Introduction
Liver cancer is the sixth most common type of cancer
worldwide and was the second most common cause of cancer-associated
mortality in 2015 (1). Hepatocytes
are the principal cellular components of the liver, followed by
other cells such as endothelial cells and bile duct cells.
Hepatocellular carcinoma (HCC), which originates in the
hepatocytes, is the most common pathological type of liver cancer,
accounting for ~80% of all liver cancer cases in adults (2). Comprehensive therapeutic strategies
have been developed, including surgical resection, radiofrequency
ablation, chemotherapy and orthotopic liver transplantation;
however, metastasis and recurrence remain major obstacles to HCC
treatment. The estimated overall five-year survival rate of
patients with liver or intrahepatic bile duct cancer is 18%
(3).
Previous studies have indicated that inflammation is
closely associated with HCC development. Persistent infection with
either hepatitis B or hepatitis C virus, one of the major risk
factors of HCC, induces chronic inflammation and subsequent
cirrhosis, thus promoting HCC initiation and progression (4,5). HCC
patients with high levels of inflammation, marked by increased
levels of inflammatory cytokines and cells, tend to have a poor
prognosis (6,7). Pro-inflammatory cytokines, including
TNF-α, interleukin (IL)-1β and IL-6, are the principal mediators of
tumor-accelerating inflammation (8–10).
Therefore, targeting tumor-accelerating pro-inflammatory cytokines
may result in HCC tumor regression.
TNF-α was initially identified by its ability to
induce lysis in tumor cells (11).
TNF-α has two forms: A cell membrane-bound form and a soluble form.
Two types of TNF-α receptors, TNF-α receptors 1 and 2, which are
either cell membrane-bound or soluble, have been classified so far
(11,12). Although TNF-α was initially
identified as a tumor-killing cytokine, further studies have
determined that it serves a more complex role in cancer development
(13). Furthermore, TNF-α is a key
mediator of the cancer-associated inflammatory networks that have
strong tumor-promoting properties (14,15).
Preclinical studies in various types of cancer, including breast,
pancreatic and blood cancer, have suggested that TNF-α stimulates
tumor growth in vivo and that anti-TNF-α treatments may
suppress tumor progression (16–18).
Previous studies have demonstrated that the level of serum TNF-α
and a number of other pro-inflammatory cytokines, were
significantly higher in patients with HCC compared with healthy
individuals, thus increased TNF-α was associated with the
occurrence of HCC (19,20). High levels of TNF-α were also
associated with increased inflammation in patients with chronic
viral hepatitis C (21). These
results suggest that TNF-α serves an important role in regulating
inflammation in HCC. However, the effects of anti-TNF-α treatments
in HCC have not yet been elucidated. The aim of the current study
was to investigate the effects of anti-TNF-α treatments on HCC,
in vitro and in vivo.
Materials and methods
Cell line culture
The human HCC cell lines HepG2 and Hep3B were
obtained from the Chinese Academy of Sciences Cell Bank (Beijing,
China) and Sigma-Aldrich (Merck KGaA, Darmstadt, Germany),
respectively. Cells were cultured in high-glucose Dulbecco's
modified Eagle's medium (DMEM) supplemented with 100 U/ml
penicillin (both from Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA), 2 mM
L-glutamine and 10% fetal bovine serum (both Thermo Fisher
Scientific, Inc.) in a humidified 5% CO2 incubator at
37°C. The cells were sub-cultured when they reached 80%
confluence.
Cell viability assay
Cell viability was evaluated using a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Cells were seeded in 96-well plates and treated with
infliximab (Janssen Pharmaceuticals, Inc., Horsham, PA, USA) and
etanercept (Enbrel®; Wyeth Pharmaceuticals; Pfizer,
Inc., New York, NY, USA) in gradient concentrations (2, 4, 8, 16 or
32 µg/ml) for 48 h. Cells were incubated with CCK-8 solution for 1
h at 37°C, then absorbance at 450 nm was measured using an MRX II
microplate reader (Dynex Technologies, Inc., Chantilly, VA, USA).
Relative cell viability was calculated as a proportion of isotype
controls according to the following formula: (Treatment -
blank)/(control - blank) (22).
Fluorescence-activated cell sorting
(FACS) analysis
Cells were harvested from fresh cell culture,
dissociated into single cells by trypsinization (Sigma-Aldrich;
Merck KGaA). Cell fixation and permeabilization were performed
using a commercial FIX & PERM® Cell Fixation and
Permeabilization kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The cells were subsequently
incubated with primary antibodies directed against TNF-α (dilution
1:200, cat. no. ab6671; Abcam, Cambridge, MA, USA) on ice for 1 h,
followed by washing with PBS once. Apoptosis was detected using an
anti-cleaved caspase-3 antibody (dilution 1:200; cat. no. ab13847;
Abcam). Incubation with primary antibodies was performed on ice for
1 h. Fluorescence-labeled secondary antibodies (dilution 1:1,000,
cat. no. A-11034; Thermo Fisher Scientific, Inc.) were subsequently
added and incubated for 15 min at room temperature. Stained cells
were washed with PBS three times prior to being analyzed with a
FACSCanto II flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). Data were visualized using Flowing 2.0 software (Turku Center
for Biotechnology, Turku, Finland).
Patient samples
Tumor and tumor-adjacent tissues were collected from
5 patients who were diagnosed with HCC at Weifang People's Hospital
(Weifang, China) between August and December 2014. A total of 2 of
the patients were female and 3 were male. The average age of the
female patients was 58.0 (55–61 years) and the average age of the
male patients was 52.0 (48–55 years). Fresh tissue samples were
collected immediately following tumor resection. They were broken
down into smaller pieces and dissociated into a single cell
suspension by incubation with collagenase type I at 37°C for 1 h.
The single cells were then immediately used for FACS analysis.
Prior to ELISA the cell suspensions were centrifuged at 1,000 × g
for 5 min at 4°C. The cell pellets were subsequently lysed by a
radioimmunoprecipitation (RIPA) buffer with a protease inhibitor
(Thermo Fisher Scientific, Inc.). No patients enrolled in this
study received pre-operative chemotherapy or radiotherapy. Informed
consent was obtained from all patients and the protocol was
approved by the Local Ethics Committee of Weifang People's Hospital
(Weifang, China).
Xenograft mouse model
A subcutaneous HCC model was established using 40,
5-week-old female BALB/c nude mice (weight, 18–20 g) purchased from
the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China), in
order to analyze the in vivo activity of TNF-α and
infliximab. All mice were kept in a specific pathogen-free
environment with 45% humidity under a 12 h light/dark cycle at room
temperature. The mice had free access to standard food and
sterilized water. A total of 5×106 HepG2 cells were
injected subcutaneously in the flanks of the mice. One week
following inoculation, all mice were randomized into 4 groups (n=10
in each group) each receiving different treatments: i) 10
mg/kg/week isotype immunoglobulin G (IgG) (control; Thermo Fisher
Scientific, Inc.); ii) 10 µg/mouse/week, TNF-α recombinant human
protein (Thermo Fisher Scientific, Inc.); iii) infliximab, 10
mg/kg/week; and iv) 10 µg/mouse/week TNF-α recombinant human
protein + 10 mg/kg/week infliximab. Tumor size and body weight were
measured every 5 days. Tumor volume was calculated according to the
following formula: Tumor volume = length × width2 × ρ/6.
The survival of all mice was documented. Excessive weight loss
(>20%), ulceration or any other symptoms of distress were
considered as the endpoint of survival analysis and observation.
Once any of these symptoms were observed the mouse was sacrificed
by cervical dislocation. As the study was performing survival
analysis, all mice were ultimately sacrificed by this method.
Antibody-dependent cell-mediated
cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC)
assays
For the ADCC assay, human HCC tumor cell lines HepG2
and Hep3B were used as target cells and peritoneal macrophages from
BALB/c mice were used as effector cells. Target cells were seeded
in a 96-well plate (2,000 cells/well) and pre-incubated with
infliximab or etanercept (2, 4, 8, 16, or 32 µg/ml) for 1 h at
37°C. In the control group, infliximab or etanercept was replaced
with IgG. Subsequently, the target and effector cells were mixed to
a ratio of 1:15 and incubated for 48 h at 37°C. For the CDC assay,
target cells were plated in a 96-well plate at a density of 5,000
cells/well. Target cells were then incubated with 5% fresh guinea
pig serum (Sigma-Aldrich; Merck KGaA) with active complements and
infliximab or etanercept for 6 h at 37°C. Cell viability was
determined using the aforementioned CCK-8 assay according to the
manufacturer's instructions. Cytotoxicity in each test was
calculated as previously described (22). Briefly, the survival rate of the two
treatment groups was calculated use the formula: 100 × (cell
concentration of treatment group/cell concentration of the control
group). The survival rate of control group was defined as 100% and
its inhibition rate was defined as 0%. The curves of the control
groups were not illustrated in the figures. The difference in
cytotoxicity between infliximab and etanercept treated groups was
analyzed by a paired t-test.
Quantification of TNF-α, IL-1β, IL-6
and IL-17 in HCC xenograft tumor tissues as measured by ELISA
Tumor tissues were dissociated into single cell
suspension and cell pellets as described above. They were lysed
with a RIPA buffer with protease inhibitor. ELISA was performed for
TNF-α, IL-1β, IL-6 and IL-17 according to the
manufacturer's instructions (cat. nos. KRC3011, EM2IL1B, EM2IL6,
and 88-7371-22, respectively) (all from Thermo Fisher Scientific,
Inc.).
Immunofluorescence
The tumor tissues were collected by resection from
the HepG2 xenograft mouse model following sacrifice. The tissues
were fixed with 10% neutral-buffered formalin followed by 70%
ethanol (each for 24 h at room temperature). The tissues were
subsequently embedded in paraffin and cut into 4-µm-thick sections.
The slides were deparaffinized with xylene and rehydrated with
gradient ethanol. Antigen retrieval was performed by steaming with
Reveal Decloaker (Biocare Medical, Concord, CA, USA) for 40 min.
Subsequently, 5% bovine serum albumin (Thermo Fisher Scientific,
Inc.) was added and incubated for 15 min at room temperature and
slides were then incubated with primary antibodies against
cleaved-caspase-3 (dilution 1:200) overnight at 4°C. Alexa
Fluor®-conjugated secondary antibodies (dilution
1:1,000, cat. no. A-11034; Thermo Fisher Scientific, Inc.) were
used to detect the primary antibodies. The slides were incubated
with the secondary antibodies for 30 min at room temperature.
Photographs of the slides were captured using an Olympus BX51
fluorescence microscope (Olympus, Tokyo, Japan).
Statistical analysis
All quantification data are expressed as the mean ±
standard error of the mean Differences between individual groups
were analyzed by Student's t-test or one-way analysis of variance
following Bonferroni's pairwise comparisons. Overall survival was
plotted using the Kaplan Meier method and the significance was
evaluated by the log-rank test. P<0.05 was considered to
indicate a statistically significant difference. All the
statistical analyses were performed using GraphPad Prism software,
version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
TNF-α is overexpressed in HCC
To understand the role of TNF-α in HCC development,
TNF-α expression was evaluated in the human HCC cell lines HepG2
and Hep3B, and tumor and tumor-adjacent healthy tissues from HCC
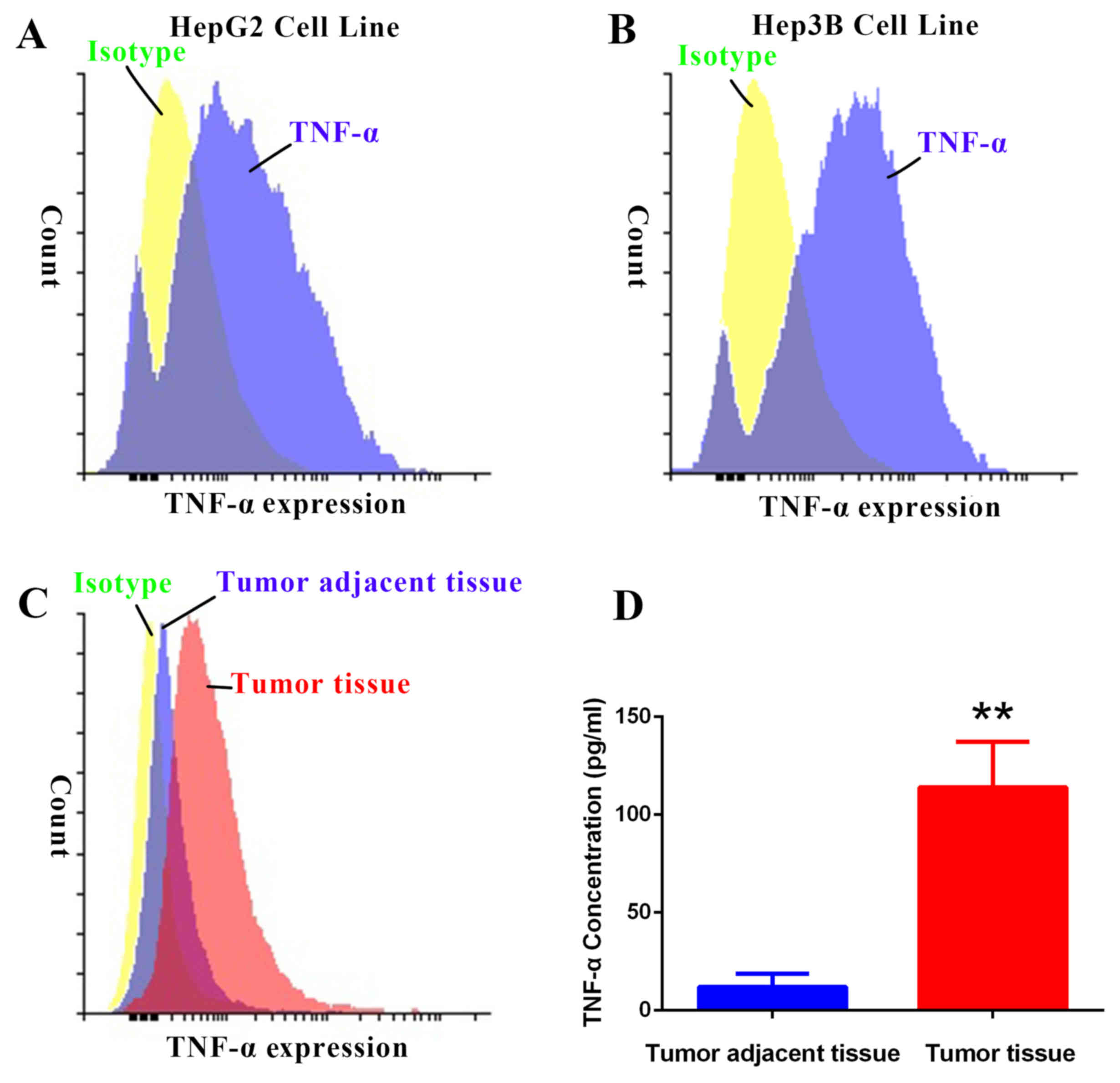
patients. Results from the FACS analysis indicated that TNF-α
expression was higher in HepG2 and Hep3B cell lines and tumor
tissues compared with control isotype expression (Fig. 1A-C). TNF-α expression was also higher
in tumor tissues compared with tumor-adjacent tissues (Fig. 1C). Quantification of TNF-α
concentration by ELISA indicated that TNF-α expression was
significantly higher in tumor tissues compared with tumor-adjacent
tissues (P<0.01; Fig. 1D). These
data demonstrate that TNF-α is overexpressed in HCC.
Inhibition of TNF-α reduces viability
of HCC cells through ADCC and CDC effects
TNF-α is overexpressed in HCC, therefore the current
study investigated whether inhibiting TNF-α expression may be
developed as a therapeutic strategy to treat HCC. Infliximab is an
anti-TNF-α monoclonal antibody and etanercept is a protein that can
bind to the TNF receptor, thus acting as a TNF-α inhibitor. In
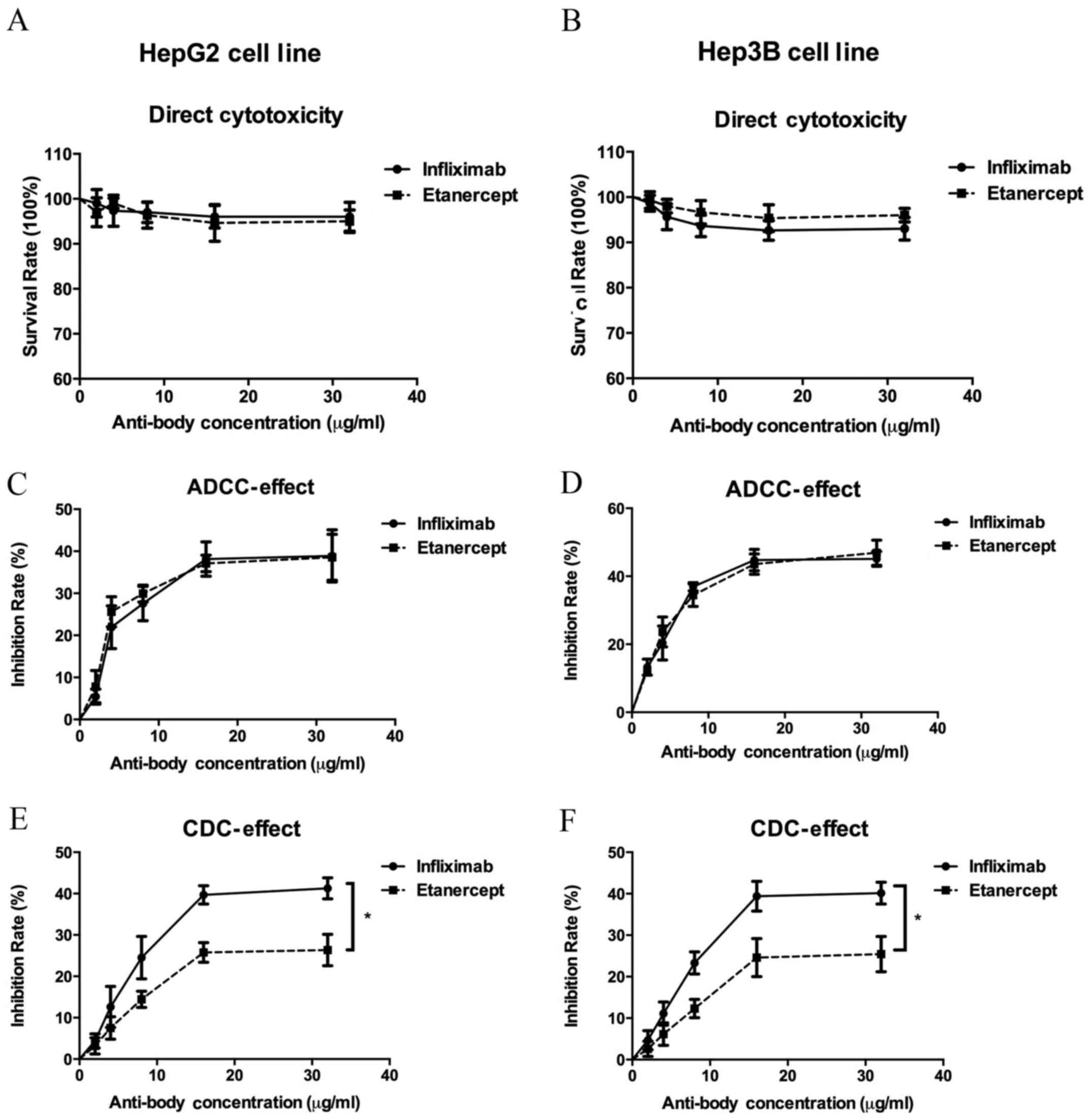
order to measure the direct cytotoxicity of infliximab and
etanercept on HepG2 and Hep3B cells, cells were treated with
gradient concentrations of each drug. Following exposure to
infliximab and etanercept, the viability of both cell lines was
only slightly decreased (Fig. 2A and
B), suggesting that inhibition of TNF-α by infliximab and
etanercept induces little direct cytotoxicity.
ADCC and CDC assays were conducted to determine the
role of anti-TNF-α treatments in HCC cells. In the ADCC assay, it
was demonstrated that anti-TNF-α treatment with infliximab or
etanercept inhibited HepG2 and HeP3B viability in the presence of
macrophages, and this effect was increased as infliximab and
etanercept concentrations increased (Fig. 2C and D). Similar effects were
observed in the CDC assay (Fig. 2E and
F). Exposed to active complements, the infliximab and the
etanercept treatments inhibited HepG2 and Hep3B cell viability.
However, both cell lines were significantly more sensitive to
infliximab treatment compared with etanercept treatment
(P<0.05). These results suggest that anti-TNF-α treatments
induced ADCC and CDC effects to inhibit HCC cell viability.
Anti-TNF-α treatment induces tumor
regression and prolongs survival in an HCC xenograft mouse
model
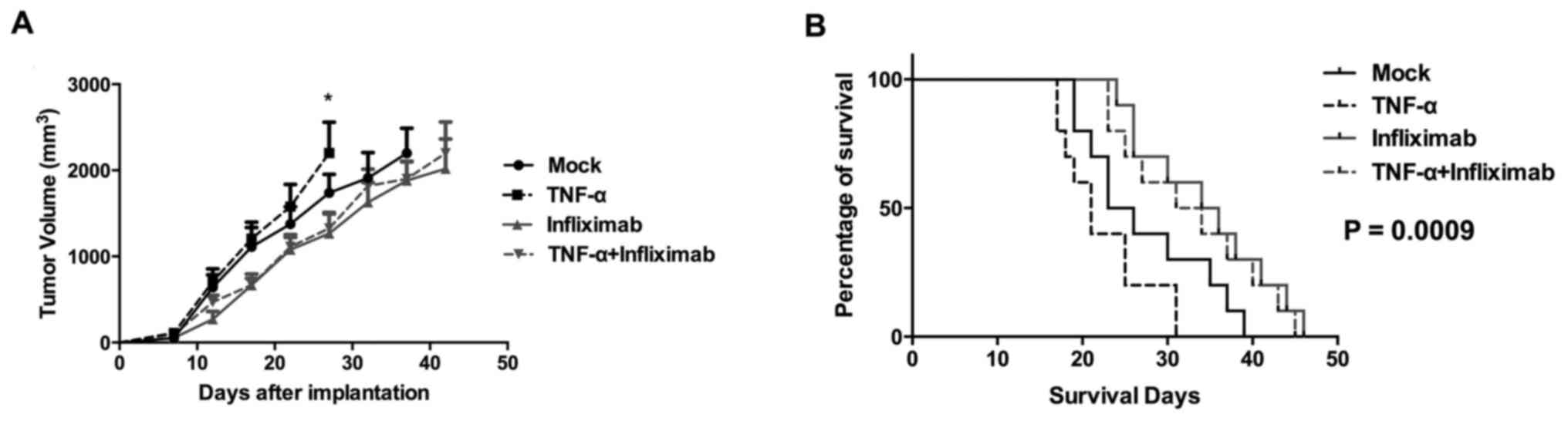
To evaluate the effects of anti-TNF-α treatment in
HCC in vivo, a xenograft mouse model was established by
subcutaneously inoculating BALB/c nude mice with HepG2 cells. The
mice were then randomized into 4 groups (n=10) to be treated with
isotype IgG, TNF-α recombinant protein, infliximab, or TNF-α
recombinant protein + infliximab. The tumor growth curve indicated
that tumors treated with TNF-α recombinant protein grew faster
compared with the mock group (Fig.
3A). The tumors of the group treated with infliximab alone grew
more slowly compared with the mock group. Furthermore, treatment
with infliximab and TNF-α recombinant protein reduced the rate of
tumor growth compared with TNF-α recombinant protein alone.
Consistent with the trends in tumor growth, the
survival of infliximab-treated mice was extended compared with the
TNF-α-treated and control groups (Fig.
3B). In the TNF-α-treated and control groups, the estimated
median survival of mice was 21 and 24.5 days, respectively.
However, in the TNF-α recombinant protein + infliximab-treated and
infliximab-treated groups, the estimated median survival times were
30 and 32.5 days, respectively. The log-rank test indicated that
the differences among all four groups were statistically
significant (P=0.0009). These data suggested that anti-TNF-α
treatment has therapeutic value in HCC pre-clinical models.
Anti-TNF-α treatment inhibits
pro-inflammatory cytokine expression
It has been established that there is an association
between inflammation and cancer, including in HCC (23,24).
TNF-α is widely accepted as a systemic pro-inflammatory cytokine
and a central modulator in inflammatory networks (25). Therefore, the current study
investigated the effects of TNF-α recombinant protein and
anti-TNF-α treatments on the expression of pro-inflammatory
cytokines in the tumor cells of a xenograft mouse model. The
cytokines evaluated were IL-1β, IL-6, IL-17 and TNF-α. It was
determined that infliximab significantly decreased the endogenous
(infliximab group vs. mock group; P<0.01) and exogenous (TNF-α
group vs. TNF-α + infliximab group; P<0.0001) TNF-α in tumor
tissues (Fig. 4A). Administration of
TNF-α significantly increased the levels of IL-1β, IL-6 and IL-17
in the tumor tissues (P<0.05; Fig.
4B-D). However, expression of these pro-inflammatory cytokines
was inhibited by infliximab treatment in the infliximab and TNF-α +
infliximab groups (Fig. 4B-D). These
data indicated that anti-TNF-α treatment might modulate
tumor-promoting inflammation in HCC.
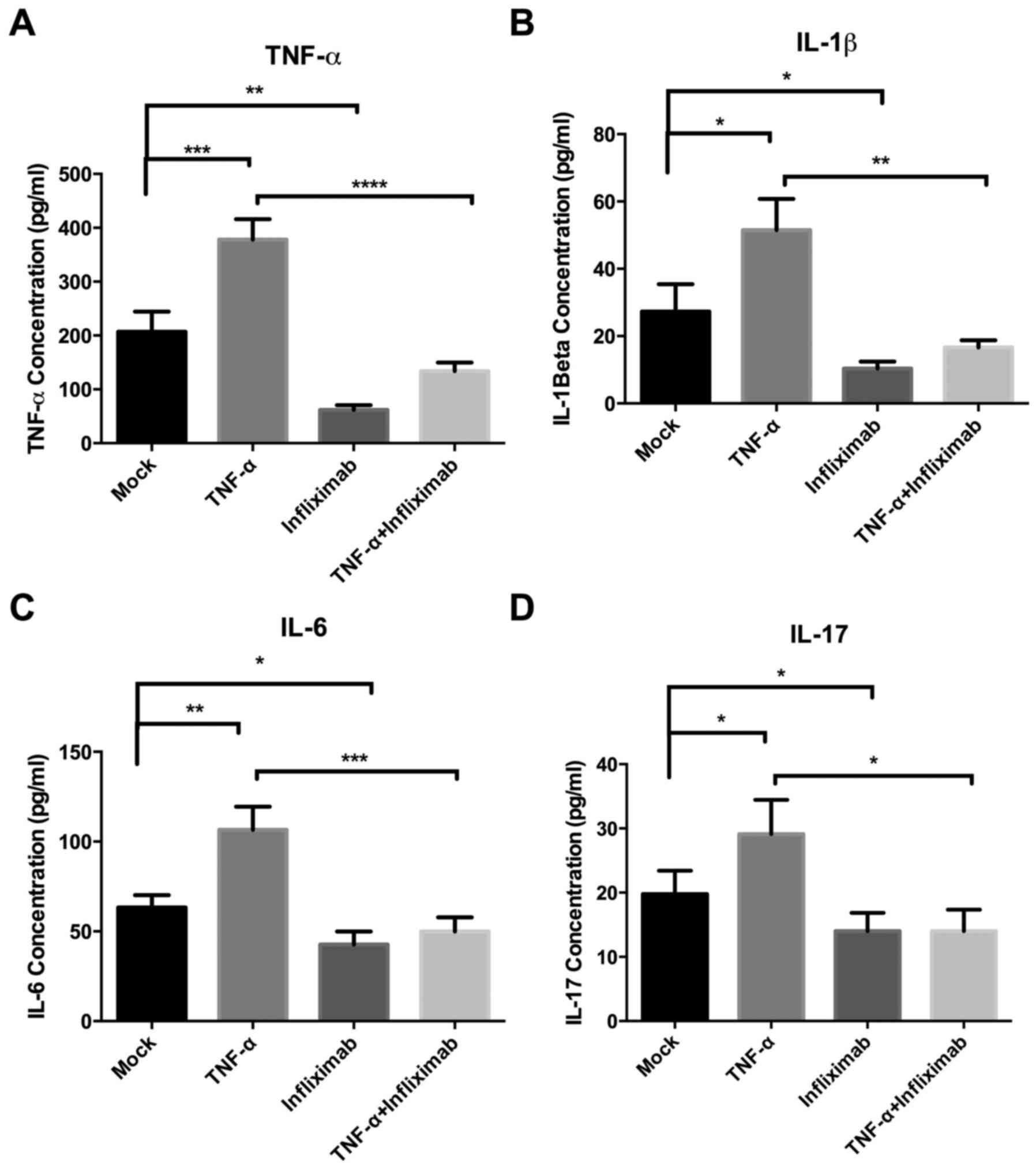 | Figure 4.Effect of anti-TNF-α treatment on the
expression of the pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and
IL-17 in a hepatocellular carcinoma xenograft mouse model. The
levels of (A) TNF-α, (B) IL-1β, (C) IL-6 and D (IL-17) were
evaluated by ELISA. TNF-α treatment promoted the expression of
these cytokines, but infliximab reversed these effects. TNF-α,
tumor necrosis factor-α; IL, interleukin; *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. |
Anti-TNF-α treatment induces apoptosis
in vitro and in vivo
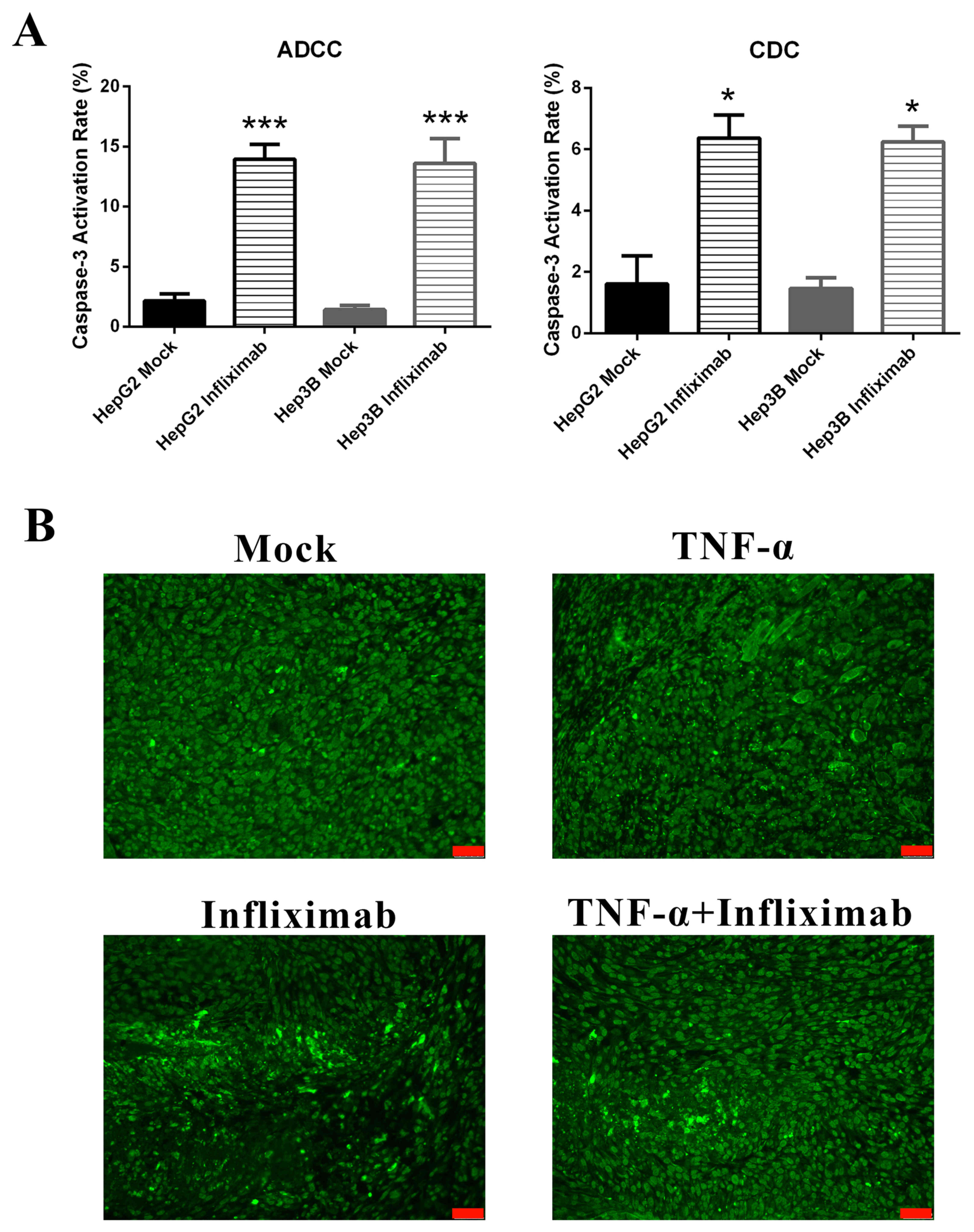
To determine whether anti-TNF-α treatment induces
apoptosis in HCC, the expression of cleaved caspase-3, which is a
common executor in the late apoptosis phase, was measured. In
vitro, infliximab-induced ADCC and CDC effects significantly
increased apoptosis rates in HepG2 and Hep3B cell lines compared
with controls (P<0.001 and P<0.05, respectively; Fig. 5A). Cleaved caspase-3 was also
evaluated by immunofluorescence in HCC tumor tissues from a
xenograft mouse model. It was observed that treatment with
infliximab markedly increased apoptosis compared with untreated
tissues (Fig. 5B). These results
suggested that apoptosis was induced by anti-TNF-α treatment in
vitro and in vivo. This is the potential mechanism of
HCC tumor regression by anti-TNF-α treatment.
Discussion
TNF-α was originally characterized by its tumor cell
necrosis effects, however it has also been suggested that TNF-α
serves a role in accelerating tumor development (26,27). In
cancer, TNF-α is secreted by tumor cells and the surrounding
stromal cells, including tumor-associated macrophages and
tumor-associated fibroblasts (27).
In multiple types of cancer, TNF-α expression is correlated with
tumor progression (22). It has
previously been reported that TNF-α is involved with the processes
of establishing the pro-inflammatory tumor microenvironment,
protecting tumor cells from apoptosis, inducing angiogenesis and
enhancing drug resistance (27). In
pancreatic and ovarian cancer, it has been demonstrated that TNF-α
inhibits cell viability in vitro but accelerates tumor
growth in vivo (17,28). Anti-TNF-α treatment with either
antibodies or genetic editing has demonstrated therapeutic effects
in pre-clinical models, including those for breast cancer,
pancreatic cancer and leukemia (17,22,28,29). The
treatment of liver cancer remains challenging, as indicated by its
low relative 5-year survival rate of <20% (3). It has not yet been investigated whether
anti-TNF-α treatment has therapeutic value for HCC patients. The
results of the current study demonstrated that TNF-α is
overexpressed in HCC. Targeting TNF-α with antibodies may have the
capacity to induce HCC cell death and compromise the
pro-inflammatory tumor microenvironment.
Tumors are made up of tumor cells, surrounding
stromal cells, extracellular components and soluble factors
including inflammatory cytokines (30,31).
Components of the tumor microenvironment are critical in tumor
development (32–35). Expression of TNF-α in tumor tissues
is elevated during tumor progression and a high level of TNF-α is
correlated with poor prognosis in multiple cancer types (18,27,36,37).
TNF-α stimulates downstream tumor promoting pathways in cancer
cells, which enhance its secretion via a positive feedback loop;
this makes it a potential target for antagonists (27). In the current study, high levels of
TNF-α were detected in the HCC cell lines HepG2 and Hep3B. FACS and
ELISA were used to evaluate TNF-α expression in HCC tumor and
tumor-adjacent tissues. A higher level of TNF-α was observed in
tumor tissues compared with tumor-adjacent tissues, suggesting that
TNF-α is involved in HCC development.
The cytotoxic impact of antibodies on cancer cells
may occur via direct cytotoxicity, ADCC or CDC effects (38). It was previously reported that
anti-TNF-α antibodies had ADCC and CDC effects on cancer cells
(39,40). However, in certain types of cancer,
infliximab had a higher CDC effect than etanercept (40). In the current study, the results
indicated that neither infliximab nor etanercept had direct cell
death-inducing functions on HCC cells. However, in the presence of
effector cells or complements, infliximab and etanercept inhibited
the viability of HCC cells and infliximab had a higher inhibitory
rate than etanercept in terms of CDC effect.
The subsequent in vivo investigation
supported the findings in vitro. Exogenous TNF-α accelerated
tumor growth in the HCC xenograft mouse model, whereas infliximab
delayed tumor growth and prolonged survival. Subsequent analyses
revealed that anti-TNF-α treatment stimulated apoptosis-mediated
cell death both in vitro and in vivo. These data are
consistent with previous studies investigating breast cancer and
leukemia (22,29).
A large number of inflammatory cells are found in
cancer (41). Previous evidence
suggests that cancer-related inflammation aids the proliferation of
malignant cells, stimulates metastasis and induces
immunosuppression (27). When
produced by malignant and stromal cells, TNF-α is a major mediator
of cancer-related inflammation (41,42). The
current data indicated that administration of exogenous TNF-α
increased the levels of pro-inflammatory cytokines including TNF-α,
IL-1β, IL-6 and IL-17 in HCC. Treatment with TNF-α antibody
markedly reduced the levels of these pro-inflammatory cytokines,
even in the presence of exogenous TNF-α. As inflammation is closely
associated with HCC, these data implied that anti-TNF-α treatment
may have therapeutic value in treating HCC by helping to neutralize
the pro-inflammatory microenvironment.
In conclusion, the current findings indicated that
TNF-α can be inhibited by antagonists in HCC, resulting in tumor
regression and improved survival in an HCC mouse model. Further
studies investigating the therapeutic value of anti-TNF-α should be
conducted in pre-clinical models and subsequent clinical
trials.
References
|
1
|
World Health Organization (WHO): Cancer
Fact Sheet. 2017
|
|
2
|
Zhang Y, Ren JS, Shi JF, Li N, Wang YT, Qu
C, Zhang Y and Dai M: International trends in primary liver cancer
incidence from 1973 to 2007. BMC Cancer. 15:942015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin MV, King LY and Chung RT: Hepatitis C
virus-associated cancer. Annu Rev Pathol. 10:345–370. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Markowitz GJ, Michelotti GA, Diehl AM and
Wang XF: Inflammatory models drastically alter tumor growth and the
immune microenvironment in hepatocellular carcinoma. Sci Bull
(Beijing). 60:762–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Fushiya N, Koike K, Nishino H and Tajiri H: Comparison of
the prognostic value of inflammation-based prognostic scores in
patients with hepatocellular carcinoma. Br J Cancer. 107:988–993.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC: An
inflammation-based prognostic score (mGPS) predicts cancer survival
independent of tumour site: A glasgow inflammation outcome study.
Br J Cancer. 104:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lasry A and Ben-Neriah Y:
Senescence-associated inflammatory responses: Aging and cancer
perspectives. Trends Immunol. 36:217–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu J, Yin Z, Cao S, Gao W, Liu L, Yin Y,
Liu P and Shu Y: Systematic review and meta-analysis on the
association between IL-1B polymorphisms and cancer risk. PLoS One.
8:e636542013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chu WM: Tumor necrosis factor. Cancer
Lett. 328:222–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Engelmann H, Holtmann H, Brakebusch C,
Avni YS, Sarov I, Nophar Y, Hadas E, Leitner O and Wallach D:
Antibodies to a soluble form of a tumor necrosis factor (TNF)
receptor have TNF-like activity. J Biol Chem. 265:14497–14504.
1990.PubMed/NCBI
|
|
13
|
Chu WM: Tumor necrosis factor. Cancer
Lett. 328:222–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mocellin S, Rossi CR, Pilati P and Nitti
D: Tumor necrosis factor, cancer and anticancer therapy. Cytokine
Growth Factor Rev. 16:35–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zins K, Abraham D, Sioud M and Aharinejad
S: Colon cancer cell-derived tumor necrosis factor-alpha mediates
the tumor growth-promoting response in macrophages by up-regulating
the colony-stimulating factor-1 pathway. Cancer Res. 67:1038–1045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hagemann T, Robinson SC, Schulz M, Trümper
L, Balkwill FR and Binder C: Enhanced invasiveness of breast cancer
cell lines upon co-cultivation with macrophages is due to TNF-alpha
dependent up-regulation of matrix metalloproteases. Carcinogenesis.
25:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Egberts JH, Cloosters V, Noack A,
Schniewind B, Thon L, Klose S, Kettler B, von Forstner C, Kneitz C,
Tepel J, et al: Anti-tumor necrosis factor therapy inhibits
pancreatic tumor growth and metastasis. Cancer Res. 68:1443–1450.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferrajoli A, Keating MJ, Manshouri T,
Giles FJ, Dey A, Estrov Z, Koller CA, Kurzrock R, Thomas DA, Faderl
S, et al: The clinical significance of tumor necrosis factor-alpha
plasma level in patients having chronic lymphocytic leukemia.
Blood. 100:1215–1219. 2002.PubMed/NCBI
|
|
19
|
Aroucha DC, do Carmo RF, Moura P, Silva
JL, Vasconcelos LR, Cavalcanti MS, Muniz MT, Aroucha ML, Siqueira
ER, Cahú GG, et al: High tumor necrosis factor-α/interleukin-10
ratio is associated with hepatocellular carcinoma in patients with
chronic hepatitis C. Cytokine. 62:421–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YY, Lo GH, Lai KH, Cheng JS, Lin CK
and Hsu PI: Increased serum concentrations of tumor necrosis
factor-alpha are associated with disease progression and
malnutrition in hepatocellular carcinoma. J Chin Med Assoc.
66:593–598. 2003.PubMed/NCBI
|
|
21
|
Avrămescu CS, Comănescu V, Popescu SN,
Turculeanu A, Bălăşoiu M, Popescu CF and Lungulescu M: Correlations
among the serum levels of some interleukins and the
histopathological aspects in chronic viral hepatitis C. Rom J
Morphol Embryol. 49:57–62. 2008.PubMed/NCBI
|
|
22
|
Yu M, Zhou X, Niu L, Lin G, Huang J, Zhou
W, Gan H, Wang J, Jiang X, Yin B and Li Z: Targeting transmembrane
TNF-α suppresses breast cancer growth. Cancer Res. 73:4061–4074.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Castello G, Scala S, Palmieri G, Curley SA
and Izzo F: HCV-related hepatocellular carcinoma: From chronic
inflammation to cancer. Clin Immunol. 134:237–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bulló M, García-Lorda P, Megias I and
Salas-Salvadó J: Systemic inflammation, adipose tissue tumor
necrosis factor, and leptin expression. Obes Res. 11:525–531. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carswell EA, Old LJ, Kassel RL, Green S,
Fiore N and Williamson B: An endotoxin-induced serum factor that
causes necrosis of tumors. Proc Natl Acad Sci USA. 72:3666–3670.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kulbe H, Thompson R, Wilson JL, Robinson
S, Hagemann T, Fatah R, Gould D, Ayhan A and Balkwill F: The
inflammatory cytokine tumor necrosis factor-alpha generates an
autocrine tumor-promoting network in epithelial ovarian cancer
cells. Cancer Res. 67:585–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou X, Zhou S, Li B, Li Q, Gao L, Li D,
Gong Q, Zhu L, Wang J, Wang N, et al: Transmembrane TNF-α
preferentially expressed by leukemia stem cells and blasts is a
potent target for antibody therapy. Blood. 126:1433–1442. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Swartz MA, Iida N, Roberts EW, Sangaletti
S, Wong MH, Yull FE, Coussens LM and DeClerck YA: Tumor
microenvironment complexity: Emerging roles in cancer therapy.
Cancer Res. 72:2473–2480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trédan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ostman A: The tumor microenvironment
controls drug sensitivity. Nat Med. 18:1332–1334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X, He Y, Gao J, Fan L, Li Z, Yang G
and Chen H: Caveolin-1 expression level in cancer associated
fibroblasts predicts outcome in gastric cancer. PLoS One.
8:e591022013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao X, He Y and Chen H: Autophagic tumor
stroma: Mechanisms and roles in tumor growth and progression. Int J
Cancer. 132:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bozcuk H, Uslu G, Samur M, Yildiz M, Ozben
T, Ozdoğan M, Artaç M, Altunbaş H, Akan I and Savaş B: Tumour
necrosis factor-alpha, interleukin-6, and fasting serum insulin
correlate with clinical outcome in metastatic breast cancer
patients treated with chemotherapy. Cytokine. 27:58–65. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karayiannakis AJ, Syrigos KN,
Polychronidis A, Pitiakoudis M, Bounovas A and Simopoulos K: Serum
levels of tumor necrosis factor-alpha and nutritional status in
pancreatic cancer patients. Anticancer Res. 21:1355–1358.
2001.PubMed/NCBI
|
|
38
|
Adams GP and Weiner LM: Monoclonal
antibody therapy of cancer. Nat Biotechnol. 23:1147–1157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitoma H, Horiuchi T, Tsukamoto H,
Tamimoto Y, Kimoto Y, Uchino A, To K, Harashima S, Hatta N and
Harada M: Mechanisms for cytotoxic effects of anti-tumor necrosis
factor agents on transmembrane tumor necrosis factor
alpha-expressing cells: Comparison among infliximab, etanercept,
and adalimumab. Arthritis Rheum. 58:1248–1257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Horiuchi T, Mitoma H, Harashima SI,
Tsukamoto H and Shimoda T: Transmembrane TNF-alpha: Structure,
function and interaction with anti-TNF agents. Rheumatology
(Oxford). 49:1215–1228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sethi G, Sung B and Aggarwal BB: TNF: A
master switch for inflammation to cancer. Front Biosci.
13:5094–5107. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou C, Nitschke AM, Xiong W, Zhang Q,
Tang Y, Bloch M, Elliott S, Zhu Y, Bazzone L, Yu D, et al:
Proteomic analysis of tumor necrosis factor-alpha resistant human
breast cancer cells reveals a MEK5/Erk5-mediated
epithelial-mesenchymal transition phenotype. Breast Cancer Res.
10:R1052008. View Article : Google Scholar : PubMed/NCBI
|