Introduction
Spinal cord injury (SCI) is a traumatic event, often
resulting in permanent motor and sensory deficits such as
paraplegia and tetraplegia (1). It
has been estimated that between 250,000 and 500,000 people sustain
a SCI each year worldwide (2). This
debilitating condition results in significant physical and
emotional burden to a substantial number of affected individuals,
and also imposes high economic costs to healthcare systems
(3). As the consequences of SCI are
severe, intense research to elucidate the underlying
pathophysiological mechanisms and to discover potential therapeutic
strategies are in demand.
The biological processes resulting in traumatic SCI
may be categorized into primary and secondary injury, followed by
regeneration and functional recovery (4). Typically, the primary injury of the
spinal cord resulting from contusion or compression is a local,
segmental-limited damage, and the secondary expansive phase results
in further destruction of neuronal and glial cells, in addition to
invasive degeneration of the surrounding spinal cord tissue
(5). The repair capacity of the
central nervous system is limited due to decreased intrinsic growth
capacity and a non-permissive environment for axonal elongation,
while regenerative processes are hindered by different factors,
such as inhibitory growth factors and the glial scar at the site of
the lesion (1). Recent neuroscience
advances have facilitated the prevention and cure for the
debilitating effects of SCI, and neuroprotection/neuroregeneration
approaches to promote axonal sprouting is a promising form of
therapy (2). An increasing body of
evidence has suggested that the predominant glial cell type
reactive astrocytes can provide essential activities that protect
tissue and preserve function after SCI (3). In addition, Davies et al
(4) found that transplantation of
specific human astrocytes could promote functional recovery after
SCI. However, the role of astrocytes has yet to be fully
elucidated.
Tacrolimus (FK506), an FDA-approved
immunosuppressive agent, is widely used to prevent the acute
rejection of allograft transplants after transplantation (5). Recently, FK506 has been reported to
exhibit both neuroprotective and neuroregenerative properties for
the treatment of peripheral nerve injuries (6). Furthermore, FK506 is able to enhance
axonal regeneration and improve functional recovery in animal
models after SCI (7). However, the
definite mechanisms of FK506 in terms of its neuroprotective and
neuroregenerative action have yet to be elucidated.
Wildering et al (8) previously demonstrated that an epidermal
growth factor (EGF) homolog could promote axonal regeneration,
substantiating existing molecular evidence that has suggested that
the EGF family is involved in peripheral nerve regeneration.
Astrocytes produce a large array of neurotrophic factors, including
EGF (9). Thus, the aim of the
present study was to assess whether FK506 is able to enhance axonal
regeneration and improve functional recovery by activating
astrocytes, and to further investigate the possible mechanisms of
action. For this purpose, a rat model of SCI was established.
Functional recovery, EGF expression levels and the length of
neuronal cells were assessed following the treatment of rats with
FK506.
Materials and methods
Animals grouping and FK506
preparation
Male Sprague-Dawley rats (n=56; age, 10 weeks;
weight, 280–320 g) were purchased from BetterBiotechnology Co.,
Ltd. (Nanjing, China) and used in the present study. The animals
were housed under a 12-h dark/light cycle at a temperature between
23 and 28°C, and had free access to food pellets and water. All
animal experiments were approved by the Animal Care and Research
Committee of the Nanjing Medical University (Nanjing, China). The
rats were randomly divided into the K506 treatment or control group
(n=28 per group) by simple random sampling without replacement
approach. The rats in each group were then separated into different
subgroups depending on the day of sacrifice with a lethal dose of
sodium pentobarbital (days 1, 3, 7 and 14; four rats per day; 100
mg/kg; Shanghai Chemical Reagent Co., Ltd., Shanghai, China)
followed by cardiac perfusion with heparinized saline and 4%
paraformaldehyde, and used for functional recovery evaluation
(n=12).
A total of 5 mg FK506 (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was dissolved in 500 µl dimethyl
sulfoxide (DMSO; Sigma-Aldrich) and stored at −20°C until use. The
final concentration of DMSO was maintained at ≤0.1%.
Surgical procedures
Acute SCI was induced as previously reported using
the New York University (NYU) weight-drop device (10). Briefly, after anesthesia by
intraperitoneal administration of 10% chloral hydrate (400 mg/kg;
Sigma-Aldrich), the spinal cords at the T10 level were exposed
after laminectomy and subjected to a weight-drop impact of a 10-g
rod falling from a height of 25 mm with the NYU impactor (11) to produce a moderate SCI model. Next,
the muscles and skin were sewn in layers. After 30 min, 0.5 mg/kg
FK506 was administered intravenously to the rats. Rats in the
control group were administered the equivalent dose of normal
saline. In addition, the bladders of these rats were emptied
manually twice a day.
Functional recovery evaluation
The open-field locomotion test was used to evaluate
the functional recovery after SCI. It was observed by two blinded
independent investigators and scored using the standardized Basso,
Beattie and Bresnahan (BBB) locomotor scoring system (12). BBB scores range between 0 (flaccid
paralysis) and 21 (normal gait). Prior to testing, rats were
acclimatized to the testing environment (90-cm diameter plastic
wading pool; 4 cm in height). The test was performed prior to
surgery and on days 3, 7, 14, 21, 28, 35 and 42 post-operation. BBB
scores were averaged for each group by both examiners.
Immunohistochemical analysis
The rats were sacrificed on days 1, 3, 7 and 14
after injury, followed by cardiac perfusion with heparinized saline
and 4% paraformaldehyde in 0.1 M phosphate buffer. Subsequent to
perfusion, the T10 region of the spinal cord was removed and fixed
in 4% paraformaldehyde overnight at 4°C. Then, the tissues were
cryoprotected with 20% and then 30% sucrose in phosphate-buffered
saline (PBS). Tissues were frozen on dry ice and cryosectioned at 8
µm using a microtome cryostat (Leica model CM1850; Leica
Microsystems, Inc., Buffalo Grove, IL, USA), and the 8 µm sections
were collected on Superfrost Plus glass slides. For the
immunohistochemical reactions, sections were rehydrated in 0.1 M
PBS, permeabilized with 0.2% Triton X-100 for 5 min, washed twice
with PBS, blocked with 5% bovine serum albumin (BSA) in PBS for 30
min at room temperature, and subsequently incubated with the
monoclonal antibody against glial fibrillary acidic protein (GFAP;
anti-GFAP-mouse-IgG; cat. no. sc-65343) and anti-EGF-rabbit-IgG
(cat. no. sc-03; both 1:100; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at 4°C. After washing 3 times for 5 min
in PBS, the samples were incubated with anti-mouse-IgG-Alexa 488
and anti-rabbit-IgG-Alexa 568 (both 1:500; Santa Cruz
Biotechnology, Inc.) dissolved in PBS for 1 h in the dark at 24°C.
Finally, the nuclei were stained with DAPI (5 mg/ml; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 5 min at room
temperature. The slides were observed under a Zeiss Axio ImagerA1
fluorescence microscopy (Carl Zeiss AG, Oberkochen, Germany) with
an attached color camera (Evolution™ MP; Media
Cybernetics, Inc., Rockville, MD, USA). The control slides
consisted of omitting the incubation with the primary antibody, and
no reactivity was observed.
Astrocytes cell culture
Primary cultures of rat spinal cord astrocytes were
prepared from two-day old Sprague-Dawley rats as previously
described, with modifications (13).
Briefly, the meninges were carefully removed and spinal cords were
dissected under sterile conditions. Spinal cords were dissociated
in 0.25% trypsin for 5 min at 37°C, and the digestion was
terminated with 1 ml fetal bovine serum (FBS). Then, the cell
suspension was centrifuged at 200 × g for 5 min at room
temperature. The cells were cultured in 30-mm Petri dishes at 37°C
in a 5% CO2 humidified atmosphere. After the
conventional trypsinization procedure, cells were seeded into 60-mm
Petri dishes and cultured until they reached confluence. Prior to
experiments, the purity of astrocytes was >95%, as determined by
immunocytochemistry with the astrocytic marker GFAP.
FK506 treatment and conditioned medium
preparation
After reaching 90–95% confluence, astrocyte
monolayers were washed with PBS, incubated with serum-free
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) for 24 h to allow cells to reach a non-dividing
G0-phase in the cell cycle (14),
then treated with or without 20 µM FK506 (Sigma-Aldrich) in
serum-free DMEM for 24 h. The conditioned media (CM) of the control
group (C-CM) and FK506-treated group (FK506-CM) were collected,
centrifuged at 7,500 × g for 20 min by an Amicon Ultra-4 3K
centrifugal filter device (Merck Millipore) to remove residual
FK506, then diluted to the initial volume with neurobasal medium
(Gibco; Thermo Fisher Scientific, Inc., Grand Island, NY, USA).
Subsequently, the conditioned media were stored at −80°C and used
within one week.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
At 8 h after the administration of FK506, total RNA
was extracted from astrocytes using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
protocol. Single-strand cDNA was synthesized from the total RNA
using the Superscript One-Step RT-PCR system (Invitrogen; Thermo
Fisher Scientific, Inc.). The obtained cDNA was used for RT-qPCR.
RT-qPCR reactions were performed using an ABI Prism 7000 sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc., Foster City, CA, USA) with the GoTaq qPCR Master Mix (Promega
Corporation, Madison, WI, USA). Primer sequences were as follows:
EGF, forward, 5′-CTTAGGGATGTGGGGGACTT-3′ and reverse,
5′-TTGGGCTGTTGGTGTTCCTC-3′ for EGF; GAPDH forward,
5′-TGAACGGGAAGCTCACTGG-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
The qPCR cycling conditions were as follows: Initial denaturation
at 95°C for 10 min, followed by 45 cycles of 15 sec denaturation at
95°C, annealing for 10 sec at 58–60°C, 20 sec extension at 72°C and
a final dissociation phase at 60–95°C. Relative mRNA levels of all
genes were normalized against the levels of GAPDH using the ΔΔCq
method (15). The experiments were
repeated three times.
DNA microarray analysis
Subsequent to treatment with 20 µM FK506 for 8 h,
astrocytes cells were charged and loaded into TRIzol at a density
of 1×106 cells/ml. Shanghai Kangcheng Biological Co.,
Ltd. (Shanghai, China) completed the follow-up experiments of gene
microarray analysis (16). In brief,
RNA was reverse-transcribed to cDNA, labeled with Cy3 dye, and then
subjected to one-color hybridization (17). Following hybridization and washing,
the slides were scanned using the Agilent DNA Microarray scanner
G2505B (Agilent Technologies, Inc., Santa Clara, CA, USA). The
resulting text files extracted by Agilent Feature Extraction
Software (version 9.5.3) were introduced into Agilent GeneSpring GX
software (version 11.0) for further analysis. The microarray
datasets were normalized and differentially expressed genes were
identified through a fold change analysis.
Enzyme-linked immunosorbent assay
(ELISA)
At 24 h post-treatment with FK506, culture media
were collected and assayed for EGF secretion. The collected media
were concentrated with centrifugal filter units (Merck Millipore)
according to the manufacturer's protocol, and cOmplete™,
Mini Protease Inhibitor Cocktail (Roche Applied Science, Penzberg,
Germany) was added to samples, as previously described (18). EGF levels were assessed in triplicate
using the Quantikine® RAT EGF Immunoassays (cat. no.
DEG00; R&D Systems, Inc., Minneapolis, MN, USA), according to
the manufacturer's protocol. Absorbance from colorimetric reactions
was determined by an ELISA reader (Biotek Instruments, Inc.,
Winooski, VT, USA), and normalized to protein content using a
standard curve for serially diluted standard recombinant EGF.
Culture and treatment of primary
neuronal cells
Spinal neurons were cultured as described previously
with modifications (19). Briefly,
the spinal cords of three fetal SD rats (BetterBiotechnology Co.,
Ltd.) were removed on embryonic day 15 (E15), and placed in dishes
containing PBS. After the removal of the meninges, the spinal cords
were dissected and incubated with 0.05% trypsin for 15 min at 37°C.
The digestion was terminated with 15% FBS DMEM/Ham's F12
(Invitrogen; Thermo Fisher Scientific, Inc.). Next, they were
dissociated using a fire-polished Pasteur pipette, centrifuged at
200 × g for 5 min at room temperature, resuspended in DMEM/Ham's
F12 containing 10% FBS, 5% horse serum, 100 U/ml penicillin and 100
µg/ml streptomycin (all Invitrogen; Thermo Fisher Scientific,
Inc.), and plated on poly-L-lysine-coated 35-mm glass bottom dish
at a density of 4×105 cells/ml in a humidified 5%
CO2 atmosphere at 37°C. At 4 h after seeding, the glial
cells were removed by washing with DMEM, and the culture medium was
exchanged for the following: i) Negative control group, neurobasal
medium + 2% B27 (Gibco); ii) positive control group, neurobasal
medium + 2% B27 + 10 ng/ml EGF; iii) C-CM; and iv) FK506-CM or v),
i.e., neutralized CM, consisting of FK506-CM and C-CM incubated in
the presence of anti-EGF neutralizing antibodies (1:100; cat. no.
MAB3214; R&D Systems) for 2 h at 37°C prior to use (20). In addition, to identify whether FK506
was able to directly promote neurite outgrowth, the total neurite
length of spinal cord neurons were cultivated in neurobasal medium
+ 2% B27 + 10 ng/ml EGF at 37°C and treated with 0, 10, 20 and 40
µM. The total neurite length was then measured following 1 and 3
days of culture.
Immunofluorescent staining
The procedure was performed as previously described
(21) with minor modifications.
Briefly, 24 h after incubation with the CM, the neuronal cells were
fixed with 4% paraformaldehyde for 30 min, washed with PBS three
times, permeabilized with 0.05% Triton X-100 for 5 min, blocked
with 5% BSA for 30 min at room temperature after washing, then
incubated with anti-MAP2-mouse-IgG (1:100; cat. no. sc-74422; Santa
Cruz Biotechnology, Inc.) overnight at 4°C. After this incubation,
the samples were extensively washed with PBS and incubated with
goat anti-mouse-IgG-conjugated with Alexa Fluor® 568
(1:100; cat. no. A11004; Invitrogen; Thermo Fisher Scientific,
Inc.) dissolved in 1% BSA for 1 h. Finally, all samples were
stained with DAPI for 5 min, rinsed with PBS, and mounted onto
microscope slides with ProLong Gold antifade reagent (Molecular
Probes; Thermo Fisher Scientific, Inc.). Negative controls were
performed by omitting the primary antibody during staining, and no
reactivity was observed. A total of 40 fields in each group were
photographed using a Laser scanning confocal microscope (Zeiss
LSM710; Carl Zeiss AG) at magnification, ×20, and images were
captured with the ZEN2009 software (version 5.5 SP1; Carl Zeiss AG,
Oberkochen, Germany). Fluorescent images of individual neurons were
obtained.
Neurite outgrowth assay
To determine neurite outgrowth, neurite length was
assessed by measuring the distance from one cell body to the end of
all neurites, in which the final length was considered as the sum
of all neurites measured from the cell body (22). Furthermore, the longest neurite
length was measured from one cell body to the end of the longest
neurite. In all groups, ≥80 randomly selected neurons were
observed, and only fluorescence-positive cells were scored and
analyzed. The neurite length of all neurons in 10 images of each
well was measured using a Zeiss LSM Image Browser software, version
4.2.0.121 (Carl Zeiss AG). The average for four wells was
calculated and recorded as the mean neurite length in each
condition.
Statistical analysis
Data are expressed as the mean ± standard deviation,
and the statistical analysis was performed using SPSS statistical
software (version 13.0; SPSS, Inc. Chicago, IL, USA) by t-tests or
one-way analysis of variance followed by the Bonferroni and
Dunnett's T3 post-hoc multiple group comparison tests. The level of
statistical significance is defined as P<0.05.
Results
FK506 could improve functional
recovery after SCI
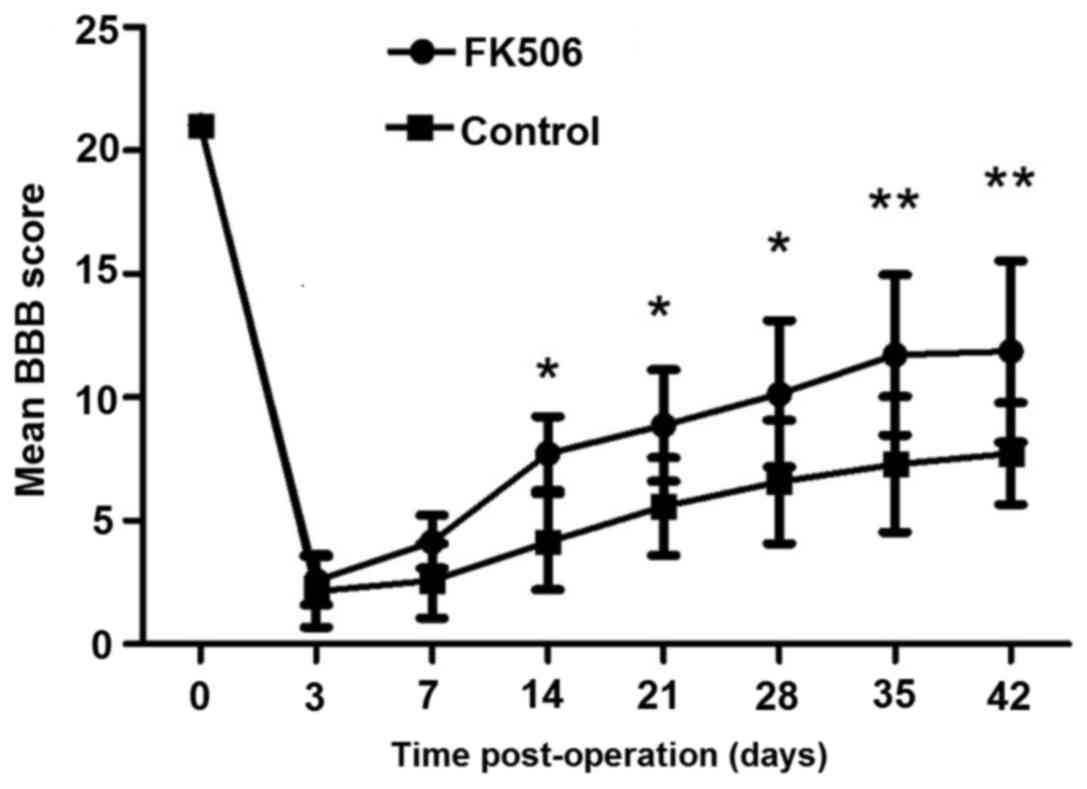
As shown in Fig. 1,
the mean BBB scores of the FK506-treated group and control group
are 21 prior to surgery, indicating normally ambulating rodents.
Mean BBB scores for all groups were recorded 2 to 3 days
post-operation. On day 14 post-operation, the FK506-treated group
showed significantly improved hindlimb performance compared with
the control group (P<0.05). Furthermore, the superior recovery
of FK506-treated group continued throughout the survival period;
with significant improvement in BBB scores at days 35 and 42
post-operation (P<0.01).
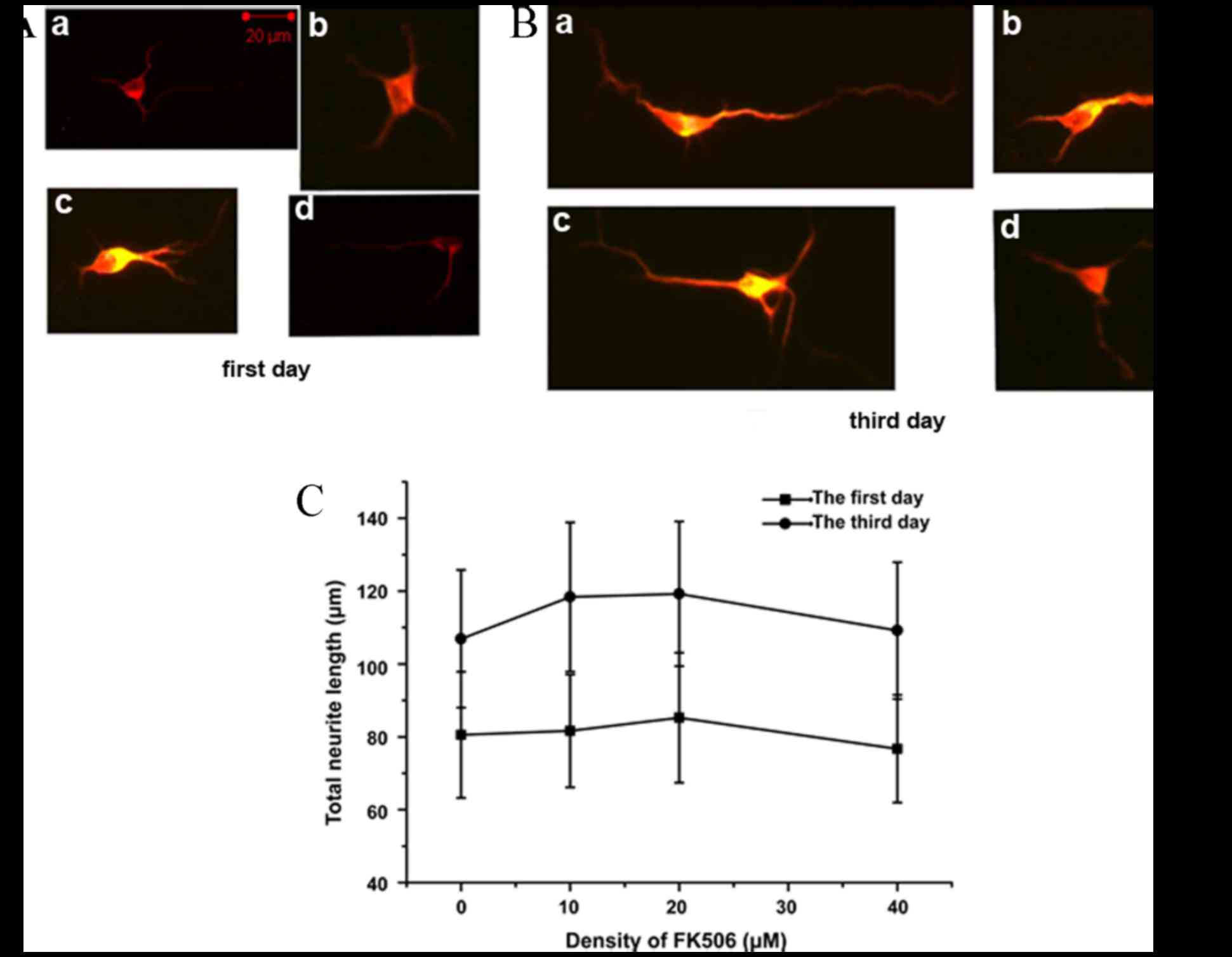
FK506 had no direct promotion effect
on neuronal cells, while EGF promoted neurite outgrowth
To identify whether FK506 could directly promote
neurite outgrowth, the total neurite length of spinal cord neurons
cultivated with 0, 10, 20 and 40 µM FK506 was measured for 1 or 3
days. After the cells were immunostained for MAP2, the neurite
length was assessed and analyzed. FK506 treatment did not
significantly increase the total neurite length on days 1 and 3
compared with the control group in vitro (Fig. 2). The mean total neurite length of
individual neurons cultured in the FK506 treatment group
(81.66±18.31 µm at 10 µM, 85.19±19.56 µm at 20 µM, and 75.32±19.99
µm at 40 µM) was similar to that of the control group (80.52±18.30
µm; P>0.05) on day 1 (Fig. 2C).
Furthermore, the mean total neurite length in the FK506 group was
not significantly different compared with the control group on day
3 (P>0.05; Fig. 2C). The results
indicated that FK506 had no direct effect on nerve cells in
promoting recovery of neurological function.
In order to verify the role of EGF to promote the
growth of neurite, the total neurite length of spinal cord neurons
after being cultivated for 4 days with 10 ng/ml EGF was measured.
The results showed that the total neurite length of individual
neurons cultured with EGF was markedly longer compared with the
control group (Fig. 3).
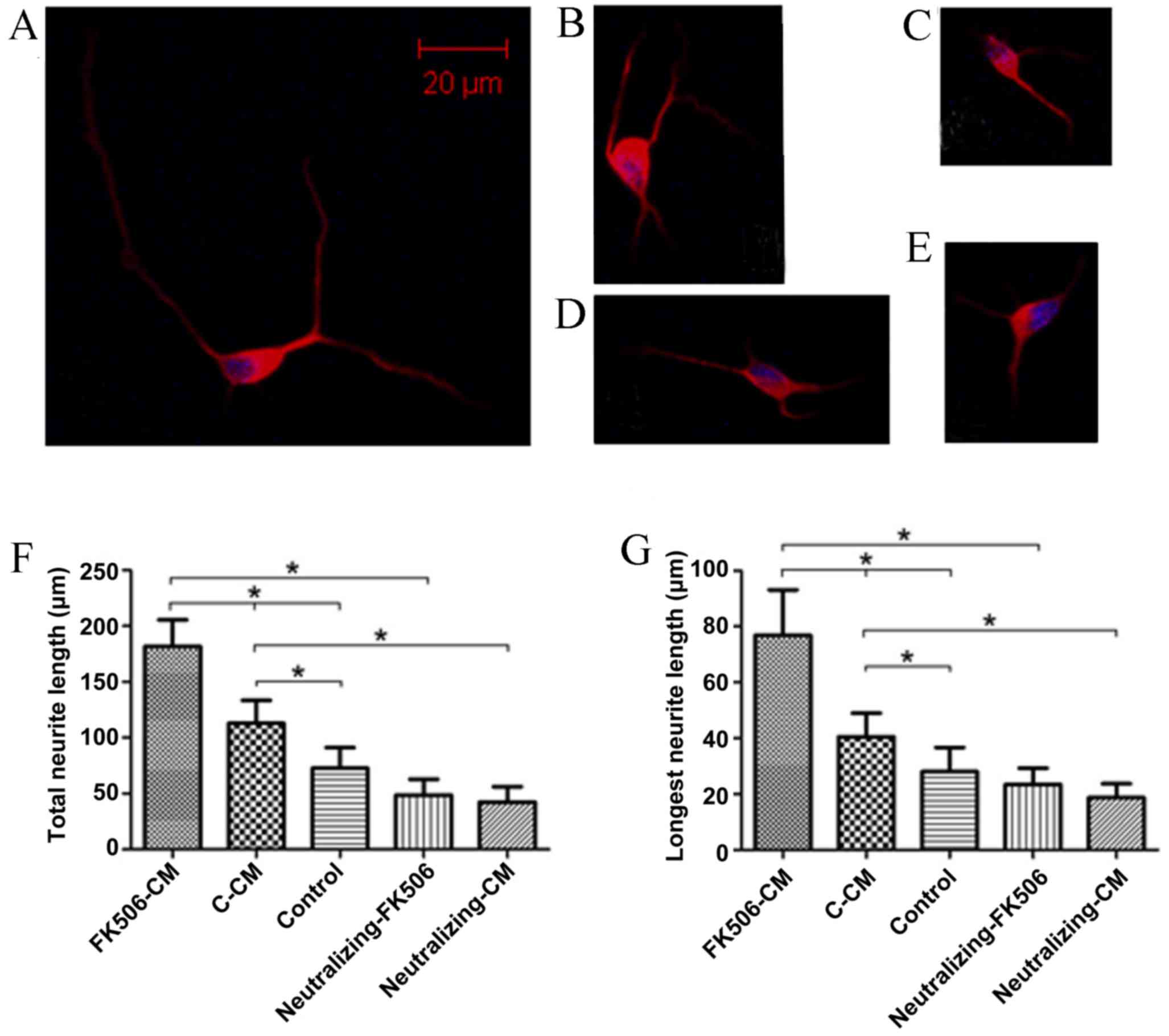
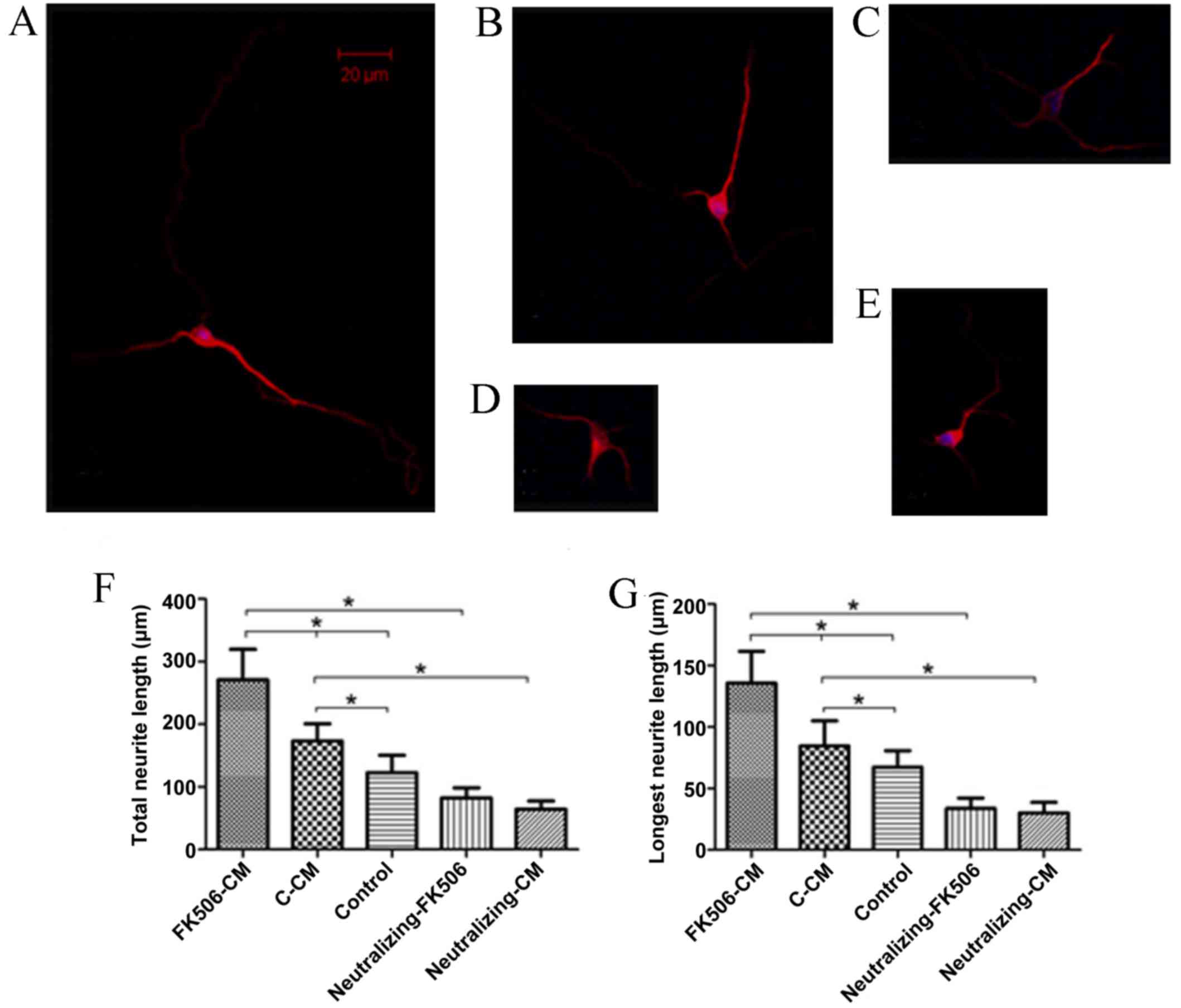
FK506-CM could increase neurite
outgrowth
To investigate the role of astrocytes as mediators
of the neuroprotective effects of FK506, the total and longest
neurite length of spinal cord neurons cultivated with various CM
for 1 and 3 days were measured. After the cells were immunostained
for MAP2, the neurite length was assessed and analyzed. The results
indicated that treatment with FK506-CM induced a 61.06% increase in
total neurite length on day 1 (Fig.
4), and 56.4% on day 3 compared with the C-CM group (Fig. 5). After incubation with CMs for one
day, the mean length of the total neurite of individual neurons
cultured in FK506-CM was 181.79±23.73 µm, which was significantly
longer than those in the C-CM group (112.88±20.48 µm; P<0.01)
and those in the control group (72.68±18.57 µm; P<0.01; Fig. 4F). By contrast, the mean lengths of
total neurites on day 3 were 270.77±48.67, 173.13±27.68 and
122.74±27.84 µm in the FK506-CM, C-CM and control groups,
respectively (P<0.01; Fig. 5F).
Similar results were observed when only the longest neurite was
measured (Figs. 4G and 5G). Thus, analysis of neuronal morphology
revealed a marked increase in the neurite outgrowth when the spinal
neurons were treated with FK506-CM.
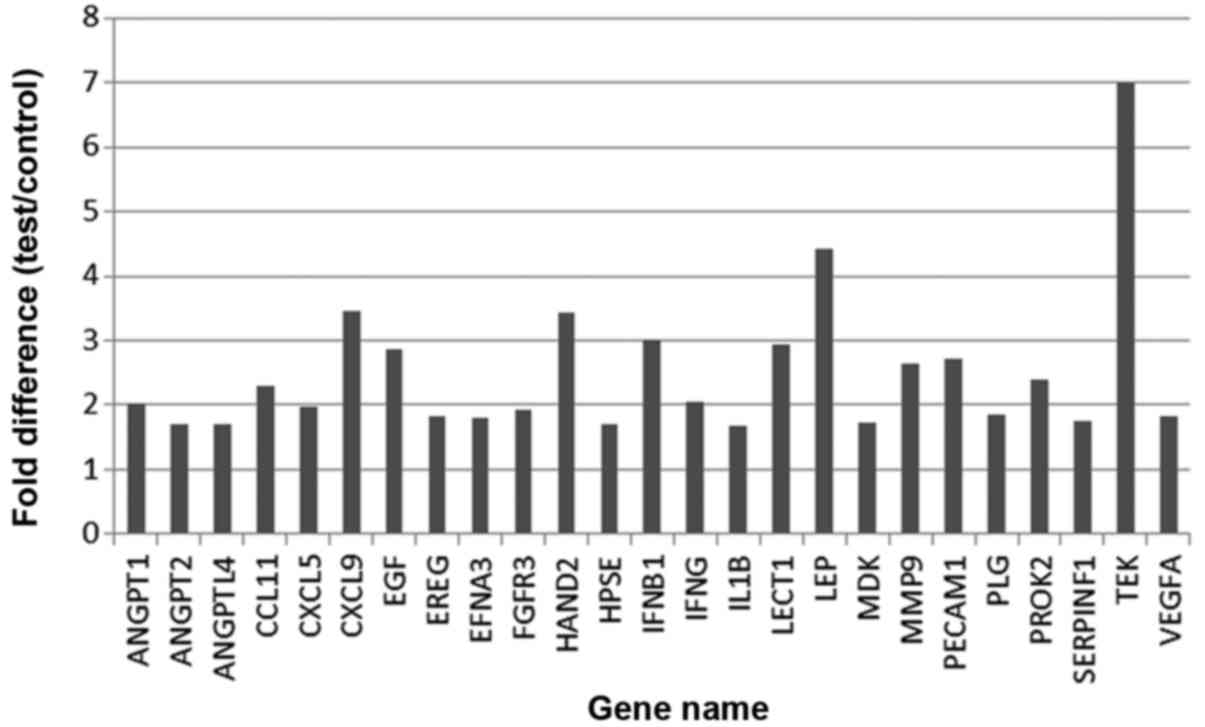
FK506 stimulated astrocyte expression
of EGF in vitro and in vivo
In the present study, gene chip detection of
astrocytes treated with FK506 was performed. The results showed
that a total of 25 significantly upregulated genes were identified
in the astrocytes treated with FK506 (P<0.05). Furthermore, EGF
displayed elevated levels of the cytokines, as shown in Fig. 6.
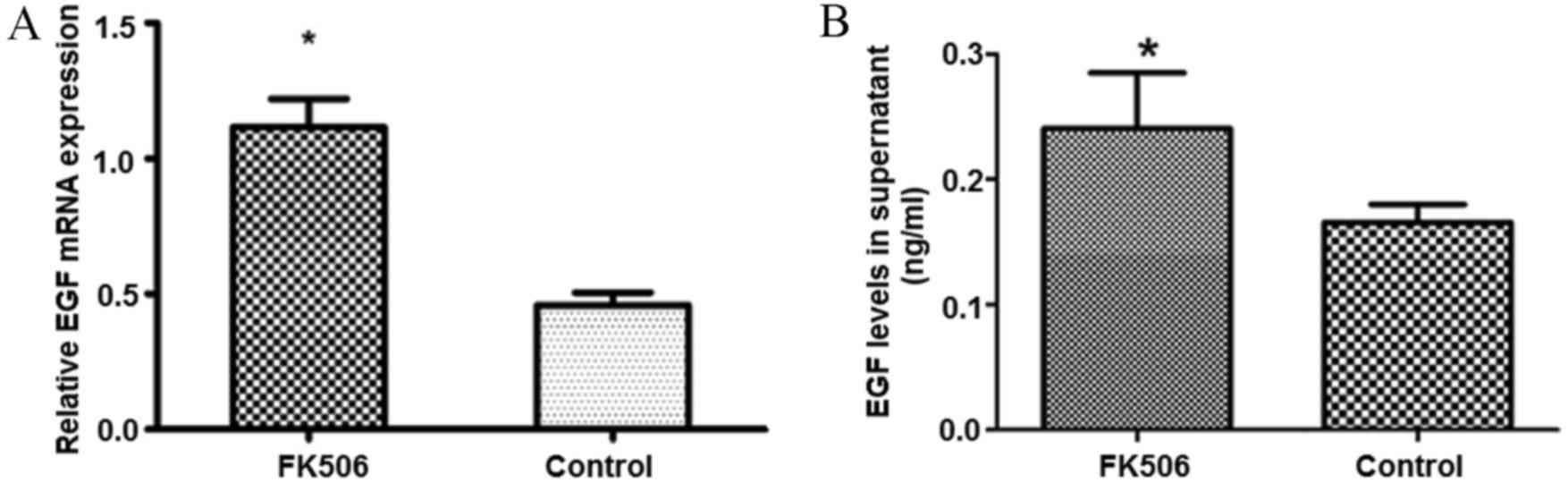
To further examine the effect of FK506 on the
expression levels of EGF, which is produced by astrocytes, total
RNA was extracted from the monolayers of astrocytes, and mRNA
expression levels of EGF were quantitatively evaluated by RT-qPCR,
and EGF protein expression levels were measured by the ELISA
method. The results indicated that the RNA expression levels of EGF
in astrocytes treated with FK506 were 2.4-times higher compared
with those of the control group, and EGF protein levels in the
supernatant were also significantly increased compared with the
control group (P<0.05; Fig.
7).
In addition, the present study examined the effect
of FK506 on the ability of astrocytes to produce EGF in vivo
(Fig. 8). Following a contusion of
the spinal cord, rats were randomly and blindly assigned to the
FK506 (0.5 mg/kg) or vehicle treatment groups. Immunohistochemical
analysis of EGF and GFAP double staining was performed. The results
indicated that sections from FK506-treated groups showed strongly
EGF-immunoreactive astrocytes. These markedly EGF-immunoreactive
astrocytes were predominantly in the vicinity of the lesion 24 h
post-injury, reaching peak levels in the initial 3 days, and
gradually decreasing until day 14 (Fig.
8A, C, E and G). By contrast, the EGF expression levels in the
control group were markedly lower compared with those in the
FK506-treated group (Fig. 8B, D, F and
H). Additionally, the present study found that the level of
EGF-immunoreactive astrocytes in the control group was perceptibly
decreased on the third day compared with those detected at 24 h.
After 7 days, few EGF-positive astrocytes remained in the control
group.
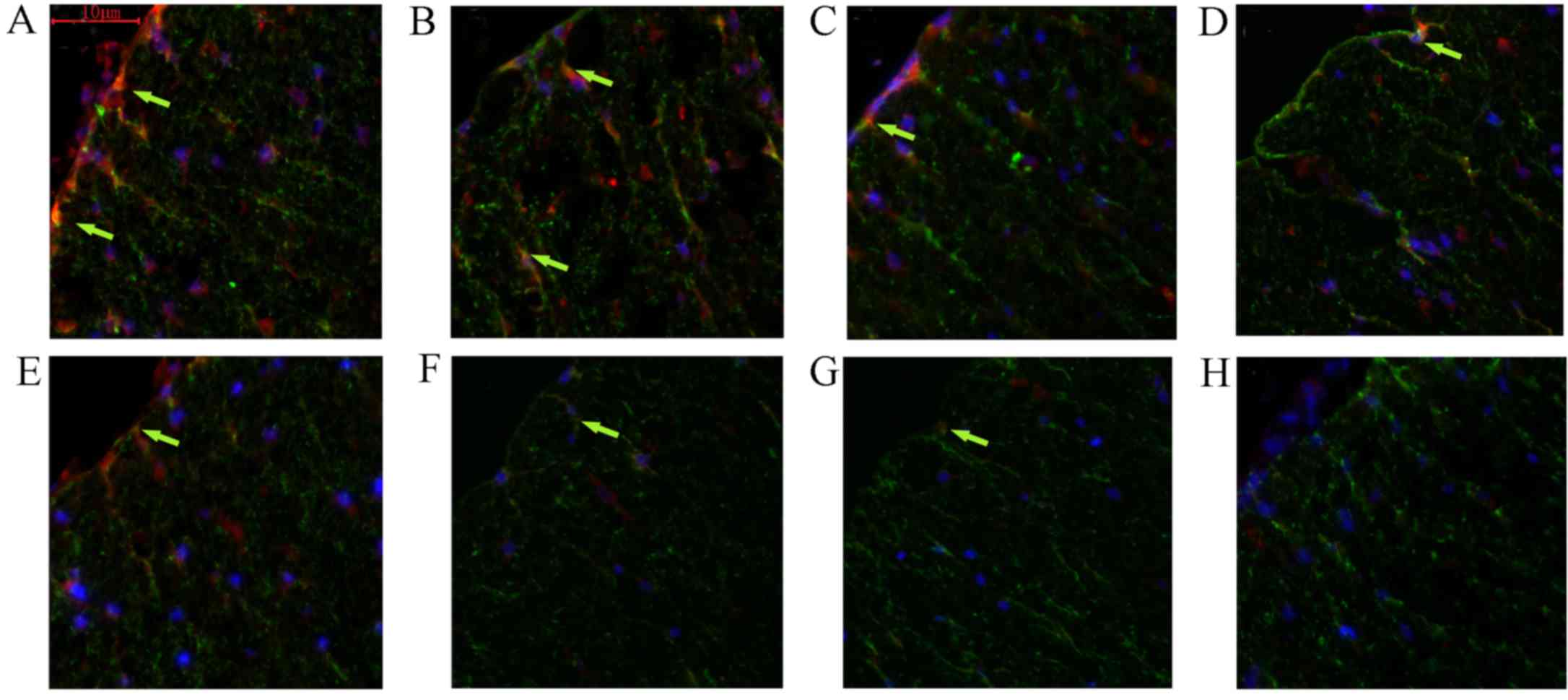 | Figure 8.FK506 modulated astrocyte production
of EGF in vivo. The expression levels of EGF in astrocytes
was markedly increased in rats treated with FK506 on days 1, 3 and
7, compared with those in the normal control. However, EGF
expression was decreased markedly after day 7 post-surgery. FK506
treated groups: (A) day 1; (B) day 3; (C) day 7; (D) day 14; and
control groups: (E), day 1; (F), day 3; (G), day 7; and (H) day 14.
Red indicates immunolabeling for EGF, green for GFAP, blue for
DAPI, and yellow for the double staining with EGF and GFAP. EGF,
epidermal growth factor; GFAP, glial fibrillary acidic protein. |
FK506-CM-induced promotion of
neuritogenesis may be interrupted by EGF neutralizing
antibodies
To further verify the involvement of
astrocyte-derived EGF on neurite outgrowth, embryonic spinal
neurons were cultured in neutralized FK506-CM and neutralized C-CM,
which had been pre-treated with EGF neutralizing antibodies, and
subsequently the length of neurites were analyzed (Figs. 4 and 5). The results demonstrated that the
increase in neurite length was reduced following treatment with
neutralized C-CM and neutralized FK506-CM, compared with the
non-neutralized C-CM and FK506-CM, on days 1 and 3. The total
length of neurites was reduced by 25.71 and 37.46% in the
neutralized FK506-CM and neutralized C-CM groups, respectively on
day 1 compared with the control. For day 3 these values were 30.52
and 36.16%, respectively. The same results were found with respect
to the longest neurite length. Furthermore, to exclude non-specific
inhibitory effects with the solution or preservatives involved in
the antibody preparation, control experiments were performed, which
showed that none of these agents altered neurite elongation (data
not shown). Thus, the results indicated that astrocytic EGF
secretion in response to FK506 treatment serves a significant role
in neurite elongation.
Discussion
In the present study it was found that that FK506
was able to enhance neurite outgrowth and improve the functional
recovery of the spinal cord by stimulating astrocytes to secrete
EGF. This is an indirect effect of FK506 on neuronal cells by an
astrocyte-mediated process.
Numerous studies support the hypothesis that FK506
is able to improve functional recovery and nerve regeneration after
nerve injury (23,24). For instance, López-Vales et al
(25) previously demonstrated that
the administration of FK506 30 min after SCI was effective in
inducing neuroprotection and functional preservation. In accordance
with previous studies, the present results showed that FK506 could
improve locomotor functional recovery of the limbs of rats after
SCI, as quantified by the BBB score. Furthermore, the study by
López-Vales et al showed that repeated treatment with FK506
could improve histologic and functional outcomes to a greater
degree than a single administration of FK506. In addition, a
bell-shaped dose-response curve of FK506 on the rate of axonal
regeneration and on neurite outgrowth has been reported (26,27). The
current study indicated little promotive effect of FK506 on neurite
outgrowth in a human neuronal cell line in vitro with
different doses (10, 20 and 40 µM) for 1 and 3 days, which
suggested that FK506 may have an indirect effect on neurite
outgrowth; however, further investigations are warranted.
Reactive astrogliosis is initiated when trigger
factors produced at the site of injury drive astrocytes to become
activated from their quiescent state (28). The reactive astrocytes are reported
to have a dual role with respect to their overall beneficial or
detrimental effect on neuroprotection, tissue regeneration and
functional recovery (28). A study
by Bush et al (29)
demonstrated that ablation of proliferating astrocytes after SCI
could lead to increased neuronal degeneration and motor deficits.
Therefore, astrocytes responses at the site of injury may
contribute to neuroprotection and functional recovery after SCI.
Additionally, Szydlowska et al (30) demonstrated that FK506 could block the
activation of extracellular signal-regulated kinases 1 and 2
signaling in glutamate-induced death of astrocytes and astrocytic
cell death in vitro and in ischemic brains. We hypothesized
that FK506 could provide a neuroprotective effect by modulating the
activity of astrocytes. Szydlowska et al (31) reported that FK506 may inhibit
glutamate-induced astrocyte death. In addition, a previous study
demonstrated that FK506 could substantially reduce the rise of the
effective concentration of the Ca2+ ionophore in
astrocytes, which is likely to be responsible for their protection
against mitochondrial depolarization and cell death (32). In the present study, spinal neuronal
cells were cultured with FK506-CM, which was the supernatant of
FK506-treated astrocyte culture, and a marked increase in neurite
length was observed. Thus, the results suggested that FK506 may
stimulate astrocytes to secrete certain cytokines, which could
significantly enhance neurite outgrowth.
Furthermore, the results of RT-qPCR and
EGF-neutralizing assessments demonstrated that the level of EGF in
the FK506-CM group is significantly higher than that of the control
group. EGF is a highly mitogenic factor in numerous mammalian cell
types (33), and is able to promote
the proliferation and differentiation of neuronal progenitors,
postmitotic neurons and glial cells in the central nervous system
(34,35). EGF is also an important neurotrophic
factor and can stimulate neurite outgrowth in a previous study
(36). In addition, studies have
reported that EGF can modulate neurite extension by stimulating
thyroid hormones and versican G3 domain (37,38).
Modulation of the expression of glial-derived neurotrophic factor
has been considered as a potential neuroprotective mechanism of
immunophilin ligands (39).
Furthermore, the results in the current study showed that
astrocytes could be stimulated to secrete EGF by treatment with
FK506, which is a potent neurotrophic factor and could enhance
neurite outgrowth in neuronal cell lines. In addition, the
neuroprotective action of EGF in the FK506-CM group is interrupted
by EGF neutralizing antibodies. Thus, we suggest that the
astrocytic EGF secretion in response to FK506 treatment serves an
important role in the neuroprotective effect of FK506.
In conclusion, the present study demonstrated that
FK506 has neuroprotective activity in repairing SCI by stimulating
astrocytes to secrete EGF. However, the current study only reported
the effects of the administration of FK506 within 30 min post-SCI
at a single dose. Further experiments and studies are required to
examine the consequence of repeated treatment with different doses
of FK506. Furthermore, the present study was not able to elucidate
the concrete mechanism underlying EGF-induced neurite outgrowth,
and further efforts are warranted to clarify this mechanism in
future investigations. Thus, the present study may provide insights
into the complexity of cell-cell interactions during SCI, and also
demonstrated a potential effective treatment strategy for SCI,
based on the promotion of neural repair and functional
recovery.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81171694).
References
|
1
|
Ibarra A and Martiñón S: Pharmacological
approaches to induce neuroregeneration in spinal cord injury: An
overview. Curr Drug Discov Technol. 6:82–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baptiste DC and Fehlings MG: Update on the
treatment of spinal cord injury. Prog Brain Res. 161:217–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faulkner JR, Herrmann JE, Woo MJ, Tansey
KE, Doan NB and Sofroniew MV: Reactive astrocytes protect tissue
and preserve function after spinal cord injury. J Neurosci.
24:2143–2155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davies S, Shih CH, Noble M, Mayer-Proschel
M, Davies JE and Proschel C: Transplantation of specific human
astrocytes promotes functional recovery after spinal cord injury.
PLoS One. 6:e173282011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Staatz CE and Tett SE: Clinical
pharmacokinetics and pharmacodynamics of tacrolimus in solid organ
transplantation. Clin Pharmacokinet. 43:623–653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klettner A and Herdegen T: FK506 and its
analogs-therapeutic potential for neurological disorders. Curr Drug
Targets CNS Neurol Disord. 2:153–162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sosa I, Reyes O and Kuffler D:
Immunosuppressants: Neuroprotection and promoting neurological
recovery following peripheral nerve and spinal cord lesions. Exp
Neurol. 195:7–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wildering WC, Hermann PM and Bulloch AG:
Lymnaea epidermal growth factor promotes axonal regeneration in CNS
organ culture. J Neurosci. 21:9345–9354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liberto CM, Albrecht PJ, Herx LM, Yong VW
and Levison SW: Pro-regenerative properties of cytokine-activated
astrocytes. J Neurochem. 89:1092–1100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuh SU, Cho YE, Yoon DH, Kim KN and Ha Y:
Functional recovery after human umbilical cord blood cells
transplantation with brain-derived neutrophic factor into the
spinal cord injured rat. Acta Neurochir (Wien). 147:985–992. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Young W: Spinal cord regeneration.
Science. 273:4511996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang A, Zhang J, Sun P, Yao C, Su C, Sui
T, Huang H, Cao X and Ge Y: EIF2alpha and caspase-12 activation are
involved in oxygen-glucose-serum deprivation/restoration-induced
apoptosis of spinal cord astrocytes. Neurosci Lett. 478:32–36.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iyer VR, Eisen MB, Ross DT, Schuler G,
Moore T, Lee JC, Trent JM, Staudt LM, Hudson J Jr, Boguski MS, et
al: The transcriptional program in the response of human
fibroblasts to serum. Science. 283:83–87. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu N, Li H, Liu K, Yu J, Cheng M, De W,
Liu J, Shi S, He Y and Zhao J: Differential expression of genes and
proteins associated with wool follicle cycling. Mol Biol Rep.
41:5343–5349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai J, Zhao XL, Liu AW, Nian H and Zhang
SH: Apigenin inhibits hepatoma cell growth through alteration of
gene expression patterns. Phytomedicine. 18:366–373. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gezginci-Oktayoglu S, Karatug A and
Bolkent S: The relation among NGF, EGF and insulin is important for
triggering pancreatic β cell apoptosis. Diabetes Metab Res Rev.
28:654–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han SS, Kang DY, Mujtaba T, Rao MS and
Fischer I: Grafted lineage-restricted precursors differentiate
exclusively into neurons in the adult spinal cord. Exp Neurol.
177:360–375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gomes FC, Maia CG, de Menezes JR and Neto
VM: Cerebellar astrocytes treated by thyroid hormone modulate
neuronal proliferation. Glia. 25:247–255. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stipursky J and Gomes FC: TGF-beta1/SMAD
signaling induces astrocyte fate commitment in vitro: Implications
for radial glia development. Glia. 55:1023–1033. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spohr E TC, Dezonne RS, Rehen SK and Gomes
FC: Astrocytes treated by lysophosphatidic acid induce axonal
outgrowth of cortical progenitors through extracellular matrix
protein and epidermal growth factor signaling pathway. J Neurochem.
119:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voda J, Yamaji T and Gold BG:
Neuroimmunophilin ligands improve functional recovery and increase
axonal growth after spinal cord hemisection in rats. J Neurotrauma.
22:1150–1161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh C, Bowers D and Hadlock TA: Effect of
FK506 on functional recovery after facial nerve injury in the rat.
Arch Facial Plast Surg. 9:333–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
López-Vales R, García-Alías G, Forés J,
Udina E, Gold BG, Navarro X and Verdú E: FK506 reduces tissue
damage and prevents functional deficit after spinal cord injury in
the rat. J Neurosci Res. 81:827–836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Udina E, Ceballos D, Verdú E, Gold BG and
Navarro X: Bimodal dose-dependence of FK506 on the rate of axonal
regeneration in mouse peripheral nerve. Muscle Nerve. 26:348–355.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gold BG, Densmore V, Shou W, Matzuk MM and
Gordon HS: Immunophilin FK506-binding protein 52 (not FK506-binding
protein 12) mediates the neurotrophic action of FK506. J Pharmacol
Exp Ther. 289:1202–1210. 1999.PubMed/NCBI
|
|
28
|
Buffo A, Rolando C and Ceruti S:
Astrocytes in the damaged brain: Molecular and cellular insights
into their reactive response and healing potential. Biochem
Pharmacol. 79:77–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bush TG, Puvanachandra N, Horner CH,
Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH and
Sofroniew MV: Leukocyte infiltration, neuronal degeneration, and
neurite outgrowth after ablation of scar-forming, reactive
astrocytes in adult transgenic mice. Neuron. 23:297–308. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Szydlowska K, Gozdz A, Dabrowski M,
Zawadzka M and Kaminska B: Prolonged activation of ERK triggers
glutamate-induced apoptosis of astrocytes: Neuroprotective effect
of FK506. J Neurochem. 113:904–918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szydlowska K, Zawadzka M and Kaminska B:
Neuroprotectant FK506 inhibits glutamate-induced apoptosis of
astrocytes in vitro and in vivo. J Neurochem. 99:965–975. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kahraman S, Bambrick LL and Fiskum G:
Effects of FK506 and cyclosporin a on calcium ionophore-induced
mitochondrial depolarization and cytosolic calcium in astrocytes
and neurons. J Neurosci Res. 89:1973–1978. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wong RW and Guillaud L: The role of
epidermal growth factor and its receptors in mammalian CNS.
Cytokine Growth Factor Rev. 15:147–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fricker-Gates RA, Winkler C, Kirik D,
Rosenblad C, Carpenter MK and Bjorklund A: EGF infusion stimulates
the proliferation and migration of embryonic progenitor cells
transplanted in the adult rat striatum. Exp Neurol. 165:237–247.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamada M, Ikeuchi T and Hatanaka H: The
neurotrophic action and signalling of epidermal growth factor. Prog
Neurobiol. 51:19–37. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goldshmit Y, Greenhalgh CJ and Turnley AM:
Suppressor of cytokine signalling-2 and epidermal growth factor
regulate neurite outgrowth of cortical neurons. Eur J Neurosci.
20:2260–2266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martinez R and Gomes FC: Neuritogenesis
induced by thyroid hormone-treated astrocytes is mediated by
epidermal growth factor/mitogen-activated protein
kinase-phosphatidylinositol 3-kinase pathways and involves
modulation of extracellular matrix proteins. J Biol Chem.
277:49311–49318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiang YY, Dong H, Wan Y, Li J, Yee A, Yang
BB and Lu WY: Versican G3 domain regulates neurite growth and
synaptic transmission of hippocampal neurons by activation of
epidermal growth factor receptor. J Biol Chem. 281:19358–19368.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zawadzka M and Kaminska B:
Immunosuppressant FK506 affects multiple signaling pathways and
modulates gene expression in astrocytes. Mol Cell Neurosci.
22:202–209. 2003. View Article : Google Scholar : PubMed/NCBI
|