Introduction
Lung cancer remains one of the major causes of
cancer-related mortality worldwide (1). In the past decade, tyrosine-kinase
inhibitors, immunotherapy and chemotherapy have been the primary
treatments for lung cancer, resulting in a median progression-free
survival time of ~6 months and a response rate of ~30%, which
appears to have reached a plateau of effectiveness in improving
survival (2). Although the survival
of patients with lung cancer has been improved with the emergence
of these treatments, novel issues continue to arise, such as drug
resistance and tumor recurrence. Therefore, significant advances
are eagerly awaited (3), and there
remains an urgent requirement to develop novel targeted drugs in
order to improve patient outcomes.
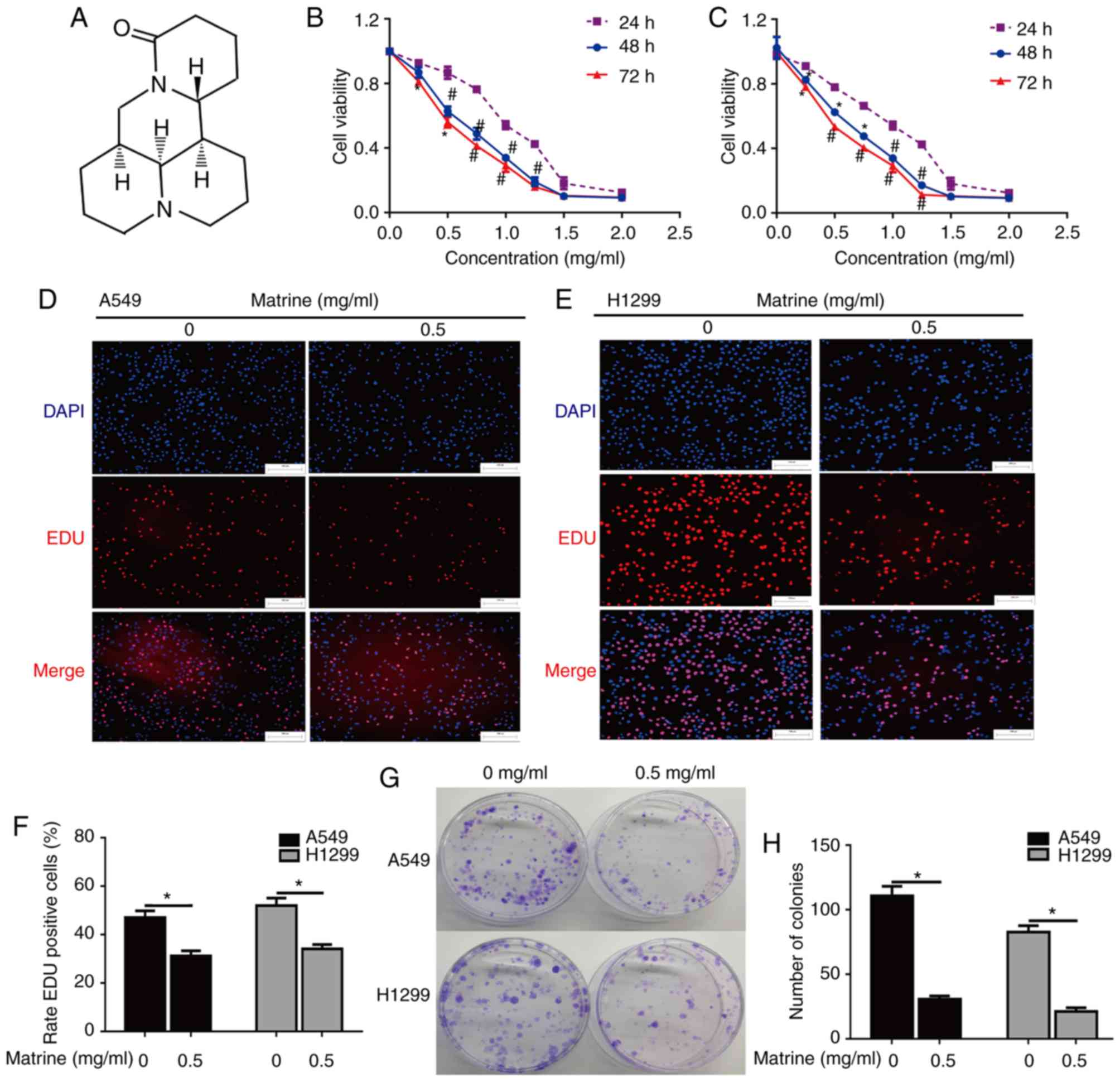
Matrine (C15H24N2O;
Fig. 1A), a natural component from
the traditional Chinese medical herb Sophora flavescens,
exhibits multiple pharmacological properties, including
anti-inflammatory, anti-tumor and anti-fibrotic effects (4–6). The
mechanisms of its anti-tumor activity are complex, and include cell
proliferation, migration and apoptosis (7). Previous studies have demonstrated that
matrine is able to negatively regulate the phosphoinositide
3-kinase (PI3K)/protein kinase B (Akt), Wnt and mitogen activated
protein kinase (MAPK) signaling pathways in a number of human tumor
types, including hepatocellular carcinoma (8), pancreatic cancer (9), and nasopharyngeal carcinoma (10). The Akt/glycogen synthase kinase-3β
(GSK-3β) signaling pathway has been demonstrated to regulate
important genes associated with proliferation and invasion, and
thus control the growth and invasion of lung cancer cells (11,12).
However, the effect of matrine on lung cancer cell growth and
invasion in vitro, and whether the anti-tumor mechanisms of
matrine are associated with the Akt/GSK-3β signaling pathway remain
unclear.
In the present study, the effect of matrine on lung
cancer was investigated in the A549 and H1299 cell lines, in order
to clarify the underlying mechanism by which matrine inhibited the
proliferation and induced the apoptotic ability of lung cancer.
Materials and methods
Reagents and antibodies
Matrine (>95% purity; Shanghai Yuanye
Biotechnology Co., Ltd., Shanghai, China) was dissolved in PBS (10
mg/ml). Antibodies against phosphorylated (p)-Akt (Thr308)
(sc-16646-R; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Akt
(2620S; Cell Signaling Technology, Inc., Danvers, MA, USA), β-actin
(66009–1-Ig; Proteintech Group, Inc., Chicago, IL, USA), GSK-3β
(9832; Cell Signaling Technology, Inc.), p-GSK-3β (9323; Cell
Signaling Technology, Inc.) were used in the present study. The
secondary anti-rabbit-horseradish peroxidase (HRP) (ab6721) or
anti-mouse-HRP antibodies (ab6728) were purchased from Abcam
(Cambridge, UK).
Cell culture
A549 and H1299 human lung cancer cell lines were
purchased from the cell bank of the Chinese Academy of Science
(Shanghai, China). Lung cancer cells were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin, at 37°C in a humidified incubator
containing 5% CO2.
Cell proliferation and colony
formation assay
A549 or H1299 cells (2,000 cells/well) were seeded
into 96-well plates and treated with matrine (0, 0.5, 1, 1.5 and 2
mg/ml) for 24, 48, and 72 h at 37°C. A Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay was
used to evaluate cell proliferation and viability according to the
manufacturer's protocol. For the colony formation assay, 1,000
cells in DMEM containing 10% FBS and 0.5 mg/ml matrine were seeded
into 35-mm plates at 37°C. After 14 days, the colonies were fixed
in 100% methanol at 25°C for 15 min, and stained with crystal
violet at 25°C for 10 min. All procedures were performed in
triplicate.
Cell proliferation
5-ethynyl-2′-deoxyuridine (EdU) assay
Proliferation of A549 and H1299 were assessed using
an EdU assay kit (Guangzhou RiboBio Co., Ltd., Guangzhou, China).
Briefly, 2×104 cells in DMEM containing 10% FBS were
treated with 0.5 mg/ml matrine or PBS in 24-well plates at 37°C for
24 h. Subsequently, the cells were incubated with EdU (50 µM) at
37°C. After 2 h, the cells were fixed in 4% formaldehyde at 25°C
for 30 min and permeabilized with 0.5% Triton X-100 at 25°C for 1
min. Following washing with PBS for 5 min, Apollo®
reaction solution from the EdU assay kit (100 µl) was added for 30
min at 25°C. Finally, the cells were incubated with Hoechst 33342
(100 µl) at 25°C for 30 min to stain the nucleus. The results of
the cell proliferation assays were quantified with Image-Pro Plus
software (6.0; Media Cybernetics, Inc., Rockville, MD, USA) and the
ratio of Apollo-positive cells to Hoechst 33342-positive cells was
recorded.
Apoptosis analysis
A549 or H1299 cells (5×104) in DMEM
containing 10% FBS were treated with matrine (0.5 mg/ml) or PBS at
37°C for 24 h. Apoptosis was detected using a Hoechst 33258
fluorescence staining kit (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocol. The effect
of matrine on cellular apoptosis was also evaluated using an
Annexin-V Apoptosis Detection kit FITC (ebioscience; Thermo Fisher
Scientific, Inc.) based on the manufacturer's protocol and
apoptosis was determined using a flow cytometer. The results of
cell apoptosis were analyzed with FlowJo software (10.0.7; FlowJo
LLC, Ashland, OR, USA).
Wound-healing, migration and invasion
assays
For the wound-healing assay, 2×105 cells
(A549 and H1299) were plated in culture-insert wells (Ibidi GmbH,
Martinsried, Germany) with DMEM containing 10% FBS at 37°C for 24
h. The culture-insert was subsequently removed and fresh DMEM
containing 0.5 mg/ml matrine or an equal amount of PBS was added.
The width of the healing monolayer wound was recorded after 24 h.
For the migration assay, 3×104 cells in DMEM were seeded
into the upper chambers of Transwell plates (Corning, Inc.,
Corning, NY, USA). Complete medium containing 10% FBS in the bottom
chamber was used as a chemoattractant, and 0.5 mg/ml matrine was
added to inhibit cell migration. For the invasion assay,
5×104 cells in DMEM were seeded in the upper chambers of
Transwell plates with 10% Matrigel (Corning, Inc.) at 37°C for 6 h.
Following, DMEM with 10% FBS was used in the bottom chamber as a
chemoattractant and 0.5 mg/ml matrine was added to assess cell
invasion. Following 24 h migration or invasion at 37°C, cells on
the lower membrane of inserts were fixed in 100% methanol at 25°C
for 15 min, stained with crystal violet at 25°C for 10 min and
counted using light microscopy (magnification, ×100).
Western blotting
Western blotting was performed as previously
described (5). Briefly, cell samples
were lysed with RIPA buffer (Beyotime, Tianjin, China) and the
concentration of total protein was determined using a BCA kit
(Beyotime). A total of 30 µg protein from each sample was separated
by 10% SDS-PAGE gels and transferred to a polyvinylidene difluoride
membrane. Membranes were blocked in 5% milk in TBS-Tween-20 (TBS-T)
at 25°C for 1 h and incubated overnight at 4°C with primary
antibodies against Akt (1:1,000), p-Akt (Thr308) (1:1,000), GSK-3β
(1:1,000), p-GSK-3β (1:1,000) and β-actin (1:2,000). Following
washing in TBS-T, membranes were incubated with HRP-conjugated
anti-mouse (1:2,000) or anti-rabbit (1:2,000) secondary antibodies
at 25°C for 2 h prior to visualization with ECL detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Densitometry
analysis was performed using Image J software (1.48; National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using Tukey's post hoc test
following one-way analysis of variance. Differences between two
groups were evaluated for significance using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Matrine inhibits lung cancer cell
proliferation and colony formation
To elucidate whether matrine inhibits the growth of
lung cancer cells, a CCK-8 assay was used to analyze the
proliferation of A549 and H1299 cells treated with different doses
of matrine for 24, 48 and 72 h. The results demonstrated that
matrine significantly inhibited cell growth, and that this
inhibition was markedly increased with increasing matrine
concentration (Fig. 1B and C). The
EdU incorporation assay was also performed for more sensitive and
specific evaluation of the effect of matrine on proliferation. As
presented in Fig. 1D-F, the number
of EdU-incorporating cells in A549 and H1299 groups exposed to 0.5
mg/ml matrine was significantly decreased compared with the
corresponding control groups, suggesting that matrine treatment
resulted in a significant suppression of cell proliferation. The
effect of matrine on colony formation was further examined in lung
cancer cells via a clonogenic assay. The results demonstrated that
matrine significantly reduced the number of colonies-formed
compared with the control groups. (Fig.
1G and H).
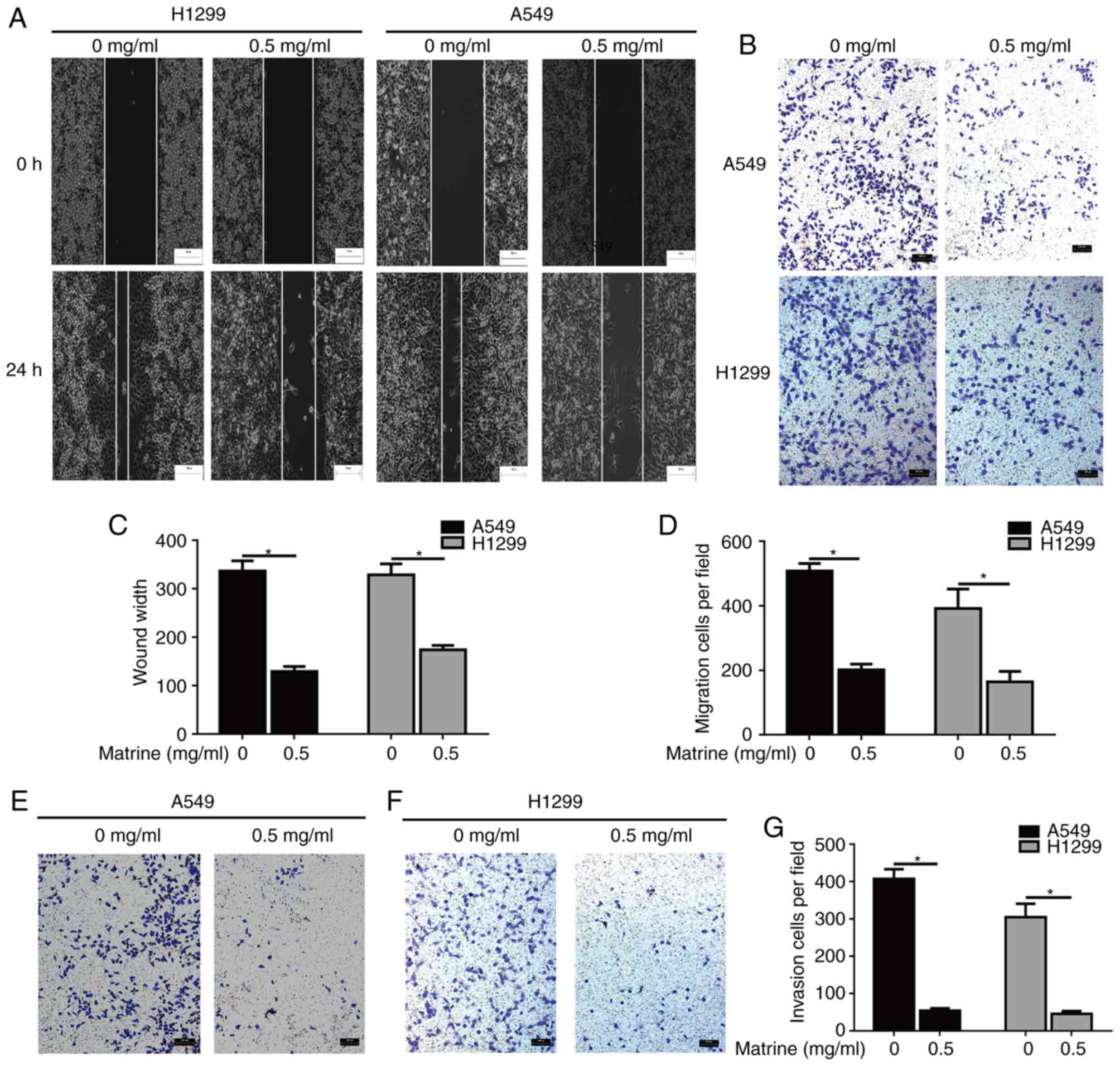
Matrine inhibits migration and
invasion in lung cancer cells
To evaluate whether matrine was able to reduce the
mobility of lung cancer cells, wound-healing and Transwell assays
were performed. The results of the wound-healing and Transwell
assays demonstrated that matrine significantly inhibited the
migration of A549 and H1299 cells (Fig.
2A-D). In addition, the effects of matrine on invasion were
also evaluated, and it was observed that matrine treatment resulted
in a significant decrease in the number of invasive lung cancer
cells (Fig. 2E-G).
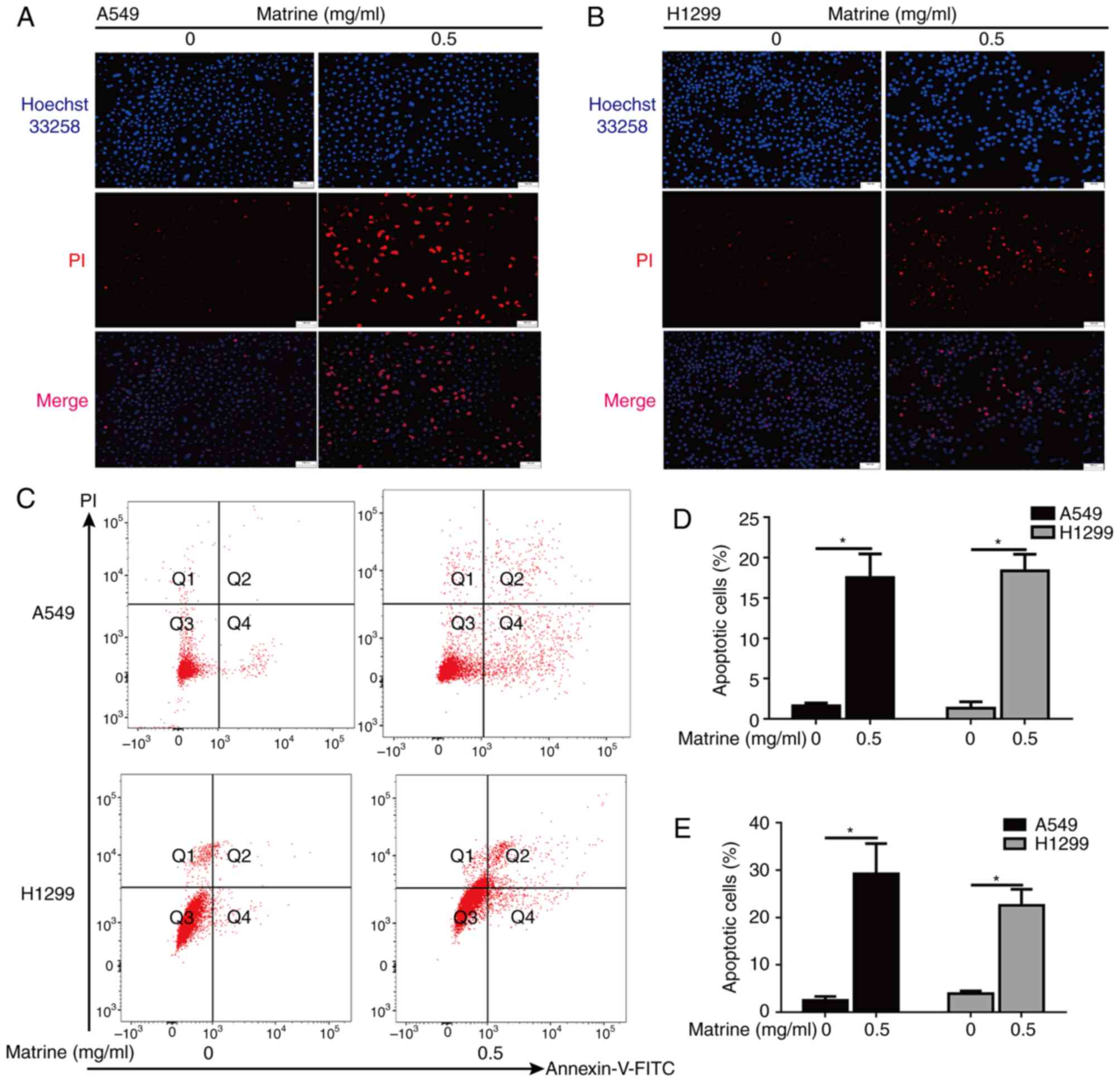
Matrine induces lung cancer cellular
apoptosis
Hoechst 33342 staining of the matrine-treated A549
and H1299 cells revealed a condensed or fragmented chromatin
staining pattern with brilliant blue fluorescent dots in the cell
nuclei, as compared with a uniformly blue staining pattern in the
control cells (Fig. 3A and B). In
addition, the effects of matrine on apoptosis were further assessed
by flow cytometry assays (Fig. 3C).
Analysis of both experiments indicated that matrine induced a
significant increase in the number of apoptotic cells (Fig. 3D and E).
Matrine inhibits the Akt GSK-3β
signaling pathway in lung cancer cells
To elucidate the molecular mechanism underlying the
suppression of proliferation and migration induced by matrine in
lung cancer cells, western blotting was performed to analyze the
classical signaling pathways associated with proliferation and
migration. The degree of Akt phosphorylation at Thr308 was
significantly inhibited in the matrine-treated cells compared with
in the control groups, whereas no significant change was observed
in the total Akt levels. Furthermore, matrine downregulated
p-GSK-3β/GSK-3β protein expression in A549 and H1299 cells
(Fig. 4). These results indicated
that the anti-cancer effects of matrine may be associated with the
Akt/GSK-3β pathway.
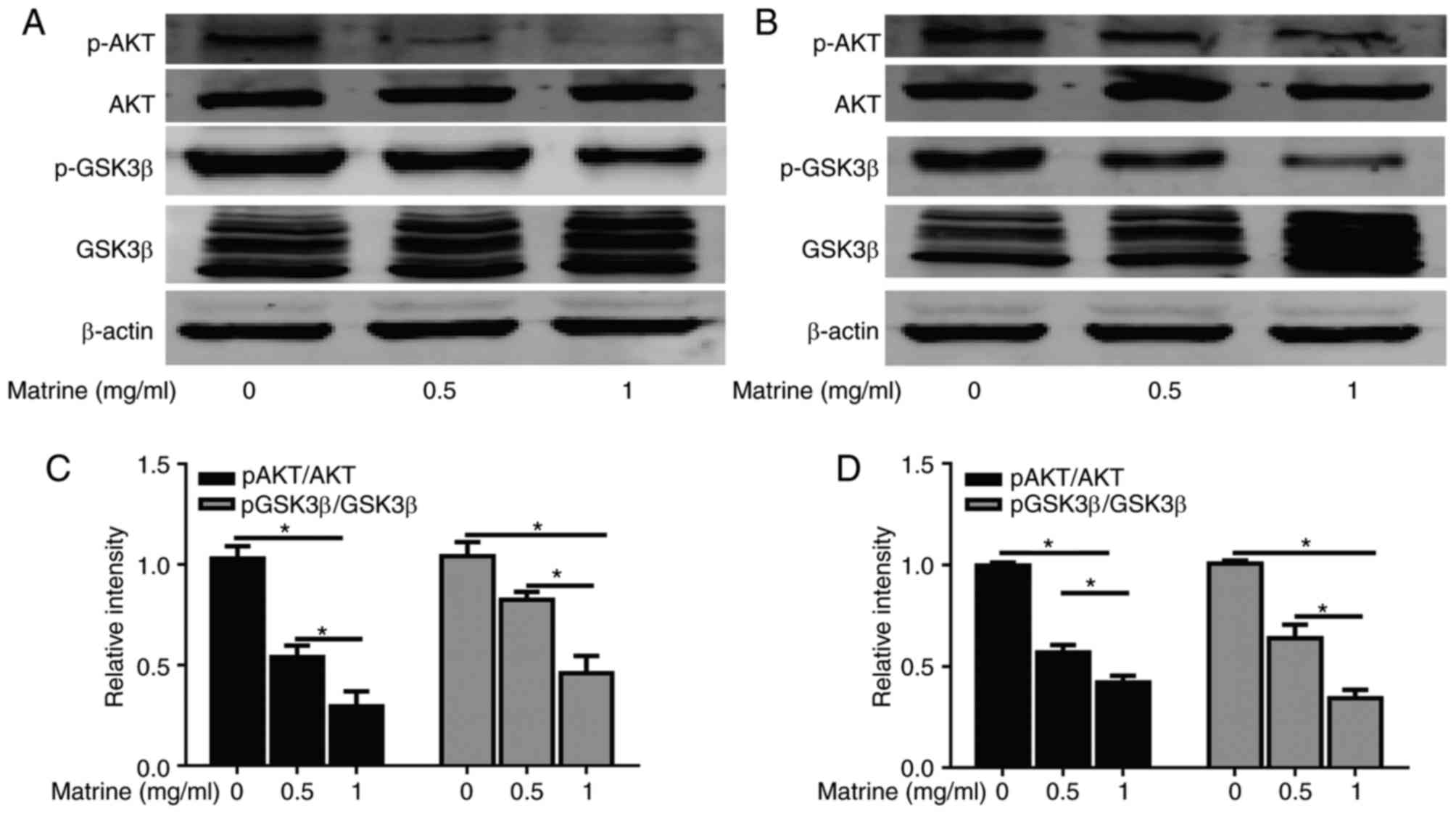 | Figure 4.Matrine regulates the Akt/GSK-3β
pathway in A549 and H1299 cells. (A) Akt, p-Akt, GSK-3β, and
p-GSK-3β (Ser9) protein expression levels in A549 cells following
the administration of various doses of matrine, evaluated by
immunoblotting with β-actin loading control. (B) Akt, p-Akt, GSK,
and p-GSK-3β (Ser9) protein levels in H1299 cells following
administration of various doses of matrine, evaluated by
immunoblotting with β-actin loading control. Quantified results of
the p-Akt/Akt and p-GSK-3β/GSK-3β ratios in (C) A549 and (D) H1299
cells following administration of matrine. Data are presented as
the mean ± standard deviation (n=3). *P<0.05. Akt, protein
kinase B; GSK-3β, glycogen synthase kinase-3β; p,
phosphorylated. |
Discussion
Matrine is an extract of Sophora flavescens,
which has been shown to have inhibitory effects on numerous tumor
types. However, the specific mechanisms underlying these inhibitory
effects remains unclear, particularly in lung cancer. In the
present study, the inhibitory effects of matrine on the human lung
cancer cell lines A549 and H1299 were evaluated, and it was
observed that matrine inhibited lung cancer cell proliferation, and
induced apoptosis by blocking the Akt/GSK-3β pathway.
Previous studies have demonstrated that the
combination of matrine and standard therapies can significantly
improve the quality of life and prognosis of patients with cancer,
suggesting its potential as an anti-cancer drug (13,14).
Additionally, it is well documented that matrine can inhibit the
proliferation of a variety of cancer cells (15,16). In
the present study, it was demonstrated that matrine significantly
inhibited the proliferation and colony formation of A549 and H1299
cells. Similar results were recently observed with the matrine
derivative YF-18 in A549, H1975 and 95D cells (17). Migration and invasion are important
indices for evaluating lung cancer malignancy, and are associated
with the adhesion between cancer cells and the basement membrane,
the depletion of the extracellular matrix and the formation of
metastases (18–20). In the present study, the effect of
matrine on the migration and invasion of lung cancer cells was
evaluated using Transwell assays, and it was demonstrated that
matrine could markedly inhibit cell mobility, as well as migration
and invasion. The results were consistent with the lower mobility
observed in prostate (4) and
cervical cancer cells (21).
However, the mechanisms underlying the inhibitory effect of matrine
on tumor invasion and migration remain unclear, and may be
associated with the following approaches: Inhibited degradation of
proteolytic enzymes on the basement membrane and extracellular
matrix (22); adhesion of lung
cancer cells to the extracellular matrix (23); tumor angiogenesis; and the
regulation-specific genes associated with cancer metastasis
(24). It was also evaluated whether
matrine was able to induce apoptosis in lung cancer cells via
Hoechst 33342 staining and FACS analysis. The results demonstrated
that the number of apoptotic A549 and H1299 cells following
exposure to 0.5 mg/ml matrine, was significantly increased,
suggesting that the anti-proliferative effect of matrine on lung
cancer cells is associated with cellular apoptosis.
The Akt/GSK-3β pathway has a critical regulatory
role in the signal transduction activity associated with cell
proliferation, apoptosis, differentiation and survival (25). Deregulation of the Akt/GSK-3β
signaling pathways has been implicated in the initiation and
progression of various human malignancies. When the MAPK and
PI3k/Akt pathways are blocked, the proliferation, invasion and
metastasis of cancer cells has been demonstrated to be
significantly inhibited (26,27). In
addition, previous studies have demonstrated that matrine regulates
the Akt signaling pathway, and inhibits hepatic stellate cell
activation and dendritic cell maturation (28,29).
Consistent with these studies, the present results demonstrated
that matrine suppresses the Akt pathway by reducing the
phosphorylation of Akt in lung cancer cells in a dose-dependent
manner, which suggests that the inhibitory effect of matrine on
these lung cancer cells was achieved via downregulation of the Akt
pathway. In addition, a recent study demonstrated that matrine also
modulates the Wnt/β-catenin self-renewal pathway, further resulting
in the increased phosphorylation of β-catenin (Ser33/Ser37/Thr41),
and that decreasing β-catenin levels, as well as its target gene
Cyclin D1, ultimately suppressed cancer proliferation (30). In the present study, the effects of
matrine on non-epidermal growth factor receptor (EGFR) mutant A549
and H1299 cell lines originated from epithelial and mesenchymal
lung adenocarcinomas was investigated (31), and it was observed that matrine was
able to inhibit the Akt pathway and subsequent GSK-3β activation,
in order to ultimately inhibit proliferation and induce apoptosis
in lung cancer cells. The phenomenon that epithelial cells
transform to mesenchymal phenotype is closely associated with
carcinogenicity, metastasis and poor prognosis in a number of tumor
types, including non-small-cell lung carcinoma (NSCLC) (32,33).
Further studies will investigate the effect of matrine on
EGFR-mutant NSCLC cells and clarify the exact role of such
downregulation in the inhibition of lung cancer cells by
matrine.
In conclusion, the present findings suggest that
matrine inhibits lung cancer cell proliferation, and induces cell
apoptosis by suppressing the Akt/GSK-3β signaling pathway, offering
possible mechanisms for its antitumor activity. The present study
provides a foundation for further preclinical and clinical
evaluations of matrine as a lung cancer therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81370174).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZG developed the idea for the study, XW, WX, JL, QL,
JH and HL did the analyses and XW and HL wrote the paper. All
authors have reviewed and approved the final version of the
manuscript and have consented to its publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hong X, Chen Q, Ding L, Liang Y, Zhou N,
Fang W, Chen X and Wu H: Clinical benefit of continuing crizotinib
therapy after initial disease progression in Chinese patients with
advanced ALK-rearranged non-small-cell lung cancer. Oncotarget.
8:41631–41640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pearce A, Bradley C, Hanly P, O'Neill C,
Thomas AA, Molcho M and Sharp L: Projecting productivity losses for
cancer-related mortality 2011–2030. Bmc Cancer. 16:8042016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Midha A, Dearden S and McCormack R: EGFR
mutation incidence in non-small-cell lung cancer of adenocarcinoma
histology: A systematic review and global map by ethnicity
(mutMapII). Am J Cancer Res. 5:2892–2911. 2015.PubMed/NCBI
|
|
4
|
Huang H, Du T, Xu G, Lai Y, Fan X, Chen X,
Li W, Yue F, Li Q, Liu L and Li K: Matrine suppresses invasion of
castration-resistant prostate cancer cells by downregulating
MMP-2/9 via NF-κB signaling pathway. Int J Oncol. 50:640–648. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Zhang Y, Tang Z, Xu J, Ma M, Pan S,
Qiu C, Guan G and Wang J: Matrine attenuates cardiac fibrosis by
affecting ATF6 signaling pathway in diabetic cardiomyopathy. Eur J
Pharmacol. 804:21–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang WC, Chan CC, Wu SJ, Chen LC, Shen
JJ, Kuo ML, Chen MC and Liou CJ: Matrine attenuates allergic airway
inflammation and eosinophil infiltration by suppressing eotaxin and
Th2 cytokine production in asthmatic mice. J Ethnopharmacol.
151:470–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yong J, Wu X and Lu C: Anticancer advances
of matrine and its derivatives. Curr Pharm Des. 21:3673–3680. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qian L, Liu Y, Xu Y, Ji W, Wu Q, Liu Y,
Gao Q and Su C: Matrine derivative WM130 inhibits hepatocellular
carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling
pathways. Cancer Lett. 368:126–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma Y, Zou F, Xiong J, Wan W, Yin L, Li X,
Bei Z, Yuan L, Meng S, Wang J and Song G: Effect of Matrine on HPAC
cell migration by down-regulating the expression of MT1-MMP via Wnt
signaling. Cancer Cell Int. 15:592015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie M, Yi X, Wang R, Wang L, He G, Zhu M,
Qi C, Liu Y, Ye Y, Tan S and Tang A: 14-Thienyl methylene matrine
(YYJ18), the derivative from matrine, induces apoptosis of human
nasopharyngeal carcinoma cells by targeting MAPK and PI3K/Akt
pathways in vitro. Cell Physiol Biochem. 33:1475–1483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei L, Yao Y, Zhao K, Huang Y, Zhou Y,
Zhao L, Guo Q and Lu N: Oroxylin A inhibits invasion and migration
through suppressing ERK/GSK-3β signaling in snail-expressing
non-small-cell lung cancer cells. Mol Carcinog. 55:2121–2134. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho MY, Liang SM, Hung SW and Liang CM:
MIG-7 controls COX-2/PGE2-mediated lung cancer metastasis. Cancer
Res. 73:439–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Mei Q, Xu YC, Du J, Wei Y and Xu
ZM: Effects of Matrine Injection on T-lymphocyte subsets of
patients with malignant tumor after gamma knife radiosurgery. Zhong
Xi Yi Jie He Xue Bao. 4:78–79. 2006.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin H, Que R, Liu C, Ji W, Sun B, Lin X,
Zhang Q, Zhao X, Peng Z, Zhang X, et al: Survivin-targeted drug
screening platform identifies a matrine derivative WM-127 as a
potential therapeutics against hepatocellular carcinoma. Cancer
Lett. 425:54–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu L, Wang G, Liu S, Wei J, Zhang S, Li M,
Zhou G and Wang L: Synthesis and biological evaluation of matrine
derivatives containing benzo-α-pyrone structure as potent anti-lung
cancer agents. Sci Rep. 6:359182016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou N, Li J, Li T, Chen G, Zhang Z and Si
Z: Matrine-induced apoptosis in Hep3B cells via the inhibition of
MDM2. Mol Med Rep. 15:442–450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu L, Wang G, Wei J, Huang N, Zhang S,
Yang F, Li M, Zhou G and Wang L: Matrine derivative YF-18 inhibits
lung cancer cell proliferation and migration through
down-regulating Skp2. Oncotarget. 8:11729–11738. 2017.PubMed/NCBI
|
|
18
|
Ali M, Wu Y, Ghosh D, Do BH, Chen K,
Dawson MR, Fang N, Sulchek TA and El-Sayed MA: Nuclear
membrane-targeted gold nanoparticles inhibit cancer cell migration
and invasion. ACS Nano. 11:3716–3726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Labernadie A, Kato T, Brugués A,
Serra-Picamal X, Derzsi S, Arwert E, Weston A, González-Tarragó V,
Elosegui-Artola A, Albertazzi L, et al: A mechanically active
heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to
drive cancer cell invasion. Nat Cell Biol. 19:224–237. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jacquemet G, Baghirov H, Georgiadou M,
Sihto H, Peuhu E, Cettour-Janet P, He T, Perälä M, Kronqvist P,
Joensuu H and Ivaska J: L-type calcium channels regulate filopodia
stability and cancer cell invasion downstream of integrin
signalling. Nat Commun. 7:132972016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Zhou J, Cai D and Li M: Matrine
inhibits the metastatic properties of human cervical cancer cells
via downregulating the p38 signaling pathway. Oncol Rep.
38:1312–1320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang SF, Yang WE, Kuo WH, Chang HR, Chu SC
and Hsieh YS: Antimetastatic potentials of flavones on oral cancer
cell via an inhibition of matrix-degrading proteases. Arch Oral
Biol. 53:287–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou LE, Wang WJ, Bai JY and Cheng GF:
Effects of ginkgolide B on arachidonic acid metabolizing enzymes
and level of intracellular calcium in rat polymorphonuclear
leukocytes. Yao Xue Xue Bao. 36:92–95. 2001.(In Chinese).
PubMed/NCBI
|
|
24
|
Li H, Tan G, Jiang X, Qiao H, Pan S, Jiang
H, Kanwar JR and Sun X: Therapeutic effects of matrine on primary
and metastatic breast cancer. Am J Chin Med. 38:1115–1130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davis NM, Sokolosky M, Stadelman K, Abrams
SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D'Assoro
A, et al: Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in
breast cancer: Possibilities for therapeutic intervention.
Oncotarget. 5:4603–4650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sagredo AI, Sagredo EA, Cappelli C, Báez
P, Rodrigo AM, Blanco C, Tapia JC, Echeverria C, Cerda O, Stutzin
A, et al: TRPM4 regulates Akt/GSK3-β activity and enhances
β-catenin signaling and cell proliferation in prostate cancer
cells. Mol Oncol. 12:151–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang PP, Wang PQ, Qiao CP, Zhang Q, Zhang
JP, Chen F, Zhang X, Xie WF, Yuan ZL, Li ZS and Chen YX:
Differentiation therapy of hepatocellular carcinoma by inhibiting
the activity of AKT/GSK-3β/β-catenin axis and TGF-β induced EMT
with sophocarpine. Cancer Lett. 376:95–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu J, Hu G, Dong Y, Ma R, Yu Z, Jiang S,
Han Y, Yu K and Zhang S: Matrine induces Akt/mTOR signalling
inhibition-mediated autophagy and apoptosis in acute myeloid
leukaemia cells. J Cell Mol Med. 21:1171–1181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niu H, Zhang Y, Wu B, Zhang Y, Jiang H and
He P: Matrine induces the apoptosis of lung cancer cells through
downregulation of inhibitor of apoptosis proteins and the Akt
signaling pathway. Oncol Rep. 32:1087–1093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hseu YC, Thiyagarajan V, Tsou HT, Lin KY,
Chen HJ, Lin CM, Liao JW and Yang HL: In vitro and in vivo
anti-tumor activity of CoQ0 against melanoma cells: inhibition of
metastasis and induction of cell-cycle arrest and apoptosis through
modulation of Wnt/β-catenin signaling pathways. Oncotarget.
7:22409–22426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu G, Yu H, Shi X, Sun L, Zhou Q, Zheng D,
Shi H, Li N, Zhang X and Shao G: Cisplatin sensitivity is enhanced
in non-small cell lung cancer cells by regulating
epithelial-mesenchymal transition through inhibition of eukaryotic
translation initiation factor 5A2. BMC Pulm Med. 14:1742014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Li YC, Wu L, Yu GT, Zhang WF,
Huang CF and Sun ZJ: TRAF6 regulates tumour metastasis through EMT
and CSC phenotypes in head and neck squamous cell carcinoma. J Cell
Mol Med. 22:1337–1349. 2018.PubMed/NCBI
|
|
33
|
Liu L, Zhou XM, Yang FF, Miao Y, Yin Y, Hu
XJ, Hou G, Wang QY and Kang J: TRIM22 confers poor prognosis and
promotes epithelial-mesenchymal transition through regulation of
AKT/GSK3β/β-catenin signaling in non-small cell lung cancer.
Oncotarget. 8:62069–62080. 2017.PubMed/NCBI
|