Introduction
Breast cancer (BC) is the most common type of
malignancy in women and poses a severe health threat. The worldwide
incidence of BC is increasing each year (1). At present, the major treatment for BC
is surgical resection, which is combined with chemoradiotherapy in
later stages, so as to reduce tumor recurrence and achieve better
overall therapeutic effects (2).
Although traditional treatment methods are effective, they still
have numerous deficiencies, e.g. cancer tissue cannot be completely
resected by surgery and chemoradiotherapy has significant side
effects (3). Chemotherapy is mainly
based on platinum drugs, including cisplatin, carboplatin or
lobaplatin (LBP). The first-generation platinum drug, cisplatin,
has a limited application due to the large dosage required and its
severe toxicity in to kidneys, digestive tract and nerves. The
second-generation platinum drug, carboplatin, also has limited
clinical efficacy and prolonged usage is required due to its wide
cross-resistance with cisplatin, as well as significant inhibitory
effects on the bone marrow. The third-generation platinum drug LBP
has high potential for the treatment of cancer due to its high
efficiency, no cross-resistance and low toxicity. Its major
mechanism of action is to form cross-links with the DNA double
helix in tumor cells, thus abrogating the replication function of
the DNA template and further inhibiting the replication of DNA,
which finally leads to the death of tumor cells. However, prolonged
use of LBP may also lead to abnormalities in the blood system
(4,5). Therefore, research has focused on
reducing the dosage of chemotherapeutic drugs, while improving the
local drug concentration, as well as on the reduction of side
effects (6). The development of a
targeted drug with low toxicity, high efficiency and high
specificity is critical for the treatment of BC.
In recent years, with the rapid development of
nanodrugs, novel methods and techniques have been applied for this
purpose, which have gained attention from the medical science
community and materials industry (7). Nanomaterials used for the in
vivo transportation of traditional drugs to improve drug
targeting and utilization, while reducing toxicity, may provide a
breakthrough toward the development of drugs to cure cancer and
other diseases (8). Carbon nanotubes
(CNTs) have attracted much attention due to their cavity-containing
structure, excellent cell penetrability and relatively large
specific surface area, which may be utilized to carry biologically
active molecules and drugs (9,10).
However, as CNTs are insoluble in physiological buffer, they can
produce a strong immunogenicity in the body, thus limiting their
application (11).
Polyethylene glycol (PEG) is neutral, non-toxic,
non-antigenic and non-immunogenic, and has unique physicochemical
properties and good biocompatibility. It may improve the
bio-solubility, compatibility and drug transitivity of CNTs when
applied as a coating (12). It may
effectively reduce the clearance of CNTs by non-specific uptake
systems in vivo (mainly in the liver and spleen) and
increase the in vivo clearance of CNTs, thus indirectly
increasing the duration of action against targeted tumors (13,14).
Estrogen, particularly β-estradiol (E2), is
essential for maintaining female reproductive development and
secondary sexual characteristics. Through binding to the estrogen
receptor (ER), E2 induces conformational changes and the release of
molecular chaperones, including heat shock protein (HSP)90, HSP70,
cyclophilin or P23; therefore, it is a necessary cofactor for the
high transcription and expression of various genes in tumor cells
(15–17). Compared with normal cells, cancer
cells have a high expression of ER (18); therefore, targeted treatment against
various hormone-sensitive tumors is also the focus of endocrine
therapy toward BC (19,20).
To improve the inhibitory effects on cancer cell
proliferation and the anti-tumor properties of LBP, the present
study selected CNTs with a high drug-loading capacity as a drug
carrier to deliver LBP. First, PEG was applied to modify the
surface of CNTs to increase their biocompatibility; E2 was then
grafted to endow CNTs with high targeting properties. Finally, the
CNT-drug complex (E2-PEG-CNT-LBP) was obtained by loading LBP onto
modified CNTs. In the present study, in vitro anti-tumor
effects of E2-PEG-CNT-LBP against human breast cancer cells (HBCCs)
and the possible adverse effects of this targeted LBP delivery
system in normal mice in vivo were investigated.
Materials and methods
Materials and animals
CNTs (purity, 99.8 wt%; internal diameter, 5-10 nm;
length, 0.8-12 µm; specific surface area, >233 m2/g),
trypsin EDTA solution, penicillin-streptomycin solution and
dimethyl sulfoxide (DMSO) were purchased from Chengdu Biological
Co. Ltd (Chengdu, China). PEG (average molecular weight, 2,500 Da;
purity, 99.0 wt%); dimethylformamide (DMF; purity, 99.5 wt%),
chloroform (CHCl3; purity, 99.0 wt%), nitric acid
(purity, 68.0 wt%) and concentrated sulfuric acid (purity, 98.0
wt%) were obtained from Sinopharm Chemical Reagent Co. Ltd,
(Shanghai, China). E2 (purity, 99.0 wt%), Propidium Iodide Staining
Kit (with 10X Buffer A), fetal bovine serum, RPMI 1640 medium and
Dulbecco's modified Eagle's medium (DMEM)/high glucose (1X) were
purchased from Boke Biobase Co. Ltd (Jinan, China). LBP (purity,
95.0 wt%) was purchased from Chang'an International Pharm Co. Ltd
(Haikou, China). The HBCC cell line MCF-7 and fetal bovine serum
were purchased from Goybiotech Co. Ltd (Shanghai, China). MTT was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). A
total of 42 healthy female C57BL/6 mice (age, 4-6 weeks; weight,
18-20 g) were provided by Beijing Vital River Laboratory Animal
Technology Co., Ltd (Beijing, China).
Preparation of E2-PEG-CNT-LBP
CNTs were first bound to PEG so as to increase the
dispersion and loading rate of the CNTs in the solvent. The complex
was then combined with E2, followed by LBP, in order to obtain the
final complex, E2-PEG-CNT-LBP.
PEG modification of CNT
First, the appropriate amount of CNTs was reacted
with concentrated nitric acid for 24 h under ultrasonic conditions,
and then reacted with a 3:1 mixture of concentrated nitric acid and
concentrated sulfuric acid for 4 h. After the reaction, a black
precipitate was observed after the mixture was allowed to stand
still. Following removal of the supernatant, the black precipitate
was washed using triple distilled water, and dried for 8 h in an
oven at 75°C. CNTs carrying-COOH moieties (CNT-COOH) were then
obtained.
Subsequently, 300 mg of CNT-COOH was refluxed with
20 ml of DMF and 5 drop of thionyl chloride (75°C) for 12 h, cooled
to room temperature, and then dried at 100°C in a vacuum to obtain
the CNTs. Next, the hydrophilic PEG was loaded onto the chlorinated
CNTs. The mixture was then reacted with 15 ml of CHCl3,
0.1 ml of PEG and 5 drop of DMF for 10 h, washed 3 times with
ethanol (5 min/wash), and dried for 8 h in an oven at 75°C to
obtain PEG-coated CNTs (PEG-CNT).
Preparation of E2-PEG-CNT
First, 5 mg E2 dissolved in 0.5 ml absolute ethanol
was ultrasonicated in an ice bath for 30 min to fully dissolve E2,
and this solution was then added to 5 mg PEG-CNTs. This mixture was
ultrasonicated in an ice bath for 15 min to fully mix E2 and
PEG-CNTs. Subsequently, an appropriate amount of triple-distilled
water was slowly added to the mixture with ultrasonication,
followed by ultrasonic crushing (10 times at 400 W, 30 sec each
time) in an ice bath. The supernatant obtained after 20 min of
centrifugation at 42,931.2 × g and 0-4°C was removed, and the black
residue was subjected to the above procedures two more times in
order to remove the remaining solvent and obtain E2-PEG-CNT.
Preparation of E2-PEG-CNT-LBP
The procedure of loading LBP onto the surface of the
E2-PEG-CNT was performed according to a previous study (21). The specific processes were as
follows: PEG-CNT (or E2-PEG-CNT) and LBP (mass ratio, 2:1) were
added to PBS (pH=7.4). The mixture was kept in an incubator
maintained at 37°C for 24 h and then centrifuged. The
ultraviolet-visible light (UV-vis) absorption spectrum of the
supernatant was then measured. The drug-loading rate was calculated
as follows:
Drug loading rate (%) = (A1-A2)/A1 ×100%
Entrapment rate % = (m2/m1) ×100%
where A1 is the absorbance of LBP, A2 is the
absorbance of the supernatant after centrifugation, m2 is the mass
of the carrier after drug loading [m2 = m1 × (1+ drug loading
rate)] and m1 is the mass of the carrier prior to drug loading. The
obtained drug-loaded complexes were labeled as PEG-CNT-LBP and
E2-PEG-CNT-LBP as appropriate.
Cell culture
The HBCC cell line MCF-7 was cultured in RPMI 1640
medium supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin at 37°C in a humidified atmosphere with 5%
CO2. Cells in the logarithmic phase were used for the
experiments.
In vitro tumor cell inhibition
experiment
The inhibitory rates of LBP, CNT-LBP, PEG-CNT,
E2-PEG-CNT and E2-PEG-CNT-LBP against the MCF-7 HBCC cell line were
detected with an MTT assay. First, the reagent solutions were
prepared at concentrations of 0, 50, 100, and 200 µg/ml in RPMI
1640 medium; the preparations were autoclaved at 121°C for 30 min
and sealed for future use. The samples were dispersed by ultrasound
for 30 min prior to use. The concentration of MTT in the MTT assay
was 5 mg/ml, which was prepared using 10× Buffer A and by 20-fold
dilution with ultra-pure water.
Following digestion with trypsin, the cells were
counted and a cell suspension (1×105 cells/ml) was
prepared, which was inoculated into 96-well culture plates (100
µl/well) overnight. Drug solutions were added to achieve final
concentrations of 50, 100 or 200 µg/ml, while the control group was
treated with RPMI 1640 medium only, and each condition was set up
in 5 wells. The cells were incubated at 37°C with 5% CO2
for 24, 48 or 72 h. MTT working solution (20 µl) was then added,
followed by further incubation for 5 h. Following the removal of
the supernatant, 150 µl of DMSO was added and the plates were
agitated until the formazan crystals were fully dissolved. The
inhibitory rate of each complex/drug on MCF-7 cells was calculated
by determining the optical density (OD) at 560 nm as follows:
Inhibitory rate (%)=[1-(OD value of the experimental
group-OD value of the blank control group)/(OD value of the control
group-OD value of the blank control group)] ×100%.
In vivo evaluation
The present study then assessed whether the targeted
drug delivery system exhibits reduced toxicity compared with that
of the drug itself in normal mice. For this, routine blood
parameters and biochemical indexes were assessed, and the heart,
liver and kidney tissues were histologically examined.
A total of 42 healthy female mice were randomly
divided into 7 groups, namely the CNT, PEG-CNT, E2-PEG-CNT,
PEG-CNT-LBP, E2-PEG-CNT-LBP, LBP and normal saline control (NS)
groups (n=6), and were administered the corresponding agents on the
1st and 7th day by injection through the tail vein. The
concentration of LBP was 0.5 mg/ml, and the total dose was 5 mg/kg
over the course of the experiment. PEG-CNT and E2-PEG-CNT were used
as LBP carriers. The entrapment rates were 115 and 120%,
respectively. To ensure that the LBP concentration in the CNT
complex suspension was 0.5 mg/ml, the total concentration of
PEG-CNT-LBP and E2-PEG-CNT-LBP was adjusted to 0.87 and 0.83 mg/ml,
respectively, so that the LBP concentration could be maintained at
5 mg/kg (22). In the PEG-CNT-LBP
and E2-PEG-CNT-LBP groups, the concentration of PEG-CNT and
E2-PEG-CNT was 0.3 and 0.25 mg/ml, respectively, and in the CNT and
LBP groups, the drug concentration was 0.5 mg/ml; the control group
was injected with the same volume of NS (22).
In order to avoid overdosing, the injected volume of
drug solution injected did not exceed 0.6 ml. Therefore, the
concentration of LBP was 0.5 mg/ml and the dose for each mouse was
30 mg/kg. According to the loading rates of PEG-CNT and E2-PEG-CNT
as the drug carriers, the drug concentrations in the mice were
required to be the identical to that of pure LBP; the drug
concentrations were thus appropriately adjusted.
On day 14, blood was sampled from the eye orbit of
each mouse, followed by the use of EOTA for anticoagulation for the
blood routine assay, as well as for determining alanine
aminotransferase (ALT), aspartate aminotransferase (AST) and
creatinine (Cr) levels. The mice were then euthanized, and the
heart, liver and kidneys were sampled for histopathological
examination, by fixing with 10% formalin, embedding in paraffin,
slicing and staining with HE in order to observe the changes in the
cells and tissue structures.
Statistical analysis
The results were statistically analyzed using SPSS
18.0 (SPSS, Inc., Chicago, IL, USA). Values are expressed as the
mean ± standard deviation, and the average values of multiple
samples were analyzed by single-factor analysis of variance
followed by a Dunn's Multiple Comparison test. P<0.05 was
considered to indicate a statistically significant difference. The
number of degrees of freedom (Df) was calculated, and F-statistics
were performed to assess heterogeneity.
Results
Water solubility
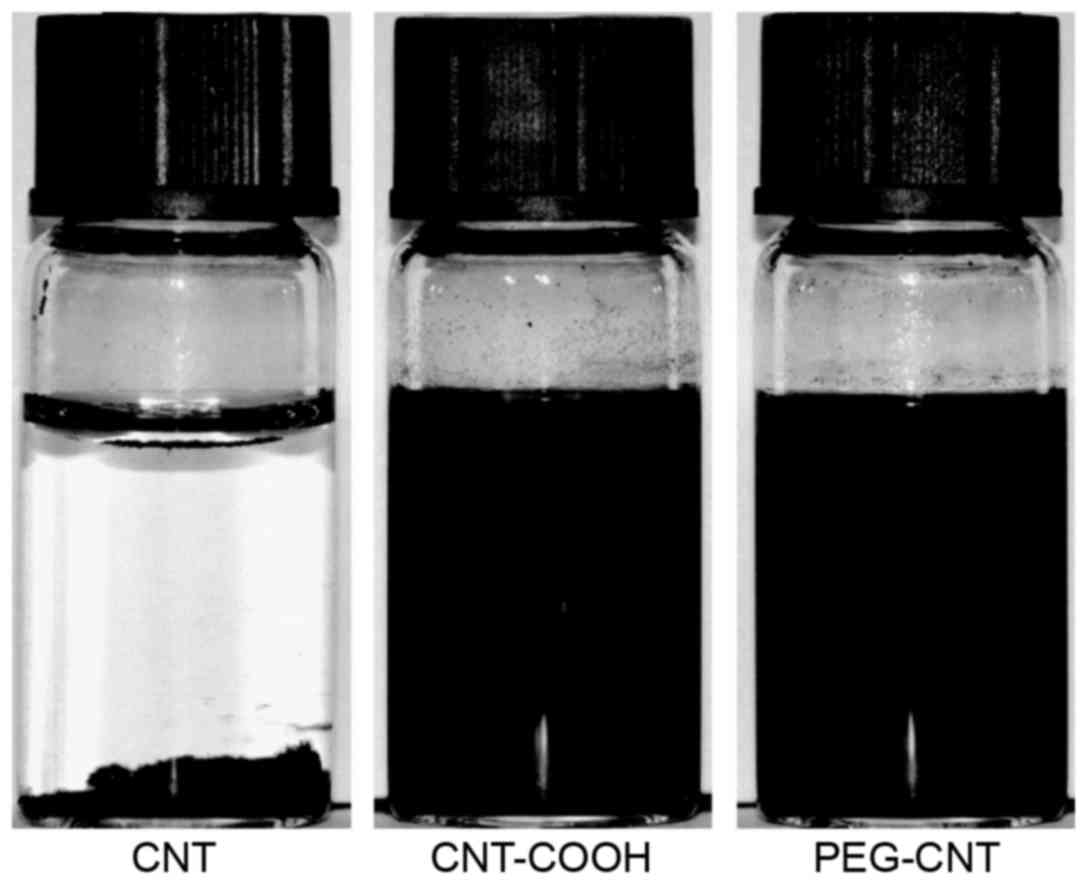
Fig. 1 presents
images of CNTs prior to and after PEG modification dispersed in
deionized water for 48 h. The images indicate that unmodified CNTs
agglomerated in water and formed a precipitate. The water
solubility of CNT-COOH was better than that of CNT, and although a
small amount of precipitate formed, the dispersion was
significantly improved. In addition, the dispersibility of CNTs
grafted by PEG was improved. Due to the grafting of PEG on the
surface of CNT, it was able to form effective electrostatic
interactions, which inhibit the aggregation of CNT.
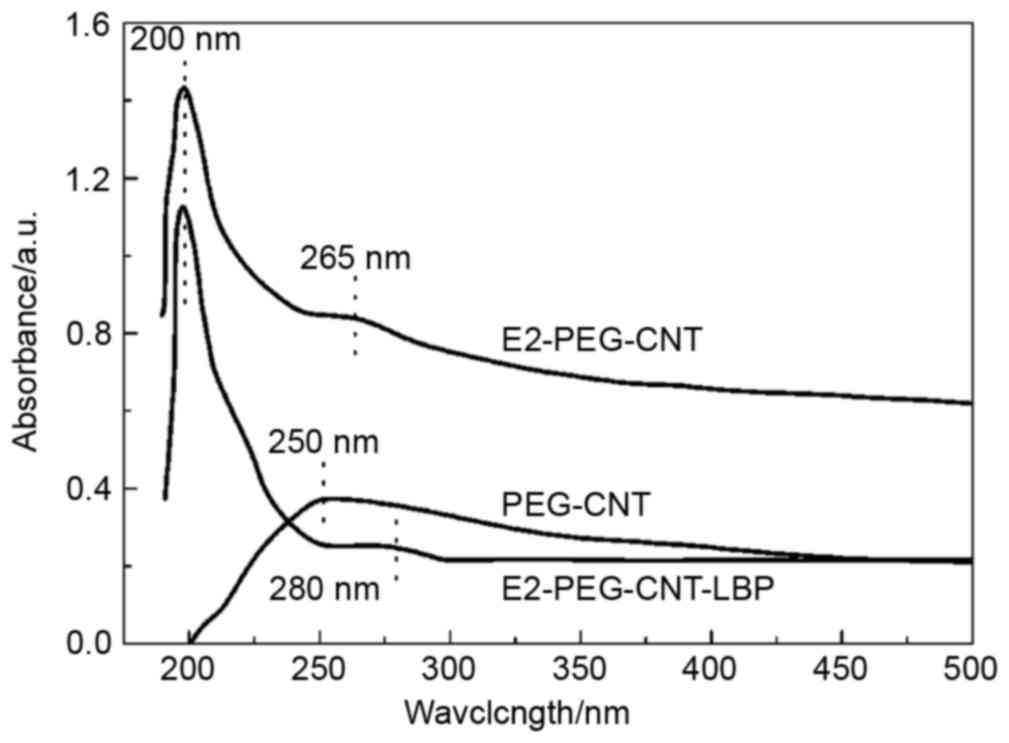
UV-vis spectra
The UV-vis spectra of PEG-CNT, E2-PEG-CNT and
E2-PEG-CNT-LBP are presented in Fig.
2. The UV-vis spectra of PEG-CNT and of E2-PEG-CNT prior to and
after LBP loading were obtained by scanning within the UV-vis
wavelength range. The absorption peak of the CNT group of PEG-CNT
was at ~250 nm, while E2-PEG-CNT exhibited a peak for E2 absorption
at ~200 nm and a peak for CNT at 265 nm; after loading with LBP,
the absorption peak of CNT shifted to ~280 nm in
E2-PEG-CNT-LBP.
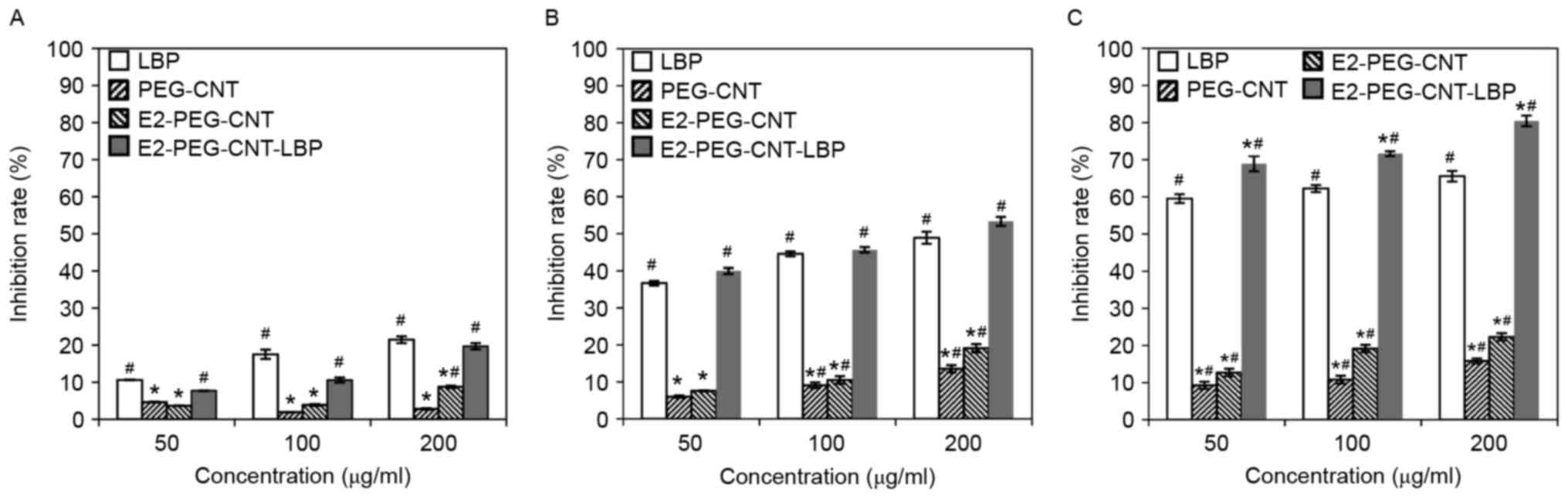
Inhibitory effects
In Fig. 3, the MCF-7
cell inhibitory rates are compared among the different compounds at
various concentrations and incubation times. The growth inhibitory
rate of MCF-7 cells in the different groups increased with the
prolongation of the interaction between the cells with LBP or the
drug-loaded complex, compared with that in the control group. The
inhibitory rate in the 200 µg/ml E2-PEG-CNT-LBP group increased
from 19.72 to 80.44% when the incubation time was markedly
increased from 24 to 72 h. Furthermore, for the same treatment
duration, the inhibitory rate increased with the drug
concentration, and this difference was marked when compared with
the control group. When MCF-7 cells were treated with the
drug-loaded complex for 24 h, the inhibition rate increased from
7.71 to 19.72% when the concentration of the complex was increased
from 50 to 200 µg/ml.
At each time point, the inhibition rates in the LBP
and E2-PEG-CNT-LBP groups were significantly greater compared with
the control group. At 72 h, the inhibition rates in the
E2-PEG-CNT-LBP groups were significantly greater compared with the
LBP. The inhibitory rate in the 200 µg/ml E2-PEG-CNT group were
significantly greater compared with the control group at each time
point. The inhibitory rate in the 100 and 200 µg/ml PEG-CNT group
were significantly greater compared with the control group at 48
and 72 h. The inhibition rates in the PEG-CNT groups were
significantly greater compared with the control group at 72 h. At
each time point, the inhibition rates in the PEG-CNT and E2-PEG-CNT
groups were significantly lower compared with the LBP group.
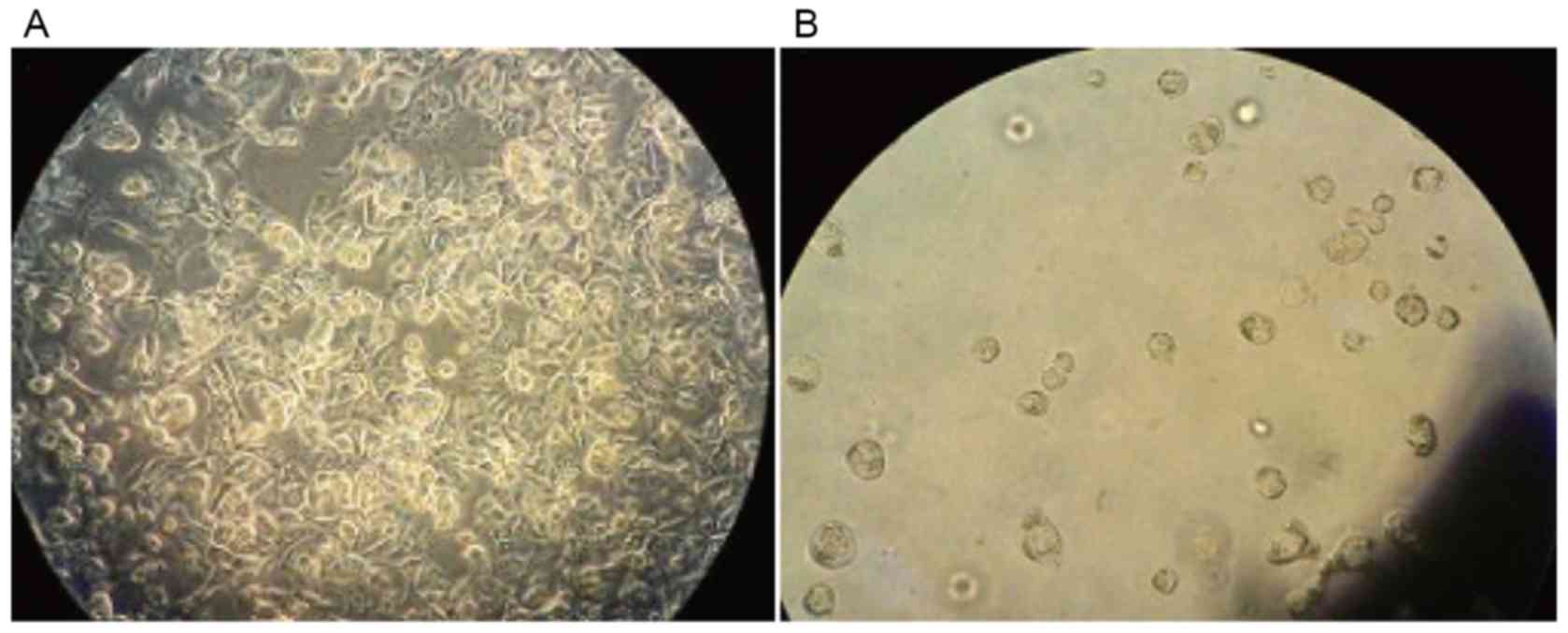
The morphology of normal MCF-7 cells (Fig. 4A) and that after culture with
E2-PEG-CNT-LBP for 48 h (Fig. 4B)
was observed using an inverted microscope (magnification, ×100).
While the untreated MCF-7 cells exhibited good adherence, had a
shuttle-like or polygonal shape and had high transparency, most of
the cells died following prolonged treatment with the drug-loaded
complex.
Blood routine and biochemical
indexes
The present study investigated the possible adverse
effects of E2-PEG-CNT-LBP in vivo, and the LBP dosage in
each group of mice was set at 5 mg/kg. Each group was injected the
respective drug on D1 and D7 through the tail vein; in order to
avoid causing excessive reactions in mice, the amount did not
exceed 0.6 ml, dose of the drug was 30 mg/kg and the injection
volume was 0.01 ml/g. According to the entrapment rates of PEG-CNT
and E2-PEG-CNT, and in order to ensure that the LBP concentration
released from the complex was equal to that of pure LBP, their
concentrations were appropriately adjusted.
Blood biochemical parameters, including blood
routine values, and parameters of liver and renal function, were
compared among the groups in order to determine whether the drugs
caused any abnormalities in organ function in the early stages
(Table I).
 | Table I.Results of blood biochemical indexes
in different groups. |
Table I.
Results of blood biochemical indexes
in different groups.
| Group | Hb (g/l) | WBC
(109/l) | PLT
(109/l) | ALT (U/l) | AST (U/l) | CR (µmol/l) |
|---|
| CNT | 134.39±6.05 | 6.29±1.20 |
360.93±87.60a |
66.16±5.81a |
165.66±4.67a | 56.16±3.48 |
| PEG-CNT | 138.09±3.68 | 6.83±1.19 |
320.00±71.22b |
48.50±6.15b |
124.00±15.08b |
62.00±3.03a,b |
| E2-PEG-CNT | 136.02±7.38 | 5.99±1.21 |
337.50±106.3b |
48.11±2.66b |
139.00±9.67b,c |
53.66±3.07a–c |
| PEG-CNT-LBP | 136.58±5.20 | 6.83±1.64 |
123.13±43.91a–d |
65.96±9.94a,c,d |
123.50±8.80a,b,d |
56.66±8.33c,d |
| E2-PEG-CNT-LBP | 137.62±6.87 | 6.21±1.32 |
101.66±18.02a–e |
54.00±8.00a–e |
134.00±14.68a–e |
58.16±4.44c,d |
| LBP | 136.42±8.26 | 7.11±1.64 |
54.00±12.13a–f |
65.65±7.41a,c,d,f |
117.50±10.13a–f |
63.16±7.33a,b,d,f |
| NS | 138.28±3.78 | 6.53±1.14 | 328.16±59.11 | 49.80±3.54 | 130.04±20.64 | 57.66±6.49 |
As presented in Table
I, it was identified that, compared with the control group,
unmodified CNT, PEG-CNT and E2-PEG-CNT did not cause any
abnormality of the WBC and Hb. Compared with the PLT count in the
control group, that in the E2-PEG-CNT-LBP, PEG-CNT-LBP and LBP
groups was significantly lower; furthermore, the PLT count in the
LBP group was significantly lower than that in the PEG-CNT-LBP and
E2-PEG-CNT-LBP groups. The level of AST in the CNT group was
significantly higher than that in the control group.
The differences in Hb, WBC, PLT, ALT, AST and Cr
levels in the C57BL/6 mice of each group were examined. The results
on PLT, ALT, AST and Cr exhibited a significant difference compared
with the control group.
Compared with the control group, the level of PLT,
ALT and AST in the CNT group was significantly higher, and CR was
significantly higher and lower in the PEG-CNT and E2-PEG-CNT group,
respectively. Compared with the CNT group, the level of PLT, ALT,
and AST was significantly lower in the PEG-CNT and E2-PEG-CNT
group, and CR was significantly higher and lower in the PEG-CNT and
E2-PEG-CNT groups, respectively. Compared with the PEG-CNT group,
the level of AST in the E2-PEG-CNT group was significantly higher.
Compared with the LBP group, the level of PLT and ALT was
significantly higher and CR was significantly lower in the
PEG-CNT-LBP and E2-PEG-CNT-LBP groups. The level of AST was
significantly higher in the LBP group and significantly lower in
the PEG-CNT-LBP group compared with the E2-PEG-CNT-LBP group.
Compared with the PEG-CNT-LBP group, the level of PLT and ALT in
the E2-PEG-CNT-LBP group was significantly lower.
Histopathology
Fig. 5A displays
histopathological images of the hearts of mice injected with the
CNT-drug complexes. The morphology of the myocardium was regular,
the nucleus exhibited integrity. The arrangement of myofibers was
normal and the structure was clear, without any oozing, hyperemia
and edema. In addition, no indication of cardiomyocyte degeneration
was observed in any group.
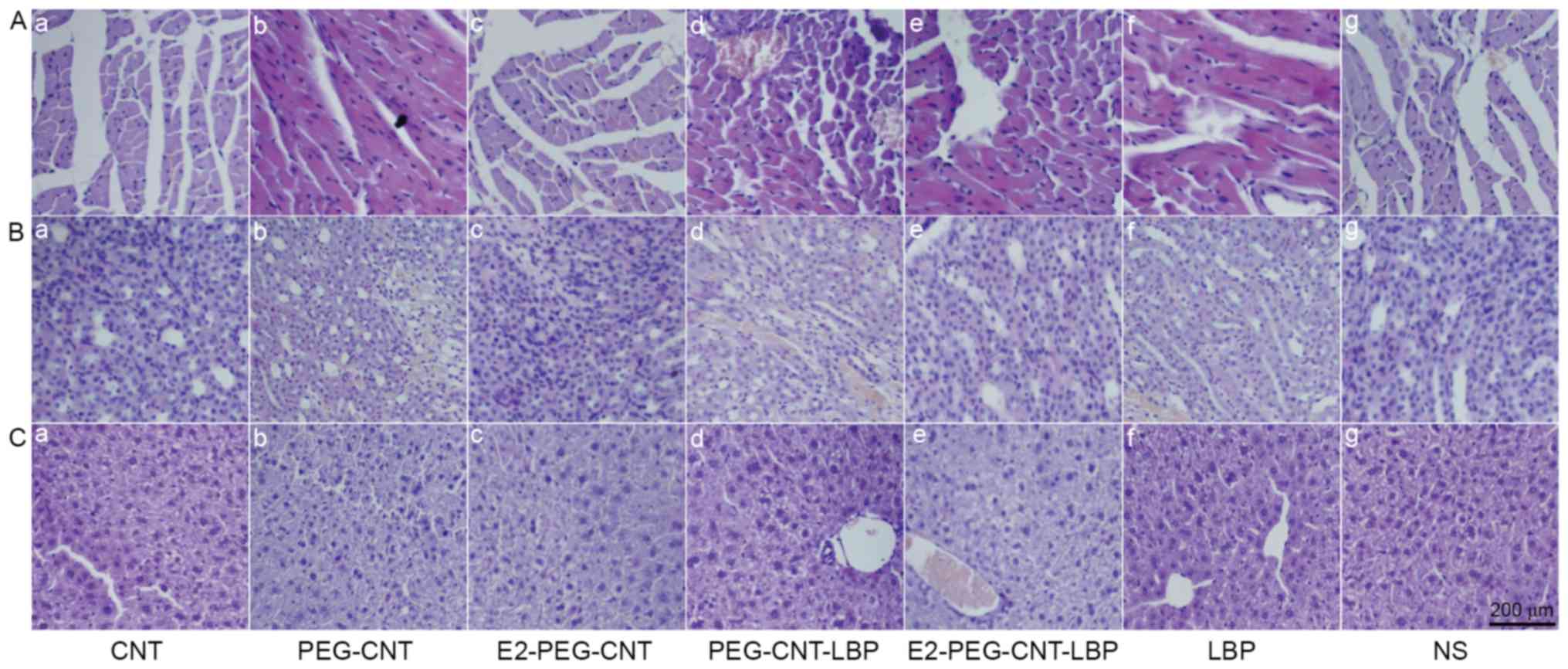 | Figure 5.Representative histopathological
images of (A) heart, (B) kidney and (C) liver tissues in groups
treated with (a) CNT, (b) PEG-CNT, (c) E2-PEG-CNT, (d) PEG-CNT-LBP,
(e) E2-PEG-CNT-LBP, (f) LBP and (g) NS (scale bar, 200 µm). CNT,
carbon nanotubes; PEG, polyethyleneglycol; E2, β-estradiol; LBP,
lobaplatin; NS, normal saline. |
Fig. 5B presents
histopathological images of the kidneys. No obvious renal tubular
epithelial cell atrophy or vacuolar changes were present, and no
protein, uric acid crystallization or congestion was observed in
the tubules in any experimental group. No interstitial inflammation
was present and the structure was normal compared with that in the
blank control group.
In Fig. 5C,
histopathological images of the liver are displayed. The liver
cells exhibited a cord-like radial pattern arrangement, with intact
morphological structure, a clear central vein, no necrosis of liver
cells or abnormal pathological changes. Compared with the NS group,
no peripheral inflammatory exudation was identified in the liver
portal area and hepatic portal tissue structure in any experimental
group.
Discussion
The improved dispersibility of CNTs by PEG may be
due to the grafting of PEG onto the CNTs, resulting in the
formation of an effective electrostatic layer in solution. This
anti-static force among the CNTs overcomes gravity and van der
Waals forces, so that the water solubility is enhanced. This result
confirms that the modification of the surface of the CNTs by PEG
improves the biocompatibility of the CNTs.
The UV-vis spectrum of E2-PEG-CNT exhibited the
characteristic absorption peaks of E2 near 200 nm and CNT at 265 nm
(23), indicating that E2 was loaded
onto the PEG-CNT surface. Compared with the UV-vis curve of
E2-PEG-CNT, the absorption peak of E2-PEG-CNT-LBP at 265 nm shifted
to ~280 nm, which may be due to the introduction of LBP, confirming
that LBP was adsorbed onto the surface of E2-PEG-CNTs.
The inhibitory rate of LBP on MCF-7 cells relative
to the control group increased in a time-dependent manner. At the
same time-point, the inhibitory rate increased with the increase in
drug concentration in a dose-dependent manner. Therefore, it may be
concluded that LBP has a significant time- and dose-dependent
inhibitory effect on BC cells. By contrast, PEG-CNT and E2-PEG-CNT
had less pronounced inhibitory effects on the proliferation of
HBCCs at any concentration, which may be due to the toxicity of
CNTs being decreased due to PEG modification. Of note, E2 is known
to affect HBCCs including MCF-7 cells as a ligand, which may
promote their proliferation, but this was apparently counteracted
by the low toxicity of CNT-PEG. By contrast, LBP and E2-PEG-CNT-LBP
significantly inhibited the proliferation of HBCCs (P<0.05).
This is due to the known cytotoxic and growth inhibitory effects of
LBP, including inhibition of cell cycle progression, and the
results further confirmed that LBP was successfully loaded onto the
surface of E2-PEG-CNT. Comparison of the inhibitory rates on HBCCs
between LBP and E2-PEG-CNT-LBP reveals that the inhibitory rate of
E2-PEG-CNT-LBP is significantly higher than that of pure LBP when
the incubation time is 72 h. This may be the result of two
responses: i) E2 can target ER in MCF-7, which increases the
inhibitory effect of LBP against the HBCCs; ii) PEG-modified CNTs
may have an increased clearance, thus indirectly increasing the
target acting time of LBP toward the BC cells. With the increased
incubation time and specific targeting of BC cells, the drug dosage
and adverse reactions were reduced, so that satisfactory results
were obtained. The apoptotic effects of E2-PEG-CNT-LBP against
MCF-7 at 72 h were further confirmed by microscopy observation.
The possible adverse effects of the drugs were then
assessed in an in vivo experiment in mice, in which blood
routine and blood biochemical parameters were measured. The results
indicated that the CNT, PEG-CNT and E2-PEG-CNT groups did not cause
any abnormalities in the WBC and Hb, when compared with the control
group. This may be due to the reason that the concentrations in
each experimental group did not reach toxic levels, which may have
caused abnormalities in the blood system, liver and renal function.
In addition to this, mice have a faster metabolism, which may lead
to faster excretion of the CNTs and the drug. Therefore, no
abnormality in the above parameters appeared. Compared with the PLT
count in the control group, that in the E2-PEG-CNT-LBP, PEG-CNT-LBP
and LBP group was significantly decreased, and the PLT count in the
LBP group was significantly lower than that in the other two
groups. These results indicated that the LBP-containing drugs
decrease the PLT count, suggesting that the major side effect of
LBP is the decrease in PLT count. However, compared with the effect
of pure LBP on the PLT count, the decrease in the PLT count in the
PEG-CNT-LBP and E2-PEG-CNT-LBP groups was smaller, which suggested
that administration of LBP as a CNT complex reduces the adverse
effect on the PLT count. This may be attributable to the fact that
PEG is neutral, non-toxic, non-antigenic and non-immunogenic, has
unique physicochemical properties and exhibits good
biocompatibility. Therefore, when it is used as a functional group
to modify CNTs, the complex slowly releases LBP, which reduces the
dose of free LBP in the body decreases the impact on the body.
Compared with the AST levels in the control group, those in the CNT
group were significantly increased, consistent with previously
reported results (24), indicating
that CNT accumulates in the liver through the blood circulation,
thus causing oxidative stress reactions in partial hepatocytes and
increasing the level of AST. The results indicated that the
PEG-modified CNTs effectively reduce the clearance of CNT in
vivo by a nonspecific uptake system (mainly the liver and
spleen) and increase the retention time in the blood circulation
system, thus indirectly increasing the action time toward the
targeted tumor (24).
In the present study, histopathological observation
of the heart, liver and kidney indicated that the toxicity of
PEG-CNT is low, and that it is therefore suitable as a targeting
drug carrier. Furthermore, LBP had no obvious adverse effects on
the heart, liver and kidneys, and mainly affected the blood
system.
In order to further validate the cancer cell
inhibition rate of E2-PEG-CNT-LBP and facilitate its examination in
preclinical studies, the optimized CNT drug-loaded complex will be
used in a clonogenic assay. Furthermore, signaling pathways
associated with the inhibition of MCF-7 cells will be assessed and
apoptosis will be examined. This will provide a broader basis for
the optimization of the material structures and properties.
Furthermore, the inhibition of BC by E2-PEG-CNT-LBP will be
investigated in tumor-bearing mice, which will lay a solid
foundation for its in vivo application.
In conclusion, in order to enhance the targeted
anti-tumor therapeutic effects of LBP, the present study coated
CNTs with PEG and loaded them with E2 and LBP to ultimately achieve
a novel CNT-drug complex, namely E2-PEG-CNT-LBP. Furthermore, the
in vitro antitumor effects of this drug-loaded complex and
in vivo effects of this drug-loaded complex in the blood and
tissues were also investigated. The conclusions are as follows: i)
The E2-PEG-CNT-LBP drug-loaded complex has higher time- and
dose-dependent cytotoxic effects on MCF-7 cells than LBP alone and
ii) blood routine, liver function, and renal function tests in
normal mice revealed no significant differences in the levels of Hb
and WBC count among different groups.
The groups administered with LBP-containing drugs
also had reduced PLT counts, indicating that the major side effect
of LBP is the reductions of the PLT count. However, compared with
the effect of pure LBP on the PLT count, the decrease in the PLT
count in the E2-PEG-CNT-LBP group was smaller, indicating that due
to PEG-modified CNT achieving a slow release of LBP, the impact of
LBP on the PLT count is reduced. In addition, the results from the
current study and a previous study (24) demonstrate that administration of the
CNTs increased the AST and ALT levels compared with those in the
control group, while, after modification with PEG, the clearance of
CNT was reduced by an in vivo nonspecific uptake system. The
CR levels were statistically significant, but the changes in each
group were small; therefore the clinical significance was small,
considering the time of observation and the dosage.
Simultaneously, it increases the retention time of
the drug in the blood circulation, thereby indirectly increasing
the action time on the target tumor. Histopathological analysis
demonstrated that the liver tissue exhibited no significant
hepatocyte necrosis or inflammatory exudation around the portal
vein, cardiac histopathology indicated no myocardial change, and
renal histopathology revealed no significant tubular necrosis. This
indicated that the platinum drugs have no significant adverse
effect on the heart, liver and kidneys, while only having a certain
impact on the blood system; at the same time, small doses of CNT
had no toxic effect.
In summary, the CNT drug-loaded complex has potent
time- and dose-dependent inhibitory effects on MCF-7 cells. In
normal mice, the adverse effects of the complex were smaller than
those of pure LBP. The present study provides a theoretical and
practical basis for effective targeted therapy with CNTs as a drug
carrier.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data are available from the corresponding author
on reasonable request.
Authors' contributions
SY was the major designer of this work and he
designed the whole scheme of the experiment. YZ performed the
inhibitory experiments of the nanomaterials on the MCF-7 cells. LC
prepared and characterized the carbon nanotubes and modified carbon
nanotubes. QL evaluated the toxicity of the nanomaterials in normal
mice by investigated their blood parameters and biochemical
indexes. JD statistically analyzed the results of inhibitory
experiments. YG histologically examined the heart, liver and kidney
tissues of mice. LZ wrote the scheme of the toxicity evaluation
in vivo, interpreted the evaluation data and drafted the
manuscript. YY involved in proposing the synthesis method of the
modified carbon nanotubes, analyzing the characterization results,
drafting the manuscript and revising it critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethical approval and consent to
participate
The animal study protocols were approved by the
Ethics Committee of Shanxi Medical University (Taiyuan, China).
Patient onsent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Grantzau T and Overgaard J: Risk of second
non-breast cancer after radiotherapy for breast cancer: A
systematic review and meta-analysis of 762,468 patients. Radiother
Oncol. 114:56–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartelink H, Maingon P, Poortmans P,
Weltens C, Fourquet A, Jager J, Schinagl D, Oei B, Rodenhuis C,
Horiot JC, et al: Whole-breast irradiation with or without a boost
for patients treated with breast-conserving surgery for early
breast cancer: 20-year follow-up of a randomised phase 3 trial.
Lancet Oncol. 16:47–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giordano SH: Breast cancer chemotherapy
cost variation substantial in the US. Pharmacoeconomics Outcomes
News. 764:9. 2016. View Article : Google Scholar
|
|
4
|
Bhatt S, Valamanesh F, Pulpytel J, Lo Dico
R, Baiyukha A, Al-Dybiat I, Pocard M, Arefi-Khonsari F and Mirshahi
M: Radio-frequency plasma polymerized biodegradable carrier for in
vivo release of cis-platinum. Oncotarget. 7:58121–58132. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guan X, Ma F, Fan Y, Zhu W, Hong R and Xu
B: Platinum-based chemotherapy in triple-negative breast cancer: A
systematic review and meta-analysis of randomized-controlled
trials. Anticancer Drugs. 26:894–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laiva AL, Venugopal JR, Karuppuswamy P,
Navaneethan B, Gora A and Ramakrishna S: Controlled release of
titanocene into the hybrid nanofibrous scaffolds to prevent the
proliferation of breast cancer cells. Int J Pharm. 483:115–123.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beck-Broichsitter M, Merkel OM and Kissel
T: Controlled pulmonary drug and gene delivery using polymeric
nano-carriers. J Control Release. 161:214–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JY, Choi WI, Kim YH and Tae G:
Brain-targeted delivery of protein using chitosan- and RVG
peptide-conjugated, pluronic-based nano-carrier. Biomaterials.
34:1170–1178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dineshkumar B, Krishnakumar K, Bhatt AR,
Paul D, Cherian J, John A and Suresh S: Single-walled and
multi-walled carbon nanotubes based drug delivery system: Cancer
therapy: A review. Indian J Cancer. 52:262–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al Faraj A, Shaik AP and Shaik AS:
Magnetic single-walled carbon nanotubes as efficient drug delivery
nanocarriers in breast cancer murine model: Noninvasive monitoring
using diffusion-weighted magnetic resonance imaging as sensitive
imaging biomarker. Int J Nanomedicine. 10:157–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Wang C, Wang X, Wang X, Cheng L, Li
Y and Liu Z: Mesoporous silica coated single-walled carbon
nanotubes as a multifunctional light-responsive platform for cancer
combination therapy. Adv Funct Mater. 25:384–392. 2015. View Article : Google Scholar
|
|
12
|
Zhang W, He J, Liu Z, Ni P and Zhu X:
Biocompatible and pH-responsive triblock copolymer
mPEG-b-PCL-b-PDMAEMA: Synthesis, self-assembly, and application. J
Polymer Sci Part A Polymer Chem. 48:1079–1091. 2010. View Article : Google Scholar
|
|
13
|
Das M, Bandyopadhyay D, Singh R, Harde H,
Kumar S and Jain SL: Orthogonal bio-functionalization of magnetic
nano-particles via ‘clickable’ poly (ethylene glycol) silanes: A
‘universal ligand’ strategy to design stealth and target-specific
nano-carriers. J Mater Chem. 22:24652–24667. 2012. View Article : Google Scholar
|
|
14
|
Moghimi SM, Hunter AC and Murray JC:
Long-circulating and target-specific nanoparticles: Theory to
practice. Pharmacol Rev. 53:283–318. 2001.PubMed/NCBI
|
|
15
|
Schiff R, Massarweh S, Shou J and Osborne
CK: Breast cancer endocrine resistance: How growth factor signaling
and estrogen receptor coregulators modulate response. Clin Cancer
Res. 9:447S–454S. 2003.PubMed/NCBI
|
|
16
|
Kurebayashi J, Otsuki T, Kunisue H, Tanaka
K, Yamamoto S and Sonoo H: Expression levels of estrogen
receptor-alpha, estrogen receptor-beta, coactivators, and
corepressors in breast cancer. Clin Cancer Res. 6:512–518.
2000.PubMed/NCBI
|
|
17
|
Horwitz KB and McGuire WL: Estrogen
control of progesterone receptor in human breast cancer:
Correlation with nuclear processing of estrogen receptor. J Biol
Chem. 253:2223–2228. 1978.PubMed/NCBI
|
|
18
|
Rai S, Paliwal R, Vaidya B, Gupta PN,
Mahor S, Khatri K, Goyal AK, Rawat A and Vyas SP: Estrogen(s) and
analogs as a non-immunogenic endogenous ligand in targeted drug/DNA
delivery. Curr Med Chem. 14:2095–2109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keely NO and Meegan MJ: Targeting tumors
using estrogen receptor ligand conjugates. Curr Cancer Drug
Targets. 9:370–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dao KL and Hanson RN: Targeting the
estrogen receptor using steroid-therapeutic drug conjugates
(hybrids). Bioconjug Chem. 23:2139–2158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Datir SR, Das M, Singh RP and Jain S:
Hyaluronate tethered, ‘smart’ multiwalled carbon nanotubes for
tumor-targeted delivery of doxorubicin. Bioconjug Chem.
23:2201–2213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu SP, Su XD, Du JL, Wang JL, Gao YD,
Zhang L, Chen L, Yang YZ and Liu XG: The cytotoxicity of water
soluble carbon nanotubes on human embryonic kidney and liver cancer
cells. New Carbon Materials. 33:35–46. 2018. View Article : Google Scholar
|
|
23
|
Carlson JC, Stefan MI, Parnis JM and
Metcalfe CD: Direct UV photolysis of selected pharmaceuticals,
personal care products and endocrine disruptors in aqueous
solution. Water Res. 84:350–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salvador-Morales C, Townsend P, Flahaut E,
Vénien-Bryand C, Vlandase A, Greena MLH and Sim RB: Binding of
pulmonary surfactant proteins to carbon nanotubes; potential for
damage to lung immune defense mechanisms. Carbon. 45:607–617. 2007.
View Article : Google Scholar
|
|
25
|
Wang DP, Li SR, Zhang M, Li ST, Zhang YP,
Geng ZX and Li RF: Determination of Hematological and Biochemical
Parameters in Three Stocks of Mice. Shiyandongwu Kexue Yu Guanli.
17:24–28. 2000.(In Chinese).
|