Introduction
Cardiovascular disease is considered to be one of
the most common non-cancer effects associated with high or medium
doses of ionizing radiation. Endothelial cells, which are critical
targets in radiation-induced cardiovascular damage, appear to serve
a key role in the development of vascular pathologies. Increasing
evidence has indicated that radiation exposure may induce premature
senescence (1,2), endothelial barrier damage or
permeability changes (1,3), cytoskeleton disruption (4) and angiogenic defects (5) in diverse endothelial models. Searching
for an effective pharmacological therapy for radiation-induced
cardiovascular damage has become an urgent task (6).
Although certain medicines, including pravastatin,
have been proven to have the ability to reduce radiation-induced
damage (7), considerable attention
has been devoted to the development of radioprotectors from natural
products in recent years, due to their cost-effectiveness and
safety. Celastrol is a pentacyclic triterpenoid originally
extracted from the root of the traditional Chinese medicinal plant
Tripterygium wilfordii (also called Thunder god vine)
(8,9). Clinical trials have demonstrated the
efficacy and safety of Tripterygium wilfordii.
Tripterygium wilfordii has been applied in the treatment of
rheumatoid arthritis (10,11) and Crohn's disease (12,13).
Celastrol has also been proven to be effective in the treatment of
asthma, chronic inflammation and neurodegenerative disease
(14–17). The biological activities of celastrol
include antioxidant (15),
anti-inflammatory (18), anticancer
(8), anti-diabetic (19), obesity-controlling (20) and insecticide (21) functions. To date, the function of
celastrol in radiation protection has seldom been investigated.
γ rays, a type of high-frequency ionizing radiation,
can penetrate the body and cause the radiolysis of water. Human
tissues contain 70–80% water. The major damage caused by γ
radiation results from free radicals, including ROS and nitric
oxide (22). ROS react with cellular
molecules, resulting in lipid peroxidation and DNA damage, and
further causing cellular dysfunction and mortality. Given its
properties of antioxidation, it was hypothesized that celastrol may
exhibit protective effects against γ irradiation-induced injury in
human umbilical vein endothelial cells (HUVECs). The present study
not only examined the protective effects of celastrol against γ
irradiation-induced cell death in HUVECs, but also explored the
possible underlying mechanisms via the inhibition of oxidative
stress. To the best of our knowledge, this is the first study to
investigate the radioprotective properties of celastrol in
endothelial cells.
Materials and methods
Reagents
Celastrol, dimethyl sulfoxide (DMSO), Tris, glycine,
sodium dodecyl sulfate (SDS), bovine serum albumin (BSA) and
diphenyl-1-pyrenylphosphine (DPPP) were purchased from J&K
Scientific Ltd. (Beijing, China).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
nuclease P1, alkaline phosphatase, β-actin antibody,
dihydroethidium (DHE), a lipid peroxidation assay kit by
malondialdehyde (MDA), CelLytic™ lysis reagent and Bradford
reagents were obtained from Sigma-Aldrich Shanghai Trading Co. Ltd.
(Shanghai, China). The Pierce Lactate Dehydrogenase (LDH)
Cytotoxicity Assay kit was from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The 2′,7′-dichlorofluorescin diacetate (DCFDA)
cellular ROS detection assay kit, Alexa Fluor®
647-conjugated anti-mouse antibody and Fluoroshield mounting medium
with DAPI were purchased from Abcam Trade Ltd. (Shanghai, China).
The genomic DNA purification kit and Griess reagent were purchased
from Promega Biotech Co. Ltd. (Beijing China). EIA kits for
8-hydroxy-2-deoxy Guanosine (8-OH-dG), and antioxidant enzyme
assays including superoxide dismutase (SOD), catalase, glutathione
peroxidase (GPx) and glutathione S-transferase (GST) were purchased
from Cayman Chemical Company (Ann Arbor, MI, USA). Antibodies
against SOD-1, SOD-2, catalase, GPx and GST, and horseradish
peroxidise (HRP)-conjugated goat anti-mouse and anti-rabbit
antibodies were purchased from Santa Cruz Biotechnology Co. Ltd.
(Shanghai, China). Anti-γH2AX antibody was from CST Biological
Reagents Company Ltd. (Shanghai, China). The nitrocellulose
membrane was obtained from Shanghai Xingya Purify Material Ltd.
(Shanghai, China). Western blotting detection ECL Reagent was
purchased from GE Healthcare China Co. Ltd. (Beijing, China).
Cell culture
HUVECs were purchased from AllCells Biotech Ltd.
(Shanghai, China), and grown in endothelial cell growth medium
(Jiangsu Promocell Biotechnology Co. Ltd., China) containing 2% FBS
at 37°C in a 5% CO2 humidified atmosphere. Cells were
passaged every three to four days. Cells at the 3rd to 8th passage
were used for the present study.
Irradiation procedure and drug
treatment
For all experiments, HUVECs were seeded in cell
culture chamber slides, 96-well plates, 100 mm2 petri
dishes or 75 cm2 cell culture flasks, and grown to
confluence prior to being exposed to γ radiation in a Gammacell 40
Exactor (Best Theratronics, Kanata, ON, Canada) using Cs-137 as a
radioactive source. Different radiation doses were applied to
generate the dose-response curve. The radiation dose rate was 2.8
Gy/min. Celastrol was dissolved in DMSO, kept at a stock solution
of 100 mM, and further diluted in medium prior to administration.
Following exposure to γ irradiation, HUVECs were treated with
celastrol at the designated concentrations for 24 h, harvested and
subjected to different assays.
Cell viability assay
The viability of HUVECs after γ irradiation with or
without celastrol treatment was tested using the colorimetric MTT
method. The assay is based on the cleavage of the yellow
tetrazolium salt MTT to purple formazan crystals by metabolically
active cells. The formazan crystals are solubilized, and then
spectrophotometrically quantified. Cells were seeded in 96-well
plates, exposed to γ irradiation at different doses, and then
incubated in medium containing different concentrations of
celastrol. After 24 h of treatment, MTT at a final concentration of
0.5 mg/ml was added to each well and incubated at 37°C for 4 h.
When the medium had been removed, HUVECs were washed twice with
PBS. DMSO was added into the wells to solubilize the blue formazan
dye and the absorbance was read at 570 nm. Cells without γ
irradiation exposure and celastrol treatment were considered to be
controls. The cell viability of the treatment groups is expressed
as a percentage of the control.
Cell cytotoxicity assay
Cytotoxicity induced by γ irradiation was determined
by LDH release. As a cytosolic enzyme present in many different
types of cells, LDH is released into the cell culture medium when
the plasma membrane is damaged. Cells were seeded in 96-well plates
and exposed to 20 Gy γ irradiation, followed by the treatment with
1.5 and 2 µM celastrol for 24 h. A volume of 50 µl medium was
loaded into a 96-well flat bottom plate in triplicate wells, and 50
µl reaction mixture was added to each well. The plate was incubated
at room temperature for 30 min in the dark and 50 µl stop solution
was added. The absorbance at 490 nm was measured using a microplate
reader (Tecan Group, Ltd., Männedorf, Switzerland). The
cytotoxicity in each group was expressed as a percentage of the
control.
Cell migration assay
Cells were seeded in Culture-Insert 2 wells in
µ-Dish 35 mm (cat. no. 81176; ibidi GmbH, Am Klopferspitz, Planegg,
Germany) with a defined 500 µm cell-free gap. Following exposure to
20-Gy γ irradiation, cell medium was removed and cells were treated
with 1.5 and 2 µM celastrol dissolved in culture medium for 24 h.
The silicone inserts were carefully removed, and the dishes were
monitored under an inverted microscope (Olympus Corp., Tokyo,
Japan). The gap distances were measured at different time points.
The data at 6 h after the removal of the inserts was used for
comparison.
Measurement of ROS
The intracellular level of ROS was measured using
the fluorescent probe H2DCFDA and DHE. DHE, upon
reaction with superoxide anions, forms a red fluorescent product,
2-hydroxyethidium. HUVECs were seeded in black-walled and
clear-bottom 96-well microplates (cat. no. M33089) and
Nunc™ Lab-Tek™ Chambered Coverglass (cat. no.
155383; both Thermo Fisher Scientific, Inc.). After exposure to
20-Gy γ irradiation and treatment with 1.5 and 2 µM celastrol,
cells were washed with PBS and stained with 5 µM DHE at 37°C for 30
min in the dark. Images of the coverglass were captured under a
Leica fluorescent microscope (Leica Biosystems, Wetzlar, Germany).
The fluorescence intensities in the 96-well plate were measured
using a fluorescent microplate reader (Tecan Infinite F200 Pro;
Tecan Group, Ltd.) at excitation and emission wavelengths of 520
and 610 nm, respectively.
ROS production was also examined by
H2DCFDA staining. As a cell-permeant and non-fluorescent
probe, H2DCFDA is converted to the highly fluorescent
2′,7′-dichlorofluorescein (DCF) upon cleavage of the acetate groups
by intracellular esterases and oxidation. Cells were treated as
stated above and stained with 25 µM H2DCFDA at 37°C for
30 min in the dark. The fluorescence intensities were measured at
excitation and emission wavelengths of 485 and 530 nm,
respectively.
Lipid peroxidation assays
Lipid peroxidation was examined using DPPP, a probe
which turns to a fluorescent diphenyl-1-pyrenylphosphine oxide
(DPPP-O) when reacting with hydroperoxides. HUVECs were seeded in
96-well plates, treated as stated above, and incubated with 50 µM
DPPP at 37°C for 60 min in the dark. The fluorescence intensities
were measured and analyzed at an excitation wavelength of 351 nm
and an emission wavelength of 380 nm using a fluorescent microplate
reader.
Lipid peroxidation was also evaluated according to
the levels of MDA. A total of 1×106 cells were
homogenized on ice in 300 µl MDA lysis buffer containing 3 µl
butylated hydroxytoluene (BHT; 100X) and centrifuged at 13,000 × g
for 10 min to remove insoluble material. A total of 200 µl
supernatant was removed into a microcentrifuge tube. A total of 600
µl thiobarbituric acid (TBA) solution was added into each tube
containing the standard and samples, and incubated at 95°C for 60
min. Following cooling to room temperature, 200 µl from each
reaction mixture was pipetted to a 96-well plate for analysis. The
absorbance at 490 nm was measured with a microplate reader. The
concentration of TBA-MDA adduct was calculated based upon the
standard curve.
DNA oxidative damage assay using an
8-OH-dG EIA kit
HUVECs were seeded in 100 mm2 Petri
dishes and treated as stated above. Genomic DNA was isolated and
purified with a commercial kit. HUVECs were harvested by
trypsinization, and treated with RNase A, proteinase K and lysis
buffer at 55°C for 10 min. The lysate was loaded into the
separation column. After the column was centrifuged and washed, the
eluted and purified DNA was collected.
An 8-OH-dG EIA kit was used to examine DNA oxidative
damage in cells according to the manufacturer's instructions.
Briefly, the aforementioned purified DNA was incubated with
alkaline phosphatase and nuclease P1 at 37°C for 30 min, and then
boiled for 10 min before being placed on ice. A total of 50 µl
sample or standard, 50 µl antibodies and 50 µl Tracer were added
into the testing plate, which was then incubated at 4°C for 18 h. A
total of 200 µl Ellman's reagent was added into the plate following
washing. The plate was shaken for 90–120 min in the dark for
optimum development. The absorbance was measured using a microplate
reader at a wavelength of 420 nm. The reading was used to calculate
the 8-OH-dG concentration based upon the standard curve.
DNA double-strand break assay by
immunofluorescence staining
DNA double-strand breaks were studied using
anti-γH2AX immunofluorescence staining. Briefly, HUVECs were grown
on chamber slides and treated as described above. Cells were fixed
in cold methanol for 10 min, washed with PBS three times, blocked
using 1% BSA for 1 h at room temperature and incubated with primary
anti-γH2AX antibody (1:200 dilution) at 4°C overnight. Following
washing, cells were incubated with secondary Alexa Fluor
647-conjugated anti-mouse antibody (1:200 dilution) for 1 h at room
temperature. Following rinsing, the nuclei were fluorescently
labelled with DAPI in mounting medium. The images were examined
under a fluorescence microscope.
Antioxidant enzyme activity assay
HUVECs were treated, harvested using a rubber
policeman, and centrifuged at 2,000 × g for 10 min. The cell
pellets were lysed in cold lysis buffer, and the protein
supernatants were collected by centrifugation at 10,000 × g at 4°C
for 15 min. The protein concentration was evaluated using the
Bradford method. Equal amounts of samples and standards were loaded
into the 96-well plates provided with the commercial kits. The
concentrations and activities of SOD, catalase, GST and GPx were
measured according to the respective protocols from the
manufacturer. The absorbance was read using a microplate reader,
and the activities of SOD, catalase, GST and GPx were calculated
using the equations obtained from their respective standard
curves.
Immunoblot analysis
Cell lysates were separated on a 10% SDS-PAGE gel
and transferred to a nitrocellulose membrane. The membranes were
blocked using 5% BSA and incubated with the respective primary
antibodies (β-actin, 1:1,000 dilution; SOD-1, SOD-2, GPx, GST and
catalase, 1:500 dilution) overnight at 4°C. Following washing, the
membrane was incubated with the respective HRP-conjugated secondary
antibodies (1:10,000 dilution) at room temperature for 1 h, and
visualized using the ECL method. Images were captured and
quantified using the Bio-Rad Laboratories, Inc. Gel Doc system.
Values were normalized to their respective loading control. The
results were calculated and expressed as a fold change relative to
control group.
Statistical analysis
Values which were normally distributed are expressed
as the mean ± SEM. One-way analysis of variance (ANOVA) was used to
determine statistical significance between groups, followed by
Tukey's post-hoc test. P 0.05 was considered to indicate a
statistically significant difference.
Results
Effects of celastrol on cell viability
and cytotoxicity in HUVECs following γ irradiation
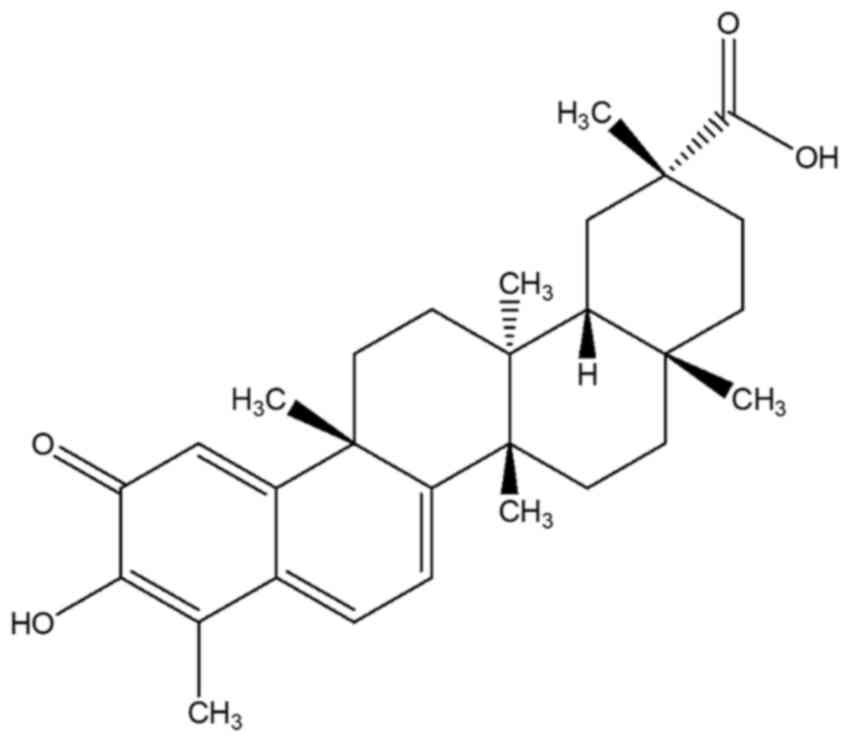
The chemical structure of celastrol is presented in
Fig. 1. HUVECs were exposed to γ
irradiation at different doses. It was observed that 10-, 20- and
40-Gy γ irradiation significantly decreased the cell viability at
24 h post-irradiation, indicative of a dose-dependent response
(Fig. 2A). Treatment of HUVECs with
1, 1.5 and 2 µM celastrol for 24 h following 20-Gy γ radiation
exposure significantly reversed the decreased cell viability
(Fig. 2B), while treatment with
celastrol at 0.5 µM did not exert any effect.
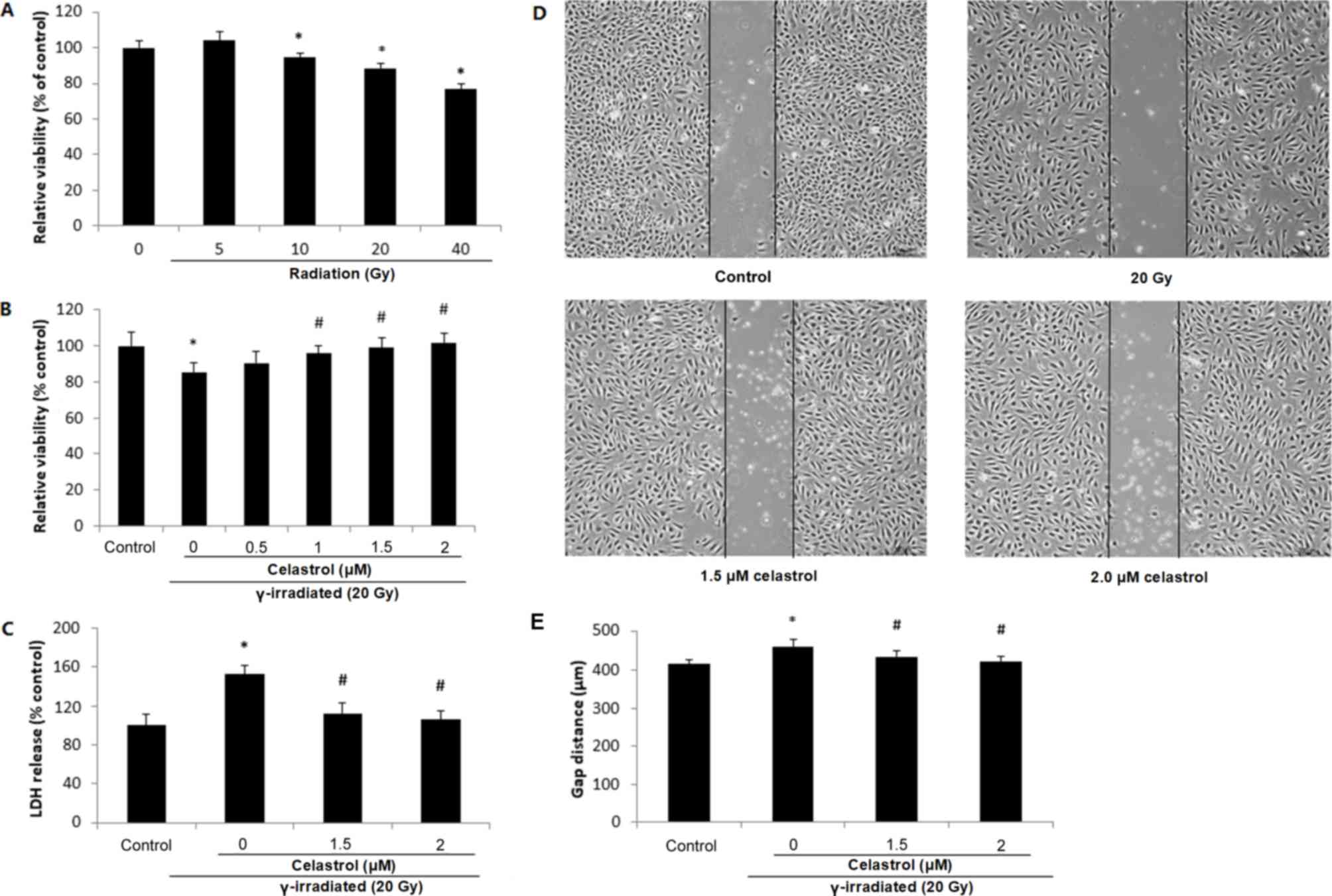 | Figure 2.Cell viability, cytotoxicity and
migratory ability of HUVECs following exposure to γ irradiation of
different doses and celastrol treatment at different
concentrations. (A) HUVECs were exposed to γ irradiation of 5 to 40
Gy. Cell viability was evaluated by MTT after 24 h. (B) HUVECs were
exposed to 20-Gy γ irradiation, followed by treatment with
celastrol at various concentrations (0, 0.5, 1, 1.5 and 2 µM). Cell
viability was evaluated by MTT after 24 h. (C) Cells were exposed
to 20-Gy γ irradiation, followed by treatment with celastrol at 1.5
and 2 µM. Cytotoxicity was determined by LDH release. HUVECs
without celastrol treatment and γ radiation served as controls.
Cell viability and cytotoxicity are expressed as a percentage of
the control. (D) Images of HUVECs in the cell migration assay at 6
h after the removal of the inserts. The dishes were monitored under
an inverted microscope (magnification, ×40). (E) Statistical
results of the cell migration assay. The gap distances at 6 h after
the removal of insert were used for comparison. All data are
expressed as the mean ± SEM. ANOVA was used to determine
statistical significance between groups followed by Tukey's
post-hoc test. *P<0.05 vs. control; #P<0.05 vs. 20
Gy without celastrol treatment group. HUVECs, human umbilical vein
endothelial cells; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; LDH,
Lactate Dehydrogenase; ANOVA, one-way analysis of variance. |
The protective effect of celastrol against γ
irradiation-induced cytotoxicity was also supported by the LDH
release assay (Fig. 2C). Compared
with the control, 20-Gy γ irradiation significantly induced LDH
release into the medium, while treatment with 1.5 and 2 µM
celastrol significantly decreased LDH release to the level of the
control group. These results indicated that celastrol could reverse
γ irradiation-induced cell death, indicating the radioprotective
ability of celastrol.
Effects of celastrol on the decreased
migratory ability of HUVECs following γ irradiation
A gap closure assay was used to examine the effects
of celastrol on the migratory potential of HUVECs following γ
irradiation. It was observed that 20-Gy γ irradiation significantly
inhibited the migratory ability of HUVECs, indicated by the
increased gap distance when compared with the control (Fig. 2D and E). Cells following treatment
with celastrol at the concentrations of 1.5 and 2 µM exhibited a
decreased gap distance, suggesting the restored migratory ability
of HUVECs (Fig. 2D and E).
Effects of celastrol on the increased
free radical production induced by γ irradiation
DHE staining illustrated elevated red fluorescence
intensity at 24 h post-exposure to 20-Gy γ irradiation in HUVECs
(Fig. 3A and B), indicating
increased ROS production. This increase was significantly blocked
by treatment with celastrol at 1.5 and 2 µM.
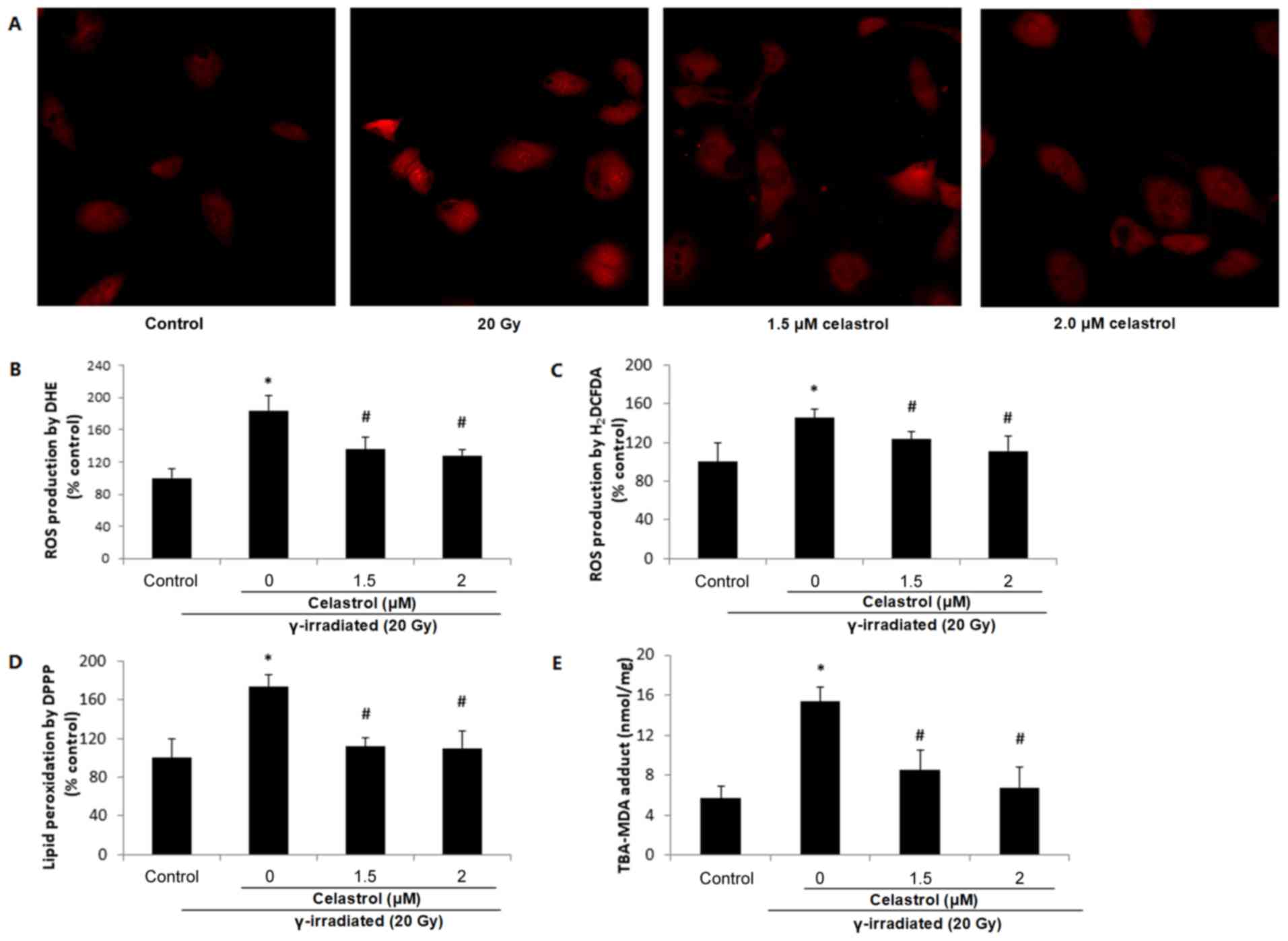 | Figure 3.Effects of celastrol on ROS
production and lipid peroxidation in HUVECs. Cells were exposed to
20-Gy γ irradiation, followed by treatment with 1.5 and 2 µM
celastrol. HUVECs without celastrol treatment and γ radiation
served as the control. (A) Images of DHE staining in HUVECs under a
fluorescence microscope (magnification, ×400). (B) Statistical
results of ROS production analysed by DHE. HUVECs were stained with
5 µM DHE at 37°C for 30 min in the dark. The fluorescence
intensities of DHE were viewed and measured an excitation and
emission wavelength of 520 and 610 nm, respectively. (C) ROS
production in HUVECs assayed by 25 µM H2DCFDA. The
fluorescence intensities of H2DCFDA were measured at an
excitation and emission wavelength of 485 and 530 nm, respectively.
(D) Lipid peroxidation assayed by 50 µM DPPP. The fluorescence
intensities of DPPP fluorescence were measured at an excitation
wavelength of 351 nm and an emission wavelength of 380 nm. (E)
Lipid peroxidation examined by MDA in HUVECs. The concentrations of
the TBA-MDA adduct were determined by the absorbance at 490 nm
based upon the standard curve. All data are expressed as the mean ±
SEM. ANOVA was used to determine statistical significance between
groups followed by Tukey's post-hoc test. *P<0.05 vs. control;
#P<0.05 vs. 20 Gy without celastrol treatment group.
HUVECs, human umbilical vein endothelial cells; DHE,
dihydroethidium; H2DCFDA, 2′,7′-dichlorofluorescin
diacetate; MDA, malondialdehyde; DPPP, diphenyl-1-pyrenylphosphine;
ANOVA, one-way analysis of variance. |
ROS production was also evaluated by
H2DCFDA. Similar results were observed, in terms of the
enhanced ROS production following γ radiation exposure which was
reversed by celastrol treatment at 1.5 and 2 µM for 24 h (Fig. 3C). These results suggested that the
protective effects of celastrol against γ irradiation-induced
injury in HUVECs are mediated by the inhibition of oxidative
stress.
Effects of celastrol on the increased
lipid peroxidation induced by γ irradiation
ROS may induce the oxidative degradation of lipids
in cell membranes, resulting in cell damage. Lipid peroxidation was
observed to increase in HUVECs at 24 h post-irradiation, indicated
by a higher fluorescence intensity of DPPP compared with the
control (Fig. 3D), while treatment
with celastrol at 1.5 and 2 µM for 24 h significantly blocked this
increase. The ability of celastrol to protect the cells against γ
irradiation-induced lipid peroxidation was also evaluated by MDA
assay (Fig. 3E). As the end-product
of lipid peroxidation, MDA is known to be a major bioactive marker
of lipid peroxidation. The TBS-MDA concentration was significantly
higher in HUVECs following exposure to 20-Gy γ irradiation in
comparison with the control. This enhancement was blocked by
treatment with celastrol at 1.5 and 2 µM for 24 h, suggesting the
protective effects of celastrol in HUVECs against cell damage from
lipid peroxidation.
Effects of celastrol on the increased
oxidative DNA damage induced by γ irradiation
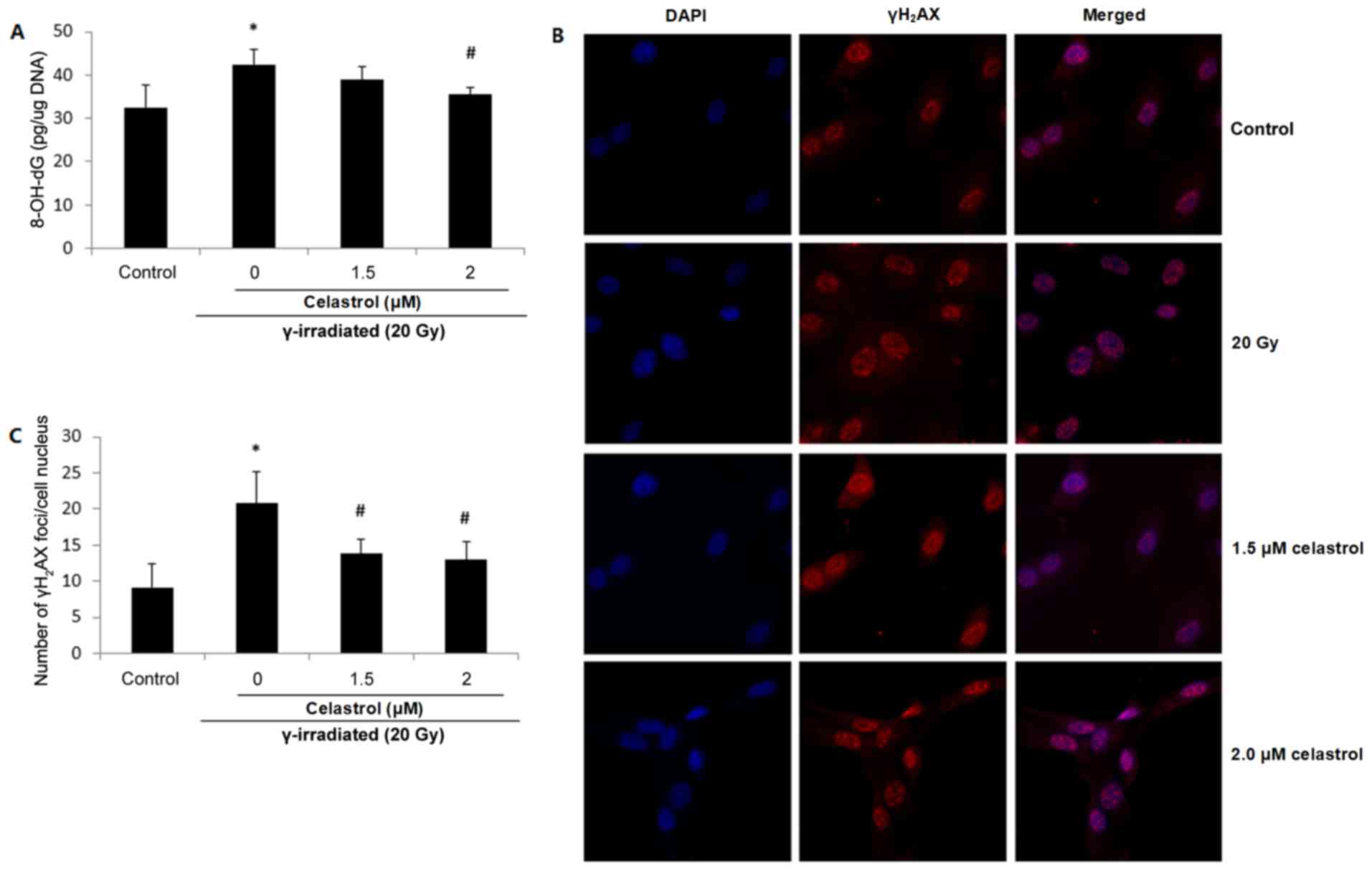
8-OH-dG is probably the most representative product
of oxidative modifications of DNA, and thus is widely used as a
non-invasive biomarker of oxidative damage to DNA. Increased
8-OH-dG concentrations were observed in HUVECs following exposure
to 20-Gy γ irradiation, suggesting elevated oxidative DNA damage
(Fig. 4A). The enhancement was
significantly blocked by 2 µM celastrol treatment for 24 h.
Treatment with 1.5 µM celastrol in HUVECs also decreased the
8-OH-dG concentration when compared with that in the 20 Gy group;
however, the difference was not statistically significant (Fig. 4A).
Oxidative stress has also been proposed to be
responsible for the production of DNA strand breaks. γH2AX is
widely used as a marker of DNA double-strand breaks. Fig. 4B presents fluorescence images of
γH2AX in HUVECs, and the number of γH2AX foci in the cell nuclei is
displayed in Fig. 4C. It was
demonstrated that compared with the control, the exposure to 20-Gy
γ irradiation significantly increased the frequency of DNA
double-strand breaks, while celastrol treatment at 1.5 and 2 µM for
24 h blocked this enhancement (Fig. 4B
and C). The above results indicated the protective activity of
celastrol against γ irradiation-induced oxidative DNA damage.
Effects of celastrol on the activity
and protein expression of antioxidant enzymes
The activities of antioxidant enzymes are presented
in Table I. 20-Gy γ irradiation
significantly decreased the activities of SOD, catalase, GPx and
GST compared with the control, while treatment with celastrol at
1.5 µM for 24 h significantly elevated the activities of these
antioxidant enzymes.
 | Table I.Antioxidant enzyme activities in
study groups. |
Table I.
Antioxidant enzyme activities in
study groups.
| Group | Superoxide
dismutase (U/mg) | Catalase
(nmol/min/mg) | Glutathione
S-transferase (nmol/min/mg) | Glutathione
peroxidase (nmol/min/mg) |
|---|
| Control | 3.23±0.85 | 20.37±2.32 | 73.82±5.98 | 58.64±7.35 |
| 20 Gy |
2.15±0.17a | 13.26±2.24
a | 42.26±3.12
a | 30.23±4.12
a |
| Celastrol+20
Gy |
3.03±0.48b |
21.25±3.39b |
65.56±6.01a,b |
46.26±3.54a,b |
In order to validate the findings, we further
investigated the protein expression of SOD-1, SOD-2, catalase, GPx
and GST in HUVECs (Fig. 5). The
expression levels of all antioxidant enzymes decreased in HUVECs
following exposure to 20-Gy γ irradiation as compared to the
control group. Treatment with celastrol significantly increased the
expression levels of these antioxidant enzymes when compared to the
20 Gy group. The expression of SOD-1, catalase and GPx was restored
to the levels of the control groups, but celastrol treatment only
partially enhanced the protein expression of SOD-2 and GST
(Fig. 5B). These western blot data
were in line with the results of the corresponding enzyme activity
assays. Taken together, these data indicated that celastrol may
enhance the activities of antioxidative systems in HUVECs following
γ irradiation.
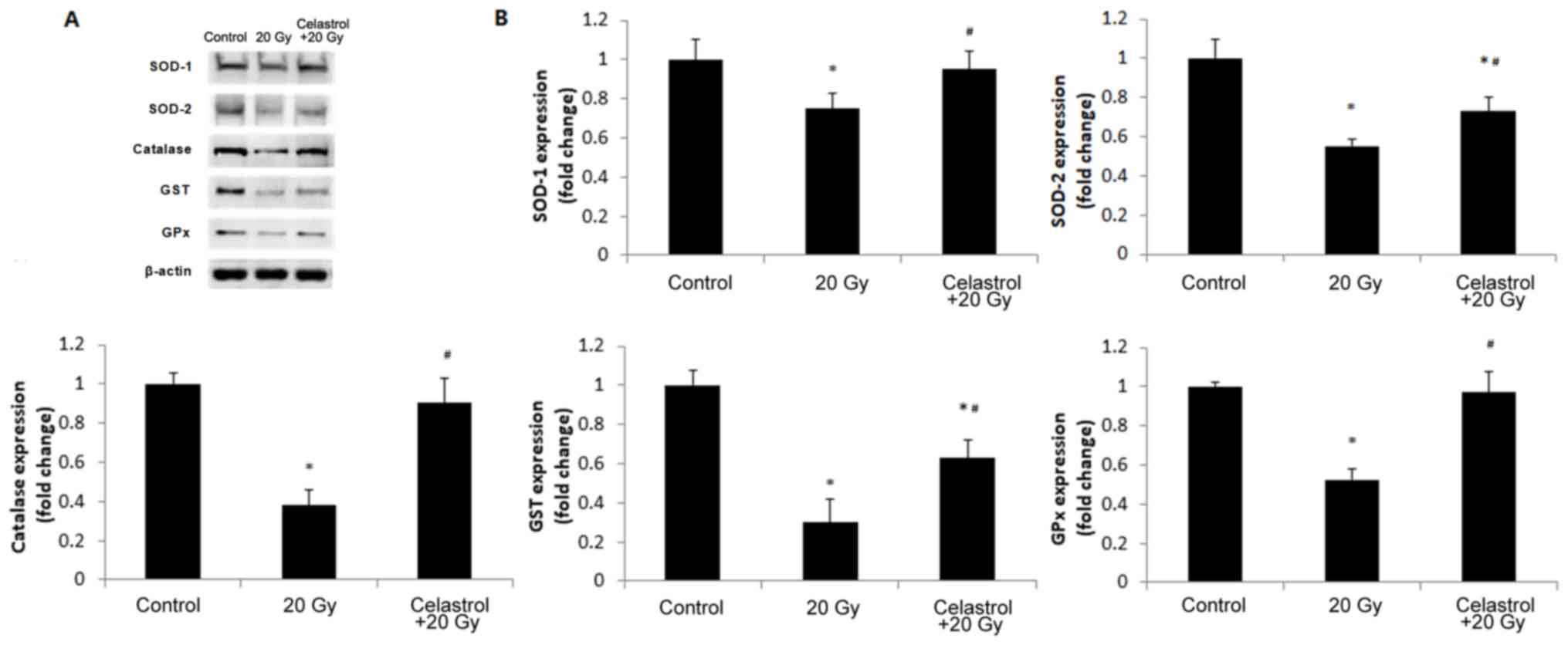 | Figure 5.Effects of treatment with celastrol
on the protein expression of antioxidant enzymes. Cells were
exposed to 20-Gy γ irradiation, followed by treatment with 1.5 µM
celastrol. HUVECs without celastrol treatment and γ radiation
served as the control. (A) Representative images of western blot
analysis illustrating the intensities of SOD-1, SOD-2, catalase,
GST, GPx and β-actin. (B) Densitometric analysis of band
intensities of antioxidant enzymes. The image was captured and
quantified using the Bio-Rad Gel Doc system. Values were normalized
to the respective loading control (β-actin). The results were
calculated and expressed as a fold change relative to the control
groups. Data are expressed as the mean ± SEM. ANOVA was used to
determine statistical significance between groups followed by
Tukey's post-hoc test. *P<0.05 vs. control;
#P<0.05 vs. 20 Gy without celastrol treatment group.
HUVECs, human umbilical vein endothelial cells; SOD, superoxide
dismutase; GPx, glutathione peroxidase; GST, glutathione
S-transferase; ANOVA, one-way analysis of variance. |
Discussion
Humans are exposed to ionizing radiation from
electronic devices, air travel, diagnostic and therapeutic
procedures, and even nuclear accidents. The increased use of
nuclear energy has made the search for safe and effective
radioprotective agents a priority (23). Although previous studies have
examined the protective effects of certain synthetic pharmaceutical
agents, including atorvastatin and recilisib sodium, against
radiation induced injury (24,25),
compounds from natural sources have still become the potential
targets due to their pharmacological properties and decreased
toxicity (26). Podophyllum
hexandrum (Himalayan mayapple) was observed to exhibit
radioprotective effects in lethally irradiated mice (27,28). Its
extracts, containing several active components, have exhibited
antioxidant activity as indicated by the inhibition of nitric oxide
production and the promotion of DNA repair (29,30).
Acorus calamus (sweet flag or calamus, from the Acoraceae
family) and its extracts were proven to scavenge free radicals and
enhance the activities of antioxidant enzymes in mouse liver
homogenates, and in mice exposed to γ irradiation (31,32).
These studies suggested the radioprotective potential of natural
products against radiation-induced damages. However, the existence
of multiple bioactive components in these plant extracts impedes
investigations into the underlying molecular and biochemical
mechanisms.
Therefore, celastrol was used in the present study,
its potential efficacy in the protection against γ
irradiation-induced cell injury was examined. To date, no study has
been conducted on the structure-activity association of celastrol
in radioprotection, to the best of our knowledge. The hydroxyl
group at position C-3 and the carboxylic group at position C-20 of
celastrol are believed to serve important roles in its activities
(33). It was demonstrated in the
present study that γ irradiation significantly decreased cell
viability and increased cytotoxicity in HUVECs, while this effect
was reversed by treatment with celastrol at 1 and 2 µM, indicative
of a dose-dependent effect of celastrol against γ
irradiation-induced cell death. To date, very little evidence has
been published on the ability of celastrol to prevent γ
radiation-induced injury, although its effects in controlling the
growth of various cancer cells have been recognized (34). It has been proven that celastrol is
able to inhibit the migration and invasion of ovarian cancer cells
by blocking the NF-κB pathway (35).
Nevertheless, the present study is the first to assess the
protective activities of celastrol on γ radiation-induced damage in
HUVECs.
Endothelial cells serve an important role in
maintaining endothelial integrity. Previous studies have suggested
a causality between high-dose radiation exposure and the
development of cardiovascular disease (36). Endothelial cells, as the most
sensitive cell type in the vasculature, are undoubtedly critical
targets in radiation-induced cardiovascular damage. In the present
study, the migratory ability of HUVECs was observed to be inhibited
by 20-Gy γ irradiation. The present results were consistent with
those from a study form Hwang et al (37), who demonstrated that far-infrared
radiation inhibited the proliferation, migration and angiogenesis
of HUVECs. The attenuated migratory ability of HUVECs was restored
by treatment with celastrol. The application of effective agents to
prevent injury in endothelial cells may have substantial
radioprotective effects against cardiovascular disease.
It is accepted today that oxidative stress is a
potent pathogenic mechanism contributing to γ radiation-induced
damage (18,38). Recent studies have proposed that γ
radiation significantly increases intracellular ROS formation, and
intracellular MDA and LDH levels, and decreases the production of
the intracellular antioxidants glutathione (GSH) and SOD in
endothelial cells (39,40). Our results indicated that 20-Gy γ
radiation significantly elevated the production of ROS and the
induction of lipid peroxidation. It is believed that ionizing
irradiation exerts its biological effects by initially generating
ROS. ROS then react with unsaturated lipids, alter membrane
permeability and induce lipid peroxidation (38). A study by Hu et al (41) demonstrated that acute γ radiation
dose-dependently increased intracellular ROS levels in HUVECs at 24
h post-irradiation. ROS and MDA production was found to be enhanced
after UVB exposure in HUVECs, suggesting the oxidative effects of
ionizing radiation on membrane lipids. Single or fractionated
irradiations with low-dose X-rays were demonstrated to induce ROS
generation in HUVECs, even when these irradiations did not affect
cell viability and DNA repair (42).
ROS may also cause the progressive modification of cellular DNA.
Such cumulative and deleterious effects induced by ROS can lead to
cell function loss and cell death. Our study showed that γ
irradiation not only increases the concentration of 8-OH-dG in
HUVECs, but also induces more DNA double strand breaks. This
finding was consistent with other literature. Olteanu et al
(43) demonstrated that UVB exposure
induced DNA damage, as indicated by the increased expression of
γ-H2AX in HUVECs, accompanied by increased apoptosis and
cell death. Chronic low-dose ionizing radiation was also
demonstrated to induce DNA damage and oxidative stress in HUVECs
(2). All of this evidence not only
supports our finding that 20-Gy γ irradiation induced oxidative
damage in HUVECs, but also suggested that a suitable radioprotector
should possess the ability to ameliorate oxidative stress, prevent
peroxidation and restore the endogenous antioxidant system
following radiation exposure.
Celastrol treatment was observed to exhibit
protective effects in HUVECs in the present study by inhibiting ROS
production, lipid peroxidation generation and oxidative DNA damage,
indicative of the antioxidative stress properties of celastrol in
radioprotection. Our results are in accordance with a report from
Stankova et al (9), which
demonstrated the antioxidative effects of celastrol in human
peripheral blood mononuclear cells exposed to γ radiation. The
action of celastrol in attenuating oxidative stress has been
investigated in cells and animal models of diabetes, colitis,
atherosclerosis and hypertension (18,44–46).
Furthermore, celastrol was demonstrated to be the most active
compound among eight sesquiterpene esters in inhibiting NF-κB
activation and NO production (47).
The excessive production of NO in LPS-stimulated microglial cells
was also inhibited by celastrol treatment (48). The inhibitory effect of celastrol on
lipid peroxidation was demonstrated in rat mitochondria (49). The anti-peroxidative property of
celastrol, together with its functions in the inhibition of ROS and
NO production, supports our findings that celastrol suppressed γ
irradiation-induced oxidative stress in HUVECs.
ROS produced following γ irradiation may act via the
NADPH and xanthine oxidase pathways. NADPH oxidase is the major
source of superoxide production. NADPH oxidase DUOX1 was reported
to promote the long-term persistence of oxidative stress in a human
thyroid cell line and primary thyrocytes after exposure to
radiation (50). Xanthine oxidase is
a type of enzyme that generates ROS and nitric oxide. It was
demonstrated that exposure of rats to γ radiation increased the
levels of NO and xanthine oxidase activity, and decreased the GSH
level, and SOD and CAT activity (51). Both pathways contribute to the
generation of ROS following γ radiation, which may negatively
impact antioxidant defence mechanisms, reduce the intracellular
concentrations of GSH and decrease the activities of antioxidant
enzymes including SOD, catalase, GST and GPx. The imbalance in
intracellular redox status will result in oxidative injury and cell
death. In our study, 20-Gy γ irradiation was demonstrated to
significantly decrease the activities of antioxidant enzymes
compared with the control. This decrease may be due to the elevated
utilisation of the antioxidant system during the detoxification of
γ irradiation-induced free radicals. The treatment with celastrol
was observed to reverse the decreased activities of antioxidant
enzymes in HUVECs. The ability of celastrol to increased
antioxidant capacities was also proven in pulmonary fibrosis and
obesity (52,53). Furthermore, HUVECs exhibited
significantly decreased expression of SOD-1, SOD-2, catalase, GST,
and GPx after 20-Gy γ irradiation compared with the control. The
treatment with celastrol completely restored the expression of
SOD-1, catalase and GPx to the control levels, and partially
recovered the protein levels of SOD-2 and GST. These protein
expression results further support the antioxidative stress
properties of celastrol in γ irradiation-induced cell damage in
HUVECs.
In summary, celastrol protects HUVECs against γ
radiation-induced cell death and migratory ability loss by
decreasing ROS production and lipid peroxidation, mitigating
oxidative DNA damage, and restoring the activity and protein
expression of antioxidant enzymes. Given its antioxidative
efficacy, pharmaceutical properties and low toxicity, celastrol may
represent a potential novel agent in the protection against γ
irradiation-induced injury in endothelial cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by Jilin Provincial
Key Laboratory Program, the Research Foundation of Jilin Provincial
Science and Technology Development (grant no. 20150622024JC).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author upon
request.
Authors' contributions
FL and XBH conceived and designed the experiments,
and performed the experiments. XBH, YQF and YT analyzed the data.
XBH and YQF contributed reagents/materials/analysis tools. XBH, YT,
YQF and FL wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors report no conflicts of interest. The
authors alone are responsible for the content and writing of the
paper.
References
|
1
|
Ungvari Z, Podlutsky A, Sosnowska D,
Tucsek Z, Toth P, Deak F, Gautam T, Csiszar A and Sonntag WE:
Ionizing radiation promotes the acquisition of a
senescence-associated secretory phenotype and impairs angiogenic
capacity in cerebromicrovascular endothelial cells: Role of
increased DNA damage and decreased DNA repair capacity in
microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci.
68:1443–1457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yentrapalli R, Azimzadeh O, Barjaktarovic
Z, Sarioglu H, Wojcik A, Harms-Ringdahl M, Atkinson MJ, Haghdoost S
and Tapio S: Quantitative proteomic analysis reveals induction of
premature senescence in human umbilical vein endothelial cells
exposed to chronic low-dose rate gamma radiation. Proteomics.
13:1096–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young EF and Smilenov LB: Impedance-based
surveillance of transient permeability changes in coronary
endothelial monolayers after exposure to ionizing radiation. Radiat
Res. 176:415–424. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gabryś D, Greco O, Patel G, Prise KM,
Tozer GM and Kanthou C: Radiation effects on the cytoskeleton of
endothelial cells and endothelial monolayer permeability. Int J
Radiat Oncol Biol Phys. 69:1553–1562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park MT, Oh ET, Song MJ, Lee H and Park
HJ: Radio-sensitivities and angiogenic signaling pathways of
irradiated normal endothelial cells derived from diverse human
organs. J Radiat Res. 53:570–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson MA: Maintaining a proper
perspective of risk associated with radiation exposure. J Nucl Med
Technol. 29:137–142; quiz 148–150. 2001.PubMed/NCBI
|
|
7
|
Doi H, Matsumoto S, Odawara S, Shikata T,
Kitajima K, Tanooka M, Takada Y, Tsujimura T, Kamikonya N and
Hirota S: Pravastatin reduces radiation-induced damage in normal
tissues. Exp Ther Med. 13:1765–1772. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuchta K, Xiang Y, Huang S, Tang Y, Peng
X, Wang X, Zhu Y, Li J, Xu J, Lin Z and Pan T: Celastrol, an active
constituent of the TCM plant Tripterygium wilfordii Hook.f.,
inhibits prostate cancer bone metastasis. Prostate Cancer Prostatic
Dis. 20:156–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stankova K, Ivanova K, Nikolov V, Aneva N,
Georgieva R and Boteva R: Proteasome inhibition protects human
peripheral blood mononuclear cells from radiation-induced oxidative
stress. Int J Radiat Biol. 89:493–500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang M, Zha Q, Zhang C, Lu C, Yan X, Zhu
W, Liu W, Tu S, Hou L, Wang C, et al: Predicting and verifying
outcome of Tripterygium wilfordii Hook F. based therapy in
rheumatoid arthritis: From open to double-blinded randomized trial.
Sci Rep. 5:97002015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv QW, Zhang W, Shi Q, Zheng WJ, Li X,
Chen H, Wu QJ, Jiang WL, Li HB, Gong L, et al: Comparison of
Tripterygium wilfordii Hook F with methotrexate in the
treatment of active rheumatoid arthritis (TRIFRA): A randomised,
controlled clinical trial. Ann Rheum Dis. 74:1078–1086. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun J, Shen X, Dong J, Wang H, Zuo L, Zhao
J, Zhu W, Li Y, Gong J and Li J: Tripterygium wilfordii Hook
F as maintenance treatment for Crohn's disease. Am J Med Sci.
350:345–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu W, Li Y, Gong J, Zuo L, Zhang W, Cao
L, Gu L, Guo Z, Li N and Li J: Tripterygium wilfordii Hook.
f. versus azathioprine for prevention of postoperative recurrence
in patients with Crohn's disease: A randomized clinical trial. Dig
Liver Dis. 47:14–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Venkatesha SH and Moudgil KD: Celastrol
and its role in controlling chronic diseases. Adv Exp Med Biol.
928:267–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Allison AC, Cacabelos R, Lombardi VR,
Alvarez XA and Vigo C: Celastrol, a potent antioxidant and
anti-inflammatory drug, as a possible treatment for Alzheimer's
disease. Prog Neuropsychopharmacol Biol Psychiatry. 25:1341–1357.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cleren C, Calingasan NY, Chen J and Beal
MF: Celastrol protects against MPTP- and 3-nitropropionic
acid-induced neurotoxicity. J Neurochem. 94:995–1004. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Venkatesha SH, Yu H, Rajaiah R, Tong L and
Moudgil KD: Celastrus-derived celastrol suppresses autoimmune
arthritis by modulating antigen-induced cellular and humoral
effector responses. J Biol Chem. 286:15138–15146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Tao W, Jiang F, Li C, Lin J and Liu
C: Celastrol attenuates hypertension-induced inflammation and
oxidative stress in vascular smooth muscle cells via induction of
heme oxygenase-1. Am J Hypertens. 23:895–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bakar Abu MH, Sarmidi MR, Tan JS and Rosdi
Mohamad MN: Celastrol attenuates mitochondrial dysfunction and
inflammation in palmitate-mediated insulin resistance in C3A
hepatocytes. Eur J Pharmacol. 799:73–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Lee J, Hernandez Salazar MA,
Mazitschek R and Ozcan U: Treatment of obesity with celastrol.
Cell. 161:999–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Avilla J, Teixidò A, Velázquez C,
Alvarenga N, Ferro E and Canela R: Insecticidal activity of
Maytenus species (Celastraceae) nortriterpene quinone methides
against codling moth, Cydia pomonella (L.) (Lepidoptera:
Tortricidae). J Agric Food Chem. 48:88–92. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Sethi G, Loke WK and Sim MK:
Des-aspartate-angiotensin i attenuates mortality of mice exposed to
gamma radiation via a novel mechanism of action. PLoS One.
10:e01380092015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Howard BJ, Fesenko S, Balonov M, Pröhl G
and Nakayama S: A comparison of remediation after the Chernobyl and
Fukushima Daiichi accidents. Radiat Prot Dosimetry. 173:170–176.
2017.PubMed/NCBI
|
|
24
|
Ghosh SP, Kulkarni S, Perkins MW, Hieber
K, Pessu RL, Gambles K, Maniar M, Kao TC, Seed TM and Kumar KS:
Amelioration of radiation-induced hematopoietic and
gastrointestinal damage by Ex-RAD(R) in mice. J Radiat Res.
53:526–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ran XZ, Ran X, Zong ZW, Liu DQ, Xiang GM,
Su YP and Zheng HE: Protective effect of atorvastatin on
radiation-induced vascular endothelial cell injury in vitro. J
Radiat Res. 51:527–533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Desouky W, Hanafi A and Abbas MM:
Radioprotective effect of green tea and grape seed extracts mixture
on gamma irradiation induced immune suppression in male albino
rats. Int J Radiat Biol. 93:433–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lata M, Prasad J, Singh S, Kumar R, Singh
L, Chaudhary P, Arora R, Chawla R, Tyagi S, Soni NL, et al: Whole
body protection against lethal ionizing radiation in mice by
REC-2001: A semi-purified fraction of Podophyllum hexandrum.
Phytomedicine. 16:47–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sankhwar S, Gupta ML, Gupta V, Verma S,
Suri KA, Devi M, Sharma P, Khan EA and Alam MS: Podophyllum
hexandrum-mediated survival protection and restoration of other
cellular injuries in lethally irradiated mice. Evid Based
Complement Alternat Med. 2011:1751402011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dutta A, Verma S, Sankhwar S, Flora SJ and
Gupta ML: Bioavailability, antioxidant and non toxic properties of
a radioprotective formulation prepared from isolated compounds of
Podophyllum hexandrum: A study in mouse model. Cell Mol Biol
(Noisy-le-grand). 58 Suppl:OL1646–OL1653. 2012.PubMed/NCBI
|
|
30
|
Saini R, Verma S, Singh A and Gupta Lata
M: Role of active principles of podophyllum hexandrum in
amelioration of radiation mediated lung injuries by reactive
oxygen/nitrogen species reduction. CellBio. 2:pp105–116. 2013.
View Article : Google Scholar
|
|
31
|
Sandeep D and Nair CK: Protection of DNA
and membrane from γ-radiation induced damage by the extract of
Acorus calamus Linn.: An in vitro study. Environ Toxicol
Pharmacol. 29:302–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sandeep D and Nair CK: Protection from
lethal and sub-lethal whole body exposures of mice to γ-radiation
by Acorus calamus L.: Studies on tissue antioxidant status
and cellular DNA damage. Exp Toxicol Pathol. 64:57–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan H, Ashour A, Katakura Y and Shimizu K:
A structure-activity relationship study on antiosteoclastogenesis
effect of triterpenoids from the leaves of loquat (Eriobotrya
japonica). Phytomedicine. 22:498–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang WJ, Wang J, Tong X, Shi JB, Liu XH
and Li J: Design and synthesis of celastrol derivatives as
anticancer agents. Eur J Med Chem. 95:166–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Z, Zhai Z and Du X: Celastrol
inhibits migration and invasion through blocking the NF-κB pathway
in ovarian cancer cells. Exp Ther Med. 14:819–824. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schultz-Hector S and Trott KR:
Radiation-induced cardiovascular diseases: Is the epidemiologic
evidence compatible with the radiobiologic data? Int J Radiat Oncol
Biol Phys. 67:10–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hwang S, Lee DH, Lee IK, Park YM and Jo I:
Far-infrared radiation inhibits proliferation, migration, and
angiogenesis of human umbilical vein endothelial cells by
suppressing secretory clusterin levels. Cancer Lett. 346:74–83.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sinha M, Das DK, Manna K, Datta S, Ray T,
Sil AK and Dey S: Epicatechin ameliorates ionising
radiation-induced oxidative stress in mouse liver. Free Radic Res.
46:842–849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu J, Zhu X, Qi X, Che J and Cao B:
Paeoniflorin protects human EA.hy926 endothelial cells against
gamma-radiation induced oxidative injury by activating the
NF-E2-related factor 2/heme oxygenase-1 pathway. Toxicol Lett.
218:224–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu J, Piao BK, Pei YX, Qi X and Hua BJ:
Protective effects of tetrahydropalmatine against gamma-radiation
induced damage to human endothelial cells. Life Sci. 87:55–63.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu S, Gao Y, Zhou H, Kong F, Xiao F, Zhou
P and Chen Y: New insight into mitochondrial changes in vascular
endothelial cells irradiated by gamma ray. Int J Radiat Biol.
93:470–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cervelli T, Panetta D, Navarra T,
Andreassi MG, Basta G, Galli A, Salvadori PA, Picano E and Del
Turco S: Effects of single and fractionated low-dose irradiation on
vascular endothelial cells. Atherosclerosis. 235:510–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Olteanu D, Baldea I, Clichici S, Bolfa P,
Cenariu M, Schrepler-Perde M, Alupei M, Muresan A and Filip A: In
vitro studies on the mechanisms involved in chemoprevention using
Calluna vulgaris on vascular endothelial cells exposed to
UVB. J Photochem Photobiol B. 136:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y,
Meng G, Xie L, Wang J, Xiao Y, et al: Celastrol prevents
atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS
One. 8:e654772013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guan Y, Cui ZJ, Sun B, Han LP, Li CJ and
Chen LM: Celastrol attenuates oxidative stress in the skeletal
muscle of diabetic rats by regulating the AMPK-PGC1α-SIRT3
signaling pathway. Int J Mol Med. 37:1229–1238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shaker ME, Ashamallah SA and Houssen ME:
Celastrol ameliorates murine colitis via modulating oxidative
stress, inflammatory cytokines and intestinal homeostasis. Chem
Biol Interact. 210:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jin HZ, Hwang BY, Kim HS, Lee JH, Kim YH
and Lee JJ: Antiinflammatory constituents of Celastrus orbiculatus
inhibit the NF-kappaB activation and NO production. J Nat Prod.
65:89–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jung HW, Chung YS, Kim YS and Park YK:
Celastrol inhibits production of nitric oxide and proinflammatory
cytokines through MAPK signal transduction and NF-kappaB in
LPS-stimulated BV-2 microglial cells. Exp Mol Med. 39:715–721.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sassa H, Takaishi Y and Terada H: The
triterpene celastrol as a very potent inhibitor of lipid
peroxidation in mitochondria. Biochem Biophys Res Commun.
172:890–897. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ameziane-El-Hassani R, Talbot M, de Souza
Dos Santos MC, Al Ghuzlan A, Hartl D, Bidart JM, De Deken X, Miot
F, Diallo I, de Vathaire F, et al: NADPH oxidase DUOX1 promotes
long-term persistence of oxidative stress after an exposure to
irradiation. Proc Natl Acad Sci USA. 112:5051–5056. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shaban NZ, Zahran Ahmed AM, El-Rashidy FH
and Kodous Abdo AS: Protective role of hesperidin against
γ-radiation-induced oxidative stress and apoptosis in rat testis. J
Biol Res (Thessalon). 24:52017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Divya T, Dineshbabu V, Soumyakrishnan S,
Sureshkumar A and Sudhandiran G: Celastrol enhances Nrf2 mediated
antioxidant enzymes and exhibits anti-fibrotic effect through
regulation of collagen production against bleomycin-induced
pulmonary fibrosis. Chem Biol Interact. 246:52–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang C, Shi C, Yang X, Yang M, Sun H and
Wang C: Celastrol suppresses obesity process via increasing
antioxidant capacity and improving lipid metabolism. Eur J
Pharmacol. 744:52–58. 2014. View Article : Google Scholar : PubMed/NCBI
|