Introduction
Cerebral ischemia/reperfusion (I/R) injury is an
irreversible event in the resistance of neuron-favorable
contributions generated by the revascularization therapeutic
strategy for ischemic stroke (1–4).
Lowering I/R-led detrimental outcomes is of clinical importance
because of the influences of these outcomes on hemorrhagic
transformation and blood-brain barrier destruction (1–5).
Although pathophysiological mechanisms, such as apoptosis, have
been proposed to be involved in cerebral I/R damage, to date, few
evidence-based strategies for the reduction of cerebral I/R injury
have been explored (1–6). Thus, expanded investigations of
effective drugs or interventions to protect neurons from I/R injury
are required to achieve greater clinical benefits.
Recently, a broader concept highlighted that
neuronal apoptosis induced by endoplasmic reticulum (ER) stress
(ERS) serves as the pivotal pathogenesis of I/R injury, which is
characterized by elevations in C/EBP homologous protein (CHOP) and
glucose-regulated protein-78 (GRP78), in concert with upregulated
apoptotic caspase cascades (1–7). Once ER
homeostasis is disturbed and sustained in response to I/R injury,
the inaccurate synthesis and assemblage of proteins are drastically
initiated (1–8). This change leads to the substantial
accumulation of mis-folded proteins and causes unfolded protein
response (UPR) within the ER, collectively referred to as ERS
(6–8). Despite the affirmed benefits against
neuronal I/R injury that developed from ERS inhibition, more
precise molecular mechanisms and available therapeutic avenues for
the dynamic regulation of ERS-associated apoptosis remain largely
obscure in cerebral I/R injury.
Nobiletin (NOB) is a ubiquitous bioflavonoid and
polyphenolic compound isolated from citrus fruits peels that has
been verified to participate in a diverse array of pathological
events such as apoptosis, inflammation and angiogenesis (9–12). For
instance, Wu et al indicated that NOB exerts protective
effects against I/R injury after liver transplantation mainly in an
inflammation-diminished manner (10). Yasuda et al previously
provided clues suggesting that the anti-apoptotic action is
regarded as a neuroprotective mechanism of NOB in transient middle
cerebral artery-occluded rats (12).
As such, the I/R-protective roles of NOB have raised a great deal
of attention. However, the exact contributions of NOB in cerebral
I/R injury are far from clear, and the potential pathogenesis
implicated in ERS-related apoptosis remains completely unknown. An
additional study found that NOB could lower myocardial apoptosis in
pressure overload-induced cardiac hypertrophy, in which the
mechanisms are implied with NOB's ERS-repressing action (13). Intriguingly, a number of
investigations have shown the crucial involvement of the
phosphoinositide 3-kinase (PI3K) and serine/threonine kinase (AKT)
pathway in the multiple bioactivities of NOB as a compelling
approach to anticancer effects (14,15).
According to the above evidence with respect to ERS-repressing and
I/R-reducing activities, the aim of the present study was to detect
the potential protective effects of NOB in response to I/R injury
in a PI3K/AKT-dependent manner, and its interventions in
ERS-induced apoptosis, by using a model of OGD/R injury in neuronal
PC12 cell in vitro.
Materials and methods
Chemicals and reagents
NOB was purchased from Shanghai Winherb Medical
Science Co., Ltd. (Shanghai, China), and the purity of NOB higher
than 98% was detected by high-performance liquid chromatography
(HPLC) analysis as previous demonstrations (11,13).
PC12 cells were obtained from the American Type Cell Culture
Collection (ATCC; Manassas, VA) (16). Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), 0.25% trypsin, and PBS were
obtained from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). A Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). A lactate
dehydrogenase (LDH) commercial assay kit was purchased from
Shanghai Enzyme-Linked Biotechnology (Shanghai, China). Annexin
V-APC/7-AAD reagents were purchased from Beijing Biosea
Biotechnology (Beijing, China). LY294002 (a PI3K inhibitor) was
obtained from Selleck Chemicals (Houston, TX, USA). Primary
antibodies against the following proteins were from Cell Signaling
Technology, Inc. (Danvers, MA, USA): phosphorylated (p)-PI3K (no.
4228, 1:600 dilution), AKT (no. 9272, 1:600 dilution) and CHOP (no.
2895, 1:600 dilution). Antibodies against GRP78 (no. ab21685, 1:500
dilution), p-AKT (ab32509, 1:1,000 dilution) and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) (no. ab181602, 1:1,000 dilution)
were purchased from Abcam (Cambridge, UK). The horseradish
peroxidase (HRP)-conjugated rabbit anti-rat IgG secondary
antibodies were from BIOSS (Beijing, China). The BCA protein assay
kit was obtained from Pierce; Thermo Fisher Scientific, Inc.
Cell culture
The PC12 cells were routinely cultured in DMEM/F12
medium supplemented with 10% FBS, 100 U/ml of penicillin, and 100
mg/ml of streptomycin in a humidified atmosphere of 5%
CO2 and 95% O2 at 37°C (16–18). The
cells were fed every 2-3 days and sub-cultured once they reached
70-80% confluent. The PC12 cells were incubated on 96-well plates
or 6-well plates at a density appropriate to the experimental
requirements.
OGD/R injury and experimental
designation
To mimic the I/R conditions in vitro, the
PC12 cells were subjected to OGD/R treatment according to a prior
demonstrations with minor modifications (16–18). In
detail, the cells were washed two times with glucose-free Earle's
balanced salt solution, and were maintained in glucose-free DMEM
without FBS. Then, the cells were moved into an anaerobic chamber
containing a gas mixture composed of 5% CO2 and 95%
N2 for 4 h at 37°C. Following the OGD treatment, the
cells were subsequently returned to normal media and were incubated
in a normal incubator for an additional 24 h. Control cells were
not exposed to OGD/R and were incubated under normal conditions
continuously.
To determine the possible functions of NOB in PC12
cells during OGD/R injury, four concentrations of NOB (1, 10, 20
and 50 µM) were performed preliminarily to detect the most
effective dosage against OGD/R-caused damages of PC12 cells. In the
following study, PC12 cells were pretreated with NOB (50 µM) for 24
h and then subjected to OGD/R injury. The cells were randomly
divided into four groups: i) Control+vehicle; ii) Control+NOB; iii)
OGD/R+vehicle; and iv) OGD/R+NOB. Moreover, in the mechanistic
determinations, a PI3K/AKT inhibitor (LY294002) (10 µM) was added 1
h prior to NOB treatment (19). Each
group included at least 3 samples.
Assessment of cell viability
Cell viability was determined using the CCK-8 assay
as a previous method (16–18). The PC12 cells were seeded into
96-well plates at a density of 6×103 cells/well for 24 h
of incubation. Following the indicated procedures, 20 µl of CCK-8
reagent was added into each well followed by continuous incubation
at 37°C for 4 h. The optical density values were measured with a
microplate spectrophotometer (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) at a wavelength of 450 nm. Cell viabilities were
determined as the percentages relative to the control group.
Cellular injury assay
When cells are damaged by the OGD/R injury,
intracellular LDH is rapidly released into the culture supernatant.
Cellular injury was measured using LDH assay (16–18).
Briefly, after the indicated treatments, the supernatant of the
cultured PC12 cells was harvested, centrifuged at 3,000 × g for 10
min and then subjected to a commercial kit using standard protocols
according to the manufacturer's protocols. The absorbance was
detected at 450 nm, and the data were determined in international
units per litre.
Cell apoptosis
Apoptosis of the PC12 cell was determined using a
flow cytometric assay with a commercial apoptosis detection kit
(16–18). According to the manufacturer's
instruction, the cells were harvested after treatment and washed
twice with cold PBS. Then, the cells were incubated in 100 µl of
binding buffer containing 5 µl of Annexin V-APC and 5 µl of 7-AAD
at room temperature in the dark for 10 min. Then, the stained cells
were analyzed using a flow cytometer (Beckman Coulter, Inc., Brea,
CA, USA) within 1 h. Cells that stained positively for Annexin
V-APC or 7-AAD were considered to be undergoing apoptosis.
Western blotting assay
Western blotting was performed as previously
described (16–19). PC12 cells were solubilized in lysing
buffer. Protein samples (30 µg) were separated by electrophoresis
on 12 and 10% SDS-PAGE gels using a slab gel apparatus, and were
then transferred to PVDF membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% skim milk for 1 h at room
temperature, and were incubated with the primary antibodies CHOP,
GRP78, p-PI3K, PI3K AKT and p-AKT overnight at 4°C. Next, the
membranes were incubated with the corresponding secondary
antibodies at room temperature for 1 h and were rinsed three times
(5 min/time) with tris buffered saline tween (TBST). GAPDH was used
as an internal reference. Proteins were visualized using ECL
reagent, and the blots were quantified using BandScan 5.0 software
(Glyko, Inc., Novato, CA, USA).
Statistical analysis
All the data are presented as means ± standard
deviation (SD). The statistical comparisons among multiple groups
were performed using analysis of variance (ANOVA) followed by a
post hoc Tukey's test. A Student's t-test was performed for
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference. The data were
analyzed using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
NOB alleviated the OGD/R injury in
PC12 cells
We detected cell viability in each group via a CCK-8
assay. None of the four concentrations of NOB (1, 10, 20 or 50 µM)
affected the viability of PC12 cells and showed much lower
cytotoxicity on normal PC12 cells (Fig.
1A). To explore the potential contributions of NOB on neuronal
OGD/R injury, the PC12 cells were subjected to 4 h of OGD and 24 h
of reoxygenation. As shown in Fig.
1B, in comparison with the control group, the cell viability
after OGD/R was decreased by 44.78%. Moreover, the gradient doses
of NOB (1, 10, 20 and 50 µM) increased cell viability in a
concentration-dependent way. Cell viability in the 10 and 20-µM
groups was increased by 15.84 and 18.90%, respectively, and the
best result was achieved at 50 µM showing a 40.24% increase,
relative to the OGD/R group. In addition, LDH release in the
cellular supernatant, as a marker of cellular damage, was
determined to estimate PC12 cells death. As demonstrated in
Fig. 1C, the LDH release induced by
OGD/R was repressed by NOB at concentrations starting at 10 µM,
with the minimum result at 50 µM, relative to the OGD/R group. In
addition, there exhibited no significant difference in the cell
viability and LDH release between the 1-µM group and the OGD/R
group. Thus, 50 µM of NOB was selected for the subsequent study.
These results indicate that NOB could enhance cell survival and
limit cell damage in PC12 cells in response to OGD/R injury.
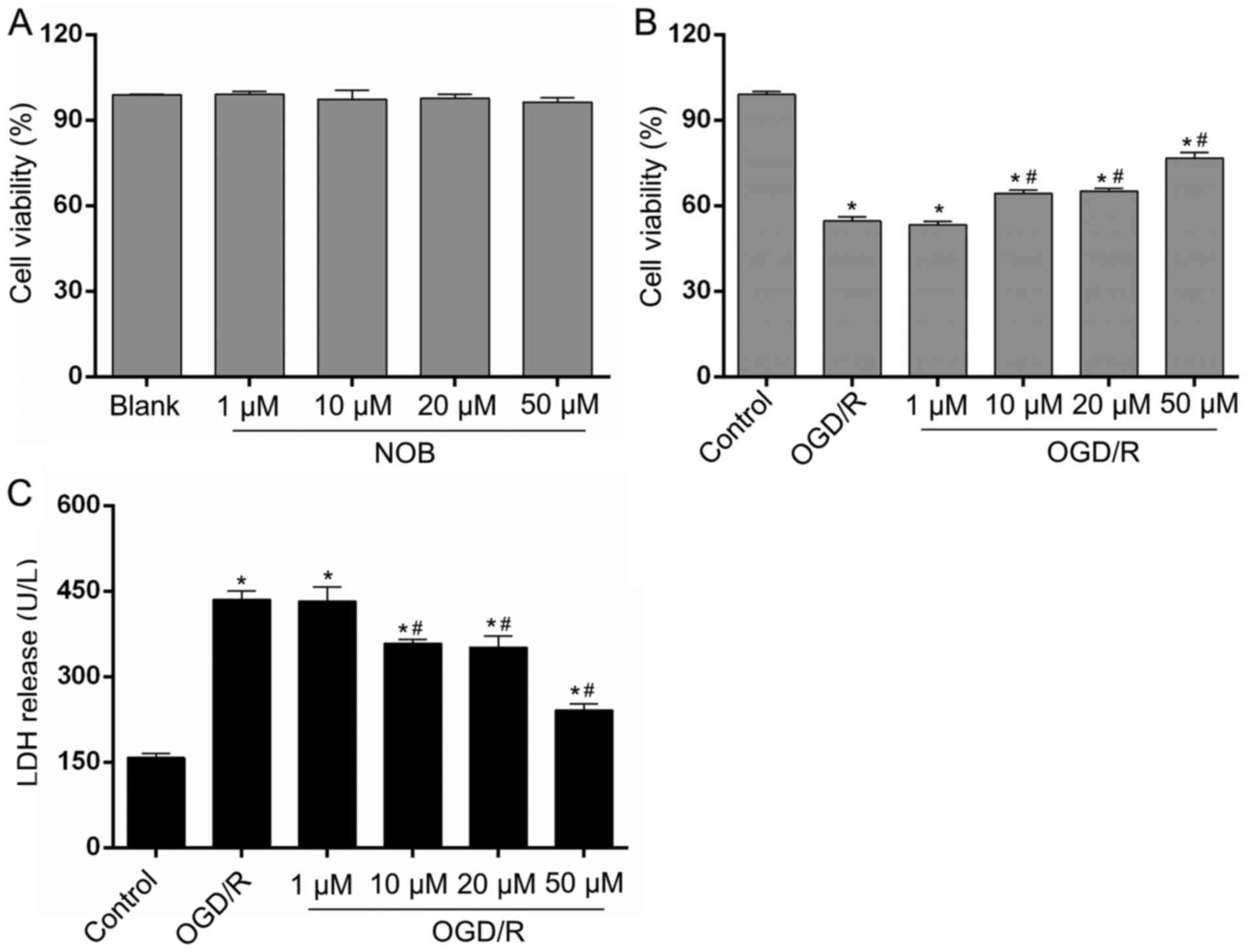 | Figure 1.NOB alleviated the OGD/R injury in
PC12 cells. (A) The cytotoxic effects of NOB (1, 10, 20, and 50 µM)
on PC12 cells were evaluated using a CCK-8 assay. The four
concentration of NOB displayed no cell toxicity on PC12 cells. The
effects of NOB (1, 10, 20, and 50 µM) on the cell viability (B) and
the LDH release (C) after OGD/R insult. Data are expressed as the
mean ± standard deviation (n=3). *P<0.05, compared with the
control; #P<0.05, compared with the OGD/R. NOB,
nobiletin; OGD/R, oxygen-glucose deprivation and reoxygenation;
LDH, lactate dehydrogenase; CCK-8, Cell Counting Kit-8. |
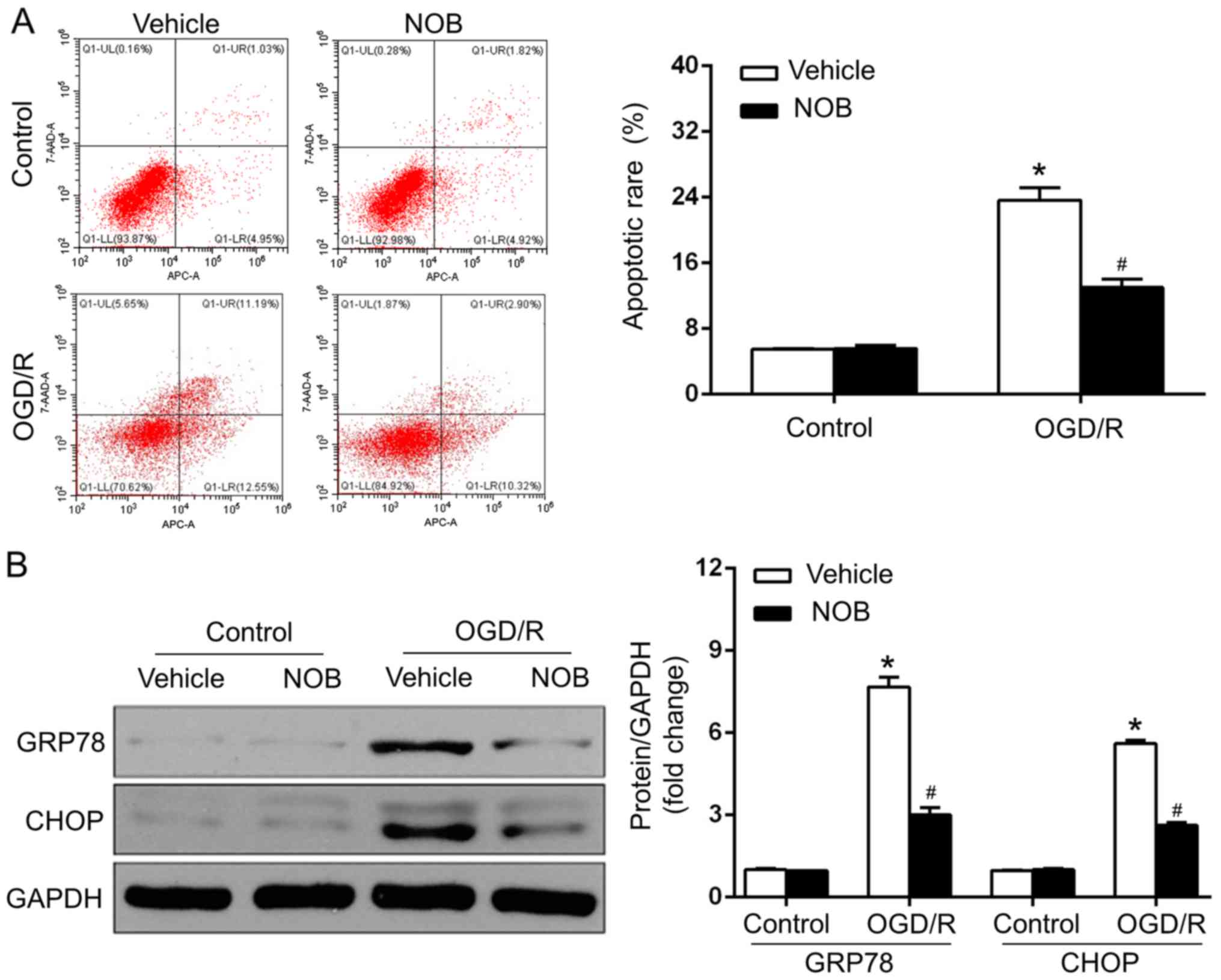
NOB treatment repressed ERS-caused
apoptosis in OGD/R injury
OGD/R injury is closely associated with the
stimulation of ERS-caused apoptosis, and NOB has been reported to
mediate the process of ERS (1–5,13). GRP78, as an ER chaperone, is pivotal
in cell apoptosis in cerebral OGD/R, and ERS drives apoptosis, in
particular, through the CHOP-dependent pathway (1–5). To
estimate whether NOB (50 µM) affected the neuronal OGD/R injury in
an ERS-associated manner, the apoptosis of PC12 cells was measured
by a flow cytometric assay, and the protein levels of ERS markers,
such asGRP78 and CHOP. were determined by western blotting. The
results demonstrated that the apoptotic rate (Fig. 2A) and GRP78/CHOP levels (Fig. 2B) did not differ between the
vehicle-control and NOB-control groups. In addition, OGD/R-suffered
PC12 cells with vehicle treatment exhibited marked increases in
apoptosis and in the GRP78/CHOP expression, compared with those in
the control group. In contrast, NOB treatment led to the mitigation
of ERS, as reflected by the dramatic decreases in apoptosis and in
the GRP78/CHOP levels, compared with that in the vehicle-treated
cells after OGD/R (Fig. 2A and B).
Therefore, NOB treatment represses OGD/R-induced ERS and the
associated apoptosis in PC12 cells.
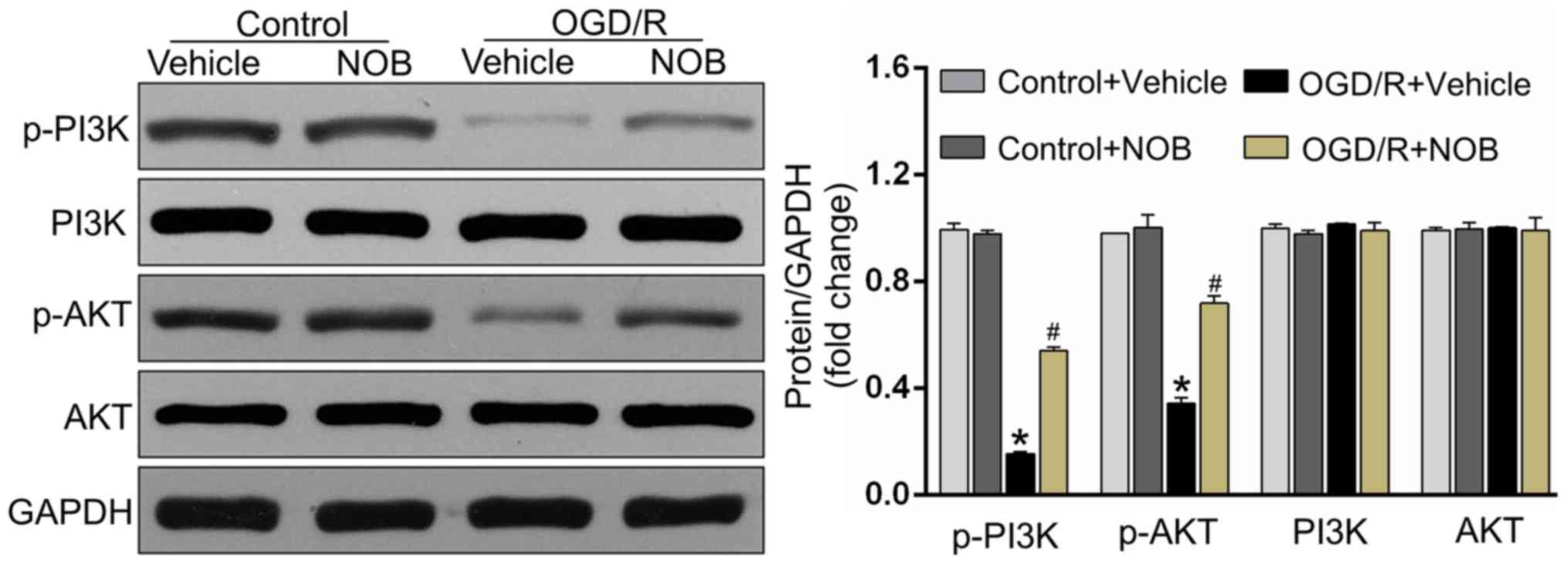
NOB activated the PI3K/AKT signaling
pathways in OGD/R injury
The PI3K/AKT pathway acts as one of the pivotal
pro-survival mediators and is fundamental in reducing apoptosis in
OGD/R-treated PC12 cells (16,20). To
detect whether the PI3K/AKT pathway was involved in the
OGD/R-reducing effects of NOB, the following study detected the
expressions of the PI3K/AKT pathway by Western blotting. As
demonstrated in Fig. 3, OGD/R
significantly repressed the activations of the phosphorylated PI3K
and AKT in PC12 cells relative to the control cells. Importantly,
the drastic increases of the level of p-PI3K/AKT were detected in
NOB-treated cells but not in vehicle-operated cells undergoing
OGD/R stimulation. Meanwhile, there displayed no significant
difference in the p-PI3K/AKT levels between the NOB-treated control
and the vehicle-treated control.
The PI3K/AKT inhibition blunted the
OGD/R-repressing effects of NOB
To further examine if the PI3K/AKT signaling acted
as a causative roles in the anti-OGD/R effect of NOB, the PC12
cells were subjected to a specific PI3K/AKT inhibitor (LY294002)
before the OGD/R insult. As expected, OGD/R-induced cell damages
and ERS-induced apoptosis were restored by LY294002, as reflected
by the reinforced LDH activity (Fig.
4B), apoptosis (Fig. 4C) and
GRP78/CHOP levels (Fig. 4D) and the
repressed cell viability (Fig. 4A).
Importantly, NOB could not further reverse these alternations
following treatment with LY294002 during OGD/R insult (Fig. 4A-D). Of note, no significant
difference were observed in these changes in the presence of
LY294002 with or without NOB treatment. Together, these findings
demonstrated that NOB ameliorates ERS-caused apoptosis in PC12
cells following OGD/R injury through activating the PI3K/AKT
pathway.
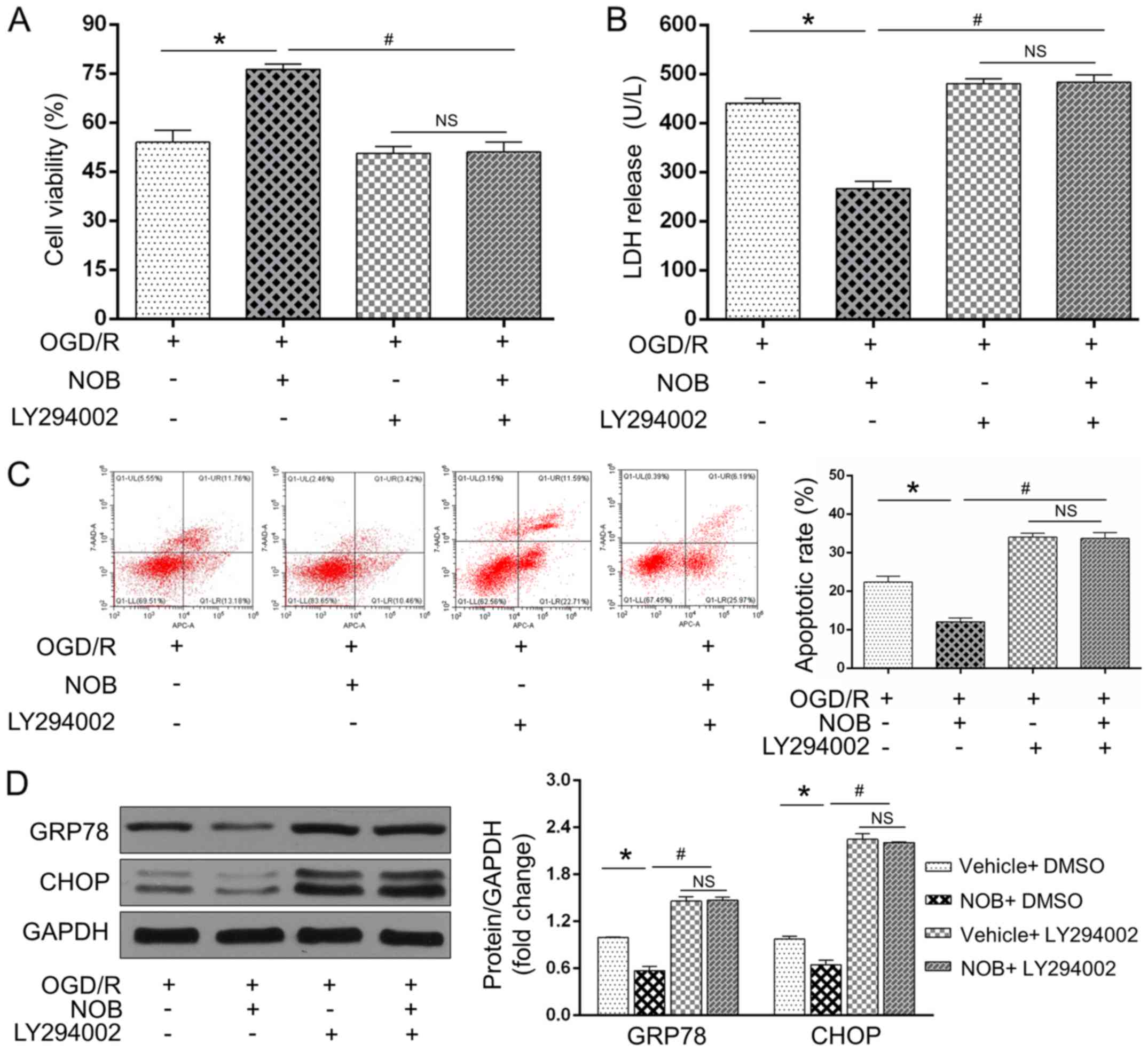 | Figure 4.The PI3K/AKT inhibition blunted the
OGD/R-repressed effects of NOB. (A) Cell viability and (B) LDH
release were detected using a CCK-8 assay and ELISA, respectively,
in the PC12 cells with or without LY294002 during OGD/R injury. The
apoptotic cells were examined by (C) flow cytometry, and GRP78/CHOP
expression levels were detected by (D) western blotting. Data are
expressed as the mean ± standard deviation (n=3). *P<0.05,
compared with the OGD/R; #P<0.05, compared with the
NOB-OGD/R. NS, not significant; NOB, nobiletin; OGD/R,
oxygen-glucose deprivation and reoxygenation; PI3K,
phosphatidylinositol 3-kinase; AKT, serine/threonine kinasel; LDH,
lactate dehydrogenase. |
Discussion
The pathophysiology of neuronal I/R injury is
intimately implicated in the stimulation of ERS and subsequent
apoptosis (1–5). A flavonoid, NOB, possesses anti-ERS and
anti-apoptotic properties (11,13).
However, the knowledge of the beneficial contributions of NOB on
neuronal I/R injury and the underlying mechanisms are still
lacking. Our studies herein proved that pretreatment with NOB
significantly reduced neuronal damages and impacted ERS-caused
apoptosis, thus lowering the susceptibility of PC12 cells to OGD/R
injury in vitro. This appealing concept, largely developed
from a series of novel evidences, was exhibited by the following:
i) First, NOB administrations followed by OGD/R injury rescued cell
viability and depressed LDH release in a dose-dependent manner in
PC12 cells; ii) next, NOB treatment prior to OGD/R injury reversed
apoptosis in concert with reductions of ERS-related markers
(CHOP/GRP78); iii) then, NOB pretreatment potentiated the PI3K/AKT
pathway during OGD/R, while blockage of the PI3K/AKT axis offset
the neuro-protective and ERS-repressing effects of NOB, in
particular. To the best of our knowledge, this is the first data
that has linked the anti-I/R roles of NOB to a PI3K/AKT-associated
and ERS-dependent approach, which supports that NOB is a promising
therapeutic option to mitigate cerebral I/R injury.
NOB is a ubiquitous bioflavonoid and polyphenolic
ingredient isolated from the peels of citrus fruit (9–12). It
has been emphasized that NOB exerts pleiotropic biological
activities, including the anti-inflammation, anti-tumor and
cardiovascular-protections (9–13). Of
note, a previous study revealed the ability of NOB to repress the
apoptotic process in I/R-exposed Kupffer cells after liver
transplantation (10). Recently,
Zhang et al provided evidence that NOB protects the
myocardium against pressure overload-caused hypertrophy and
fibrosis, in which ERS-initiated apoptosis has been substantially
implicated (13). However, there is
a paucity of data regarding whether NOB slows the progression of
cerebral I/R injury, particularly in an apoptosis-limited manner.
Based on prior investigations, we thus hypothesized that NOB may
act on neuronal PC12 cells and render anti-apoptotic effect in
response to OGD/R injury. Accordingly, our study found that NOB
exerts beneficial contributions on injured PC12 cells, which
expanded the potential activity of NOB in protecting against
cerebral I/R injury.
Timely and successful revascularization serves as
the cornerstone for the treatment of ischemic stroke (1–5).
Nevertheless, the ischemic brain is susceptible to secondary
reperfusion injury, which minimizes the benefits of blood
re-establishment itself (1–5). In spite of considerable advances in the
elucidation of the molecular mechanisms of cerebral I/R injury,
there is still a lack of efficient methods to eliminate the
detrimental outcomes of this injury. The investigations of more
effective drugs or interventions to protect neurons from I/R injury
are required to generate greater clinical benefits (1–5). Plenty
of studies have verified that the ERS-regulated apoptotic cascade
acts as the central etiology in the pathogenesis of cerebral I/R
injury, in which this cascade triggers cell death irrespective of
mitochondria-dependent and death receptor-relevant apoptosis
(1). The ER is an endogenous
self-protective organelle responsible for cellular protein
biosynthesis, folding and transportation (1–5).
Adaptive ER stress helps in the obliteration of mis-folded
proteins, namely, via the UPR. However, sustained over-activation
of ERS induced by OGD/R provokes the above process upon adaption
failing and drives ERS-related apoptotic cascade events
subsequently. In this way, ERS substantially switches from a
protective response to a pro-apoptotic process with cerebral I/R
progression (1–5). There exist numerous ERS-related
mediators that have been implicated in cerebral I/R injury, and
GRP78/CHOP essentially determine the initiation and extension of
ERS-caused apoptosis (1–5). As was set forth, punctual interventions
that focuse on prolonged ERS appear to be a promising way to blunt
cerebral I/R lesions, despite the current limitations in lowering
I/R impairments. It has been reported that the alleviation of ERS
and apoptosis yielded by NOB results from the diminishments of CHOP
and GRP78 (13). In line with
previous studies, our findings provided evidences that NOB
supplement at the beginning of OGD/R was available to reduce GRP78
and CHOP expressions, with improvement of cell viability and the
repression of both LDH release and neuronal apoptosis in PC12
cells. NOB has thus garnered attention to further examine the
precise molecular mechanisms in the context of cerebral I/R
injury.
It is well known that the PI3K and AKT pathway is
the important survival mediator in cerebral I/R injury, and
PI3K/AKT deactivation seems to be able to explain neuronal
vulnerability to I/R insult (19–21).
Indeed, the activation of the PI3K/AKT pathway participates in
multiple pathologic processes such as the myocardial and
choriocarcinoma cell I/R injury, in which the compromised
ERS-induced apoptosis has been found to be a predominant factor
underlying these protections (22,23).
However, there is still a lack of clear evidence derived from
ERS-induced apoptosis during cerebral I/R injury via the
PI3K/AKT-dependent pathway. A previous study confirmed the
neuroprotective effect of PI3K/AKT activation against brain I/R
injury, and its anti-apoptotic contribution was greatly highlighted
(19–21). Intriguingly, Yuan et al
produced clues indicating that the activities of the PI3K/AKT
pathway are causatively involved in the anti-I/R effects generated
by cerebral ischemic postconditioning in an ERS-related way, thus
leading to GRP78/CHOP repression and apoptosis reduction (24). In the current study, we suggested
that NOB treatment antagonized the apoptotic process that was
observed in OGD/R-suffered PC12 cells and promoted protein
expression of p-PI3K/AKT while simultaneously suppressing the ERS
markers CHOP and GRP78. Additionally, by applying an PI3K/AKT
inhibitor, it was found that ERS-limited and OGD/R-attenuated
outcomes were significantly blocked in PC12 cells. Therefore, these
data suggested the presence of anti-ERS-induced apoptosis in
response to the PI3K/AKT stimulation during cerebral I/R, and more
importantly, NOB lower ERS-caused neuronal damages in PC12 cells
via the PI3K/AKT-dependent way, as expected.
In conclusion, the present study demonstrated, for
the first time, that NOB possesses a strong effect against ERS and
subsequent apoptosis in neuronal PC12 cells through the activation
of the PI3K/AKT pathway under OGD/R conditions. Thus, these results
provided novel insights into the molecular mechanisms underpinning
cerebral I/R injury. Although the protective effects of NOB were
merely measured in the in vitro model, our findings raised
the appealing possibility that NOB may represent a novel
therapeutic strategy for cerebral I/R injury. More investigations
will be conducted to evaluate the role of NOB in other pathogenic
mechanism of cerebral I/R injury, such as autophagy, inflammation
and mitochondrial-dependent apoptosis, irrespective of
ERS-associated apoptosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZRL made substantial contributions to the conception
and design of the present study. LY, JZ, YZ and ZNL performed the
experiments including cell culture, OGD/R establishment, apoptotic
detection and western blotting assay, and were involved in data
interpretation manuscript revision.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NOB
|
nobiletin
|
|
ERS
|
endoplasmic reticulum stress
|
|
OGD/R
|
oxygen-glucose deprivation and
reoxygenation
|
|
GRP78
|
glucose-regulated protein-78
|
|
CHOP
|
C/EBP homologous protein
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
AKT
|
serine/threonine kinase
|
References
|
1
|
Gong L, Tang Y, An R, Lin M, Chen L and Du
J: RTN1-C mediates cerebral ischemia/reperfusion injury via ER
stress and mitochondria-associated apoptosis pathways. Cell Death
Dis. 8:e30802017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai XH, Li XC, Jin SW, Liang DS, Wen ZW,
Cao HC, Mei HF, Wu Y, Lin ZD and Wang LX: Endoplasmic reticulum
stress plays critical role in brain damage after chronic
intermittent hypoxia in growing rats. Exp Neurol. 257:148–156.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen
X, Chen J and Bai B: Endoplasmic reticulum stress in cerebral
ischemia. Neurochem Int. 68:18–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiu B, Hu S, Liu L, Chen M, Wang L, Zeng X
and Zhu S: CART attenuates endoplasmic reticulum stress response
induced by cerebral ischemia and reperfusion through upregulating
BDNF synthesis and secretion. Biochem Biophys Res Commun.
436:655–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Park JH, Maharjan S, Park JA,
Choi KS, Park H, Jeong Y, Ahn JH, Kim IH, Lee JC, et al: Sac-1004,
a vascular leakage blocker, reduces cerebral ischemia-reperfusion
injury by suppressing blood-brain barrier disruption and
inflammation. J Neuroinflammation. 14:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwon SK, Ahn M, Song HJ, Kang SK, Jung SB,
Harsha N, Jee S, Moon JY, Suh KS, Lee SD, et al: Nafamostat
mesilate attenuates transient focal ischemia/reperfusion-induced
brain injury via the inhibition of endoplasmic reticulum stress.
Brain Res. 1627:12–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Min HM, Wang Y, Ren DY, Cheng X, Li J,
Jiang XQ, Min LQ and Bao CF: Protective effect of 2-deoxy-D-glucose
on the brain tissue in rat cerebral ischemia-reperfusion models by
inhibiting Caspase-apoptotic pathway. Histol Histopathol. 32:57–67.
2017.PubMed/NCBI
|
|
8
|
Nakka VP, Gusain A and Raghubir R:
Endoplasmic reticulum stress plays critical role in brain damage
after cerebral ischemia/reperfusion in rats. Neurotox Res.
17:189–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sp N, Kang DY, Joung YH, Park JH, Kim WS,
Lee HK, Song KD, Park YM and Yang YM: Nobiletin inhibits
angiogenesis by regulating Src/FAK/STAT3-mediated signaling through
PXN in ER+ breast cancer cells. Int J Mol Sci.
18:E9352017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Y, Zhang W, Li M, Cao D, Yang X and
Gong J: Nobiletin ameliorates ischemia-reperfusion injury by
suppressing the function of Kupffer cells after liver
transplantation in rats. Biomed Pharmacother. 89:732–741. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang N, Yang Z, Xiang SZ, Jin YG, Wei WY,
Bian ZY, Deng W and Tang QZ: Nobiletin attenuates cardiac
dysfunction, oxidative stress, and inflammatory in streptozotocin:
Induced diabetic cardiomyopathy. Mol Cell Biochem. 417:87–96. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yasuda N, Ishii T, Oyama D, Fukuta T,
Agato Y, Sato A, Shimizu K, Asai T, Asakawa T, Kan T, et al:
Neuroprotective effect of nobiletin on cerebral
ischemia-reperfusion injury in transient middle cerebral
artery-occluded rats. Brain Res. 1559:46–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang N, Wei WY, Yang Z, Che Y, Jin YG,
Liao HH, Wang SS, Deng W and Tang QZ: Nobiletin, a polymethoxy
flavonoid, protects against cardiac hypertrophy induced by
pressure-overload via inhibition of NAPDH oxidases and endoplasmic
reticulum stress. Cell Physiol Biochem. 42:1313–1325. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi MD, Liao YC, Shih YW and Tsai LY:
Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways
in HGF-treated liver cancer HepG2 cells. Phytomedicine. 20:743–752.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YC, Cheng TH, Lee JS, Chen JH, Liao
YC, Fong Y, Wu CH and Shih YW: Nobiletin, a citrus flavonoid,
suppresses invasion and migration involving FAK/PI3K/Akt and small
GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell
Biochem. 347:103–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang JF, Zhang L, Shi LL, Zhao ZH, Xu H,
Liang F, Li HB, Zhao Y, Xu X, Yang K and Tian YF: Parthenolide
attenuates cerebral ischemia/reperfusion injury via Akt/GSK-3β
pathway in PC12 cells. Biomed Pharmacother. 89:1159–1165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mo ZT, Li WN, Zhai YR and Gao SY: The
effects of icariin on the expression of HIF-1α, HSP-60 and HSP-70
in PC12 cells suffered from oxygen-glucose deprivation-induced
injury. Pharm Biol. 55:848–852. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Zhu X, Chen M, Ge Q, Shen Y and Pan
S: Resveratrol protects PC12 cells against OGD/R-induced apoptosis
via the mitochondrial-mediated signaling pathway. Acta Biochim
Biophys Sin (Shanghai). 48:342–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang ZG, Cheng Y, Yu XC, Ye LB, Xia QH,
Johnson NR, Wei X, Chen DQ, Cao G, Fu XB, et al: bFGF protects
against blood-brain barrier damage through junction protein
regulation via PI3K-Akt-Rac1 pathway following traumatic brain
injury. Mol Neurobiol. 53:7298–7311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Ren Z, Wei X, Wang S, Wang Y,
Cheng Y, Gao H and Liu H: Losartan protects against cerebral
ischemia/reperfusion-induced apoptosis through β-arrestin1-mediated
phosphorylation of Akt. Eur J Pharmacol. 815:98–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Q, An R, Tian X, Yang M, Li M, Lou
J, Xu L and Dong Z: β-caryophyllene pretreatment alleviates focal
cerebral ischemia-reperfusion injury by activating PI3K/Akt
signaling pathway. Neurochem Res. 42:1459–1469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Wang Y, Ye J, Lu X, Cheng Y, Xiang
L, Chen L, Feng W, Shi H, Yu X, et al: bFGF attenuates endoplasmic
reticulum stress and mitochondrial injury on myocardial
ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J
Cell Mol Med. 19:595–607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yung HW, Korolchuk S, Tolkovsky AM,
Charnock-Jones DS and Burton GJ: Endoplasmic reticulum stress
exacerbates ischemia-reperfusion-induced apoptosis through
attenuation of Akt protein synthesis in human choriocarcinoma
cells. FASEB J. 21:872–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan Y, Guo Q, Ye Z, Pingping X, Wang N
and Song Z: Ischemic postconditioning protects brain from
ischemia/reperfusion injury by attenuating endoplasmic reticulum
stress-induced apoptosis through PI3K-Akt pathway. Brain Res.
1367:85–93. 2011. View Article : Google Scholar : PubMed/NCBI
|