Introduction
Arrhythmia recurrence is the most common
electrophysiological complication following atrial fibrillation
(AF) ablation, which occurs in up to 31% of patients undergoing
this procedure and frequently requires repeated ablation procedures
(1). Organized atrial tachycardias
(ATs) developing during ablation of AF are common, particularly
when extensive ablation strategies are employed (2–4). It is
important to understand the underlying mechanisms of tachycardia
for treatment and development of preventative strategies.
Macro-reentrant arrhythmias are the most common forms of ATs,
occurring in 34% of patients during ablation of persistent AF
(5). The most frequent mechanisms
are mitral annular and gap-associated macro-reentrant flutter
(6,7). Previous studies suggested
ridge-associated reentry of ATs and ATs utilizing the ligament of
Marshall as possible mechanisms (8,9). In
addition to macro-reentrant tachycardia, localized reentry or focal
ATs are relatively uncommon in patients following AF ablation
(10–13). Foci do not occur randomly throughout
the atria, but exhibit a tendency to cluster at characteristic
anatomical locations. A total of 63% of ATs originate from the
right atrium, while 37% are from the left atrium (13). Sites of origin within the right
atrium include: The crista terminalis, the tricuspid annulus, the
right atrial appendage, the ostium of the coronary sinus, and
within perinodal locations (14). In
the left atrium, the ostia of the pulmonary vein is a common
location for foci, exhibiting smaller numbers at the superior
aspect of the mitral annulus and body of the coronary sinus
(15). Additionally, left atrial
appendage (LAA) may be implicated in the maintenance of AF and ATs
(16–19).
The aims of AT mapping are to identify foci or
reentrant circuits of arrhythmia. The underlying mechanism of AT
can be inferred from activation maps. However, low voltage and
fractionated electrograms, commonly found in atria of redo-AF
patients are prone to incorrect assignment of local activation time
(20). Although tachycardia may be
recognized by entrainment, this risks its transformation or
termination, making local capture within scarred areas difficult
(21). A combined strategy using
activation mapping and entrainment is regularly utilized to
overcome these individual limitations (20).
Anatomic studies have demonstrated that the LA wall
is usually smooth, with the pectinate muscles being contained
mostly within the LAA (22–24). The response to RF applications in
pectinate muscle areas differ from those in smooth muscle areas due
to a lack of contact with the tissue and the thermal homeostatic
effect of blood on the pectinate muscles (19). However, the LAA has a very thin wall
and may be prone to perforation. Thus, caution should be exercised
when LAA ablation is performed.
In the present study, local reentrant tachycardia
within the LAA, either occurring spontaneously or induced during
repeated ablation of AF, is described. The findings of the current
study support the proposal that in selected patients with AF, LAA
may serve as a source to harbor ATs and ablation may be performed
successfully in these patients.
Materials and methods
Study population
A total of 76 patients (age range, 56–78 years, 22
female and 54 male) undergoing repeated catheter ablation (21
paroxysmal, 55 persistent) for symptomatic and drug-resistant
recurrent AF in the Cardiology Department of Xiamen Cardiovascular
Hospital (Xiamen, China) from July 2010 to April 2016. Baseline
characteristics of the patients, including age, gender, left atrium
size, left ventricular ejection fraction (EF) and presence of
structural heart disease, were collected from medical records
(Table I). Exclusion criteria were
defined as follows: Patients <18 years old, patients with LA/LAA
thrombus, patients with structural heart disease or unwillingness
to participate. Antiarrhythmic medications, except amiodarone for
>4 weeks, were discontinued for five half-lives prior to
surgery. The current study was approved by the Institutional Ethics
Committee of Xiamen Cardiovascular Hospital (Xiamen, China;
XMCH-014-173) and was in compliance with national legislation and
the Declaration of Helsinki guidelines. Patients provided written
informed consent.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Paroxysmal AF | Persistent AF | P-value |
|---|
| Numbers | 21 | 55 |
|
| Male/Female | 14/7 | 30/25 | >0.05 |
| Age (years) | 62.1±9.9 | 65.3±8.7 | >0.05 |
| LA diameter
(mm) | 42.4±4.3 | 47.2±3.8 | >0.05 |
| EF (%) | 63.6±6.2 | 57.8±7.3 | >0.05 |
| Presence of
structure heart disease | 2 | 5 | >0.05 |
| Duration of AF
(years) | 3.4±0.9 | 4.2±0.7 | >0.05 |
Electrophysiology study
Transesophageal echocardiography and cardiac
computed tomography were performed in all the patients. Three
femoral venous approaches were performed. A 6-Fr decapolar catheter
(Bard Dynamic Tip; Boston Scientific Co., Marlborough, MA, USA) was
positioned in the coronary sinus. Double transseptal access was
achieved using an 8.5-Fr non-steerable sheath (SL1; St. Jude
Medical, Inc., Saint Paul, MN, USA). Intravenous heparin (100 U/kg;
Shenzhen Hai Purui Pharmaceutical Co., Ltd., Shenzhen, China) was
administered to achieve an activated clotting time of 300–400 sec.
A LassoNAV catheter (Biosense Webster, Inc., Irvine, CA, USA) was
used for mapping and recording of pulmonary vein (PV) potentials.
Surface and intracardiac electrocardiograms were recorded (Prucka
CardioLab EP System; GE Healthcare, Chicago, IL, USA).
Radiofrequency ablation was delivered using an open irrigated 7F,
3.5-mm-tip, pressure-sensitive ablation catheter (Biosense Webster,
Inc.). Ablation lesions were delivered at 25–35 W, for 30–60 sec at
43°C using a temperature-controlled mode with an irrigation rate of
17–30 ml per min. When ablating in the coronary sinus, 25 W for 25
sec with an irrigating rate of 30 ml was used.
A three-dimensional rendering of the left atrium
(LA) was created from a computed tomography image of the LA and
integrated with an electroanatomical map to guide ablation catheter
navigation (CARTO MERGE; Biosense Webster, Inc.). Patients with
paroxysmal AF underwent PV isolation (PVI) only. While a fixed
ablation approach, consisting of circumferential PVI and three
linear ablation lesion sets across the mitral isthmus, left atrial
roof and cavotricuspid isthmus, was performed in patients with
persistent AF. Introduction tests were then conducted in all
patients. The introduction protocol was rapid pacing initiated with
a cycle length of 250 msec progressively shortening by 10 to 180
msec or refractoriness. Inducibility was defined as atrial
arrhythmia >1 min. Activation mapping using the CARTO mapping
system and entrainment mapping were undertaken as diagnostic
techniques to differentiate focal, localized reentry and
macro-reentry ATs. For rapid distinction of left and right ATs,
entrainment was performed at the high right atrium (RA), proximal
coronary sinus (CS) and distal CS. Postpacing interval
(PPI)-tachycardia cycle length (TCL) differences <50 msec at the
high RA suggested RA reentrant circuits, while >50 msec
suggested LA circuits. For LA circuits, PPI-TCL difference at the
proximal and distal CS distinguished perimitral reentry from other
reentries within the LA. When left ATs were suggested, activation
mapping of LA was performed. Rove catheters (Biosense Webster,
Inc., Irvine, CA, USA) were deployed through the long sheath to
acquire stable tissue contact at each location and to create
activation maps. Potential critical isthmuses, often containing
fractionated electrocardiograms, were identified as areas of
constrained activation, resulting from idiopathic or iatrogenic
scars and anatomic barriers. Once electrical isolation in all PVs
was confirmed and the wave fronts of atrial activation were
identified by activation mapping, entrainment-pacing maneuvers were
performed from the ablation catheter with a cycle length 20–30 msec
less than the tachycardia cycle length. The entrainments were as
follows: The first entrainment was performed at the anterior and
posterior wall of the LA, then the left atrial ridge, PV, roof and
septal area of LA and LA anterior wall (LAAW) near the mitral
annulus (Fig. 1). The site was
considered part of the circuit if the PPI measured from the
stimulation artifact to the return atrial electrocardiogram on the
ablation catheter was within 20 msec of the TCL.
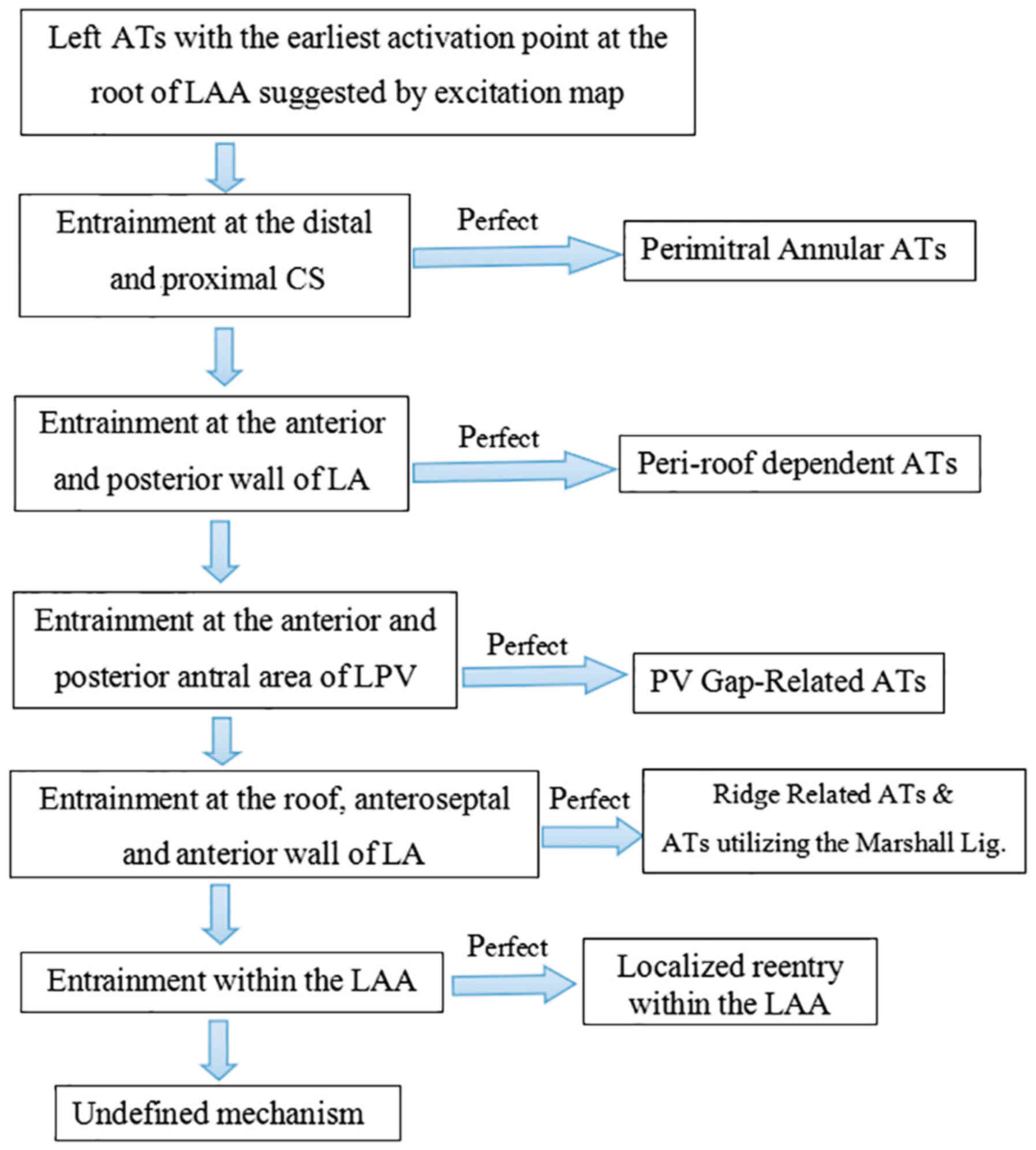 | Figure 1.Scheme of entrainment. For patients
undergoing ablation for symptomatic atrial fibrillation, activation
and entrainment mapping were undertaken as diagnostic techniques to
differentiate focal, localized and macro-reentry AT. For rapid
distinction of left and right ATs, entrainment was performed at the
high RA, proximal and distal CS, respectively. For LA circuits,
PPI-TCL difference at the proximal and distal CS distinguished
perimitral reentry from other reentries within the LA. When left
ATs were indicated, entrainments were as follows: First entrainment
was performed at the anterior and posterior wall of the LA, left
atrial ridge, PV, roof and septal area of LA and LAAW near the
mitral annulus; AT, atrial tachycardia; RA, right atrium; CS,
coronary sinus; LA, left atrium; PPI-TCL, postpacing
interval-tachycardia cycle length; PV, pulmonary vein; LAAW, left
atrial anterior wall; LAA, left atrial appendage; LPV, left PV. |
Follow-up
Patients were assessed at 3, 6 and 12 months
following the procedure and underwent the transthoracic
echocardiography and ambulatory monitoring. Postprocedural
anticoagulation was continued for ≥3 months in the absence of
arrhythmia recurrence or for a longer period otherwise.
Statistical analysis
Data are reported as the mean ± standard deviation.
Comparisons between groups were performed with Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Study Population
Sustained ATs within LAA were identified in 3
patients (age range, 63–74; 66% male) from a cohort of 76 patients
undergoing repeated ablation for symptomatic AF. Circumferential
PVI was successfully performed in all paroxysmal AF patients during
the first procedure. A fixed ablation approach consisting of
circumferential PV anteroom isolation (PVAI) and three linear
ablation lesion sets across the mitral isthmus, left atrial roof
and cavotricuspid isthmus had been performed in persistent AF,
during first time ablation. There was no statistically significant
difference in age, sex, AF history, EF, structural heart diseases
and LA diameters among the groups as presented in Table I. One patient with previous
persistent AF had presented electrical isolation of all four PVs at
the time of AT occurrence. All patients undergoing ablation had
failed treatments with ≥1 anti-arrhythmic agent.
Arrhythmia characteristics
Macro-reentries including mitral annular, ring
gap-dependent, roof-dependent and cavotricuspid isthmus-dependent
flutters were observed in this cohort. Mitral annular atrial
flutter was reported for 15 (19.7%) patients, and left atrial
flutter sustained by reentry through ≥2 gaps in the isolation ring
of PV was observed in 6 (7.8%) patients. Typical right atrial
flutter, which was anticlockwise around the tricuspid annulus and
dependent on the cavotricuspid isthmus, was observed in 2 (2.6%)
patients and an additional 2 patients (2.6%) were observed to have
a roof dependent flutter. Local reentry within the LAA was
recognized in 3 patients (3.9%). In 2 (2.6%) patients, the
tachycardia occurred spontaneously and in 1 patient (1.3%),
arrhythmia was induced by burst pacing. The mean atrial cycle
length of the tachycardia was 264±15 msec (range 250–280 msec). The
tachycardia demonstrated a characteristic P-wave morphology. The
P-wave was highly positive in inferior leads of all patients. Lead
V1 displayed upright or biphasic (±) components. Lead V2-V6
exhibited isoelectric component or upright components with low
amplitude. A characteristically high negative P-wave was detected
in lead I in all 3 patients with local reentry within the LAA.
Endocardial mapping
Activation mapping was performed in the 3 patients
with local reentry within the LAA and the earliest endocardial
activation site was at the root of LAA. In 1 patient, due to the
unsuccessful ablation of mitral isthmus, an anterior wall ablation
line was created (Fig. 2A). During
the additional ablation, inadvertent isolation of the LAA was
noted, which was confirmed by ATs contained entirely within the
LAA, as recorded by lasso catheter (Fig.
2C). Fig. 2B presents an EKG
exhibiting a characteristic positive P-wave in the inferior lead
and a negative P-wave in lead I and the avL lead. Focal ablations
were applied to terminate AT (Fig.
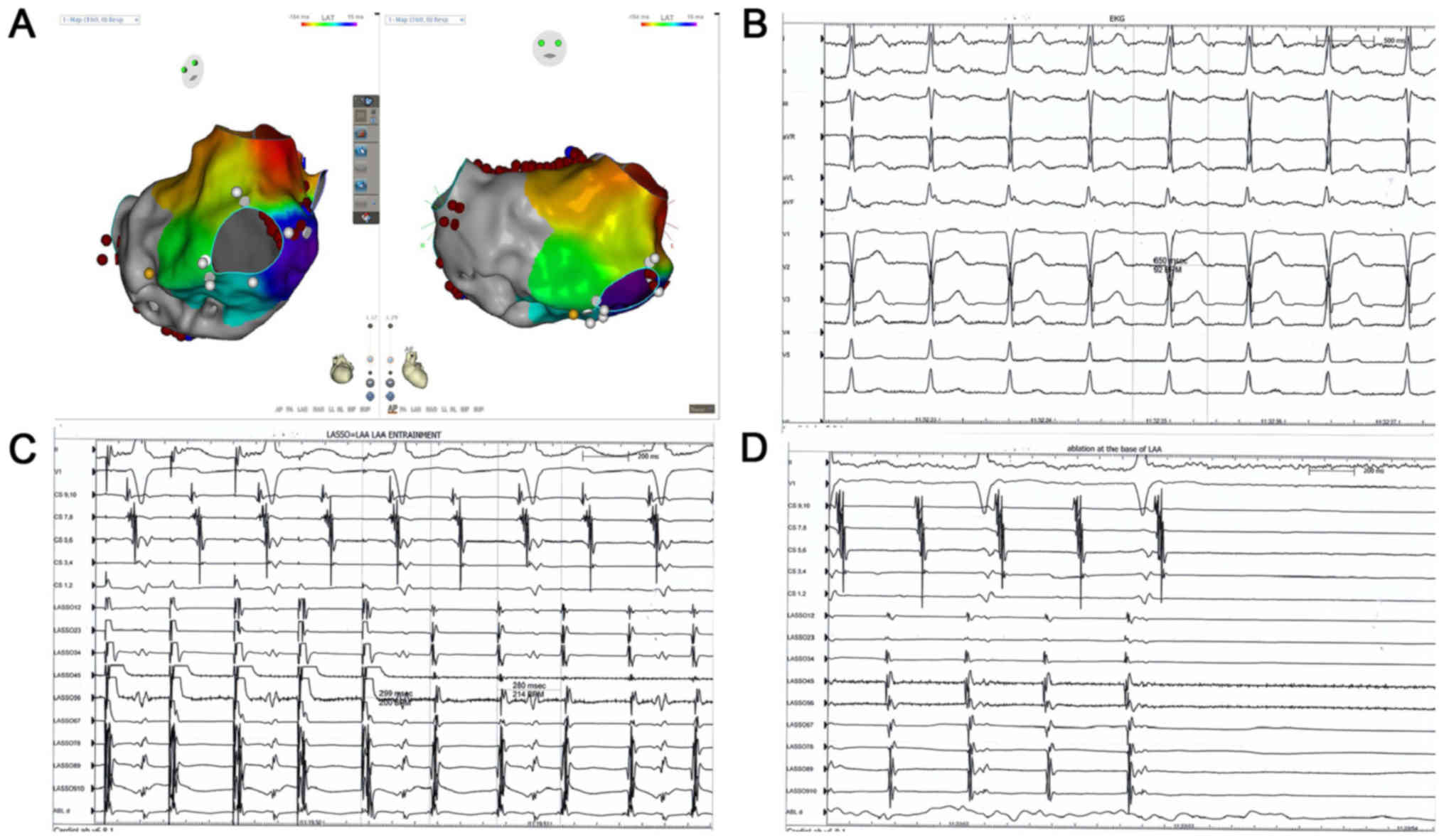
2D). In another patient, distinguishing local reentry from the
macro-reentry ATs was affected by the presence of ablation lines
(Fig. 3A). AT demonstrated a
characteristic P-wave morphology, idicating an LAA origin (Fig. 3B). In addition, entrainment at the
base of LAA indicated a localized reentry (Fig. 3C) and ablation at the base of LAA
successfully terminated the AT (Fig.
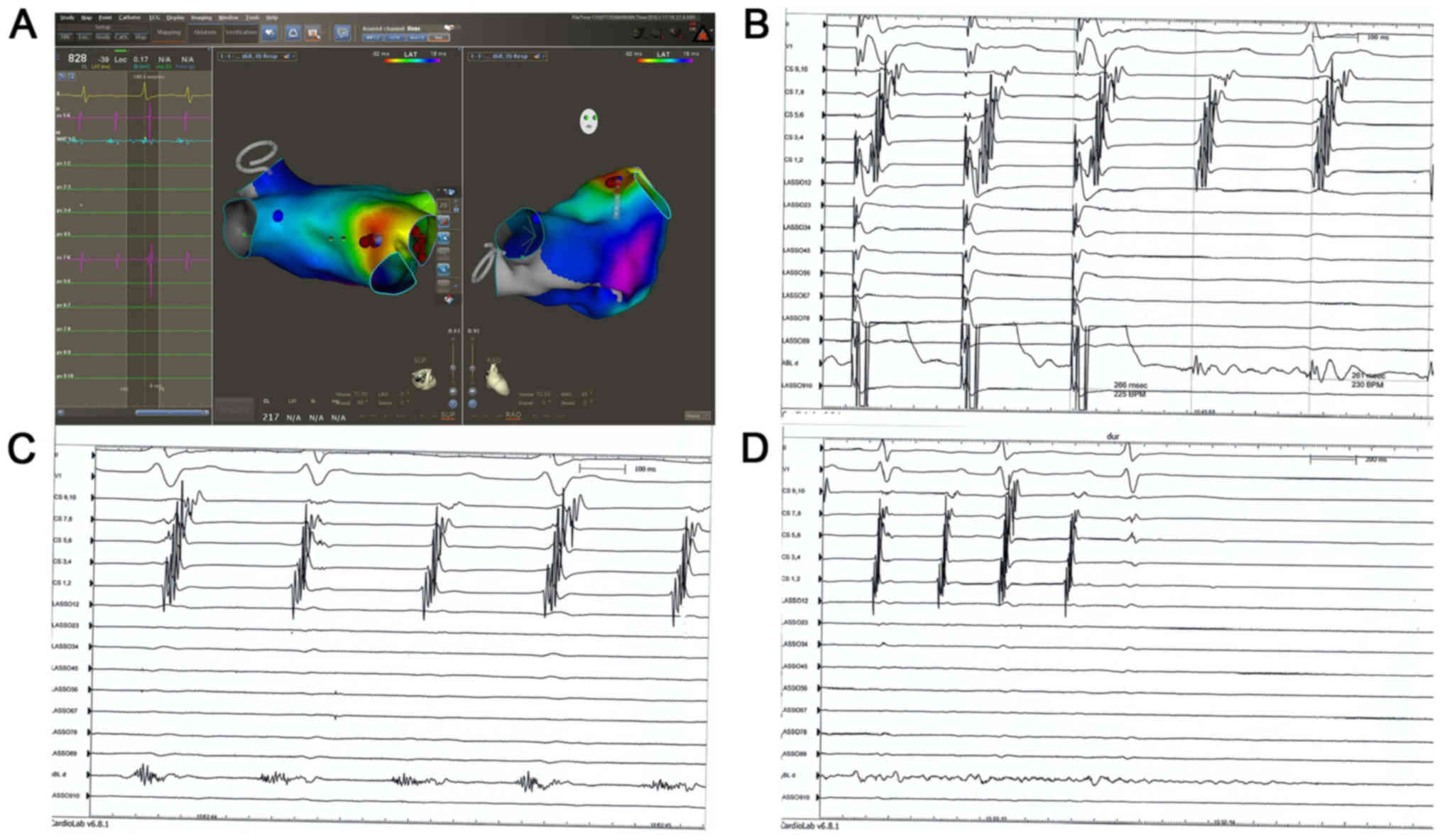
3D). Focal reentry within the LAA in paroxysmal AF was
confirmed in 1 patient (Fig. 4A).
Local reentry within LAA was confirmed by local entrainment
(Fig. 4B). A low-amplitude and long
fractionated electrocardiogram was recorded (Fig. 4C), which indicated the critical
isthmus of the arrhythmia. Ablation applied at the same time point
successfully terminated the arrhythmia that was exhibited (Fig. 4D).
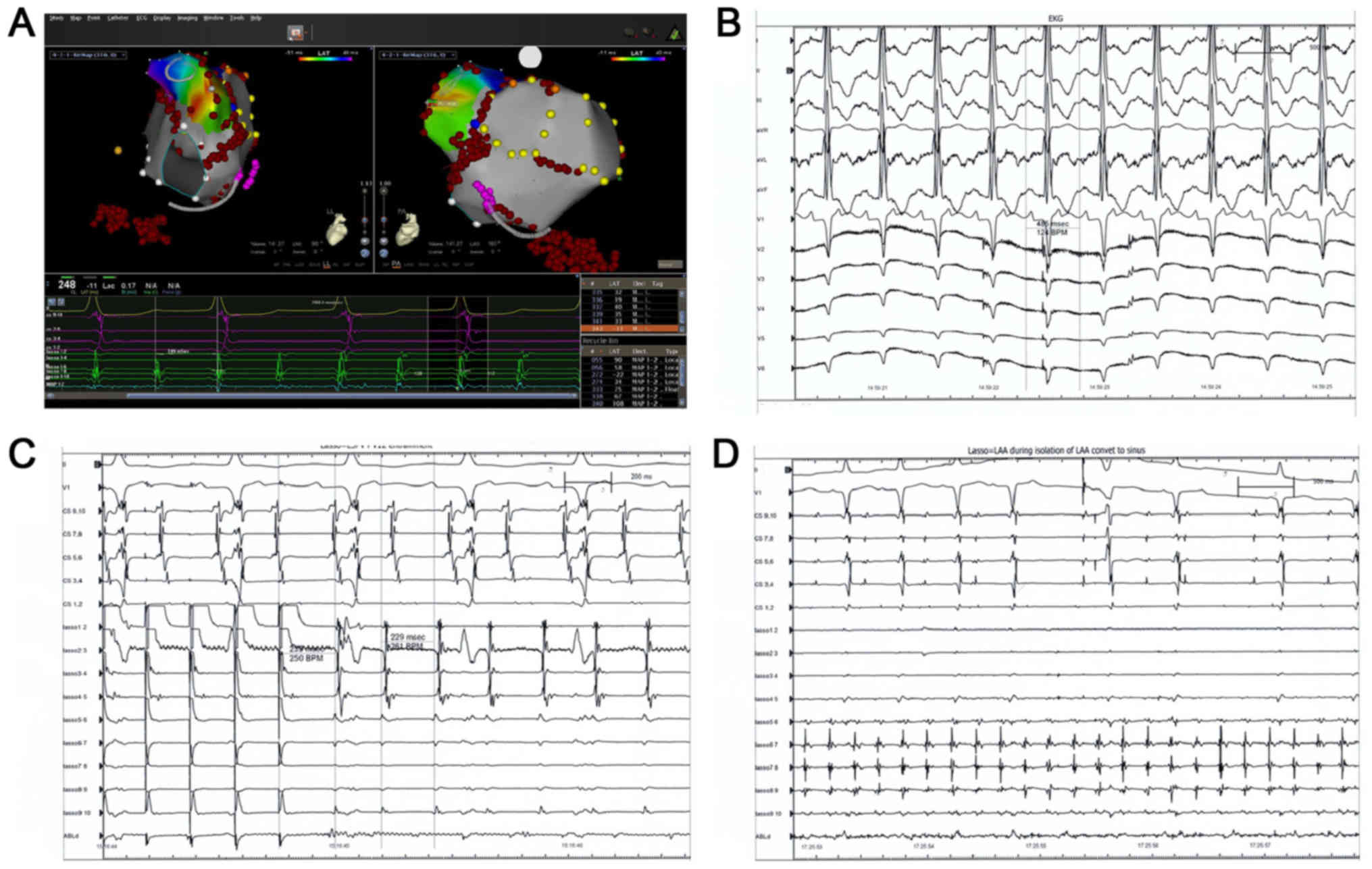 | Figure 2.An 80-year-old female patient admitted
for repeat RFCA with persistent atrial fibrillation following a
fixed ablation approach consisting of circumferential pulmonary
vein anteroom isolation and three linear ablation lesion sets
across the mitral isthmus, left atrial roof and cavo-tricuspid
isthmus. During the ablation of mitral isthmus, the patient
converted back to sinus rhythm. As a response to bidirectional
mitral isthmus conduction block, an additional ablation line was
created at the anterior wall. During this procedure, an organized
AT was observed. (A) First endocardial post diastolic activation
site was observed at the base of the LAA. (B) EKG displaying a
characteristic positive P-wave in inferior lead and negative P-wave
in lead I and lead avL. (C) Entrainments were performed at the base
of LAA, distal and proximal coronary sinus, roof and anteroseptal
parts of left atrium. Tachycardia cycle length was 229 msec and
postpacing interval was 250 msec at the base of the LAA, suggesting
the pacing site was anatomically adjacent to the circuit. (D) AT
completely contained within the LAA as confirmed by lasso catheter.
Additional ablation at the base of the LAA eliminated the AT. RFCA,
radiofrequency catheter ablation; AT, atrial tachycardia; LAA, left
atrial appendage; EKG, electrocardiogram. |
Successful ablation sites
In the present study, ablation was not performed
circumferentially and was targeted to the segment of interest in an
attempt to avoid severely delayed LAA activation or complete
isolation. In each case, the successful ablation site was at the
base of LAA with long fractionated or mid-diastolic
electrocardiograms and resulted in tachycardia termination.
Discussion
It has long been suggested that the potential
importance of LAA is not only in triggering, but in long-term
maintenance of atrial arrhythmias (25–28).
Vazquez et al (29) reported
a case with sustained fibrillation contained entirely within the
LAA, which continued even following electrical isolation of the
LAA. It supported the concept that in patients with AF, LAA
isolation should be performed to minimize AF recurrence. ATs
following persistent AF ablation are common (7). The most common mechanism of ATs is
macro-reentry, which includes mitral annular, ring gap-dependent
and roof-dependent flutter (30,31).
Other previously described mechanisms include cavotricuspid
isthmus-dependent flutters, ridge-associated reentrant ATs and ATs
utilizing the ligament of Marshall (31). LAA was suggested as an uncommon site
of origin for ATs following AF ablation (18). LAA appears to be responsible for
arrhythmias in 27% of patients who undergo repeated procedures
(28). The present study discovered
that 4.7% of patients with paroxysmal AF and 3.6% of patients with
persistent AF had recurrent ATs due to localized reentry within the
LAA.
ATs are classified into focal, local reentry and
macro-reentry types (32).
Activation and entrainment mapping are useful diagnostic techniques
in differentiating post-ablation ATs. Activation mapping is useful
for precise positioning and facilitating ablation (33). Activation mapping by itself may not
be able to differentiate a focal tachycardia mimicking
macro-reentry from a true macro-reentry (34). When tachycardia arises from a focus
near a completely blocked ablation line, activation mapping may
present an activation sequence similar to macro-reentry. In those
cases, additional entrainment mapping is required to differentiate
between these types of tachycardia (34). In the current study, a fixed ablation
approach was selected, consisting of circumferential PVAI and three
linear ablation lesion sets across the mitral isthmus, left atrial
roof and cavotricuspid isthmus in persistent AF. Induction of ATs
was performed in all AF ablation patients. Following induction of
ATs, wave fronts of atrial activation and potential isthmus sites
were described using activation maps. The majority of interventions
are used to perform entrainment pacing only at the isthmus, as
indicated by the activation map, instead of multiple pacing in left
atrium to verify the mechanism of ATs. Using this method, the
mechanism of certain critical ATs may be misidentified. A sustained
AT within the vicinity of LAA occurring following a fixed ablation
approach may be an example for this, as it may be mistaken as
mitral annual flutter. Therefore, combining activation and
entrainment mapping may be important in approaching ATs. In
patients with repeated AF ablation, identification of the atrial
wave may prove difficult due to the low voltage of the left atrium.
Furthermore, it may lead to a false atrial activation mapping
result in cases with local long-range CFAE or unsuccessful setting
of the window of interest. In previous studies, mistakes when only
applying entrainment mapping of the key isthmus according to the
indication of activation mapping were presented (34). A strategy to confirm the results by
programmed entrainment mapping may be favorable. The current study
demonstrated that AT diagnosed by both activation and entrainment
mapping may be verified during ablation.
The mechanism of ATs in the LAA remains unknown;
however, it has been demonstrated that the LAA is a remnant of the
original embryonic atrium and may be the source of arrhythmia.
Iatrogenic ATs have been reported following ablation of either
paroxysmal or persistent AF (35).
In paroxysmal AF, the mechanism of post-ablation ATs is usually
focal and associated to PVs (36).
In persistent AF, substrate modification of the left atrium by
linear ablation or CFAE ablation may complicate the situation. The
post-ablation ATs in persistent AF largely depend on the ablation
performed in previous procedures and are variable between cases
(37). Anatomic approaches have been
reported with a higher prevalence of macro-reentry, while the
complex fractionated electrocardiogram ablation strategies may
increase the prevalence of focal ATs by up to 50% of total
post-ablation ATs (38). The
prevalence of LAA-associated ATs raised the question of whether
this type of arrhythmia is a primary change or an iatrogenic origin
(22). The current study observed a
case of AT located at the vicinity of ablation line surrounding the
left ipsilateral PV. Although AT was confirmed within the LAA by
activation mapping and left atriogram, there was a possibility that
this type of AT was iatrogenic.
To improve the success rates of AF treatment during
repeat procedures, Di Biase et al (28) proposed a strategy for the isolation
of the LAA rather than focal ablation. Normal contraction of the
LAA and adequate blood flow within the LAA may be lost, which
increases the risk for the formation of thrombi inside the cavity
and impaired function of the LA (17,39,40). In
the present study, ablation was not performed circumferentially and
was targeted to the segment of interest in an attempt to avoid
severely delayed LAA activation or complete isolation. Previous
studies were highly effective in acute and medium-term elimination
of arrhythmias within the LAA following ablation targeting the site
with long fractionated or mid-diastolic LAA electrocardiograms
(17). In the present study, no AT
recurrence was recorded during follow-up in the 3 patients with
local reentry within the LAA following successful ablations.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JCG wrote the manuscript and assisted with
preoperative preparation. WBH and FGZ performed radiofrequency
ablation. JH collected and analyzed the data. YW designed the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval for the study was provided by the
Institutional Ethics Committee of Xiamen Cardiovascular Hospital
(Xiamen, China; XMCH-014-173) and it was in compliance with
national legislation and the Declaration of Helsinki guidelines.
Patients provided written informed consent.
Consent for publication
Patient consent was obtained for publication of this
manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Du X, Guo L, He X, Jia Y, Wu J, Long D, Yu
R, Sang C, Liu X, Yin H, et al: A comparison of the real world
effectiveness of catheter ablation and drug therapy in atrial
fibrillation patients in a Chinese setting. BMC Cardiovasc Disord.
17:2042017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haïssaguerre M, Hocini M, Sanders P,
Sacher F, Rotter M, Takahashi Y, Rostock T, Hsu LF, Bordachar P,
Reuter S, et al: Catheter ablation of long-lasting persistent
atrial fibrillation: Clinical outcome and mechanisms of subsequent
arrhythmias. J Cardiovasc Electrophysiol. 16:1138–1147. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng L, Yao Y, Zhang S, Chen W, Zhang K,
Wang F, Chen X, He DS and Kadish AH: Organized left atrial
tachyarrhythmia during stepwise linear ablation for atrial
fibrillation. J Cardiovasc Electrophysiol. 20:499–506. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ju W, Yang B, Chen H, Zhang F, Zhai L, Cao
K and Chen M: Localized reentry as a novel type of the
proarrhythmic effects of linear ablation in the left atrium. Pacing
Clin Electrophysiol. 34:919–926. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chugh A, Oral H, Lemola K, Hall B, Cheung
P, Good E, Tamirisa K, Han J, Bogun F, Pelosi F Jr and Morady F:
Prevalence, mechanisms, and clinical significance of macroreentrant
atrial tachycardia during and following left atrial ablation for
atrial fibrillation. Heart Rhythm. 2:464–471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sawhney N, Anand K, Robertson CE, Wurdeman
T, Anousheh R and Feld GK: Recovery of mitral isthmus conduction
leads to the development of macro-reentrant tachycardia after left
atrial linear ablation for atrial fibrillation. Circ Arrhythm
Electrophysiol. 4:832–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chae S, Oral H, Good E, Dey S, Wimmer A,
Crawford T, Wells D, Sarrazin JF, Chalfoun N, Kuhne M, et al:
Atrial tachycardia after circumferential pulmonary vein ablation of
atrial fibrillation: Mechanistic insights, results of catheter
ablation, and risk factors for recurrence. J Am Coll Cardiol.
50:1781–1787. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takatsuki S, Fukumoto K, Igawa O, Kimura
T, Nishiyama N, Aizawa Y, Tanimoto Y, Tanimoto K, Miyoshi S and
Fukuda K: Ridge-related reentry: A variant of perimitral atrial
tachycardia. J Cardiovasc Electrophysiol. 24:781–787. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chik WW, Chan JK, Ross DL, Wagstaff J,
Kizana E, Thiagalingam A, Kovoor P and Thomas SP: Atrial
tachycardias utilizing the Ligament of Marshall region following
single ring pulmonary vein isolation for atrial fibrillation.
Pacing Clin Electrophysiol. 37:1149–1158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haïssaguerre M, Hocini M, Sanders P,
Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu LF, Jonsson A,
O'Neill MD, et al: Localized sources maintaining atrial
fibrillation organized by prior ablation. Circulation. 113:616–625.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yokokawa M, Latchamsetty R, Ghanbari H,
Belardi D, Makkar A, Roberts B, Saint-Phard W, Sinno M, Carrigan T,
Kennedy R, et al: Characteristics of atrial tachycardia due to
small vs large reentrant circuits after ablation of persistent
atrial fibrillation. Heart Rhythm. 10:469–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wasmer K, Mönnig G, Bittner A, Dechering
D, Zellerhoff S, Milberg P, Köbe J and Eckardt L: Incidence,
characteristics, and outcome of left atrial tachycardias after
circumferential antral ablation of atrial fibrillation. Heart
Rhythm. 9:1660–1666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biviano AB, Bain W, Whang W, Leitner J,
Dizon J, Hickey K and Garan H: Focal left atrial tachycardias not
associated with prior catheter ablation for atrial fibrillation:
Clinical and electrophysiological characteristics. Pacing Clin
Electrophysiol. 35:17–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kistler PM and Kalman JM: Locating focal
atrial tachycardias from P-wave morphology. Heart Rhythm.
2:561–564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho SY, Anderson RH and Sánchez-Quintana D:
Atrial structure and fibres: Morphological basis of atrial
conduction. Cardiovasc Res. 54:325–336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krul SP, Berger WR, Smit NW, van
Amersfoorth SC, Driessen AH, van Boven WJ, Fiolet JW, van Ginneken
AC, van der Wal AC, de Bakker JM, et al: Atrial fibrosis and
conduction slowing in the left atrial appendage of patients
undergoing thoracoscopic surgical pulmonary vein isolation for
atrial fibrillation. Circ Arrhythm Electrophysiol. 8:288–295. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hocini M, Shah AJ, Nault I, Sanders P,
Wright M, Narayan SM, Takahashi Y, Jaïs P, Matsuo S, Knecht S, et
al: Localized reentry within the left atrial appendage:
Arrhythmogenic role in patients undergoing ablation of persistent
atrial fibrillation. Heart Rhythm. 8:1853–1861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng XF, Lu SB, Wang J and Li YG: Atrial
fibrillation arising from the left atrial appendage. Intern Med.
54:3157–3160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi Y, Sanders P, Rotter M and
Haïssaguerre M: Disconnection of the left atrial appendage for
elimination of foci maintaining atrial fibrillation. J Cardiovasc
Electrophysiol. 16:917–919. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luther V, Linton NW, Koa-Wing M, Lim PB,
Jamil-Copley S, Qureshi N, Ng FS, Hayat S, Whinnett Z, Davies DW,
et al: A prospective study of ripple mapping in atrial
tachycardias: A novel approach to interpreting activation in
low-voltage areas. Circ Arrhythm Electrophysiol. 9:e0035822016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schaeffer B and Stevenson WG: Entrainment
mapping: Theoretical considerations and practical implementation. J
Cardiovasc Electrophysiol. 29:204–213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Saady NM, Obel OA and Camm AJ: Left
atrial appendage: Structure, function, and role in thromboembolism.
Heart. 82:547–554. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alli O and Holmes D Jr: Evaluation of the
WATCHMAN left atrial appendage closure device. Expert Rev Med
Devices. 11:541–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ho SY and Sánchez-Quintana D: The
importance of atrial structure and fibers. Clin Anat. 22:52–63.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo XG, Zhang JL, Ma J, Jia YH, Zheng Z,
Wang HY, Su X and Zhang S: Management of focal atrial tachycardias
originating from the atrial appendage with the combination of
radiofrequency catheter ablation and minimally invasive atrial
appendectomy. Heart Rhythm. 11:17–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Q, Ma J, Zhang S, Hu JQ and Liao ZL:
Focal atrial tachycardia originating from the distal portion of the
left atrial appendage: Characteristics and long-term outcomes of
radiofrequency ablation. Europace. 14:254–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosso R, Morton JB, Aggarwal A and Kalman
JM: Image integration to guide ablation of incessant left atrial
appendage tachycardia. Heart Rhythm. 7:1913–1914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Di Biase L, Burkhardt JD, Mohanty P,
Sanchez J, Mohanty S, Horton R, Gallinghouse GJ, Bailey SM,
Zagrodzky JD, Santangeli P, et al: Left atrial appendage: An
underrecognized trigger site of atrial fibrillation. Circulation.
122:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vazquez J, B Biviano A, Garan H and Whang
W: Sustained fibrillation within the left atrial appendage during
catheter ablation for recurrent atrial tachyarrhythmia. J Atrial
Fibrillation. 5:5812012.
|
|
30
|
Patel AM, D'avila A, Neuzil P, Kim SJ,
Mela T, Singh JP, Ruskin JN and Reddy VY: Atrial tachycardia after
ablation of persistent atrial fibrillation: Identification of the
critical isthmus with a combination of multielectrode activation
mapping and targeted entrainment mapping. Circ Arrhythm
Electrophysiol. 1:14–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gerstenfeld EP and Marchlinski FE: Mapping
and ablation of left atrial tachycardias occurring after atrial
fibrillation ablation. Heart Rhythm. 4(3 Suppl): S65–S72. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee G, Sanders P and Kalman JM: Catheter
ablation of atrial arrhythmias: State of the art. Lancet.
380:1509–1519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao L, Wu S, Jiang W, Zhou L, Gu J, Wang
Y, Liu Y, Zhang X and Liu X: Differential clinical characteristics
and prognosis of patients with longstanding persistent atrial
fibrillation presenting with recurrent atrial tachycardia versus
recurrent atrial fibrillation after first ablation. J Cardiovasc
Electrophysiol. 25:259–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ejima K, Shoda M, Miyazaki S, Yashiro B,
Wakisaka O, Manaka T and Hagiwara N: Localized reentrant
tachycardia in the aorta contiguity region mimicking perimitral
atrial flutter in the context of atrial fibrillation ablation.
Heart Vessels. 28:546–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakahara S, Hori Y, Hayashi A, Kobayashi
S, Nakamura H, Okumura Y and Takayanagi K: Impact of left atrial
appendage ridge ablation on the complex fractionated electrograms
in persistent atrial fibrillation. J Interv Card Electrophysiol.
41:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin YJ, Tai CT, Kao T, Tso HW, Higa S,
Tsao HM, Chang SL, Hsieh MH and Chen SA: Frequency analysis in
different types of paroxysmal atrial fibrillation. J Am Coll
Cardiol. 47:1401–1407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Scherr D, Khairy P, Miyazaki S,
Aurillac-Lavignolle V, Pascale P, Wilton SB, Ramoul K, Komatsu Y,
Roten L, Jadidi A, et al: Five-year outcome of catheter ablation of
persistent atrial fibrillation using termination of atrial
fibrillation as a procedural endpoint. Circ Arrhythm
Electrophysiol. 8:18–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ouyang F, Antz M, Ernst S, Hachiya H,
Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, et al:
Recovered pulmonary vein conduction as a dominant factor for
recurrent atrial tachyarrhythmias after complete circular isolation
of the pulmonary veins: Lessons from double Lasso technique.
Circulation. 111:127–135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chugh A and Oral H: Delayed activation of
the left atrial appendage following catheter ablation of persistent
atrial fibrillation: A cause for concern? Pacing Clin
Electrophysiol. 33:649–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang YL, Li XB, Quan X, Ma JX, Zhang P, Xu
Y, Zhang HC and Guo JH: Focal atrial tachycardia originating from
the left atrial appendage: Electrocardiographic and
electrophysiologic characterization and long-term outcomes of
radiofrequency ablation. J Cardiovasc Electrophysio. 18:459–464.
2007. View Article : Google Scholar
|