Introduction
Osteoarthritis is a degenerative disease with
clinical manifestations, including joint pain, tenderness,
stiffness, joint swelling, restricted movement and joint
deformities (1). In recent years,
the incidence of osteoarthritis has increased and presents as a
serious threat to human health and quality of life (2–4). The
causes of osteoarthritis are complex, therefore it is difficult to
develop a comprehensive classification system and its pathogenesis
remains unclear (5). Osteoarthritis
is divided into primary and secondary osteoarthritis depending on
the presence of local and systemic risk factors, including high
bone mass and metabolic disorders (6). It is frequently diagnosed in the clinic
as rheumatoid arthritis and ankylosing spondylitis (7). Previous studies have demonstrated that
rheumatoid arthritis is the most common manifestation of
osteoarthritis in patients; however, an effective treatment
strategy for rheumatoid arthritis remains unknown (8,9). Thus,
further investigations into efficient treatments for osteoarthritis
with minimal side effects are required.
Platelet-rich plasma (PRP) is a multifunctional
platelet concentrate of the blood that may be used for the
treatment of the manifestations of osteoarthritis, including
osteonecrosis of the femoral head, cartilage injury and rheumatoid
arthritis (10). It has been
demonstrated previously that PRP improves the repair of articular
cartilage injury in patients with joint disease by removing harmful
inflammation factors (11). It was
also demonstrated that PRP reduces the level of inflammatory factor
synovial fluid in rheumatoid arthritis without exhibiting side
effects (12). Treatment-emergent
adverse events of PRP have not been previously reported. The
isolation of PRP, including blood product rich in cytokines, growth
factors and other bio-active molecules from autologous peripheral
blood mononuclear cells, is an efficient and innovative treatment
strategy (13). Furthermore, Sadabad
et al (14) investigated the
efficiency of PRP vs. hyaluronic acid (HA) for the treatment of
knee osteoarthritis and Khoshbin et al (15) conducted a systematic review of PRP as
a therapeutic intervention in the management of symptomatic knee
osteoarthritis. These reports demonstrated that intravenous
injection of PRP repaired the damage to the tendon and articular
bone and reduced inflammation, which may serve a key function in
maintaining the morphology, collagen microarchitecture and
mechanical properties of the injected vein.
HA is a high molecular weight glucosamine
(5–7×106 kD) synthesized by chondrocytes, fibroblasts
and synoviocytes (16). It is
responsible for viscoelasticity and lubricance in the synovial
fluid and extracellular matrix (17). It has been demonstrated that there is
a higher concentration of HA (2.5–4.0 mg/ml) in the synovial fluid,
while there is a decreased concentration level in patients with
osteoarthritis (18). Therefore, the
concentration of HA is an indicator of the prognosis of patients
with osteoarthritis. The efficacy of HA treatment in improving
osteoarthritis symptoms has been widely studied and the clinical
outcomes for patients with osteoarthritis are positive (19–21).
The current study investigated the efficacy and
outcomes of PRP and HA combination treatment in patients with knee
osteoarthritis aged 22–68 years. The clinical data demonstrated
that intra-articular injections of PRP were more successful in
recovering articular function, alleviating symptoms and reducing
arthralgia and body pain compared with HA treatment. PRP and HA
combination treatment significantly improved arthralgia, reduced
humoral and cellular immune responses and promoted angiogenesis,
which led to an improvement in histological parameters, compared
with PRP or HA injections alone. These results suggest that PRP and
HA serve a critical therapeutic role in knee osteoarthritis
progression and highlights their potential for the treatment of
knee osteoarthritis in the future.
Materials and methods
Patients
Patients with knee osteoarthritis (age, 22–72 years;
170 females and 190 males) with a Karnofsky performance status of
≥80% (patients have difficulty walking by themselves and have knee
pain) (22) were randomly divided
into four groups and once-weekly, double-blind trials were
conducted in Xi'an Jiangtong University College of Medicine (Xi'an,
China). The inclusion/exclusion criteria, and allocation method are
described in previously published studies (23,24).
Patients with knee osteoarthritis received PRP (2, 4, 8, 10, 12 and
14 ml, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), HA (0.10,
0.15, 0.20, 0.25 and 0.30 mg, Sigma-Aldrich; Merck KGaA),
combination treatment or placebo (normal saline, Sigma-Aldrich;
Merck KGaA) through intralesional injections as referenced
previously (23). The current
phase-III study (XAJT006999781) was carried out in strict
accordance with the recommendations in the Guide for Xi'an
Jiangtong University College of Medicine between February 2009 and
October 2014 (25). All patients
were required to review trial protocols, amendments and provide
informed consent. Ethical approval was granted by the Ethics
Committee of Xi'an Jiangtong University College of Medicine
(24).
Study design
The double-blind study was carried out in three
phases: Baseline stage, the double-blind treatment phase (4-week
dose-titration treatment, PRP, 2, 4, 8, 10, 12 and 14 ml, HA, 0.10,
0.15, 0.20, 0.25 and 0.30 mg) and 52-week post-treatment (PRP, 8ml,
HA, 0.2 mg) for patients with knee osteoarthritis who volunteered
to complete the ongoing extension study. Patients were randomly
sorted into groups where they underwent once-weekly, double-blind
treatment with HA (n=88), PRP (n=104), combination therapy of HA
and PRP (n=96) or a placebo (n=72). Patients with knee
osteoarthritis continued treatment with PRP (8 ml), HA (0.20 mg),
combination (PRP: 8 ml, HA: 0.20 mg) or placebo throughout the
maintenance period (52 weeks).
Outcomes measures
A Western Ontario and McMaster Universities
Arthritis Index (WOMAC) questionnaire (26) for pain, two items for stiffness and
17 items for assessing functional limitation and the function of
patients with knee osteoarthritis. The data was recorded and the
degree of lesion was calculated.
ELISA
Plasma samples were prepared immediately using
centrifugation at 2,000 × g at 4°C for 10 min. Serum levels of
TNF-α (cat no. MBS6080, Thermo Fisher Scientific, Inc., Waltham,
MA, USA), IL-1β (cat no. MBS700340, Thermo Fisher Scientific,
Inc.), IL-6 (cat no. MBS3205, Thermo Fisher Scientific, Inc.),
IL-17A (cat no. DY-5194, Bio-Rad Laboratories, Inc., Hercules, CA,
USA), RANKL (cat no. DY626, Bio-Rad Laboratories, Inc.), PD-ECGF
(cat no. DY229-05, Bio-Rad), VEGF (cat no. DVE00, Bio-Rad
Laboratories, Inc.) and IL-10 (cat no. MBS910284, Thermo Fisher
Scientific, Inc.) were analyzed using ELISA kits according to the
manufacturer's protocol. The serum concentration levels of these
cytokines were measured using micro-plate reader at a wavelength of
570 nm.
Efficacy and safety assessments
Efficacy assessments, including WOMAC scores or
Karnofsky performance were analyzed in patients with knee
osteoarthritis at baseline, during the 52-week and double-blind
period in the PRP (8 ml), HA (0.20 mg), combination (PRP: 8 ml, HA:
0.20 mg) or placebo treatment groups. In addition, the overall
safety and pharmacokinetic analysis were conducted according to
previous clinical studies (25–27).
Safety assessments of the most frequent treatment-emergent adverse
events were evaluated in all randomized patients who received the
study drug and had undergone at least one post-dose safety
assessment. Dose-response analysis was conducted at the time of the
last drug injection. Common Toxicity Criteria grades for
hypertension and proteinuria were determined by the National
Institute Common Terminology Criteria (28).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using a Student's t-test for
unpaired data. Comparisons of data between multiple groups were
performed using one-way analysis of variance followed by a
Dunnett's t test. Treatment effect was presented as median
reduction in knee osteoarthritis over the treatment period. Robust
nonparametric Responder rates and treatment-emergent adverse events
were analyzed using a χ2 test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
There were 360 patients with knee osteoarthritis
(mean age, 48 years) who were candidates for intra-articular
injection in the present study. All patients were randomly divided
into four groups and treated with HA (n=88), PRP (n=104),
combination therapy of HA and PRP (n=96) or a placebo (n=72). The
numbers of male and female patients were approximately equal. The
characteristics of patients with knee osteoarthritis are presented
in Table I. Overall, 277 (75%)
patients with knee osteoarthritis completed the maintenance period
of the phase III study, the other 25% stopped the study due to side
effects.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Study groups |
|---|
|
|
|
|---|
| Characteristic | PRP | HA | Combination | Placebo |
|---|
| Number, n (%) | 104 (28.9) | 88 (24.4) | 96 (26.7) | 72 (20.0) |
| Age, mean ± SD | 46.2±8.6 | 51.5±9.3 | 46.5±7.5 | 56.2±8.4 |
| Gender (%) |
|
|
|
|
| Female, n
(%) | 54
(51.9) | 40 (45.5) | 46 (47.9) | 30 (41.7) |
| Male, n (%) | 50
(48.1) | 48 (54.5) | 50 (52.1) | 42 (58.3) |
Duration of treatment, dose-limiting
toxicities and maximum tolerated dose
Median overall duration of PRP and HA treatments was
8 weeks. Patients underwent treatments with at least one of the
following doses: 2, 4, 8, 10, 12 and 14 ml for PRP; and 0.10, 0.15,
0.20, 0.25 and 0.30 mg for HA. The data presented in Table II demonstrated that 12 ml PRP once a
week was identified as the maximum tolerated dose (MTD) and 16 ml
of PRP once a week was identified as dose-limiting toxicity (DLT)
(29). Doses of 0.25 and 0.30 mg HA
were identified as the MTD and DLT, respectively (Table III). The common treatment-emergent
adverse events of PRP or HA injection were hypertension, diarrhea,
vomiting, rash, proteinuria, fatigue, constipation,
hypertriglyceridemia and edema peripheral. A total of 60 patients
with knee osteoarthritis required a reduction in drug dose for
cumulative toxicity following treatment with MTD dose. Therefore,
most patients were enrolled at a dose of 8 ml PRP and 0.20 mg HA
for further examination of the tolerability and therapeutic effects
of patients with knee osteoarthritis.
 | Table II.Treatment-emergent adverse events
following platelet-rich plasma (PRP) treatment. |
Table II.
Treatment-emergent adverse events
following platelet-rich plasma (PRP) treatment.
| Adverse event | Total (n=28) | PRP (2–4 ml;
n=8) | PRP (8–12 ml;
n=12) | PRP (14 ml;
n=8) |
|---|
| Hypertension | 4 | 1 | 1 | 2 |
| Diarrhea | 3 | 1 | 1 | 1 |
| Proteinuria | 5 | 1 | 2 | 2 |
| Vomiting | 1 | 0 | 1 | 0 |
| Rash | 2 | 0 | 1 | 1 |
| Fatigue | 3 | 0 | 1 | 2 |
| Constipation | 4 | 1 | 1 | 2 |
|
Hypertriglyceridemia | 3 | 1 | 1 | 1 |
| Edema
peripheral | 3 | 1 | 1 | 1 |
 | Table III.Treatment-emergent adverse events
following hyaluronic acid (HA) treatment. |
Table III.
Treatment-emergent adverse events
following hyaluronic acid (HA) treatment.
| Adverse event | Total (n=30) | HA (0.10–0.15 mg;
n=8) | HA (0.20–0.25 mg;
n=15) | HA (0.30 mg;
n=7) |
|---|
| Hypertension | 5 | 1 | 3 | 2 |
| Diarrhea | 2 | 0 | 1 | 1 |
| Proteinuria | 4 | 1 | 1 | 2 |
| Vomiting | 2 | 0 | 1 | 1 |
| Rash | 2 | 0 | 1 | 1 |
| Fatigue | 2 | 0 | 1 | 1 |
| Constipation | 3 | 1 | 1 | 1 |
|
Hypertriglyceridemia | 3 | 0 | 1 | 2 |
| Edema
peripheral | 2 | 0 | 1 | 1 |
Treatment-emergent adverse events of
PRP, HA and combination treatment
Patients with knee osteoarthritis who received at
least one dose of study therapy with a post-baseline safety
evaluation were included in the safety population. Following the
last dose of PRP, it was observed that the most common
treatment-emergent adverse events of PRP, HA and combination
treatment (PRP, 8 ml; HA, 0.20 mg) were hypertension and
proteinuria (≥10% each; Table IV).
The data for the 12 ml (n=28) and 14 ml (n=18) PRP doses are not
shown as there were more side effects, including hypertension,
proteinuria, constipation and diarrhea and few patients were
treated at these dose levels. Of the 360 patients enrolled in the
current study, 118 patients with knee osteoarthritis completed the
overall maintenance period of the phase III study.
 | Table IV.Common Toxicity Criteria grades for
hypertension and proteinuria. |
Table IV.
Common Toxicity Criteria grades for
hypertension and proteinuria.
| Adverse event | Total (n=72) | PRP (n=28) | HA (n=30) | Combination
(n=14) |
|---|
| Hypertension | 11 | 4 | 5a | 2 |
| Grade
1 | 5 | 2 | 2 | 1 |
| Grade
2 | 4 | 1 | 2 | 1 |
| Grade
3 | 2 | 1 | 1 | 0 |
| Proteinuria | 11 |
5b |
4b | 2 |
| Grade
1 | 5 | 2 | 2 | 1 |
| Grade
2 | 3 | 1 | 1 | 1 |
| Grade
3 | 3 | 2 | 1 | 0 |
Efficacy of combination PRP and HA
treatment
The clinical outcomes of combination treatment of
PRP and HA were analyzed. Preliminary clinical analyses indicated
that pain was markedly improved in drug-treatment groups compared
with the placebo group. Pain was reduced more in patients who
received PRP treatment compared with patients who received HA
treatment in the 52-week observation (P<0.01). Combination
treatment of PRP and HA improved pain, physical function, stiffness
and total WOMAC score compared with PRP treatment or HA treatment
alone compared to the baseline (Table
V). These clinical outcomes indicate that PRP (8 ml) and HA
(0.20 mg) combination therapy improves the clinical features of
knee osteoarthritis.
 | Table V.WOMAC scores during the study
period. |
Table V.
WOMAC scores during the study
period.
|
| WOMAC score, mean
(SD) |
|
|---|
|
|
|
|
|---|
| Group | Pain | Stiffness | Physical
function | Total |
|---|
| PRP |
|
|
|
|
|
Baseline | 8.86 (3.14) | 2.67 (1.76) | 24.68 (12.63) | 36.21 (17.53) |
| Week
52 | 4.15 (3.08) | 1.44 (0.84) | 14.66 (11.28) | 20.25 (15.20) |
|
Changes | 4.67
(3.07)g | 1.19
(1.26)g | 9.98
(11.92)g | 15.84
(16.59)h |
|
P-value |
<0.0001a |
<0.0001a |
<0.0001a |
<0.0001a |
| HA |
|
|
|
|
|
Baseline | 8.91 (3.82) | 2.78 (1.58) | 25.72 (12.32) | 37. 41 (16.72) |
| Week
52 | 5.26 (3.75) | 2.25 (1.38) | 18.89 (12.85) | 26.40 (16.98) |
|
Changes | 3.61 (3.75) | 0.49 (1.44) | 6.79
(12.55) | 10.89 (17.74) |
|
P-value | 0.01 | 0.01 | 0.01 | 0.01 |
| Combination |
|
|
|
|
|
Baseline | 8.94 (5.12) | 2.81 (1.47) | 26.46 (10.66) | 38.21 (17.25) |
| Week
52 | 3.32 (2.44) | 0.85 (0.68) | 10.23 (8.61) | 14.40 (11.73) |
|
Changes | 5.58
(2.64)c,e | 1.92
(1.04)f | 16.19
(9.60)d,f | 23.69
(13.28)d,f |
|
P-value |
<0.0001b |
<0.0001b |
<0.0001b |
<0.0001b |
Anti-inflammation effects of
combination PRP and HA treatment for patients with knee
osteoarthritis
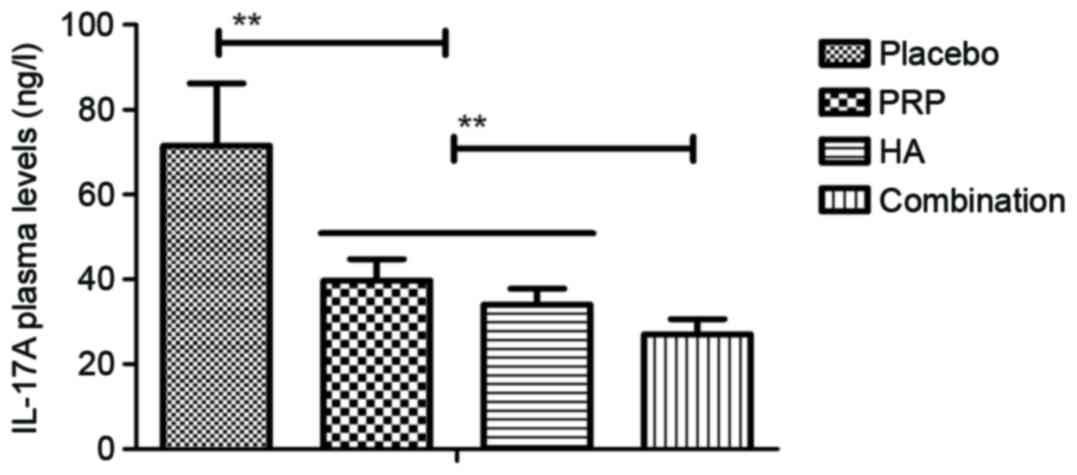
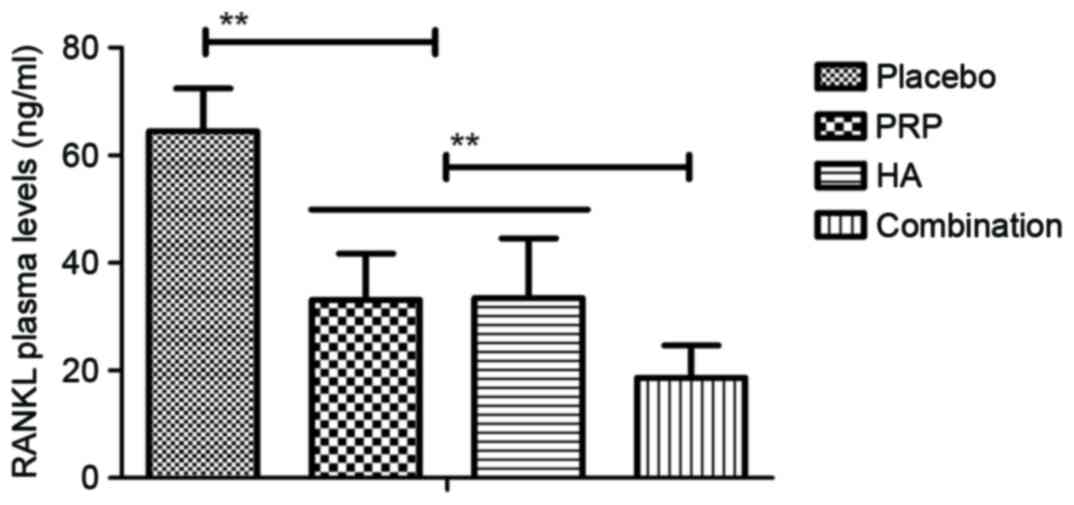
The level of serum inflammatory cytokines in
patients with knee osteoarthritis following PRP and/or HA
treatments was then measured. Plasma concentration levels of
interleukin (IL)-17A, tumor necrosis factor-α, IL-1β and receptor
activator of nuclear factor κ-B ligand were downregulated in
patients with knee osteoarthritis following treatment with PRP or
HA and further downregulated following combination treatment with
PRP and HA (all P<0.01; Figs.
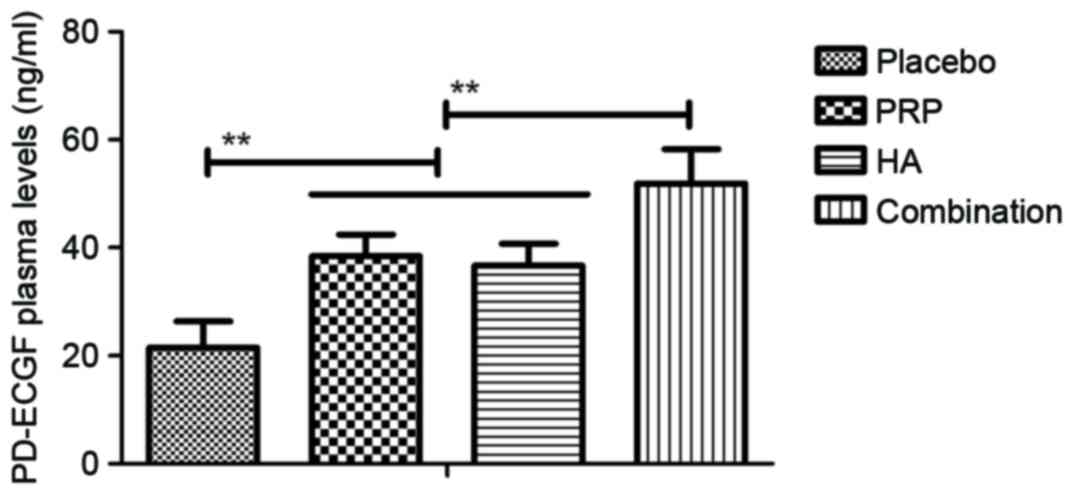
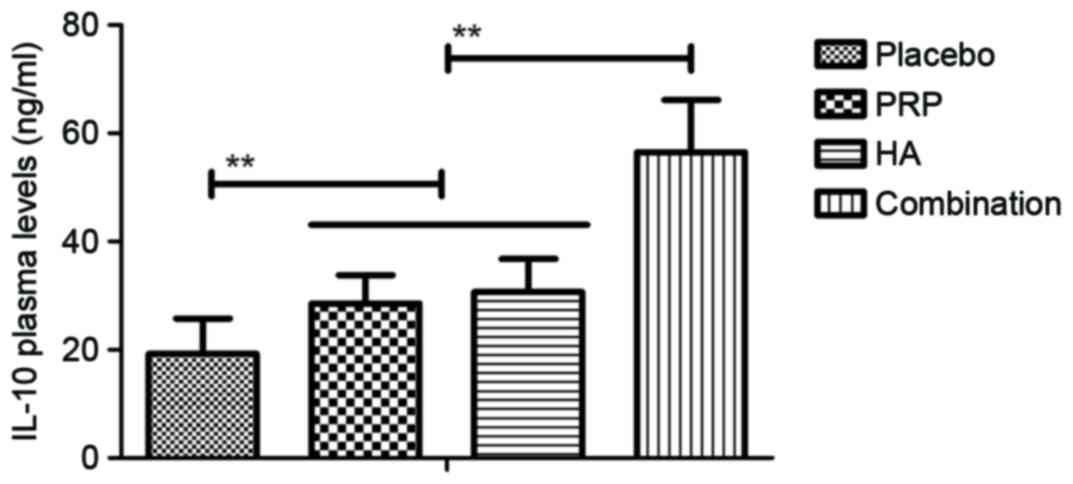
1–4). The plasma concentration
of platelet derived-endothelial cell growth factor, IL-6, vascular
endothelial growth factor and IL-10 were upregulated in patients
with knee osteoarthritis following treatment with PRP or HA and
were further upregulated following combination treatment with PRP
and HA (all P<0.01; Figs.
5–8). These results suggest that
combination PRP and HA treatment inhibits inflammation for patients
with knee osteoarthritis.
Discussion
Reports have indicated that inflammatory cytokines
serve a critical function in the induction and development of
osteoarthritis (30,31). PRP is an autologous and
multifunctional platelet concentrate of the blood that contains
highly concentrated platelets and high levels of cell growth
factors. PRP promotes synovial cell proliferation and
differentiation, and promotes recovery of cartilage morphology.
Clinically, Battaglia et al (32) has demonstrated the efficacy of
ultrasound-guided intra-articular injections of PRP vs. HA for hip
osteoarthritis. Laudy et al (33) has suggested that PRP injections are
beneficial to patients with knee osteoarthritus based on a
reduction in pain, improvement in function, global assessment and
changes regarding joint imaging. Meheux et al (34) demonstrated that treatment with PRP
injection significantly improved validated patient-reported
outcomes in patients with symptomatic knee osteoarthritis at 6 and
12 months post-injection, and indicated similarities and
differences in outcomes based on the PRP formulations used in the
analyzed studies. These clinical reports suggest that PRP exhibited
a potential efficacy for treating osteoarthritis.
HA is responsible for the viscoelastic and lubricant
capabilities of the synovial fluid in joints. It serves a key
function in metabolism in the joints and mechanical support, which
stimulates chondrocyte metabolism and cartilage matrix components
synthesis as well as inflammatory processes (35). Clinical research has indicated that
intra-articular HA injections in patients with knee osteoarthritis
are associated with pain relief, quality of life, survival time,
clinical effect and a longer period of time prior to the onset of
knee arthroplasty (16). In
addition, the efficacy and safety of HA in the management of
osteoarthritis has been investigated using real-life setting trials
and surveys (17). However, single
intra-articular HA injections did not achieve the ideal therapeutic
effect for patients with osteoarthritis.
Previous studies have compared the clinical efficacy
of PRP vs. HA for treatment of knee osteoarthritis and determined
that PRP presented more notable improvements in physical function,
stiffness and total WOMAC (14,36).
Coincidentally, Kon et al (37) investigated PRP intra-articular
injection vs. HA viscosupplementation in treatments for cartilage
pathology from early degeneration to osteoarthritis, and outcomes
suggested that PRP was superior to HA treatment for patients with
cartilage pathology. However, the efficacy and safety of
combination treatment with PRP and HA for patients with knee
osteoarthritis remains unknown.
In the present study, the clinical efficacy of
combination treatment with PRP and HA for patients with knee
osteoarthritis was investigated in a phase-III clinical study.
Following an 8-week baseline period, patients with knee
osteoarthritis were randomized into groups undergoing once-weekly,
double-blind treatment with PRP, HA, combined therapy or a placebo.
Although previous studies indicated that patients with knee
osteoarthritis treated with PRP or HA exhibited regulated plasma
concentrations of inflammatory factors and pro-angiogenic factors,
the clinical outcomes of combined PRP and HA have not been
investigated (27,38). The current study was performed to
evaluate the clinical application of combination treatment with PRP
and HA. Responses to treatment were assessed by median percent
reduction in arthralgia, which was improved with PRP and/or HA
treatments compared with the placebo group. Hypertension and
proteinuria were the treatment-emergent adverse events with the
highest incidence following treatment with PRP or HA alone. The
results demonstrated that PRP and/or HA alleviated knee
osteoarthritis and reduced the humoral and cellular immune
responses, which subsequently led to beneficial effects on
histological parameters. The clinical outcomes revealed a
significant improvement in all the variables of WOMAC following
combination treatment with PRP and HA.
In conclusion, although the direct effects of
different drugs on knee osteoarthritis have been demonstrated
previously, it is critical that the overall role of PRP and HA in
affecting entire joint cytokines homeostasis is investigated
(39). Clinical outcomes of the
current study demonstrated that PRP and HA are potential novel
therapeutic options for treating knee osteoarthritis and an
increasing number of clinical reports continue to indicate
promising results. Of note, the results of the current study
suggest that pharmacokinetic interactions of PRP and HA are
important determinants in optimizing therapies for treating knee
osteoarthritis. Therefore, clinicians are required to monitor
clinical responses and tolerability when patients are treated with
PRP and HA. The results indicate that patients with knee
osteoarthritis treated with PRP and HA exhibited beneficial effects
on body pain, and alleviated arthralgia, cartilage destruction and
bone damage.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Onuora S: Osteoarthritis: Molecular
imaging detects activated macrophages. Nat Rev Rheumatol.
12:3132016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma YW, Jiang DL, Zhang D, Wang XB and Yu
XT: Radial extracorporeal shock wave therapy in a person with
advanced osteonecrosis of the femoral head. Am J Phys Med Rehabil.
95:e133–e139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee GW, Park KS, Kim DY, Lee YM,
Eshnazarov KE and Yoon TR: Results of total hip arthroplasty after
core decompression with tantalum rod for osteonecrosis of the
femoral head. Clin Orthop Surg. 8:38–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mutlu H, Karaca H, Akca Z and Torun YA:
Should fish test be performed to all patients with breast cancer?
Med Sci (Turkey). 2:539–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ,
Eckstein F, Grago J, Boudreau RM, Englund M and Guermazi A: Partial
meniscectomy is associated with increased risk of incident
radiographic osteoarthritis and worsening cartilage damage in the
following year. Eur Radiol. 27:404–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang H, He S, Zhang X, Luo S, Zhang B,
Duan X, Zhang Z, Wang W, Wang Y and Sun Y: A network pharmacology
approach to uncover the pharmacological mechanism of XuanHuSuo
powder on osteoarthritis. Evid Based Complement Alternat Med.
2016:32469462016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poquet N, Williams M and Bennell KL:
Exercise for osteoarthritis of the hip. Phys Ther. 96:1689–1694.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maricar N, Callaghan MJ, Parkes MJ, Felson
DT and O'Neill TW: Clinical assessment of effusion in knee
osteoarthritis-A systematic review. Semin Arthritis Rheum.
45:556–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beumer L, Wong J, Warden SJ, Kemp JL,
Foster P and Crossley KM: Effects of exercise and manual therapy on
pain associated with hip osteoarthritis: A systematic review and
meta-analysis. Br J Sports Med. 50:458–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smyth NA, Haleem AM, Ross KA, Hannon CP,
Murawski CD, Do HT and Kennedy JG: Platelet-rich plasma may improve
osteochondral donor site healing in a rabbit model. Cartilage.
7:104–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bembo F, Eraud J, Philandrianos C,
Bertrand B, Silvestre A, Veran J, Sabatier F, Magalon G and Magalon
J: Combined use of platelet rich plasma & micro-fat in sport
and race horses with degenerative joint disease: Preliminary
clinical study in eight horses. Muscles Ligaments Tendons J.
6:198–204. 2016.PubMed/NCBI
|
|
12
|
Fu CJ, Sun JB, Bi ZG, Wang XM and Yang CL:
Evaluation of platelet-rich plasma and fibrin matrix to assist in
healing and repair of rotator cuff injuries: A systematic review
and meta-analysis. Clin Rehabil. 31:158–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vannini F, Di Matteo B and Filardo G:
Platelet-rich plasma to treat ankle cartilage pathology-from
translational potential to clinical evidence: A systematic review.
J Expe Orthop. 2:22015. View Article : Google Scholar
|
|
14
|
Sadabad HN, Behzadifar M, Arasteh F,
Behzadifar M and Dehghan HR: Efficacy of platelet-rich plasma
versus hyaluronic acid for treatment of knee osteoarthritis: A
systematic review and meta-analysis. Electron Physician.
8:2115–2122. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khoshbin A, Leroux T, Wasserstein D, Marks
P, Theodoropoulos J, Ogilvie-Harris D, Gandhi R, Takhar K, Lum G
and Chahal J: The efficacy of platelet-rich plasma in the treatment
of symptomatic knee osteoarthritis: A systematic review with
quantitative synthesis. Arthroscopy. 29:2037–2048. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ong KL, Anderson AF, Niazi F, Fierlinger
AL, Kurtz SM and Altman RD: Hyaluronic acid injections in medicare
knee osteoarthritis patients are associated with longer time to
knee arthroplasty. J Arthroplasty. 31:1667–1673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maheu E, Rannou F and Reginster JY:
Efficacy and safety of hyaluronic acid in the management of
osteoarthritis: Evidence from real-life setting trials and surveys.
Semin Arthritis Rheum. 45 4 Suppl:S28–S33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altman R, Lim S, Steen RG and Dasa V:
Correction: Hyaluronic acid injections are associated with delay of
total knee replacement surgery in patients with knee
osteoarthritis: Evidence from a large U.S. health claims database.
PLoS One. 11:e01485912016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Witteveen AG, Hofstad CJ and Kerkhoffs GM:
Hyaluronic acid and other conservative treatment options for
osteoarthritis of the ankle. Cochrane Database Syst Rev.
10:CD0106432015.
|
|
20
|
Panuccio E, Memeo A and Richetta S:
Evaluation of the combined treatment of oral viscosupplementation
with hyaluronic acid intra-articular injection on symptomatic knee
osteoarthritis. Clin Ter. 166:e321–e326. 2015.(In Italian).
PubMed/NCBI
|
|
21
|
Lubowitz JH: Editorial commentary: Knee
hyaluronic acid viscosupplementation reduces osteoarthritis pain.
Arthroscopy. 31:20462015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okita Y, Narita Y, Miyahara R, Miyakita Y,
Ohno M and Shibui S: Health-related quality of life in long-term
survivors with grade II gliomas: The contribution of disease
recurrence and Karnofsky performance status. Jpn J Clin Oncol.
45:906–913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raeissadat SA, Rayegani SM, Hassanabadi H,
Fathi M, Ghorbani E, Babaee M and Azma K: Knee osteoarthritis
injection choices: Platelet-rich plasma (PRP) versus hyaluronic
acid (A one-year randomized clinical trial). Clin Med Insights
Arthritis Musculoskeletal Disord. 8:1–8. 2015. View Article : Google Scholar
|
|
24
|
Rodriguez-Merchan EC: Intraarticular
injections of platelet-rich plasma (PRP) in the management of knee
osteoarthritis. Arch Bone Jt Surg. 1:5–8. 2013.PubMed/NCBI
|
|
25
|
Filardo G, Kon E, DI Matteo B, DI Marino
A, Sessa A, Merli ML and Marcacci M: Leukocyte-poor PRP application
for the treatment of knee osteoarthritis. Joints. 1:112–120.
2014.PubMed/NCBI
|
|
26
|
Hmamouchi I, Allali F, Tahiri L, Khazzani
H, Mansouri LE, Alla Ali Ou S, Abouqal R and Hajjaj-Hassouni N:
Clinically important improvement in the WOMAC and predictor factors
for response to non-specific non-steroidal anti-inflammatory drugs
in osteoarthritic patients: A prospective study. BMC Res Notes.
5:582012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gobbi A, Lad D and Karnatzikos G: The
effects of repeated intra-articular PRP injections on clinical
outcomes of early osteoarthritis of the knee. Knee Surg Sports
Traumatol Arthrosc. 23:2170–2177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nations KR, Bursi R, Dogterom P,
Ereshefsky L, Gertsik L, Mant T and Schipper J: Maximum tolerated
dose evaluation of the AMPA modulator Org 26576 in healthy
volunteers and depressed patients: A summary and method analysis of
bridging research in support of phase II dose selection. Drugs R D.
12:127–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hodge JA, Kawabata TT, Krishnaswami S,
Clark JD, Telliez JB, Dowty ME, Menon S, Lamba M and Zwillich S:
The mechanism of action of tofacitinib-an oral Janus kinase
inhibitor for the treatment of rheumatoid arthritis. Clin Exp
Rheumatol. 34:318–328. 2016.PubMed/NCBI
|
|
31
|
van der Goes MC, Jacobs JW and Bijlsma JW:
Rediscovering the therapeutic use of glucocorticoids in rheumatoid
arthritis. Curr Opin Rheumatol. 28:289–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Battaglia M, Guaraldi F, Vannini F, Rossi
G, Timoncini A, Buda R and Giannini S: Efficacy of
ultrasound-guided intra-articular injections of platelet-rich
plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics.
36:e1501–e1508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Laudy AB, Bakker EW, Rekers M and Moen MH:
Efficacy of platelet-rich plasma injections in osteoarthritis of
the knee: A systematic review and meta-analysis. Br J Sports Med.
49:657–672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meheux CJ, McCulloch PC, Lintner DM,
Varner KE and Harris JD: Efficacy of intra-articular platelet-rich
plasma injections in knee osteoarthritis: A systematic review.
Arthroscopy. 32:495–505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Altman RD, Manjoo A, Fierlinger A, Niazi F
and Nicholls M: The mechanism of action for hyaluronic acid
treatment in the osteoarthritic knee: A systematic review. BMC
Musculoskeletal Disord. 16:3212015. View Article : Google Scholar
|
|
36
|
Ahadi T and Abtahi M: Platelet-rich plasma
versus hyaluronic acid. Arthroscopy. 28:1585author reply 1585.
–1586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kon E, Mandelbaum B, Buda R, Filardo G,
Delcogliano M, Timoncini A, Fornasari PM, Giannini S and Marcacci
M: Platelet-rich plasma intra-articular injection versus hyaluronic
acid viscosupplementation as treatments for cartilage pathology:
From early degeneration to osteoarthritis. Arthroscopy.
27:1490–1501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lùrati A, Laria A, Mazzocchi D, Re KA,
Marrazza M and Scarpellini M: Effects of hyaluronic acid (HA)
viscosupplementation on peripheral Th cells in knee and hip
osteoarthritis. Osteoarthritis Cartilage. 23:88–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Görmeli G, Görmeli CA, Ataoglu B, Çolak C,
Aslantürk O and Ertem K: Multiple PRP injections are more effective
than single injections and hyaluronic acid in knees with early
osteoarthritis: A randomized, double-blind, placebo-controlled
trial. Knee Surg Sports Traumatol Arthrosc. 25:958–965. 2017.
View Article : Google Scholar : PubMed/NCBI
|