Introduction
Venous ulcers (VUs) are ulcerative changes caused by
chronic venous insufficiency. These types of ulcers have a high
recurrence rate with no simple treatment determined for an
effective short-term outcome. This severely influences the
patients' life quality and causes a heavy burden following a long
treatment period (1). Therapy plans
for VUs typically include drugs, skin transplantation and surgery
(2). Drug therapy works slowly,
whereas the therapeutic effect is frequently not obvious, though it
is defined as a basic treatment for VUs (2). Skin transplantation provides a
promising outcome within a short period, yet severe surgical trauma
is inevitable (2). Furthermore,
dermal biomaterial are expensive and often unaffordable for
patients. The amniotic membrane (AM) has advantages of
epithelialization stimulation, anti-bacterial properties and
definite mechanical strength. Consequently, the use of the AM has a
well-established history in healing burn wounds and corneal ulcers
(2). Cases of adopting the AM to
heal VUs have also been reported worldwide due to its beneficial
attributes (2–4). However, the AM is not convenient to
store, as it requires a temperature of −80°C and a sterile vial
(3,4). Hence, expensive medical costs are
incurred by the patients. In contrast, the human acellular amniotic
membrane (HAAM) has overcome this disadvantage. HAAM is the natural
extracellular matrix of the human amnion membrane, which contains
fibronectin, laminin, elastin, proteoglycans, hyaluronan, collagens
I, III, IV, V, and VII, basic-fibroblast growth factor, epidermal
growth factor and transforming growth factor-β, with multiple
bioactive factors (2,4). HAAMs retain effective components and
exclude the majority of cells, which results in weak antigenicity
(2). Additionally, HAAM was
demonstrated to be anti-inflammatory, anti-microbial and
non-tumorigenic, producing few ethical issues (3). Furthermore, the remaining basal
membrane layer and strata compactum are easy to store, transport
and use (2). Furthermore,
theoretically, the HAAM is safer than the AM, yet it is equally
effective (2–4). All these parameters have made HAAM a
cyto-compatible membrane with various bioactive factors, which is
widely applied in tissue engineering and within clinics. The HAAM
is isolated from the AM via cleansing the cellular components
(4). Thus, a pilot study regarding
the efficiency and safety of HAAM adopted for the treatment of VUs
was conducted.
Patients and methods
Clinical data
A total of 4 patients, who received HAAM therapies
in West China Hospital (Chengdu, China) from January-April 2013 and
were diagnosed with VU of the lower limbs were included in the
present pilot study. The patients' left extremities had swollen to
varying degrees. All ulcers had medium-considerable purulence and
considerable secretion. The area surrounding the ulcers appeared
painful. Furthermore, long-term drug therapy had been ineffective
in the 4 patients prior to admission. Following admission, all
patients underwent an ultrasound examination. Consequently, 2
patients were diagnosed with left lower extremity varicose veins
(stage C6) and the remaining 2 patients were diagnosed with
post-thrombotic syndrome (PTS; stage 4b). All 4 patients, who
received HAAM treatment, were followed up for ~6 months. The study
protocol conformed to the ethical guidelines of the 1975
Declaration of Helsinki, with approval granted by the Human
Research Review Committee at West China Hospital, Sichuan
University. All patients provided written informed consent.
Preparation of HAAM
HAAM were prepared by chemical detergent-enzymatic
extraction at the Stem Cells and Tissue Engineering Laboratory of
the West China Hospital of Sichuan University. Fresh human amnion
was cross-linked with glutaraldehyde, then shaken in 0.5% SDS for
24 h at 4°C, and finally treated with 0.25% trypsin for 4 h at
37°C. The product was freeze-dried and sterilized using ethylene
oxide. Human fibroblasts were isolated from embryos, expanded in
vitro using an amplification kit (Turely Cell VGA kit performed
according to the manufacturer's protocol; (Sichuan Neo-Life Stem
Cell Biotech, Inc., Sichuan, China) for 48 h at 37°C and seeded in
HAAM. The HAAMs were stained for 4 h at 4°C with hematoxylin and
eosin and were examined under light microscopy (magnification,
×400). Then using Mallory's stain, samples were fixed in 3%
glutaraldehyde with 0.1 M phosphate buffer (pH 7.2) for 120 min at
4°C. Samples were post-fixed in 1% osmium tetroxide for 60 min at
room temperature, and dehydrated in 50, 70, 80, 90, and 100%
ethanol for 10 min. The samples were then air-dried, mounted,
sputter coated with gold and visualized using a Hitachi S-4800
scanning electron microscope (magnification, ×500; Hitachi, Ltd.,
Tokyo, Japan). It was confirmed that there were no cell residues in
the HAAM. One side of the HAAM had a reticular and porous
structure, whereas the other side had a compact, fibrous structure.
The pore diameter ranged from 10-80 nm.
Protocol for use of HAAM
Patients initially received drug therapy and
symptomatic treatment, including 500 mg Alvenor (Servier
Laboratories, Nueilly-sur-Seine, France) twice daily with elevation
of the affected limb. Subsequently, the VU was disinfected with
povidone-iodine solution prior to the application of HAAM, in order
to ensure the VU did not become infected.
Once no purulence or secretion exuded from the VU
was observed, a suitably sized HAAM (Chengdu Qingshan Likang
Pharmaceutical Co., Ltd., Chengdu, China) was used to completely
cover the VU (the first layer). An asepsis gauze saturated with
heparinized water (100 mg/500 ml) was placed on the HAAM (the
second layer), then covered with an oiled gauze (the third layer)
and, finally, another asepsis gauze was deposited on the oiled
gauze (the fourth layer). The dressing materials were replaced
(except the first layer) every 2 days. No iodine solution or ethyl
was used to disinfect the inner layer, which was soaked with normal
saline instead. Furthermore, no scraping was performed. Replacement
of the outer dressing materials was repeated every 2 days for the
initial 14 days.
Evaluation index for ulcer
treatment
The evaluations were carried out on the 3rd day and
at the end of the 1st, 2nd and 3rd week, and the 2nd and 3rd month
after the HAAMs were adopted. The size and depth of the VU
(determined based on whether the depth of VU reaches the tibial
plane), the proportion of granulation tissue (whether >50%) and
the degree of secretion (measured by asessing the degree of
satuation in the outer gauze) and infection (assessed qualitatively
via the appearance of purulence or peripheral swelling) were
evaluated. If the size and depth of the ulcer increased during
therapy, the HAAM treatment was considered to have failed, whereas
if the size and depth of the ulcer did not change, the HAAM
treatment was deemed null. Pain scores were also assessed at the
same intervals using a visual analog scale (4–6), where 0
was no pain and 10 was the worst pain imaginable. Patients chose a
suitable score to represent and quantify the pain they suffered on
admission and during therapy. Secretion evaluation was measured by
the saturation degree of the outer gauze. Infection was
qualitatively assessed via the appearance of purulence or
peripheral swelling (4).
Results
Patient characteristics
As presented in Table
I, the 4 included patients were all male, with a median age of
62.3±2.2 years. All received drug therapy. A total of 2 patients
were diagnosed with left lower extremity varicose veins coupled
with VU, whereas the remaining 2 patients exhibited PTS coupled
with VU. The sizes of VU ranged from 3.2×0.9 to 5.1×2.5
cm2, which are presented in Table I. One VU accessed the tibia and the
other three were confined to the tissue.
 | Table I.Baseline characteristics of included
patients. |
Table I.
Baseline characteristics of included
patients.
| Patient | Gender | Age (years) | Size of VU (cm ×
cm) | Depth of VU | History | Diagnosis |
|---|
| Case 1 | Male | 60 | 3.4×2.7 | No access to
tibia | Appeared for >6
months and not healed | Left lower extremity
varicose vein coupled with VU |
| Case 2 | Male | 61 | 2.8×2.3 | No access to
tibia | Appeared for >4
months and not healed | PTS coupled with VU
Case 3 |
| Case 3 | Male | 63 | 5.1×2.5 | No access to
tibia | Appeared for >5
months and not healed | Left lower extremity
varicose vein coupled with VU |
| Case 4 | Male | 65 | 3.2×0.9 | Access to tibia | Appeared for >12
months and not healed | PTS coupled with
VU |
Evaluation of HAAM treatment
effect
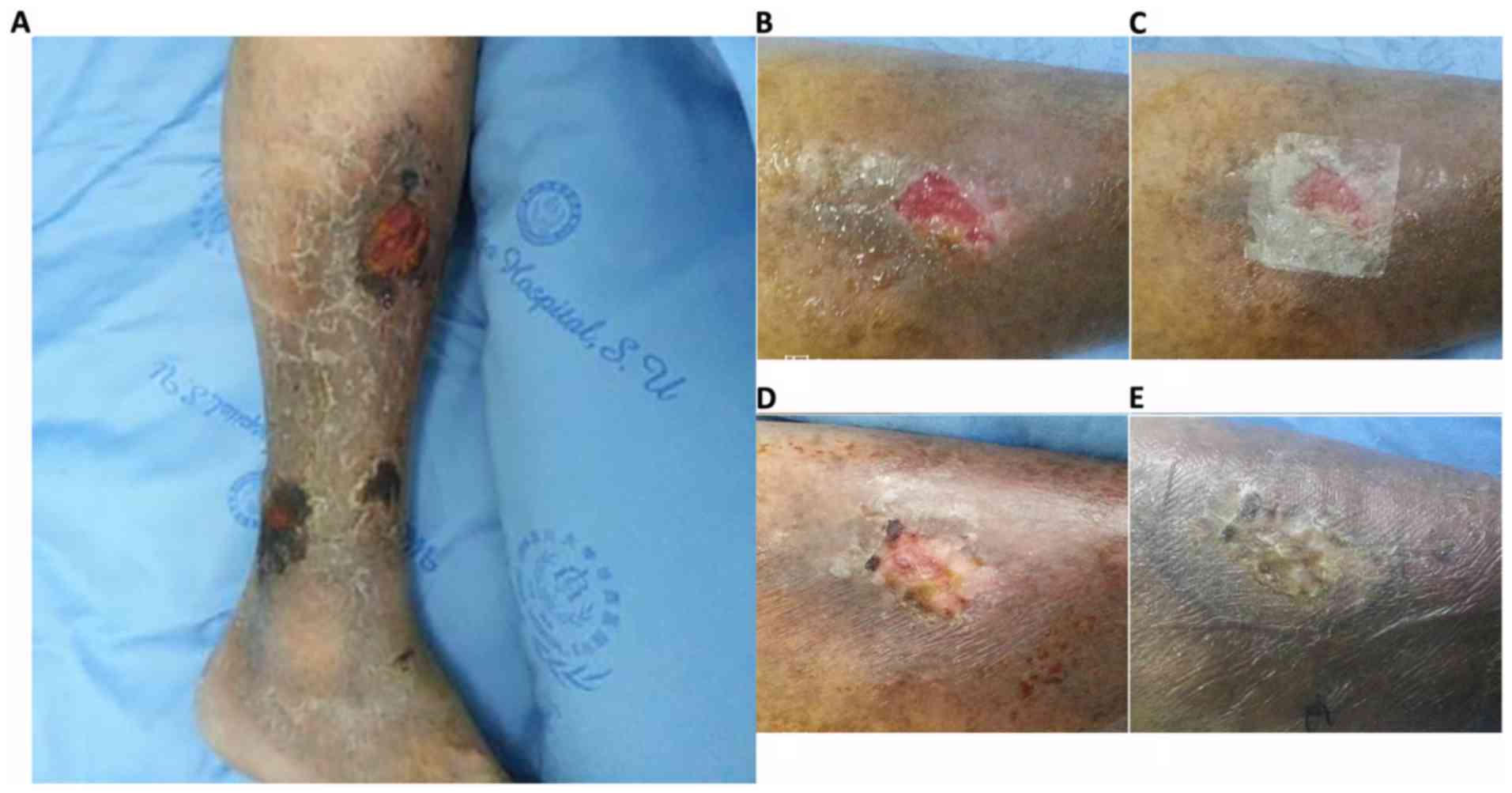
On the 3rd day after HAAM was adopted to treat the
VUs, the HAAM had firmly adhered to the surface of the VU. A
condition evaluation revealed 100% adherence rate, ensuring
long-term HAAM efficacy. Complete epithelialization (healed tissue)
occurred in 2 cases (case 1 and 2): The first at the end of the 3rd
week and the second, at the 2nd month following HAAM induction. In
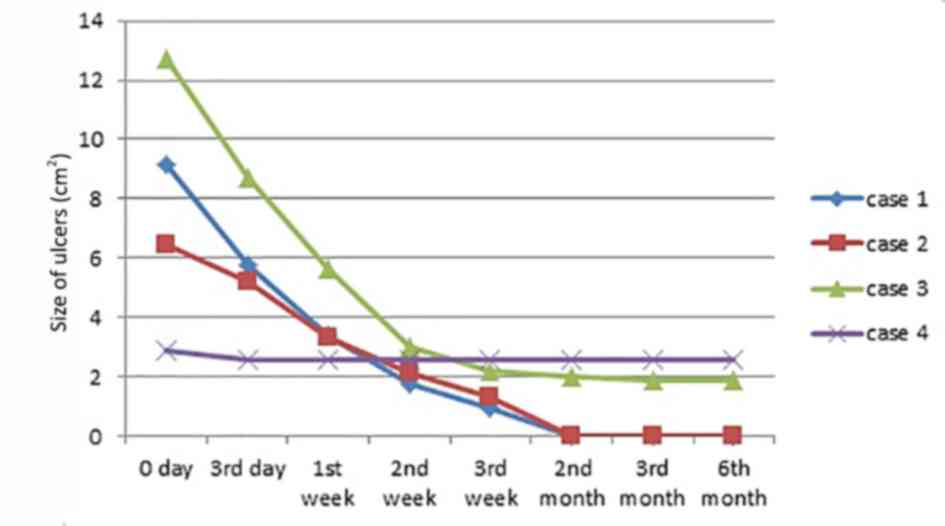
case 3, ulcer size was reduced by >60%; however, the ulcer size
of the remaining case (case 4) only reduced by <20%. Overall,
the mean size of ulcer reduction in each case was >50%, case 1
and 2 decreased to 1.2×1.1 and 1.4×0.4 cm, and case 3 and 4
decreased to 1.3×1.8 and 2.3×1.4, respectively, with evident
decreases in ulcer depth at the end of 3rd week. The proportion of
granulation tissue in each case was >50%. Nearly all ulcers had
decreased in size and/or depth at the end of the 1st week after the
HAAM was applied, except for case 4. The onset of the HAAM effect
was apparent within a shorter time for the smaller VUs compared
with the larger ones. Consequently, the therapeutic effect,
measured by size and depth change per day, was observed earlier for
the smaller VUs, whereas relatively larger sized VUs improved
slowly. The detailed changes in all four patients are presented in
Figs. 1 and 2.
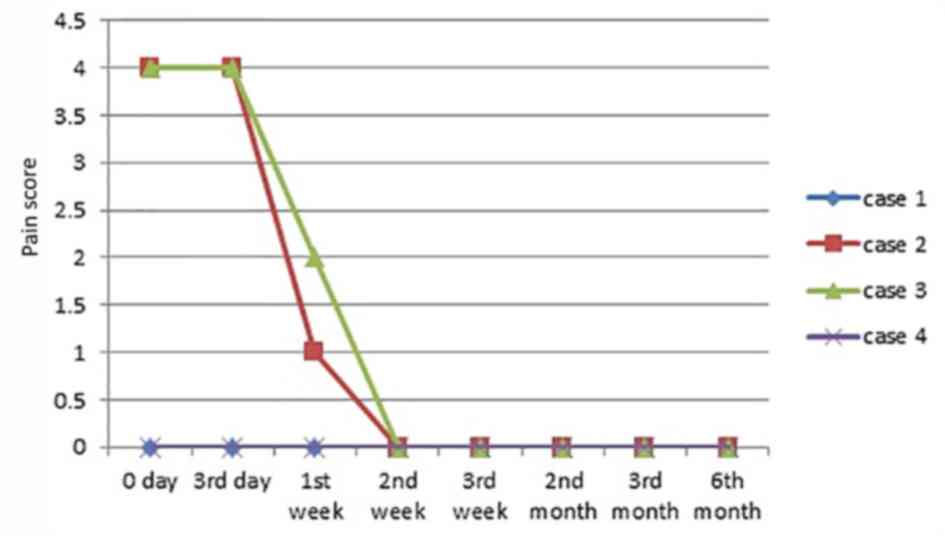
Symptoms associated with VUs
On the 3rd day following HAAM therapy, no secretion
was observed in 3 cases. Some exudation occurred in 1 case but
there was no purulence and at 1 week of therapy, this ulcer became
dry, with no exudation evident. A total of 2 patients (case 1 and
4) did not feel pain surrounding the ulcer during HAAM therapy.
Also, the pain was relieved in the remaining 2 patients (case 2 and
3), with the pain scores reduced by 3 and 2 points to 1 and 2
point(s), respectively, at 1-week of therapy, followed by 0 pain in
both patients. Detailed changes in all the cases are presented in
Fig. 3.
Safety and convenience of HAAM
No uncomfortable symptoms occurred during the HAAM
therapy. No local or general inflammation or allergic reactions
were noted. In addition, the HAAM therapy protocol was simple to
manage: 2 patients (case 3 and 4) dressed the wound by themselves
following discharge.
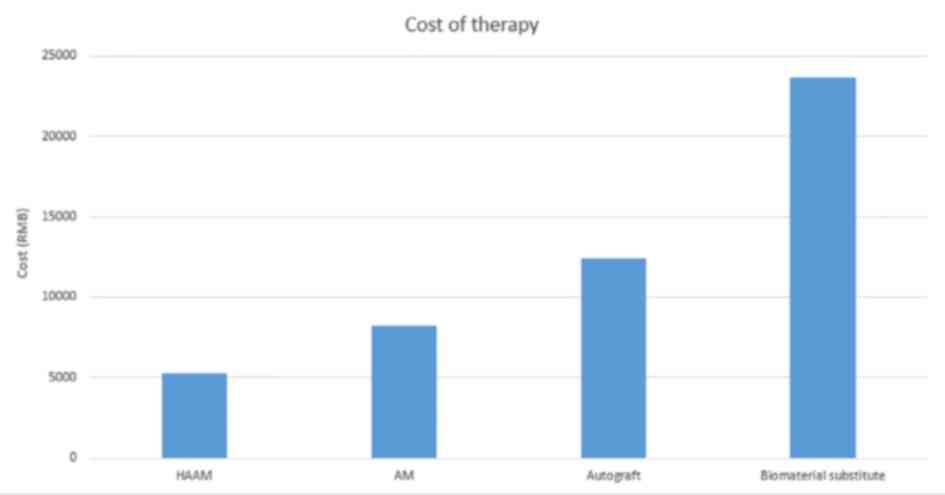
Medical cost of HAAM
The mean medical cost of AM graft treatment is
estimated at ¥8,256, whereas HAAM treatment cost for each patient
at the West China Hospital is ¥5,276 (7). In comparison, the mean cost of an
autograft and biomaterial substitute is ¥12,435 and ¥23,643,
respectively (7). Thus, HAAM's
cost-efficiency is markedly high. A detailed comparison of the mean
costs of these treatments is presented in Fig. 4.
Discussion
The efficacy of the AM, to treat various kinds of
ulcers, has been previously confirmed (5), yet the application of the AM to treat
VUs of the lower limbs has only emerged more recently (4,6). In
addition, although AM is an alloplastic biomaterial, any untoward
reaction of the AM is rarely reported. Hence, considering its
safety and efficiency, the use of AMs to treat VUs is clinically
recommended (2,6). In the present study, the safety and
efficacy of HAAM was explored. Furthermore, to the best of our
knowledge, the present study is the first to detail practice
experience regarding HAAM applied in VU treatment. In the present
study, it was evident that the size and/or depth of most ulcers
improved at 1-week following HAAM treatment, irrespective of the VU
size. Particularly, when the ulcer was relatively small, the
therapeutic effect of HAAM is more impressive; namely, the ulcers
healed completely (epithelia appeared and granulation tissue
formed) between 1 and 2-week post-treatment. Despite this effect,
we found the HAAM is mostly effective in the first two weeks, which
was confirmed by the previous study by Mermet et al
(4). The present data indicated that
the decrease in ulcer size range for the 3 cases (excluding case 4)
was more intense in the first 2 weeks than during the subsequent
duration. The total size decrease of the four ulcers was estimated
as 21.77 cm2 in the first 2 weeks, which is
substantially greater than 4.99 cm2, which was recorded
in the final 2 weeks of therapy. It was demonstrated that the depth
of the wound may affect the healing speed; the deeper the wound,
the longer time it takes to heal. The size reduction of three
ulcers (cases 1-3) was evident, with all decreasing by >80%
(85-100%). In contrast, in case 4, the ulcer size decreased by
<10%. However, its depth and secretion reduced dramatically. It
has been reported that HAAM is particularly effective in shrinking
shallow ulcers, rather than deep ulcers (6). This was also reported in Sawhney's
research on the AM as a biological dressing in the management of
burns (8). The mean healing time was
reported to be significantly faster in all groups with amnion
coverage than in controls (superficial, 9.3 vs. 12.5 days;
intermediate, 15.7 vs. 23.9 days; and deep, 27.5 vs. 37.5 days).
The authors concluded that as the depth of the wound increased,
healing time increased, although this needs to be validated in a
future study. The current study provided conclusive evidence that
HAAM therapy was able to shrink the VUs, while simultaneously
maintaining the ulcers free from secretion and purulence.
The underlying pathophysiology of unhealed VU is
repeated inflammatory reaction. Fortunately, anti-inflammation and
anti-bacterial properties are main characteristics of HAAM
(9,10). Owing to the existence of T lymph
cells, lysozymes, and thrombin, the HAAM has a powerful
anti-bacterial property and ability to absorb wound secretion
(11,12). Although numerous cellular components
are excluded from the AM in preparing the HAMM, the therapeutic
effect of the HAAM is not affected (11). During HAAM therapy, the pain score
was lower than that of dressing therapy (12), which may help to ensure patients'
compliance. The possible reasons for the low pain score can be
attributed to various factors. First, the key premise of successful
HAAM treatment and lower pain score rely on the prior preparation
of the ulcers, which would create an initial optimal environment
for HAAM. Second, the absence of scraping assists to preserve the
integrity of the HAAM, which is essential for its efficacy. Third,
the anti-inflammation function of the HAMM, which decreases and
weakens the stimulation of local nerve endings, may be another
crucial reason contributing to the low pain scores recorded.
Compared with the AM, weak antigenicity is the major
advantage of the HAAM (7). In the
present study, no local or general allergic reaction occurred in
any of the evaluated cases. Removing serum human leukocyte antigen
(HLA)-I from the HAAM is paramount (13), to avoid allergic reactions and ensure
safety. Convenience and low cost are additional benefits of the
HAAM compared with the AM. The AM is fragile, requiring particular
storage conditions, thus AM appliance incurs a relatively higher
cost than the HAAM (9,10). An AM graft is estimated to cost
¥2,122 (AM therapy typically requires needs 2-3 pieces of AM; the
mean medical fee is ¥8,256, ranging from ¥7,580-10,500), whereas
the HAAM costs patients <¥1,000 (HAAM therapy requires 1-3
pieces of HAAM; the mean medical fee is ¥5,276, ranging from
¥4,700-6,700), at the West China Hospital. Also, compared with the
mean cost of an autograft (¥12,435) and biomaterial substitute
(¥23,643), the HAAM has high cost-efficiency. A similar cost trend
is observed in Spain (6). However,
the main limitation of the present study is the lack of serum HLA-I
detection in the patients. The HAAM is acquired by removing the
majority of the cells from the AM, therefore, a loss of growth
factors and precursor cells is inevitable, which may have affected
the results. Therefore, a direct comparison between the HAAM and AM
is not feasible, so it cannot be concluded whether HAAM is more
effective than the AM. Nevertheless, in the present study, no
treatment fails were recorded in all four cases evaluated.
Furthermore, the effect is similar to the AM in terms of the wound
healing (9,10). Accordingly, the present findings
suggest that HAAM has a promising effect.
The present study explored the therapeutic effect of
the HAAM, adopted to treat VUs via analyzing a number of cases.
Although the present study cannot prove whether HAAM has a
determined effect on VUs, the HAAM displayed an impressive tendency
for the treatment of VUs, providing a short therapy duration, low
medical cost and simple dressing procedure. However, regardless of
the HAAM efficacy, drug therapy and compression stockings remain
the fundamental methods for VU treatment. Therapy for VU also
includes surgery, skin autografts and biomaterial transplantation.
The HAAM is an innovative way to clinically treat VUs and provides
epithelial stimulation, pain relief and a simple dressing
procedure, as well as lower cost compared with the AM, autograft or
biomaterial transplantation. Thus, future studies on adopting HAMMs
to treat ulcers is worthwhile for physicians and researchers.
Acknowledgements
Not applicable.
Funding
The presnt study was supported by the Technology
Research and Development Program of Sichuan Province, China
(grant.no. 2016SZ0060).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DY designed the present study; ZW and XL wrote the
manuscript and collected data; DY and JZ supervised experiements to
ensure they were conducted correctly and revised the manuscript. JZ
also contributed to the conception and design of the study,
manuscript revision and final approval of the version to be
published.
Ethics approval and consent to
participate
The study protocol conformed to the ethical
guidelines of the 1975 Declaration of Helsinki, with approval
granted by the Human Research Review Committee at West China
Hospital, Sichuan University (Chengdu, China). All patients
provided written informed consent.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Patel NP, Labropoulos N and Pappas PJ:
Current management of venous ulceration. Plast Reconstr Surg. 117
Suppl:S254–S260. 2006. View Article : Google Scholar
|
|
2
|
Lo V and Pope E: Amniotic membrane use in
dermatology. Int J Dermatol. 48:935–940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Litwiniuk M, Bikowska B, Niderla-Bielińska
J, Jóźwiak J, Kamiński A, Skopiński P and Grzela T: Potential role
of metalloproteinase inhibitors from radiation sterilized amnion
dressings in the healing of venous leg ulcers. Mol Med Rep.
6:723–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mermet I, Pottier N, Sainthillier JM,
Malugani C, Cairey-Remonnay S, Maddens S, Riethmuller D, Tiberghien
P, Humbert P and Aubin F: Use of amniotic membrane transplantation
in the treatment of venous leg ulcers. Wound Repair Regen.
15:459–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh R, Chouhan US, Purohit S, Gupta P,
Kumar P, Kumar A, Chacharkar MP, Kachhawa D and Ghiya BC: Radiation
processed amniotic membranes in the treatment of non-healing ulcers
of different etiologies. Cell Tissue Bank. 5:129–134. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gutiérrez-Moreno S, Alsina-Gibert M,
Sampietro-Colom L, Pedregosa-Fauste S and Ayala-Blanco P:
Cost-benefit analysis of amniotic membrane transplantation for
venous ulcers of the legs that are refractory to conventional
treatment. Actas Dermosifiliogr. 102:284–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo JC, Li XQ and Yang ZM: Preparation of
human acellular amniotic membrane and its cytocompatibility and
biocompatibility. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
18:108–111. 2004.PubMed/NCBI
|
|
8
|
Sawhney CP: Amniotic membrane as a
biological dressing in the management of burns. Burns. 15:339–342.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beele H, de la Brassine M, Lambert J, Suys
E, De Cuyper C, Decroix J, Boyden B, Tobback L, Hulstaert F, De
Schepper S, et al: A prospective multicenter study of the efficacy
and tolerability of cryopreserved allogenic human keratinocytes to
treat venous leg ulcers. Int J Low Extrem Wounds. 4:225–233. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bailo M, Soncini M, Vertua E, Signoroni
PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P,
et al: Engraftment potential of human amnion and chorion cells
derived from term placenta. Transplantation. 78:1439–1448. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walker AB, Cooney DR and Allen JE: Use of
fresh amnion as a burn dressing. J Pediatr Surg. 12:391–395. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ganatra MA: Amniotic membrane in surgery.
J Pak Med Assoc. 53:29–32. 2003.PubMed/NCBI
|
|
13
|
Kubo M, Sonoda Y, Muramatsu R and Usui M:
Immunogenicity of human amniotic membrane in experimental
xenotransplantation. Invest Ophthalmol Vis Sci. 42:1539–1546.
2001.PubMed/NCBI
|