-cadherin staining was increased in
patients without DM compared with those with DM (Fig. 1A). Furthermore, the results of
western blotting confirmed that the expression of E-cadherin
protein was lower in patients with DM compared with patients
without DM (Fig. 1B); however, the
expression of vimentin protein was significantly higher in patients
with DM compared with those without DM (Fig. 1B). HCT-116 and HT-29 cells were
exposed to HG for 4 days and it was demonstrated that HG reduced
the expression of E-cadherin protein, whereas the expression of
vimentin protein was increased (Fig.
1C). These results suggest that HG serves an important role in
the EMT of CRC cells.
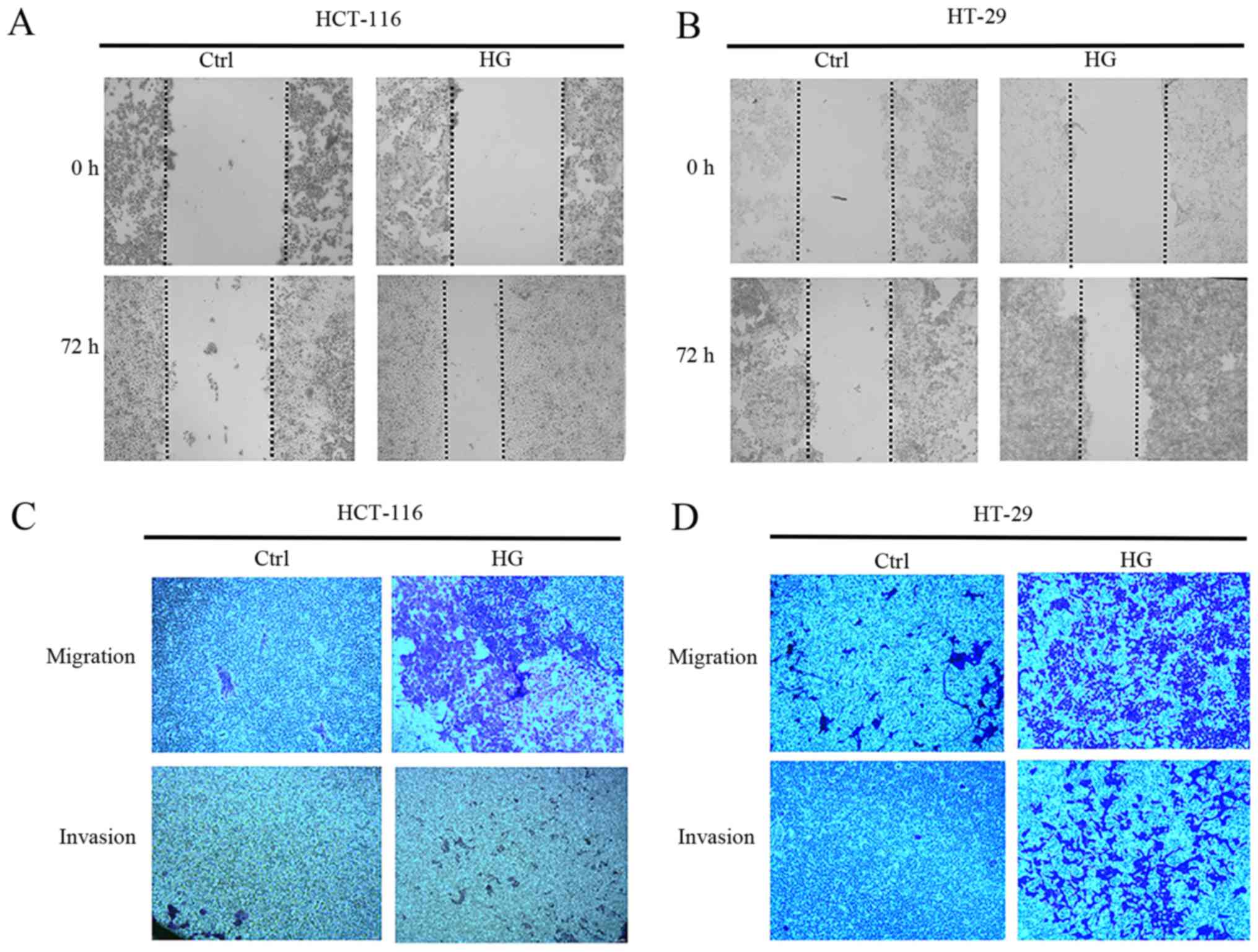
HG promotes the migration and invasion
of CRC cells
EMT is characterized by a loss of cell-to-cell
adhesion and increased cell migration and invasion (23). As such, the effect of HG on the
metastatic capability of CRC cells was investigated. Scratch assays
revealed that wound healing was faster in HCT-116 and HT-29 cells
grown in HG conditions compared with those grown in NG (Fig. 2A and B). Furthermore, HG accelerated
the cells ability to invade and migrate compared with NG (Fig. 2C and D). These results suggest that
HG is able to promote the invasion and migration of CRC cells.
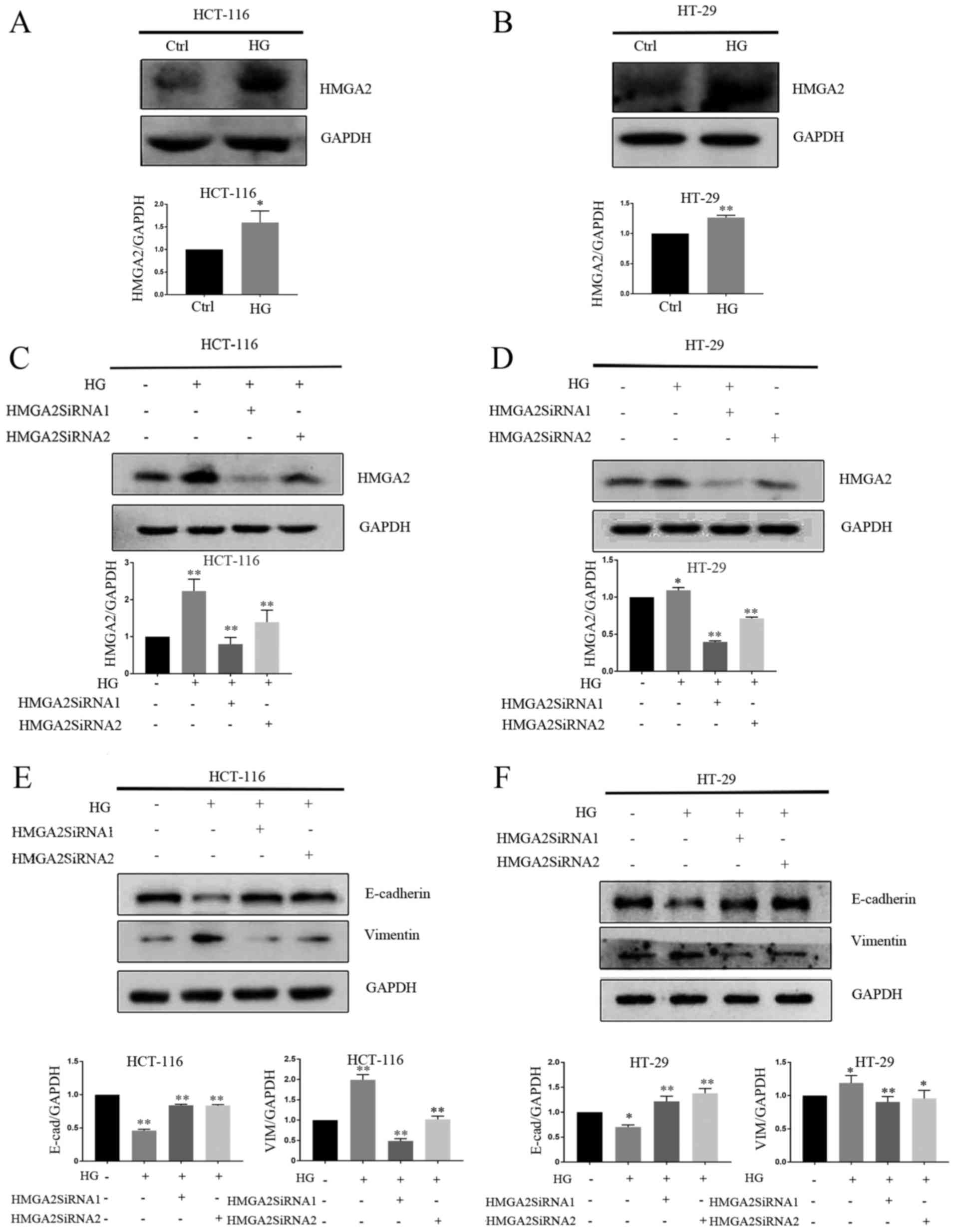
HG promotes EMT by increasing the
level of HMGA2 protein
HMGA2 is known to control the expression of a
diverse set of transcription factors associated with the regulation
of E-cadherin transcription (24,25).
HMGA2 has been reported to regulate EMT in gastric cancer (26,27),
tongue squamous cell carcinoma (28)
and prostate cancer cells (29). As
such, it was hypothesized that HMGA2 may regulate HG-induced EMT
and the expression of HMGA2 in CRC cells exposed to HG or NG for 4
days was assessed. HMGA2 was significantly upregulated in
HG-stimulated cells compared with those treated with NG (Fig. 3A and B). HMGA2 expression was knocked
down in HCT-116 and HT-29 cells and confirmed used western blotting
(Fig. 3C and D). The results
revealed that HMGA2 knockdown significantly increased E-cadherin
protein expression and decreased vimentin protein expression in
HG-stimulated cell compared with those treated with NG (Fig. 3E and F).
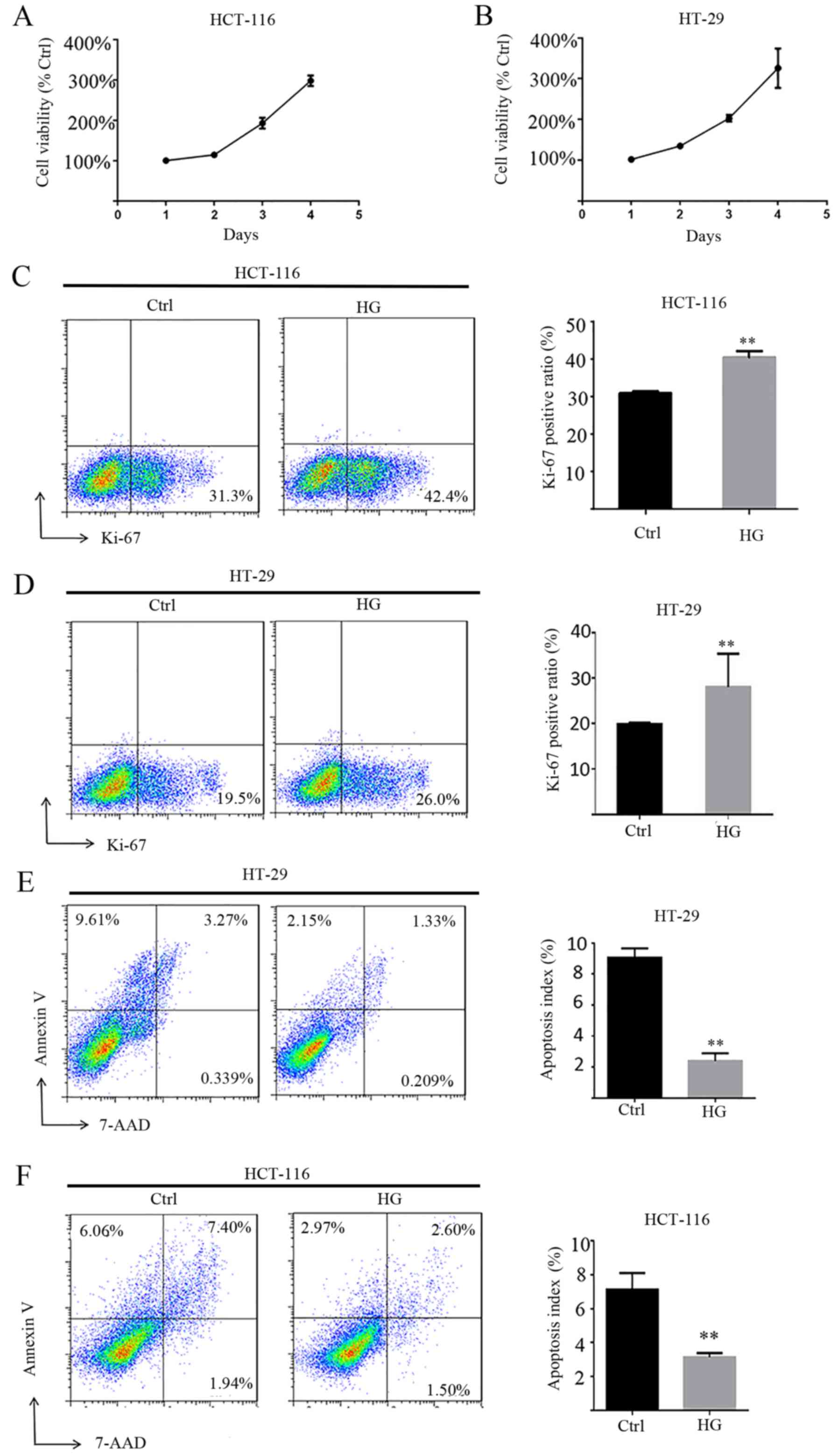
HG enhances cell viability and
suppresses apoptosis in CRC cells
To characterize the functional roles of HG in cell
proliferation, MTT assays were performed and Ki-67 was measured.
The results revealed that HG enhances the viability of HCT-116 and
HT-29 cells in a time-dependent manner (Fig. 4A and B). Ki-67 is a nuclear antigen
present only in proliferating cells and is one of the most widely
used proliferation-associated markers in cancer cells (30). Ki-67 staining demonstrated that HG
enhances the expression of Ki-67 and therefore the proliferation of
HCT-116 and HT-29 cells compared with NG (Fig. 4C and D). The role of HG on apoptosis
in HCT-116 and HT-29 cells was also assessed and it was revealed
that HG significantly decreased apoptosis compared with HG
(Fig. 4E and F).
Discussion
Impaired metabolism and unlimited growth are two
hallmarks of cancer and serve an important role in cancer
progression (31) and DM promotes
the growth and metastasis of tumor cells (32). The results of the present study
demonstrate that HG increases HMGA2 expression and induces EMT in
CRC cells.
The invasive and migratory capabilities of CRC cells
were significantly enhanced by HG, while transfection with HMGA2
siRNA suppressed HG-induced EMT in HCT-116 and HT-29 cells. In
addition, HG enhanced the proliferation and reduced the apoptosis
of CRC cells. These results suggest that DM causes EMT and promotes
metastasis in CRC cells. As such, DM may induce CRC tumor
growth.
DM has been reported to have pro-migratory and
pro-invasive effects in both normal (33) and cancer cells (34–38).
Epidemiological studies have previously established an association
between inflammation and DM (39–41). The
chronic inflammatory response may contribute to DM development by
causing insulin resistance, which in turn intensifies hyperglycemia
to promote long-term complications of diabetes (42). Furthermore, inflammation induces EMT
in CRC (43,44). Previous research has verified that HG
induces EMT in pancreatic and breast cancers (45). Similarly, the results of the present
study demonstrate that DM is associated with the downregulation of
E-cadherin and upregulation of vimentin in patients with CRC.
Meanwhile, HG induces EMT in CRC cells in vitro.
Downregulated E-cadherin expression is associated with lymph node
metastases, poor tumor differentiation and worse prognosis in
patients with CRC (46,47). Conversely, increased vimentin
expression is significantly associated with lymph node metastasis
and poor prognosis in CRC (48). The
results of the present study demonstrated that the invasion and
migration capabilities of CRC cells were enhanced by the occurrence
of EMT. HMGA2 is a chromatin remodeling factor that is able to
alter chromatin architecture to activate transcriptional enhancers
(49). High expression of HMGA2 is
associated with cell proliferation and increased metastasis in a
number of cancers (50). The results
of the present study are consistent with a number of previous
studies in which it was reported that HMGA2 activates EMT in cancer
cells (51,52). At least 11 EMT-associated molecular
pathways have been reported in the literature about CRC cells,
including β-catenin-associated EMT, transforming growth factor-β
and Wnt pathway-associated EMT and aberrant NOTCH-1 signaling
associated EMT (53) Future studies
should aim to elucidate whether there any other signaling pathways
are associated with HG-induced EMT.
HG in patients with DM may alter the expression of
genes that promote cell proliferation in the colon (32–37,54). The
rate of proliferating cell nuclear antigen-positive cells is higher
in patients with CRC and DM compared with patients with CRC alone
(55). HG conditions enhance cell
proliferation via decreasing the population of cells arrested in
the G0/G1 phase (56). In accordance
with the results of the present study, HG has previously been
reported to increase the proliferation of CRC cells (57).
The present study is not without limitations. The
effect of HG, which is the main feature of DM, was studied in
isolation. T2DM is typically accompanied by other metabolic
abnormalities, including hyperlipidemia and hyperinsulinemia
(58,59). These abnormalities should be
considered in future studies.
In summary, the results of the present study
indicate that hyperglycemia is associated with a reduction in
epithelial markers and an increase mesothelial markers in CRC. The
HG-induced enhanced migratory and invasive abilities of CRC cells
may be attributed to EMT via the upregulation of HMGA2. The results
of the present study may provide novel insights into the
association between DM and CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ningbo
Science and Technology Innovation Team Program (grant nos.
2014B82002 and 2015B11050), the Public Benefit Technology and
Society Development Program of Zhejiang Province (grant no.
2015C33309), the National Natural Science Foundation of China
(grant nos. 81370165, 81501421 and 31301068) and the Fang Runhua
Fund of Hong Kong, K. C. Wong Magna Fund in Ningbo University
(grant no. NBU2013001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HS collected patient tumor tissue samples, JW and JC
performed experimental work and conceived ideas. YX, FW, LL, YZ, XH
and SB conceived ideas and evaluated the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Ethics Committee of the Ningbo Urology and Nephrology Hospital and
informed consent was taken from all patients.
Consent for publication
Patient provided written informed consent for the
publication of all associated data and images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DM
|
diabetes mellitus
|
|
CRC
|
colorectal cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HMGA2
|
high-mobility group A protein 2
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
References
|
1
|
McGuire S: World cancer report 2014.
Geneva, Switzerland: World health organization, international
agency for research on cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan N and Mukhtar H: Cancer and
metastasis: Prevention and treatment by green tea. Cancer
Metastasis Rev. 29:435–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoon SS and Tanabe KK: Surgical treatment
and other regional treatments for colorectal cancer liver
metastases. Oncologist. 4:197–208. 1999.PubMed/NCBI
|
|
6
|
Puavilai G, Chanprasertyotin S and
Sriphrapradaeng A: Diagnostic criteria for diabetes mellitus and
other categories of glucose intolerance: 1997 criteria by the
expert committee on the diagnosis and classification of diabetes
mellitus (ADA), 1998 WHO consultation criteria, and 1985 WHO
criteria. world health organizatio. Diabetes Res Clin Pract.
44:21–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Larsson SC, Orsini N and Wolk A: Diabetes
mellitus and risk of colorectal cancer: A meta-analysis. J Natl
Cancer Inst. 97:1679–1687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gabriel B, Pinsard N, Gerard R and Louchet
E: Association of myocardiopathy and spino-cerebellar degeneration
(Friedreich's disease) Apropos of a case. Pediatrie. 29:367–377.
1974.(In French). PubMed/NCBI
|
|
10
|
Mills KT, Bellows CF, Hoffman AE, Kelly TN
and Gagliardi G: Diabetes mellitus and colorectal cancer prognosis:
A meta-analysis. Dis Colon Rectum. 56:1304–1319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stein KB, Snyder CF, Barone BB, Yeh HC,
Peairs KS, Derr RL, Wolff AC and Brancati FL: Colorectal cancer
outcomes, recurrence, and complications in persons with and without
diabetes mellitus: A systematic review and meta-analysis. Dig Dis
Sci. 55:1839–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flood A, Mai V, Pfeiffer R, Kahle L,
Remaley AT, Lanza E and Schatzkin A: Elevated serum concentrations
of insulin and glucose increase risk of recurrent colorectal
adenomas. Gastroenterology. 133:1423–1429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jullumstro E, Kollind M, Lydersen S and
Edna TH: Diabetes mellitus and outcomes of colorectal cancer. Acta
Oncol. 48:361–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noh GY, Hwang DY, Choi YH and Lee YY:
Effect of diabetes mellitus on outcomes of colorectal cancer. J
Korean Soc Coloproctol. 26:424–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-Iike cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Natalwala A, Spychal R and Tselepis C:
Epithelial-mesenchymal transition mediated tumourigenesis in the
gastrointestinal tract. World J Gastroenterol. 14:3792–3797. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calangiu CM, Simionescu CE, Stepan AE,
Cernea D, Zavoi RE and Margaritescu C: The expression of CK19,
vimentin and E-cadherin in differentiated thyroid carcinomas. Rom J
Morphol Embryol. 55:919–925. 2014.PubMed/NCBI
|
|
19
|
Loboda A, Nebozhyn MV, Watters JW, Buser
CA, Shaw PM, Huang PS, Van't Veer L, Tollenaar RA, Jackson DB,
Agrawal D, et al: EMT is the dominant program in human colon
cancer. BMC Med Genomics. 4:92011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flores-Lopez LA, Martinez-Hernandez MG,
Viedma-Rodriguez R, Diaz-Flores M and Baiza-Gutman LA: High glucose
and insulin enhance uPA expression, ROS formation and invasiveness
in breast cancer-derived cells. Cell Oncol (Dordr). 39:365–378.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He L, Lou W, Ji L, Liang W, Zhou M, Xu G,
Zhao L, Huang C, Li R, Wang H, et al: Serum response factor
accelerates the high glucose-induced Epithelial-to-Mesenchymal
Transition (EMT) via snail signaling in human peritoneal
mesothelial cells. PLoS One. 9:e1085932014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gou W, Zhou X, Liu Z, Wang L, Shen J, Xu
X, Li Z, Zhai X, Zuo D and Wu Y: CD74-ROS1 G2032R mutation
transcriptionally up-regulates Twist1 in non-small cell lung cancer
cells leading to increased migration, invasion, and resistance to
crizotinib. Cancer Lett. 422:19–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tam L and Weinberg A: The Epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thuault S, Tan EJ, Peinado H, Cano A,
Heldin CH and Moustakas A: HMGA2 and Smads co-regulate SNAIL1
expression during induction of epithelial-to-mesenchymal
transition. J Biol Chem. 283:33437–33446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan EJ, Thuault S, Caja L, Carletti T,
Heldin CH and Moustakas A: Regulation of transcription factor Twist
expression by the DNA architectural protein high mobility group A2
during epithelial-to-mesenchymal transition. J Biol Chem.
287:7134–7145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong J, Wang R, Ren G, Li X, Wang J, Sun
Y, Liang J, Nie Y, Wu K, Feng B, et al: HMGA2-FOXL2 axis regulates
metastases and epithelial-to-mesenchymal transition of
chemoresistant gastric cancer. Clin Cancer Res. 23:3461–3473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Wang Z, Zha L, Kong D, Liao G and Li
H: HMGA2 regulates epithelial-mesenchymal transition and the
acquisition of tumor stem cell properties through TWIST1 in gastric
cancer. Oncol Rep. 37:185–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grossarth-Maticek R and Eysenck HJ: Length
of survival and lymphocyte percentage in women with mammary cancer
as a function of psychotherapy. Psychol Rep. 65:315–321. 1998.
View Article : Google Scholar
|
|
29
|
Shi Z, Wu D, Tang R, Li X, Chen R, Xue S,
Zhang C and Sun X: Silencing of HMGA2 promotes apoptosis and
inhibits migration and invasion of prostate cancer cells. J Biosci.
41:229–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alco G, Bozdogan A, Selamoglu D, Pilanci
KN, Tuzlali S, Ordu C, Igdem S, Okkan S, Dincer M, Demir G and
Ozmen V: Clinical and histopathological factors associated with
Ki-67 expression in breast cancer patients. Oncol Lett.
9:1046–1054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ryu TY, Park J and Scherer PE:
Hyperglycemia as a risk factor for cancer progression. Diabetes
Metab J. 38:330–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abhijit S, Bhaskaran R, Narayanasamy A,
Chakroborty A, Manickam N, Dixit M, Mohan V and Balasubramanyam M:
Hyperinsulinemia-induced vascular smooth muscle cell (VSMC)
migration and proliferation is mediated by converging mechanisms of
mitochondrial dysfunction and oxidative stress. Mol Cell Biochem.
373:95–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beckner ME, Stracke ML, Liotta LA and
Schiffmann E: Glycolysis as primary energy source in tumor cell
chemotaxis. J Natl Cancer Inst. 82:1836–1840. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rose DP and Vona-Davis L: The cellular and
molecular mechanisms by which insulin influences breast cancer risk
and progression. Endocr Relat Cancer. 19:R225–R241. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Joshi S, Liu M and Turner N: Diabetes and
its link with cancer: Providing the fuel and spark to launch an
aggressive growth regime. Biomed Res Int. 2015:3908632015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masur K, Vetter C, Hinz A, Tomas N,
Henrich H, Niggemann B and Zanker KS: Diabetogenic glucose and
insulin concentrations modulate transcriptome and protein levels
involved in tumour cell migration, adhesion and proliferation. Br J
Cancer. 104:345–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang X, Kong F, Wu X, Ren Y, Wu S, Wu K,
Jiang Z and Zhang W: High glucose promotes tumor invasion and
increases metastasis-associated protein expression in human lung
epithelial cells by upregulating heme oxygenase-1 via reactive
oxygen species or the TGF-β1/PI3K/Akt signaling pathway. Cell
Physiol Biochem. 35:1008–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lontchi-Yimagou E, Sobngwi E, Matsha TE
and Kengne AP: Diabetes mellitus and inflammation. Curr Diab Rep.
13:435–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu B, Meigs B, Li Y, Rifai N and Manson E:
Inflammatory markers and risk of developing type 2 diabetes in
women. Diabetes. 53:693–700. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Han TS, Sattar N, Williams K,
Gonzalez-Villalpando C, Lean ME and Haffner SM: prospective study
of C-reactive protein in relation to the development of diabetes
and metabolic syndrome in the Mexico City Diabetes study. Diabetes
Care. 25:2016–2021. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-alpha:
Direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rokavec M, Oner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng XS, Li YF, Tan J, Sun B, Xiao YC,
Fang XB, Zhang XF, Li Q, Dong JH, Li M, et al: CCL20 and CXCL8
synergize to promote progression and poor survival outcome in
patients with colorectal cancer by collaborative induction of the
epithelial-mesenchymal transition. Cancer Lett. 348:77–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li W, Zhang L, Chen X, Jiang Z, Zong L and
Ma Q: Hyperglycemia promotes the epithelial-mesenchymal transition
of pancreatic cancer via hydrogen peroxide. Oxid Med Cell Longev.
2016:51903142016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pena C, Garcia JM, Silva J, Garcia V,
Rodriguez R, Alonso I, Millan I, Salas C, de Herreros AG, Munoz A
and Bonilla F: E-cadherin and vitamin D receptor regulation by
SNAIL and ZEB1 in colon cancer: Clinicopathological correlations.
Hum Mol Genet. 14:3361–3370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He X, Chen Z, Jia M and Zhao X:
Downregulated E-cadherin expression indicates worse prognosis in
Asian patients with colorectal cancer: Evidence from meta-analysis.
PLoS One. 8:e708582013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Toiyama Y, Yasuda H, Saigusa S, Tanaka K,
Inoue Y, Goel A and Kusunoki M: Increased expression of Slug and
Vimentin as novel predictive biomarkers for lymph node metastasis
and poor prognosis in colorectal cancer. Carcinogenesis.
34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Boo LM, Lin HH, Chung V, Zhou B, Louie SG,
O'Reilly MA, Yen Y and Ann DK: High mobility group A2 potentiates
genotoxic stress in part through the modulation of basal and DNA
damage-dependent phosphatidylinositol 3-kinase-related protein
kinase activation. Cancer Res. 65:6622–6630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fusco A and Fedele M: Roles of HMGA
proteins in cancer. Nat Rev Cancer. 7:899–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang Y, Le Y, Xue JY, Zheng ZJ and Xue YM:
Let-7d miRNA prevents TGF-beta1-induced EMT and renal fibrogenesis
through regulation of HMGA2 expression. Biochem Biophys Res Commun.
479:676–682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao XP, Zhang H, Jiao JY, Tang DX, Wu YL
and Pan CB: Overexpression of HMGA2 promotes tongue cancer
metastasis through EMT pathway. J Transl Med. 14:262016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Matejka M, Finek J and Kralickova M:
Epithelial-mesenchymal transition in tumor tissue and its
metastatic spread of cancer. Klin Onkol Winter. 30:20–27. 2017.(In
Czech). View Article : Google Scholar
|
|
54
|
Tomas NM, Masur K, Piecha JC, Niggemann B
and Zanker KS: Akt and phospholipase Cgamma are involved in the
regulation of growth and migration of MDA-MB-468 breast cancer and
SW480 colon cancer cells when cultured with diabetogenic levels of
glucose and insulin. BMC Res Notes. 5:2142012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang B, Huang CZ, Yu T, Zhou SN, Liu Q,
Liu GJ, Chen S and Han FH: Metformin depresses overactivated
Notch1/Hes1 signaling in colorectal cancer patients with type 2
diabetes mellitus. Anticancer Drugs. 28:531–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang IP, Tsai HL, Huang CW, Lu CY, Miao
ZF, Chang SF, Juo SH and Wang JY: High blood sugar levels
significantly impact the prognosis of colorectal cancer patients
through down-regulation of microRNA-16 by targeting Myb and VEGFR2.
Oncotarget. 7:18837–18850. 2016.PubMed/NCBI
|
|
57
|
Lee SK, Moon JW, Lee YW, Lee JO, Kim SJ,
Kim N, Kim J, Kim HS and Park SH: The effect ofhigh glucose levels
on the hypermethylation of protein phosphatase 1 regulatory subunit
3C (PPP1R3C) gene in colorectal cancer. J Genet. 94:75–85. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Haffner M: Diabetes, hyperlipidemia, and
coronary artery disease. Am J Cardiol. 83:17F–21F. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
O'Dea K, Lion RJ, Lee A, Traianedes K,
Hopper JL and Rae C: Diabetes, hyperinsulinemia, hyperlipidemia in
small aboriginal community in northern Australia. Diabetes Care.
13:830–835. 1990. View Article : Google Scholar : PubMed/NCBI
|