Introduction
Non-alcoholic fatty liver disease (NAFLD) is
characterized by excessive fat deposited in hepatocytes (1). NAFLD has a high prevalence both in both
developed and developing countries (2). In a recent publication, National Health
and Nutrition Examination Survey (NHANES) announced a NAFLD
prevalence of 30% in the United States (3). In a large population prospective
cross-sectional study including 2,493 volunteers recruited from the
general population and the Red Cross Transfusion Center in Hong
Kong, the prevalence of NAFLD in the general Chinese population was
42% (4). In patients with metabolic
syndrome (MetS), the NAFLD prevalence is particularly high, and
approximately 45% of patients with MetS have fatty liver disease
(5). In total, 1.2 and 0.002% of
NAFLD patients progress to liver fibrosis and cirrhosis,
respectively (4).
Traditionally, the development of NAFLD has been
associated with genetics, systolic blood pressure, serum
cholesterol, fasting glucose, gender, age, and waist circumference
(6). In recent years, it was
gradually realized that gut microbiota and related inflammatory
process are, to a great extent, also involved in the development of
NAFLD (7). Nod-like receptor protein
3 (NLRP3) inflammasome, which has the ability to sense
intracellular danger signals, is highly linked to alterations in
the gastrointestinal microflora (7).
NLRP3 recognizes gut microbial, stress and damage signals,
resulting in direct activation of caspase-1, thereby leading to the
secretion of potent pro-inflammatory cytokines and pyroptosis
(8). Thus, NLRP3 inflammasome partly
aggravates the progression of NAFLD/non-alcoholic steatohepatitis
(NASH), and causes MetS via a cell-extrinsic effect of modulation
of gut microbiota through activation of the effector protein IL-1β
(9). In mice, NLRP3 inflammasome
gain of function leads to early and severe onset of methionine and
choline-deficient (MCD) diet-induced steatohepatitis (10–12).
Furthermore, NLRP3 inflammasome activation is required in the
development of fibrosis in NAFLD (13).
In our previous in vivo and in vitro
studies (14–18),
2,3,5,4′-tetrahydroxy-stilbene-2-O-β-D-glucoside (TSG), a naturally
occurring stilbenoid, showed beneficial lipid accumulation
regulation effects in hepatocytes. The data showed that TSG could
partly cut off free fatty acid (FFA) supply, regulated the balance
of gut microbiota, improved intestinal mucosal barrier function,
and reduced the content of serum lipopolysaccharide (LPS).
Prevention of high fat diet (HFD)-induced NAFLD as induced by TSG
was mediated by modulation of the gut microbiota via gut-liver
axis. Furthermore, TSG suppressed the activation of Toll-like
receptor 4/nuclear factor-κB (TLR4/NF-κB) signaling pathway, which
may alleviate chronic low grade inflammation by reducing exogenous
antigen load on the host.
However, activities and capabilities of TSG on NAFLD
induced by factors other than HFD are presently unknown. Whether
TSG affects the activation of NLRPs, related effector proteins,
activation of caspase-1 and further pyroptosis remains to be
elucidated. Therefore, we established an MCD diet-induced
NAFLD/NASH model to investigate the anti-NAFLD activity of TSG.
In this study, the effects of TSG and resveratrol on
regulating NAFLD were compared. Resveratrol, which has a similar
backbone as TSG, has been widely recognized for its great
bioactivity. Previous studies demonstrated that
resveratrol-mediated improvements in glycemic control and NAFLD
were associated with alterations in hepatic metaflammation that was
related to the NLRP3 inflammasome (19). Thus, this study may provide more
powerful evidence for anti-NAFLD activities of TSG compared to
resveratrol.
Materials and methods
Chemicals
TSG was purchased from Nanjing Jingzhu
Bio-technology Co., Ltd., (Nanjing, China). TSG was purified using
a high performance liquid chromatography-diode array detector and
the purity was over 98%. Fenofibrate (Laboratoires Fournier S.A.,
Dijon, France) was used as positive control for serum and hepatic
lipid regulation.
In all liver homogenates samples levels of aspartate
aminotransferase (AST), alanine aminotransferase (ALT),
triglyceride (TG), total cholesterol (TC), low-density lipoprotein
cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C),
very low-density lipoprotein cholesterol (VLDL-C), and FFA were
measured by assay kits purchased from Cusabio Biotech Co., Ltd.
(Wuhan, China) and Nanjing Jiancheng Bioengineering Institute, Co.,
Ltd. (Nanjing, China). Antibodies directed against NLRP3,
apoptosis-associated speck-like protein containing a C-terminal
caspase recruitment domain (ASC) and caspase-1 were purchased from
Proteintech Group, Inc. (Chicago, IL, USA). IL-18 and IL-1β were
detected by ELISA kits purchased from Cusabio Biotech Co., Ltd.
MCD diet and its control, methionine and choline
supplemented (MCS) diet, were purchased from Trophic Animal Feed
High-tech Co., Ltd. (Nantong, China).
Animals and treatments
Seventy C57BL/6 male mice (20±2 g, approximately
6-week-old) were provided by Beijing HFK Bioscience Co., Ltd.
(Beijing, China). Mice (n=10 per cage) were housed in
stainless steel cages, containing sterile paddy husk bedding in
ventilated animal rooms (temperature 22±1°C; 60±10% humidity; and a
12/12 h light/dark cycle), and had free access to water. All animal
experiments were performed in compliance with Institutional Ethical
Committee on Animal Care and Experimentations of Yunnan
University of Traditional Chinese Medicine (Kunming, China;
R-0620150019). The ethics committee provided ethical approval for
the animal experiments performed in this study. All reasonable
efforts were made to minimize the animals' suffering.
After adaptive feeding for three days, mice were
randomly assigned to 7 groups (n=10 per group): A, normal
control group; B, MCD diet-induced NAFLD model group; C, low dosage
of TSG (TSG.L, 17.5 mg/kg); D, middle dosage of TSG (TSG.M, 35
mg/kg); E, high dosage of TSG (TSG.H, 70 mg/kg); F, fenofibrate (26
mg/kg) served as a positive control; G, resveratrol (35 mg/kg)
(Table I). Mice in groups B to G
were fed with MCD diet for 0–7 weeks and MCS diet for 8–9
weeks.
 | Table I.Animal groupings and treatments. |
Table I.
Animal groupings and treatments.
|
| Diet | Treatment |
|---|
|
|
|
|
|---|
| Groups | 0–7 weeks | 8–9 weeks | 0–9 weeks | Dosage (mg/kg) |
|---|
| A | MCS | MCS | – | – |
| B | MCD | MCS | – | – |
| C | MCD | MCS | TSG | 17.5 |
| D | MCD | MCS | TSG | 35.0 |
| E | MCD | MCS | TSG | 70.0 |
| F | MCD | MCS | Fenofibrate | 26.0 |
| G | MCD | MCS | Resveratrol | 35.0 |
Mice were fasted for 2 h every day before
administration of TSG, fenofibrate and resveratrol. Then, all TSG,
fenofibrate and resveratrol treatment was administered (0.2 ml/20
g) by gavage.
Morphology observations
At termination of the experiment, mice were
sacrificed by cervical dislocation. For light microscopic
observations, liver samples were fixed in formalin fixative and
embedded in paraffin following a routine procedure. Next, 5
µm-thick sections were cut, stained with hematoxylin and eosin
(H&E), and examined using a light microscope.
Evaluation of liver TC, TG,
lipoprotein, IL-1β, and IL-18 levels
Mice were sacrificed by cervical dislocation and
liver tissue samples were harvested and immediately processed for
biochemical analysis. A total of 100 mg of liver sample from mice
in each group were cut into pieces and homogenized on ice using 1
ml of physiological saline. Homogenates were centrifuged at 9,600 ×
g for 10 min at 4°C. The supernatants were stored at −80°C
until further analysis. Levels of AST, ALT, TG, TC, LDL-C, HDL-C,
VLDL-C, and FFA were determined in liver homogenates by enzymatic
colorimetric method using commercial standard enzymatic assay kits.
Moreover, levels of IL-1β and IL-18 in the supernatant were
measured by commercial Elisa kits (Cusabio Biotech Co., Ltd.).
Western blot analysis
Liver tissue was minced and homogenized in a glass
homogenizer on ice. Homogenates were centrifuged at 9,600 ×
g for 10 min at 4°C. The supernatants were stored at −80°C
until further evaluated by Western blot analysis. Protein levels in
the supernatant were measured by the bicinchoninic acid (BCA)
method. In brief, 10–50 mg of total protein per sample was
separated on a 10–15% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to polyvinilide fluoride
(PVDF) membranes. Membranes were blocked by incubation with 5% skim
milk in TBS at room temperature for 1 h, followed by incubating
with primary antibodies directed against NLRP3, ASC, caspase-1,
IL-18, and IL-1β for overnight at 4°C, respectively. Next,
membranes were washed three times with TBS/Tween-20 (TBST) at room
temperature and incubated with secondary antibodies (Proteintech
Group, Inc.). Protein bands were visualized using an enhanced
chemiluminescence (ECL) detection system and quantified with ImageJ
software.
Overall structural changes of gut
microbiota
Feces were collected at the last day of the
experiment and stored in sterilized centrifuge tubes at −80°C. All
feces samples of mice in the same group were mixed, transferred
into a mortar and grinded to a fine powder. DNA was extracted
according to the instruction of test kits (Omega Bio-Tek, Inc.,
Norcross, GA, USA).
To determine the diversity and composition of the
bacterial species in the feces samples from each group, we used the
protocol as described by Caporaso et al (20). PCR amplifications were conducted with
the 515f/806r primer set, which allowed for the V4 region of the
16S rDNA gene to be amplified. The reverse primer contained a 6-bp
error-correcting barcode that was unique for each sample. DNA was
amplified following a protocol described previously (21). Sequencing was conducted on an
Illumina MiSeq platform (NoVogene, Beijing, China).
Statistical analysis
Data were expressed as the mean ± SD. One-way
analysis of variance (ANOVA) was employed to analyze the data when
multiple group comparisons were performed. Graphics were created
using Origin 6.1 software (MicroCal Software, Northampton, MA,
USA). Operational taxonomic units (OTUs) clustering was analyzed by
the software system of Mev Development Team (TMEV) Clustering.
Results
Effects of TSG on the general
physiological features of NAFLD mice
At the end of the experiment, the body weights of
mice in the MCD diet groups were significantly lower compared to
that of mice in the regular feed group. However, treatment with
TSG.L alleviated this weight reduction (Fig. 1A). Entirely different from
HFD-induced NAFLD, fatty liver induced by MCD diet possessed a
similar liver index when compared to normal mice (Fig. 1B), indicating that liver atrophy was
not significantly different in mice in the MCD group. Treatment
with TSG, fenofibrate, and resveratrol effectually attenuated this
liver atrophy (Fig. 1B). Morphology
observations indicated that in our study, an MCD diet-induced NAFLD
model was successfully established. TSG effectively relieved lipid
accumulation in the liver of mice (Fig.
1C). Resveratrol also decreased lipid accumulation induced by
MCD, however, lipid drops in hepatic cells could also be observed
in resveratrol group (Fig. 1C).
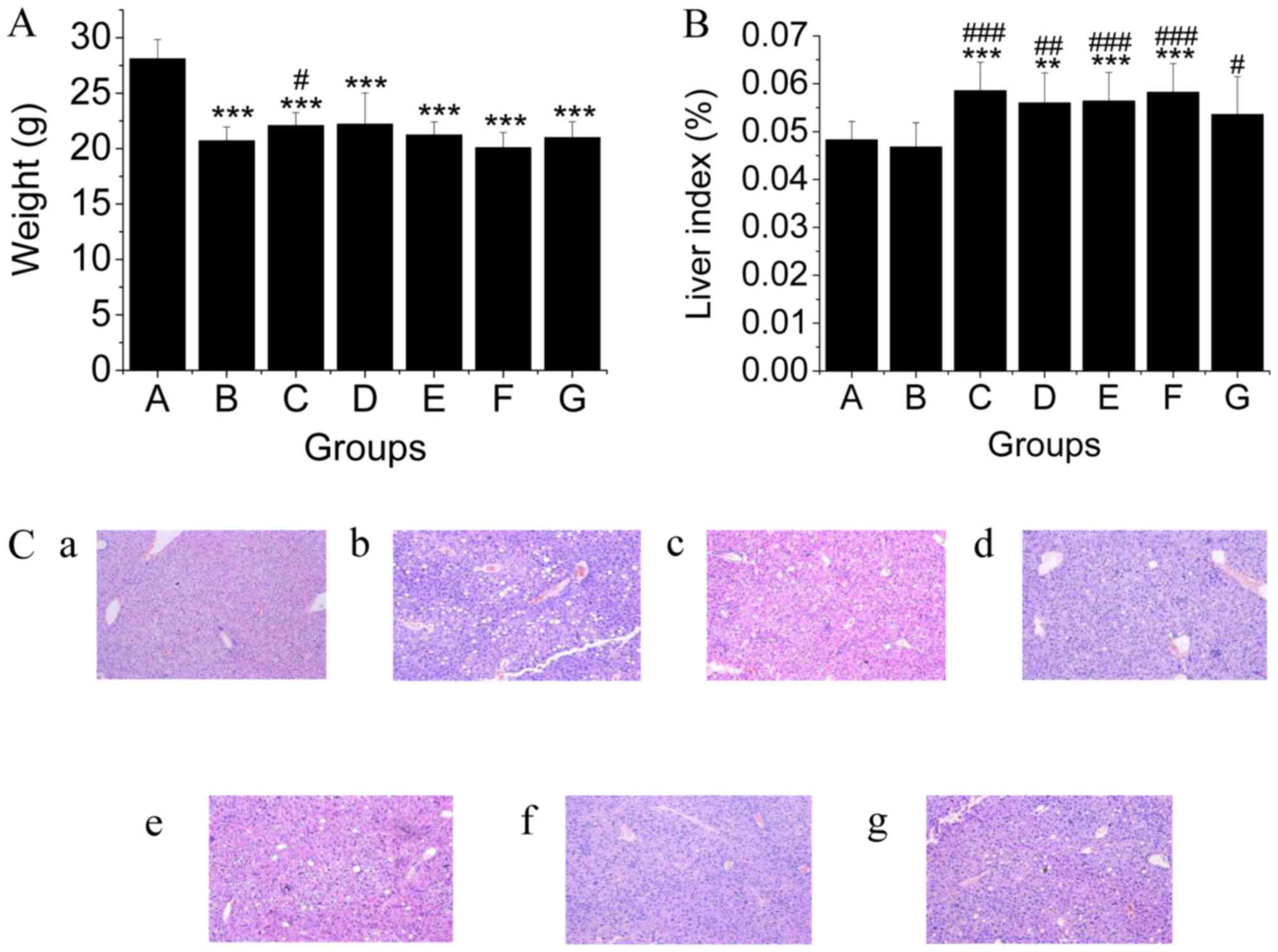 | Figure 1.Shown are (A) body weight, (B) liver
index, and (C) microscopic morphology of liver of mice in all
groups. In this study, the changes of (A) body weight, (B) liver
index and (C) microscopic morphology of liver in groups were
evaluated, 10 times of magnification. (C-a) Normal control group
(C-b) MCD diet group, (C-c) low dosage of TSG (17.5 mg/kg) group,
(C-d) middle dosage of TSG (35 mg/kg) group, (C-e) high dosage of
TSG (70 mg/kg) group, (C-f) fenofibrate (26 mg/kg) group, (C-g)
resveratrol(35 mg/kg) group. Liver atrophy and fat drop
accumulation were apparent in mice in the MCD group compared with
the normal group. TSG (TSG.L, 17.5 mg/kg; TSG.M, 35 mg/kg; and
TSG.H, 70 mg/kg) significantly reduced the degree of fatty liver
degeneration. Fenofibrate also effectively decreased the fatty
degeneration of liver cells. **P<0.01, ***P<0.001 (*,
compared with the control group). #P<0.05,
## P<0.01, ###P<0.001 (#,
compared with the model group). MCD, methionine and
choline-deficient; TSG,
2,3,5,4′-tetrahydroxy-stilbene-2-O-β-D-glucoside. |
Subsequently, we determined the levels of AST, ALT,
TG, TC, LDL-C, VLDL-C, and FFA in liver tissue (Fig. 2). Levels of TC and TG in mic in the
NAFLD group were higher compared to that of mice in the normal
group. The levels of TC and TG were significantly decreased in TSG
treatment groups (P<0.05). TSG treatment significantly reduced
TC and TG accumulation in the liver in a dose dependent manner.
Moreover, TSG treatment demonstrated a more attractive hepatic
lipid control effect compared to fenofibrate and resveratrol. LDL-C
contents were increased in MCD diet-induced NAFLD mice. This
increase was inhibited by treatment with TSG.M, TSG.H, and
fenofibrate. The mechanism for stetosis in mice on a MCD diet
appeared to be related with impaired VLDL-C secretion due to the
lack of phosphatidyl choline synthesis (22). Our results confirmed that VLDL-C
content of mice in the MCD diet-induced NAFLD group was reduced by
about 50%. Unfortunately, none of these treatments could reverse
this VLDL-C reduction. In NAFLD mice, hepatic FFA levels
dramatically increased about 243.5%. This increase was reduced
after treatment of TSG.M, TSG.H, fenofibrate, and resveratrol.
Thus, these results confirmed that TSG could partly reduced FFA
supply as presented in our previous study (14).
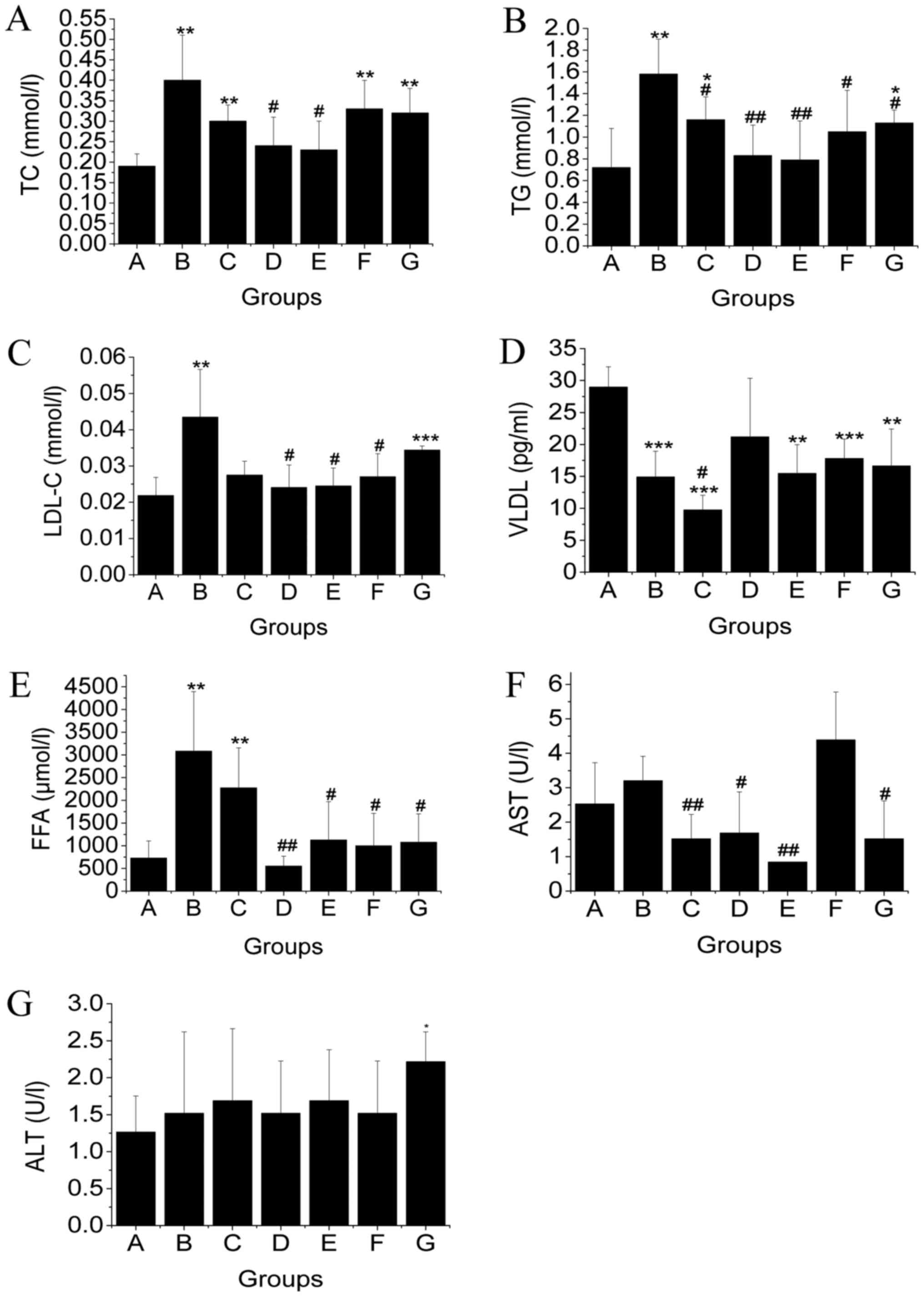 | Figure 2.Levels of (A) TC, (B) TG, (C) LDL-C,
(D) VLDL-C, (E) FFA, (F) AST, and (G) ALT in liver tissue of mice
in the different groups (mean ± SD, n=10). In this study,
levels of TC, TG, LDL-C, VLDL-C, FFA, AST, and ALT were measured.
The contents of TG, TC, LDL-C, AST, and FFA were significantly
increased in mice that were fed an MCD diet. Moreover, TSG
treatment effectively reduced the contents of TG, TC, LDL-C, AST,
and FFA in the liver. #P<0.05; ##P<0.01
(#, compared with MCD diet-induced NAFLD model group).
*P<0.05; **P<0.01; ***P<0.001 (*, compared with the
control group). TC, total cholesterol; TG, triglyceride; LDL-C,
low-density lipoprotein cholesterol; VLDL-C, very low-density
lipoprotein cholesterol; FFA, free fatty acid; AST, aspartate
aminotransferase; ALT, alanine aminotransferase. |
To evaluate the possible effect of MCD diet and
treatments on mouse hepatic function, hepatic AST and ALT levels
were measured. AST levels were slightly elevated after mice were
fed an MCD diet, however, the difference was not significant.
Interestingly, the increase in AST levels was inhibited by
treatment with TSG and resveratrol. In contrast, hepatic ALT levels
remained at relative stable levels and were unrelated to the MCD
diet. Treatment with TSG, fenofibrate, and resveratrol did not
influence hepatic ALT levels.
Effects of TSG treatment on regulation
of NLRP3 inflammasome and hepatic metaflammation
The regulatory effects of TSG treatment on levels of
NLRP3, ASC, and caspase-1 in liver were investigated by Western
blot analysis. As shown in Fig. 3,
MCD diet fed mice showed an increase in protein expression levels
of NLRP3, ASC, and caspase-1 compared with mice in the control
group. TSG treatment had no significant effect on the level of
NLRP3 (Fig. 3B). However, TSG.M
treatment reduced the level of ASC to a similar level of mice in
the control group (Fig. 3C). The
level of caspase-1 was also reduced by TSG.M treatment. These
results showed that TSG.M, TSG.H, fenofibrate, and resveratrol
down-regulated the ASC content. Compared to model group, treatment
with TSG.M and resveratrol resulted in caspase-1 lowering
activities (Fig. 3D).
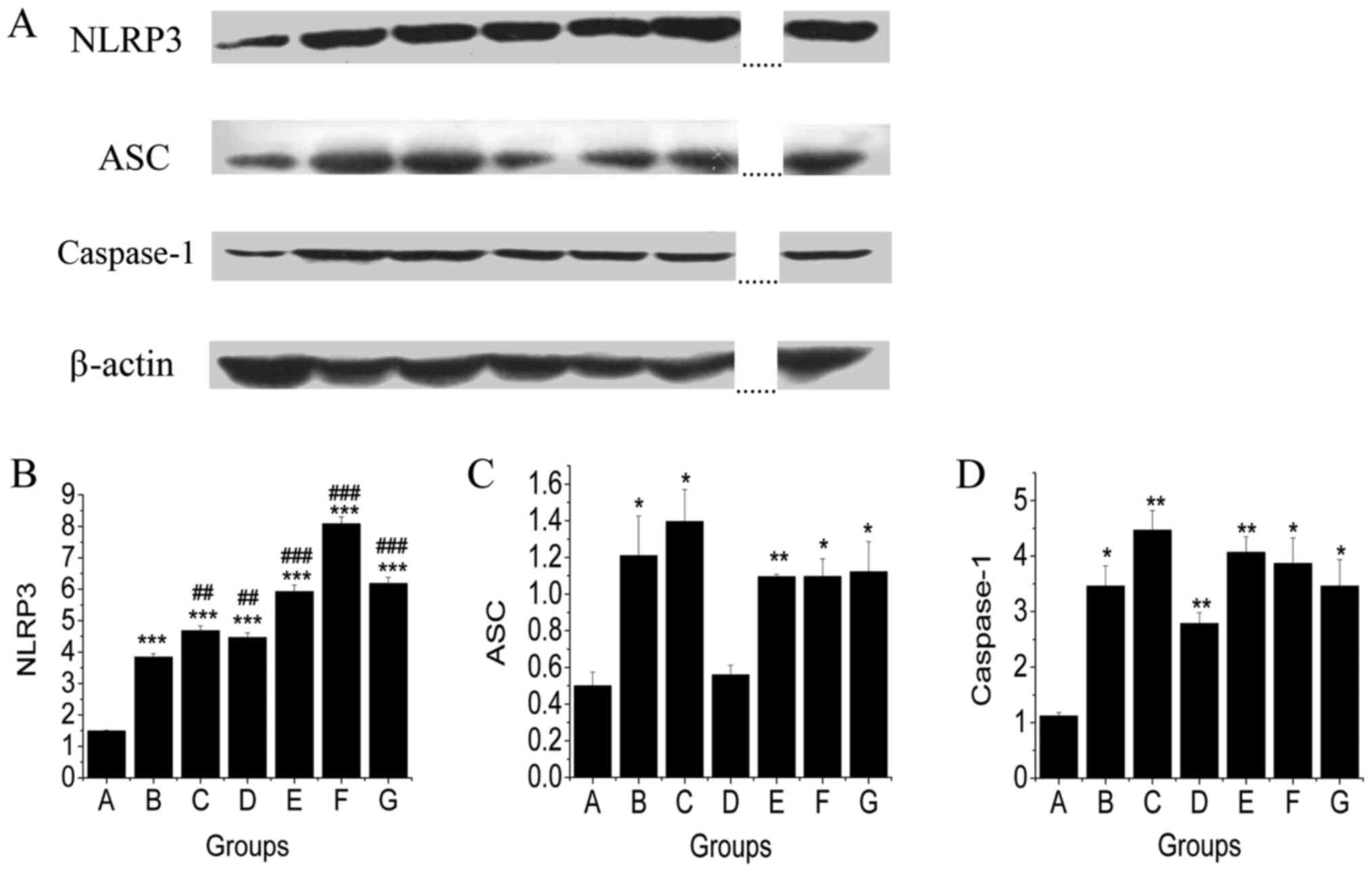 | Figure 3.The effects of TSG on protein
expression of NLRP3, ASC and caspase-1 in livers of MCD diet fed
mice. Protein imprinting of NLRP3, ASC, and caspase-1. (A) As a
matter of fact, nine samples were included in the original
gel/experiment. The antepenultimate and penultimate pair were
omitted from the figure (as denoted by the dotted lines) since they
were not relevant for a discussion of the experiment at hand.
Densitometric analysis of (B) NLRP3, (C) ASC, (D) caspase-1. Mice
fed an MCD diet showed increased levels of NLRP3, ASC and caspase-1
protein expressions compared with mice in the control diet-fed
group. The results showed that the middle dosage of TSG
downregulated ASC and caspase-1 levels. ##P<0.01;
###P<0.001 (#, compared with MCD
diet-induced NAFLD model group). *P<0.05; **P<0.01;
***P<0.001 (*, compared with the control group). TSG,
2,3,5,4′-tetrahydroxy-stilbene-2-O-β-D-glucoside; NLRP3, Nod-like
receptor protein 3; ASC, apoptosis-associated speck-like protein
containing a C-terminal caspase recruitment domain; MCD, methionine
and choline-deficient; NAFLD, Non-alcoholic fatty liver
disease. |
In general, pathogen-associated molecular patterns
(PAMPs) and damage-associated molecular patterns (DAMPs) are
recognized by the inflammasome, thereby activating the cysteine
protease caspase-1 that, in turn, will result in the maturation of
the proinflammatory cytokines interleukin (IL)-1β and IL-18
(23). In this study, IL-1β and
IL-18 levels were evaluated in liver tissue of mice. We found that
levels of IL-1β and IL-18 of mice in the NAFLD group were not
significantly different compared to that in normal mice. TSG.L
treatment reduced the level of IL-1β (Fig. 4A) compared to that in NAFLD mice,
whereas TSG.H treatment reduced IL-18 content (Fig. 4B).
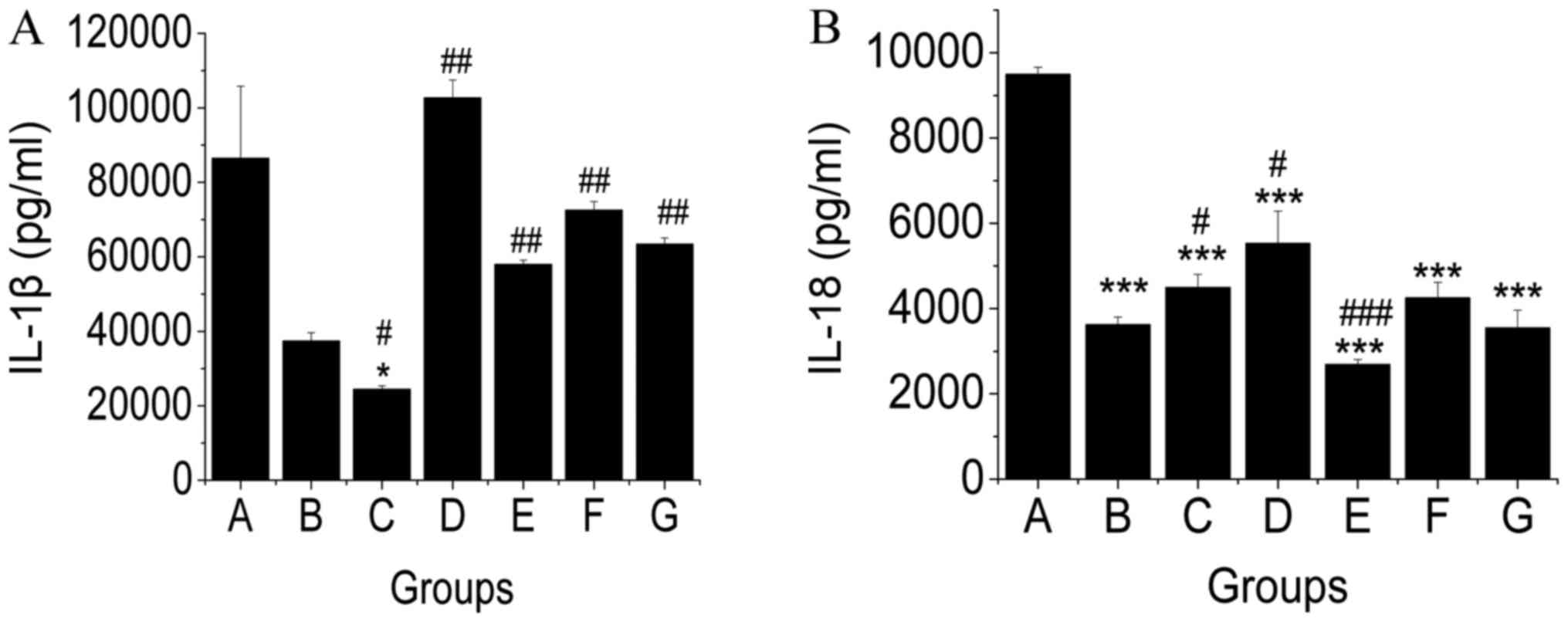 | Figure 4.Effects of TSG on the protein
expression of (A) IL-1β and (B) IL-18 in livers of MCD diet-fed
mice. At the end of the experiment, the levels of IL-1β and IL-18
in liver tissue were evaluated. Low dosage of TSG reduced the
levels of IL-1β, and high dose of TSG showed that IL-18 levels were
reduced compared with the model group. Data are shown as the mean ±
SD (#, compared with the MCD diet-induced NAFLD model
group). #P<0.05; ##P<0.01;
###P<0.001. *P<0.05; ***P<0.001 (*, compared
with the control group). TSG,
2,3,5,4′-tetrahydroxy-stilbene-2-O-β-D-glucoside; IL, interleukin;
MCD, methionine and choline-deficient; NAFLD, non-alcoholic fatty
liver disease. |
Effects of TSG treatment on regulating
relative proportions of intestinal microbial
Numerous studies have suggested that disruption of
the relative proportions of gut microbial populations may
contribute to the progress of NAFLD. Therefore, increased attention
has been paid to the status and therapy of the intestinal microbial
balance in NAFLD.
An MCD diet may modify the gut microbiota and gut
permeability (24–26). Several findings suggested that
circulating LPS levels are elevated in rodent MCD diet-induced
NAFLD (24–26).
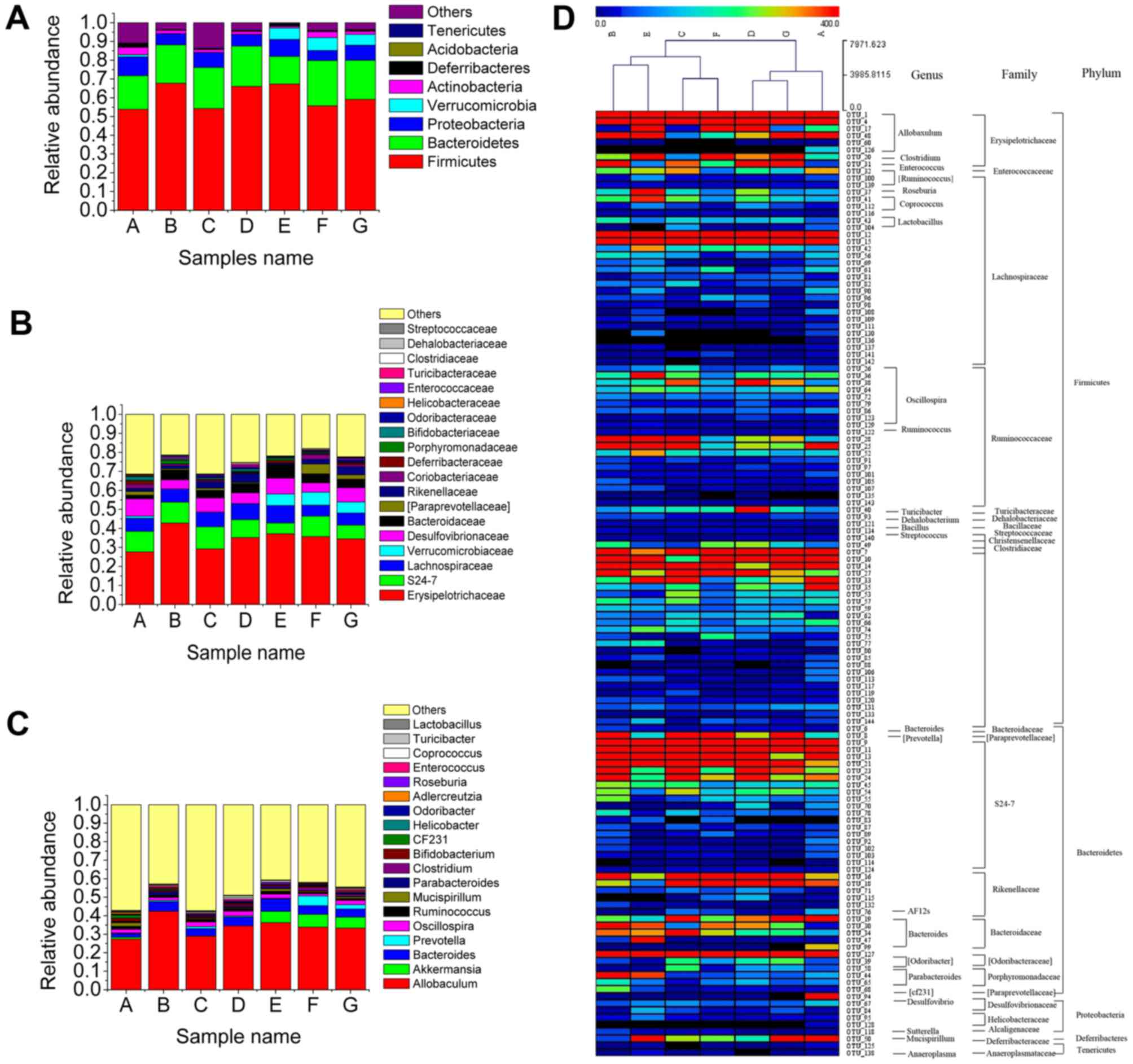
The relative abundance of microbial species in
phylum revealed that the presence of Firmicutesin MCD diet fed mice
(67.7%) was high compared with that in normal mice (53.8%). An
increase in the relative abundance of Firmicutes was also observed
in HFD-induced and genetically engineered obese mice as well as in
obese humans when compared with lean control subjects (27). Mice in Group C, D, E, F, and G showed
a reduction in relative abundance of Firmicutes (54.2, 66.1, 67.3,
55.68, and 59.10%, respectively) (Fig.
5A).
Erysipelotrichaceae abundance (42.85%) in mice in
Group B was significantly higher compared to that of mice in the
control group (27.65%) (Fig. 5B).
Several species of Erysipelotrichaceae were identified as potential
candidates to play a causal role in the pathogenesis of parenteral
nutrition-associated liver injury (PNALI) (28). Moreover, the abundance of
Erysipelotrichaceae seemed was affected after treatment with TSG.L,
TSG.M, TSG.H (29.20, 35.18, 37.15%), fenofibrate (35.70%), and
resveratrol (34.43%). It was also worth mentioning that an MCD diet
increased the relative abundance of Helicobacteraceae (0.557%)
(Fig. 5B), which was usually
considered as pathogenic species (29). Moreover, Helicobacter pylori
may activate the inflammasome and caspase-1 in antigen-presenting
and other cells, resulting in processing and release of
caspase-1-dependent cytokines (30).
Mice in groups C, D, E, F and G showed a reduction in the relative
abundance of Helicobacteraceae (0.071, 0.065, 0.15, 0.043, and
0.10%, respectively), which suggested that TSG treatment
effectively controlled Helicobacteraceae abundance. Mice in groups
D, E, F, G, H and I showed increased abundance of
Verrucomicrobiaceae (Fig. 5B). It
was previously reported that decreased Verrucomicrobia in
prediabetes was consistent with the findings by Barlow et al
that Akkermansia may play a substantial role in regulating
host adiposity and weight loss (31).
Furthermore, MCD diet intake resulted in an increase
of Allobaculum and Parabacteroides (Fig. 5C). Abundances of Allobaculum
and Parabacteroides showed a significant increase in
NAFLD/NASH patients compared with controls (29). The relative abundances of
Allobaculum and Parabacteroides in the gut negatively
correlated with the expression levels of tight junction protein
(ZO-1 and occludin) and anti-inflammatory genes, such as IL-10 and
Foxp3 (27). Treatment with TSG
effectively inhibited their abundances to a normal level (Fig. 5C).
Given that Allobaculum genera belong to the
Erysipelotrichaceae family, which belong to Firmicutesphylum, we
proposed that TSG treatment reduced the abundance of
Firmicutesphylum by affecting Erysipelotrichaceae family and
Allobaculum genera.
On the contrary, an MCD diet (0.038%) decreased the
relative abundance of Akkermansia (10.93%). Several reports
demonstrated that Akkermansia muciniphila treatment reversed
high-fat diet-induced metabolic disorders, including fat-mass gain,
metabolic endotoxemia, adipose tissue inflammation, and insulin
resistance (32). TSG.M, TSG.H,
fenofribrate, and resveratrol increased the relative abundance of
Akkermansia.
Effects of TSG treatment on regulating
intestinal microbial balance
In this study, we identified 134 key variables,
which were significantly altered after treatment with TSG and
resveratrol. C clustering analysis of 134 OTUs (Fig. 5D) showed that mice in the MCD diet
group were significantly different from the control group. TSG.M
treatment shortened its distance compared to the control group.
Thus, these results indicated that TSG treatment partially
recovered the gut microbiota equilibrium.
Comparison of TSG and resveratrol in
the treatment of NAFLD
In this study, we compared the effects of TSG and
resveratrol treatment on NAFLD regulation. Our data indicated that
TSG treatment had a better alleviating effect on liver atrophy
compared to resveratrol. The levels of TC, LDL-C, and ALT in mice
in the TSG-treated Group were lower compared to that of mice in
Group G. TG levels of mice in Group C and D were lower than those
of mice in Group G. Considering VLDL-C and FFA regulation, TSG
treatment demonstrated a better activity compared to resveratrol.
Moreover, when compared to resveratrol, treatment with TSG.L and
TSG.M displayed better NLRP3-lowering activities. Levels of ASC and
caspase-1 were significantly decreased in TSG.M-treated mice
compared to mice treated with resveratrol.
Discussion
Previous studies have suggested that TSG, a
promising anti-NAFLD candidate, prevented HFD-induced NAFLD. In
this study, we tried to investigate whether TSG could reverse NAFLD
induced by an MCD diet and if this effect was related to gut
microbiota and the NLRP3 inflammasome.
It had been reported that mice fed an MCD diet might
loss >40% of body weight and >60% of liver weight after 30
weeks. In addition, 2 weeks after switching back to the control
diet, both body and liver weights significantly recovered (33). We found that an MCD diet impaired
mitochondrial β-oxidation, which led to increased production of
reactive oxygen species (ROS), mitochondrial DNA damage, and
apoptotic cell death, in addition to hepatic stellate cell (HSC)
activation and extracellular matrix deposition (34). Furthermore, oxidative stress had been
implicated as an etiological factor in various acute and chronic
liver diseases, including NAFLD and NASH (35).
It has been shown that inflammasome-mediated
dysbiosis regulated the progression of NAFLD and obesity (6). In mice, NLRP3 inflammasome activation
resulted in hepatocyte pyroptosis, liver inflammation, and fibrosis
(36). Previous studies indicated
that resveratrol may ameliorate hepatic metaflammation and inhibit
NLRP3 inflammasome activation in HFD-induced obesity mice (19). Therefore, in this study, components
of the NLRP3 inflammasome complex and proinflammatory markers were
analyzed to test whether NLRP3 inflammasome and related hepatic
metaflammation were involved in TSG-mediated improvement of MCD
diet-induced hepatic stetosis.
Gut microbiota and related inflammatory process
played to a great extent a role in the development of MCD
diet-induced NAFLD. Moreover, gut microbiota and bacterial
endotoxin were involved in several underlying mechanisms of NAFLD
as well as in its progression to NASH. Management of gut microbiota
dysbiosis may become a cornerstone for future treatment of liver
diseases (37). Alterations in the
gut microbiota population and/or changes in gut permeability
promote microbial translocated into the portal circulation, thus
directly to the liver, via the NLRP3 inflammasome (38). Several studies have demonstrated that
activation of the NLRP3 inflammasome resulted in severe liver
inflammation and fibrosis, while hepatocyte pyroptotic cell death
was identified as a novel mechanism of NLRP3-mediated liver damage
(36).
In our study, we demonstrated that the levels of TG,
TC, LDL-C, AST, and FFA in the liver were significantly increased
in C57BL6/mice fed an MCD diet. This increase could be effectively
reduced by treatment with TSG. On the other hand, TSG reduced the
accumulation of TG in the liver by lowering the expression of
LDL-C. Moreover, MCD-diet elevated levels of AST and ALT were also
alleviated after treatment with TSG. The protein expression levels
of NLRP3, ASC, and caspase-1 were increased by MCD diet, and TSG.M
treatment reduced the levels of ASC and caspase-1.
Gut microbiota of MCD diet-induced NAFLD mice were
significantly altered by treatment with TSG. TSG decreased relative
abundances of Firmicutes phylum, Erysipelotrichaceae, and
Helicobacteraceae in MCD diet-induced NAFLD mice. However, levels
of Verrucomicrobiaceae were increased after TSG treatment.
Moreover, abundances of Allobaculum and
Parabacteroides, which may affect the incidence and progress
of NAFLD, were also raised after TSG treatment. The clustering
analysis of 134 key OTUs showed that TSG partly recovered gut
microbiota alteration induced in MCD diet-induced NAFLD mice.
However, some inadequacies also existed in our research, due to the
small sample size, the fecal samples from mice in each experimental
group were mixed (instead of analyzed in each individual mouse) and
used to determine the effect of TSG and resveratrol on the gut
microbiome.
Finally, we found that TSG possessed better effects
on MCD diet-induced NAFLD when compared to resveratrol. Although
TSG and resveratrol are both natural stilbenoids, they may regulate
NF-κB differently. Resveratrol was reported to primarily decrease
the production of pro-inflammatory factors by impairing
phosphorylation and nuclear translocation of NF-κB (39,40).
However, TSG may protect brain tissue from ischemia by impairing
the DNA binding activity of NF-κB (41).
In addition, several in vivo studies in both
animals and humans indicated a very low intestinal uptake of
resveratrol, leading to trace amounts in the bloodstream based on
extensive metabolism in the gut and liver (42). An effective approach to stabilize
resveratrol derivatives was accomplished by methylation (43) or glycosidation (44) of resveratrol to form methylated or
glycosylated resveratrol (45). TSG
possessed better stability and water solubility compared to
resveratrol. This may contribute to its increased effect on MCD
diet-induced NAFLD compared to resveratrol.
In summary, our findings demonstrated that TSG
reversed the occurrence and development of NAFLD by affecting the
intestinal microbial content and by partly regulating the
subsequent inherent immune system. Thus, TSG may be a promising
compound for NAFLD therapy.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (grant nos. 81260553 and
81460623), the Natural Science Foundation of Yunnan Province (grant
no. 2014FA035), the Southern Medicine Collaborative And Innovation
Center (30270100500), and Young and Middle-aged Academic and
Technological Leader of Yunnan (2015HB053). We thank the technical
assistance of NoVogene (Beijing, China) to determine the diversity
and composition of the bacterial communities.
References
|
1
|
Ishii KA and Takamura T: Non-alcoholic
fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)
and nutrition. Clin Calcium. 26:363–367. 2016.(In Japanese).
PubMed/NCBI
|
|
2
|
Moghaddasifar I, Lankarani KB, Moosazadeh
M, Afshari M, Ghaemi A, Aliramezany M, Gharebagh Afsar R and Malary
M: Prevalence of non-alcoholic fatty liver disease and its related
factors in Iran. Int J Organ Transplant Med. 7:149–160.
2016.PubMed/NCBI
|
|
3
|
Ruhl CE and Everhart JE: Fatty liver
indices in the multiethnic united states national health and
nutrition examination survey. Aliment Pharm Therap. 41:65–76. 2015.
View Article : Google Scholar
|
|
4
|
Fung J, Lee CK, Chan M, Seto WK, Lai CL
and Yuen MF: Hong Kong Liver Health Census Study Group: High
prevalence of non-alcoholic fatty liver disease in the
Chinese-results from the Hong Kong liver health census. Liver Int.
58:542–549. 2015. View Article : Google Scholar
|
|
5
|
Iftikhar R, Kamran SM, Sher F and Wahla
MS: Prevalence of non alcoholic fatty liver disease in patients
with metabolic syndrome. PAFMJ. 65:616–619. 2015.
|
|
6
|
Mehal WZ: The Gordian Knot of dysbiosis,
obesity and NAFLD. Nat Rev GastroHepat. 10:637–644. 2013.
|
|
7
|
Manan B and Saraswat VA: Gut-liver axis:
Role of inflammasomes. J Clin Exp Hepatol. 3:141–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Henao-Mejia J, Elinav E, Jin C, Hao L,
Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ,
et al: Inflammasome-mediated dysbiosis regulates progression of
NAFLD and obesity. Nature. 482:179–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dixon LJ, Berk M, Thapaliya S, Papouchado
BG and Feldstein AE: Caspase-1-mediated regulation of fibrogenesis
in diet-induced steatohepatitis. Lab Invest. 92:713–723. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petrasek J, Bala S, Csak T, Lippai D,
Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA and Szabo G:
IL-1 receptor antagonist ameliorates inflammasome-dependent
alcoholic steatohepatitis in mice. J Clin Invest. 122:3476–3489.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Csak T, Ganz M, Pespisa J, Kodys K,
Dolganiuc A and Szabo G: Fatty acid and endotoxin activate
inflammasomes in mouse hepatocytes that release danger signals to
stimulate immune cells. Hepatology. 54:133–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wree A, Mcgeough MD, Peña CA, Schlattjan
M, Li H, Inzaugarat ME, Messer K, Canbay A, Hoffman HM and
Feldstein AE: NLRP3 inflammasome activation is required for
fibrosis development in NAFLD. J Mol Med (Berl). 92:1069–1082.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin P, Lu J, Wang Y, Gu W, Yu J and Zhao
RH: Naturally occurring stilbenoid TSG reverses non-alcoholic fatty
liver diseases via gut-liver axis. PLoS One. 10:01403462015.
View Article : Google Scholar
|
|
15
|
Wang W, He Y, Lin P, Li Y, Sun R, Gu W, Yu
J and Zhao R: In vitro effects of active components of Polygoni
Multiflori Radix on enzymes involved in the lipid metabolism. J
Ethnopharmacol. 153:763–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin P, He YR, Lu JM, Li N, Wang WG, Gu W,
Yu J and Zhao RH: In vivo lipid regulation mechanism of polygoni
multiflori radix in high-fat diet fed rats. Evid Based Complement
Alternat Med. 2014:6420582014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li N, Chen Z, Mao XJ, Yu J and Zhao RH:
Effects of lipid regulation using raw and processed radix polygoni
multiflori in rats fed a high-fat diet. Evid Based Complement
Alternat Med. 2012:3291712012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang M, Zhao R, Wang W, Mao X and Yu J:
Lipid regulation effects of polygoni multiflori radix, its
processed products and its major substances on steatosis human
liver cell line L02. J Ethnopharmacol. 139:287–293. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang SJ and Lim Y: Resveratrol ameliorates
hepatic metaflammation and inhibits NLRP3 inflammasome activation.
Metabolism. 63:693–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
CaporasoJ G, Kuczynski J, Stombaugh J,
Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich
JK, Gordon JI, et al: QIIME allows analysis of high-throughput
community sequencing data. Nat Methods. 7:335–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Magoč T and Salzberg SL: FLASH: Fast
length adjustment of short reads to improve genome assemblies.
Bioinformatics. 27:2957–2963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao ZM and Vance DE: Reduction in VLDL,
but not HDL, in plasma of rats deficient in choline. Biochem Cell
Biol. 68:552–558. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bieghs V and Trautwein C: The innate
immune response during liver inflammation and metabolic disease.
Trends Immunol. 34:446–452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okubo H, Sakoda H, Kushiyama A, Fujishiro
M, Nakatsu Y, Fukushima T, Matsunaga Y, Kamata H, Asahara T,
Yoshida Y, et al: Lactobacillus casei strain Shirota protects
against nonalcoholic steatohepatitis development in a rodent model.
Am J Physiol Gastrointest Liver Physiol. 305:G911–G918. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Endo H, Niioka M, Kobayashi N, Tanaka M
and Watanabe T: Butyrate-producing probiotics reduce nonalcoholic
fatty liver disease progression in rats: New insight into the
probiotics for the gut-liver axis. PLoS One. 8:00633882013.
View Article : Google Scholar
|
|
26
|
Miura K and Ohnishi H: Role of gut
microbiota and Toll-like receptors in nonalcoholic fatty liver
disease. World J Gastroentero. 20:7381–7391. 2014. View Article : Google Scholar
|
|
27
|
Lee SM, Han HW and Yim SY: Beneficial
effects of soy milk and fiber on high cholesterol diet-induced
alteration of gut microbiota and inflammatory gene expression in
rats. Food Funct. 6:492–500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harris JK, El Kasmi KC, Anderson AL,
Devereaux MW, Fillon SA, Robertson CE, Wagner BD, Stevens MJ, Pace
NR and Sokol RJ: Specific microbiome changes in a mouse model of
parenteral nutrition associated liver injury and intestinal
inflammation. PLoS One. 9:01103962014. View Article : Google Scholar
|
|
29
|
Del Chierico F, Gnani D, Vernocchi P,
Petrucca A, Alisi A, Dallapiccola B, Nobili V and Lorenza P:
Meta-omic platforms to assist in the understanding of NAFLD gut
microbiota alterations: tools and applications. Int J Mol Sci.
15:684–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koch KN and Müller A: Helicobacter pylori
activates the TLR2/NLRP3/caspase-1/IL-18 axis to induce regulatory
T-cells, establish persistent infection and promote tolerance to
allergens. Gut Microbes. 6:382–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barlow GM, Yu A and Mathur R: Role of the
gut microbiome in obesity and diabetes mellitus. Nutr Clin Pract.
30:787–797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Everarda A, Belzerb C, Geurtsa L,
Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli
GG, Delzenne NM, et al: Cross-talk between Akkermansia muciniphila
and intestinal epithelium controls diet-induced obesity. Proc Natl
Acad Sci USA. 110:9066–9071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Itagaki H, Shimizu K, Morikawa S, Ogawa K
and Ezaki T: Morphological and functional characterization of
non-alcoholic fatty liver disease induced by a
methionine-choline-deficient diet in C57BL/6 mice. Int J Clin Exp
Patho. 6:2683–2696. 2013.
|
|
34
|
Leclercq IA, Farrell GC, Field J, Bell DR,
Gonzalez FJ and Robertson GR: CYP2E1 and CYP4A as microsomal
catalysts of lipid peroxides in murine nonalcoholic
steatohepatitis. J Clin Invest. 105:1067–1075. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oliveira CP, da Costa Gayotto LC, Tatai C,
Della Bina BI, Janiszewski M, Lima ES, Abdalla DS, Lopasso FP,
Laurindo FR and Laudanna AA: Oxidative stress in the pathogenesis
of nonalcoholic fatty liver disease, in rats fed with a
choline-deficient diet. J Cell Mol Med. 6:399–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wree A, Eguchi A, McGeough MD, Pena CA,
Johnson CD, Canbay A, Hoffman HM and Feldstein AE: NLRP3
inflammasome activation results in hepatocyte pyroptosis, liver
inflammation, and fibrosis in mice. Hepatology. 59:898–910. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fukui H: Gut microbiota and host reaction
in liver diseases. Microorganisms. 3:759–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bawa M and Saraswat V: Gut-liver axis:
Role of inflammasomes. J Clin Exp Hepatol. 3:141–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Capiralla H, Vingtdeux V, Zhao H,
Sankowski R, Al-Abed Y, Davies P and Marambaud P: Resveratrol
mitigates lipopolysaccharide- and Aβ-mediated microglial
inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J
Neurochem. 120:461–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yi CO, Jeon BT, Shin HJ, Jeong EA, Chang
KC, Lee JE, Lee DH, Kim HJ, Kang SS, Cho GJ, et al: Resveratrol
activates AMPK and suppresses LPS-induced NF-κB-dependent COX-2
activation in RAW 264.7 macrophage cells. Anat Cell Biol.
44:194–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang C, Wang Y, Wang J, Yao W, Chen X and
Zhang W: TSG (2,3,4′,5-tetrahydroxystilbene 2-O-β-D-glucoside)
suppresses induction of pro-inflammatory factors by attenuating the
binding activity of nuclear factor-κB in microglia. J
Neuroinflammation. 10:1292013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saiko P, Szakmary A, Jaeger W and Szekeres
T: Resveratrol and its analogs: Defense against cancer, coronary
disease and neurodegenerative maladies or just a fad? Mutat Res.
658:68–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chao JF, Li HT, Cheng KW, Yu MS, Chang RC
and Wang M: Protective effects of pinostilbene, a resveratrol
methylated derivative, against 6-hydroxydopamine-induced
neurotoxicity in SH-SY5Y cells. J Nutr Biochem. 21:482–489. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Larrosa M, Tomé-Carneiro J, Yáñez-Gascón
MJ, Alcántara D, Selma MV, Beltrán D, García-Conesa MT, Urbán C,
Lucas R, Tomás-Barberán F, et al: Preventive oral treatment with
resveratrol pro-prodrugs drastically reduce colon inflammation in
rodents. J Med Chem. 53:7365–7376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou XX, Yang Q, Xie YH, Sun JY, Qiu PC,
Cao W and Wang SW: Protective effect of tetrahydroxystilbene
glucoside against D-galactose inducedaging process in mice.
Phytochem Lett. 6:372–378. 2013. View Article : Google Scholar
|