Introduction
Traumatic optic neuropathy (TON) is an acute injury
of the optic nerve secondary to trauma; clinically, it is
characterized by vision loss, dyschromatopsia, visual field defects
and a relative afferent pupil defect (1). The causes of TON are thought to be
multifactorial. Generally, TON can be classified as direct or
indirect according to the mode of injury. Direct TON results from
anatomical disruption of the optic nerve, whereas indirect TON
results from trauma to the head or face in which the energy from
the force of impact is transmitted to the bony structures that
convey the optic nerve (2).
Regardless of whether the cause is direct or indirect, TON remains
an important cause of vision loss following traumatic head
injury.
Although various management strategies have been
investigated, including observation, corticosteroid treatment,
surgical decompression of the optic nerve and combinations of
steroids and surgical decompression (3,4), little
consensus has been reached among neuro-ophthalmologists regarding
the appropriate treatment for TON. In addition, few studies have
been published regarding the treatment of TON in children. As
children have limited language expression ability, visual loss is
not easily identified at an early stage, and visual acuity may not
be examined when a child has a head injury. The prognosis of TON is
poor, and children's lives and studies are seriously affected.
Considering this, we will summarize our experience with treating
TON in children based on 29 cases treated from April 1999 to May
2015 at the Affiliated Hospital of Qingdao University (Qingdao,
China). We hope that our study will provide a valuable reference
for the clinical treatment and prevention of TON in children.
Patients and methods
Patient enrolment
This study was approved by the Ethics Committee of
the Affiliated Hospital of Qingdao University. Informed consents
were freely given by the parents of the children before the study
and the parents of the patients provided consent for the
publication of their images.
The clinical history data of 29 children diagnosed
with TON and hospitalized from April 1999 to May 2015 in our
hospital were retrieved for this study. The inclusion criteria
included clinical findings of visual loss in children due to head
or mid-facial trauma or the presence of an ipsilateral afferent
pupillary defect with normal eyesight in the other eye. Patients
were excluded if their post-traumatic visual loss was not related
to optic nerve dysfunction. All the children underwent a baseline
neuro-ophthalmologic examination as soon as their mental state and
physical condition allowed an examination of their visual function.
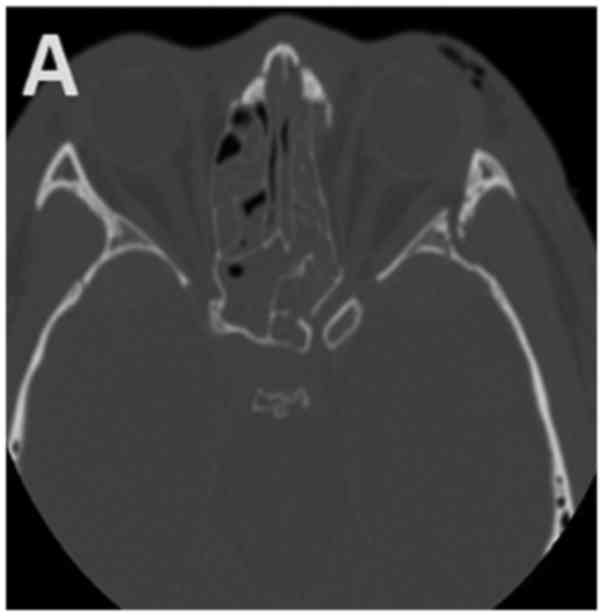
A high-resolution computed tomography (CT) scan was conducted to
evaluate the orbit and optic canal (Fig.
1).
Surgical techniques and post-operative
treatments
All patients received high-dose glucocorticoids
[GEM-P (Pfizer Manufacturing Belgium NV, Belgium), 5–10 mg/kg/day]
combined with vasodilators, neurotrophic agents and nutritional
support after admission; dehydrators were applied in some cases.
Among all the patients, 5 received drug therapy, 24 received drug
combined with surgical therapy. Among the patients who received
surgical therapy, 23 received nasal endoscopic optic decompression,
and 1 patient received nasal endoscopic orbital decompression.
The optic canal, sphenoid sinus and skull base were
examined with layer-targeted scanning CT before surgery. Routine
peripheral blood, chest and electrocardiogram examinations were
also conducted. The operations were performed using a tracheal
cannula under general anaesthesia and well-controlled reduced blood
pressure. The Messerklinger operation was adopted. Residual blood
and bone chips were cleared away. After the suprasphenoid cells or
the upper sphenoidalis sinus were exposed, the orbital apex was
located along the orbital wall, and the surface mucosa above the
optic alprotrusion was dissected to expose the canal. After that,
the canal wall was thinned with a diamond drill, bone chips were
removed with hooks and curettes, and the optic canal was dissected
to further expose the optic nerve anterior to the common tendinous
ring. Then, the hydrocele and common tendinous rings were cut along
the optic nerve, and the surface of the optic nerve was washed with
1 ml dexamethasone solution (0.5%). The optic nerve was covered,
and the sphenoid sinus and posterior ethmoid sinus were filled with
gelatin sponge soaked in dexamethasone solution (0.5%). The nasal
cavity was filled with sponge or haemostatic dressing in cases of
obvious bleeding. The filling in the nasal cavity was removed
gradually 24–48 h after the operation. Routine antibiotics were
applied to prevent infection. Glucocorticoids and neurotrophic
agents were continuously applied. Post-surgical follow-up was
routinely performed with a nasal endoscope.
Visual evaluation
Visual acuity and the pupillary response were
evaluated daily over the course of treatment and at the end of
treatment. After discharge from hospital, the patients were
regularly followed up for at least 6 months and submitted to tests
of visual acuity. The last visual acuity result recorded was
considered the final outcome. A patient's vision was considered to
have improved if the patient presented an increase of one line or
more on the Snellen visual chart or demonstrated an improvement
from no light perception to light perception, from light perception
to hand motion, or from hand motion to finger counting.
Factors evaluated at follow-up
The follow-up factors evaluated in this study
included post-treatment visual acuity (with or without light
perception, hand movement, finger counting, visual acuity chart),
position of the optic canal (sphenoid sinus, ethmoid sinus, poor
sphenoidal sinus gasification), the interval between the time of
the injury and the time of the operation, optic canal fracture,
optic nerve sheath incision, and haemorrhage within the ethmoid
and/or sphenoid sinus. Given that immediate or secondary visual
loss was not determined in the majority of patients because they
had lost consciousness or exhibited severe eyelid swelling,
immediate and secondary visual loss were not evaluated in the
study. Likewise, visual field and visual evoked potential data were
incomplete and therefore they were not assessed.
Results
The baseline characteristics of the 29 children with
TON are given in Table I. There were
29 children, aged from 4 to 17 years; 6 were between 4 and 10
years, and the remaining 23 cases were between 11 and 17 years. The
duration of trauma ranged from 15 h to 20 days; 16 cases had a
duration between 15 h and 3 days, while 13 cases had a duration
longer than 3 days. Twenty-two patients manifested with Marcus-Gunn
pupils, and 7 cases had indirect but impaired direct light
responses. Contusions around the orbital soft tissues but no eye
ball injury was evident in the subjects. Flash visual evoked
potentials (FVEPs) were measured in 13 patients. Fracture of the
optic canal was present on CT in 15 patients. Regarding other
complications, 15 patients suffered from coma; 4 had cerebrospinal
rhinorrhoea; 11 had abasal fracture; 15 presented sinus bleeding
(Fig. 1B); 3 had malar bone
fracture; 19 presented orbital fracture (Fig. 1A); 4 presented epidural haematomas; 2
presented subdural haematomas; 2 had subarachnoid haemorrhage; 1
had facial paralysis; 3 had temporal bone fracture; 1 presented
metacarpal and phalanx fractures; 3 had brain contusions and
lacerations; 1 had clavicular fracture; 1 had anasal bone fracture;
2 presented oculomotor paresis; 1 had lacrimal gland contusion; 1
presented impaired dentition and mouth-opening; 1 had femur, tibia
and fibula fractures; and 1 had lost some teeth.
 | Table I.Clinical features of patients. |
Table I.
Clinical features of patients.
| Parameters | No. of patients | Percentage (%) |
|---|
| Sex |
|
|
| Male | 23 | 79.31 |
|
Female | 6 | 20.69 |
| Injured side |
|
|
| Left | 18 | 62.07 |
|
Right | 11 | 37.93 |
|
Bilateral | 0 |
|
| Cause |
|
|
| Car
accident | 9 | 31.03 |
| Motorbike
accident | 10 | 34.48 |
|
Bicycle | 1 | 3.45 |
| Fall | 6 | 20.69 |
| Slip | 1 | 3.45 |
| Fishing
rod | 1 | 3.45 |
| Telegraph
pole | 1 | 3.45 |
| Region |
|
|
| City | 3 | 10.34 |
|
County | 1 | 3.45 |
|
Rural | 1 | 3.45 |
|
Countryside | 24 | 82.76 |
| Complications |
|
|
| Coma | 15 | 51.72 |
|
Cerebrospinalrhinorrhoea | 4 | 13.79 |
| Pre-treatment visual
acuity |
|
|
| No light
perception | 22 | 75.86 |
| Light
perception | 3 | 10.34 |
| Hand
movement | 1 | 3.45 |
| Finger
counting | 2 | 6.9 |
| Visual
acuity chart | 1 | 3.45 |
During follow-up periods lasting from 6 months to 5
years after surgery, all the lesions in each child were
epithelized, and no complications were observed. Of the 29
children, 14 showed improved visual acuity to different extents
(effectiveness rate, 48.28%): of the 5 patients who received drug
therapy, 3 showed improvement (60%). Of the 24 cases who received
drug and surgical therapy, 11 showed improvement (45.83%). Of 22
children with no light perception, 10 showed improved acuity
(effectiveness rate, 45.45%); of 7 children with acuity above light
perception, 4 showed improved acuity (effectiveness rate, 57.14%).
Among the 29 patients, post-treatment acuity was equivalent to
light perception in 6 patients, hand movement in 1 patient, finger
counting in 1 patient, visual acuity chart in 9 patients (Table II).
 | Table II.Post-treatment visual acuity and
position of the optic canal. |
Table II.
Post-treatment visual acuity and
position of the optic canal.
| Parameters | No. of patients | Percentage (%) |
|---|
| Post-treatment visual
acuity |
|
|
| No light
perception | 12 | 41.38 |
| Light
perception | 6 | 20.69 |
| Hand
movement | 1 | 3.45 |
| Finger
counting | 1 | 3.45 |
| Visual
acuity chart | 9 | 31.03 |
| Position of the optic
canal |
|
|
| Sphenoid
sinus | 20 | 68.96 |
| Ethmoid
sinus | 7 | 24.14 |
| Poor
sphenoidal sinus gasification | 2 | 6.9 |
Discussion
TON is primarily caused by indirect injury (5), during which external forces impact the
optic canal through the displacement of the craniofacial skeleton
and/or the elastic deformation of the sphenoid bone and compress
and injure the optic nerve, thus leading to secondary defects in
the nerve blood circulation and axonal transportation,
retrospective degeneration and apoptosis of retinal ganglion cells
(RGCs) and finally, visual dysfunction (6–8). The
inability of children to self-assess and report symptoms after a
traumatic injury can lead to the misdiagnosis of traumatic
neuropathy and a poor prognosis, and early diagnosis and proper
treatments are keys to a better prognosis. Thus, early
ophthalmologic examinations should be required for children with
head and face injuries. In the current study, we reported 29 cases
of children with TON in our hospital and summarized the related
treatments and prognosis, which have rarely been reported in
previous studies; thus, our retrospective study may provide
clinical experience for the treatment of children with TON caused
by related craniofacial injuries.
In our retrospective study, 79.31% of our subjects
were boys. The 68.96% of the traumatic injuries were the result of
traffic accidents, including bicycles, motorcycles, electric
vehicles and cars; 20.69% were caused by falls from heights; and
the remaining injuries were caused by other factors, such as falls
due to slipping, injury by a fishing rod and being knocked against
a wire post. Such distributions of sex and cause were consistent
with previous studies (9–11). Ford et al (10) reported 26 children with TON in
England, 81% of whom were boys, and the main causes of injuries
were falling, traffic accidents and sport-related injuries. Gupta
et al (11) reported 31
children with TON; 68% were boys, and falls from heights were the
main cause. Gender differences may result from the fact that boys
are more active than girls and are thus subject to a higher
incidence of traumatic injuries. Furthermore, the high incidence of
children from rural and countryside regions versus cities (86.21
vs. 13.79%) may be accounted for by the fact that motorbikes and
electric bikes are routine sources of transportation for teenagers
in non-city regions, and such modes of transportation offer less
protection than others. Additionally, children have weak safety
awareness; they play in high places and can suffer fall injuries as
a result.
Most children with TON also present brain injury,
and some are comatose; because the main treatment after injury is
for brain injury, visual loss is easily ignored. Eyelid swelling
and skin injury around the eyebrow caused by orbital trauma may
delay vision examinations; furthermore, because children's language
abilities are poor, they cannot state their concerns, and visual
loss may not be found early. The authors believe that vision should
be examined in children with craniofacial trauma to allow patients
with TON to be diagnosed and receive treatment as soon as
possible.
Currently, the treatment of TON is controversial,
and there are no evidence-based guidelines (12); treatment is still under exploration
and includes mainly observation and pharmacological and surgical
treatments. Routine treatment involves high-dose glucocorticoid
pulse therapy combined with circulation improvement, neurotropic
reagents and dehydration. Recently, erythropoietin and mesenchymal
stem cell-based therapies have emerged as new treatments based on
lowering tissue oedema, relieving optic nerve compression,
preventing post-injury blood vessel spasm and improving axonal
transduction (13–15). For the treatment of this disease, we
suggest personalized treatment. All of our cases received drug
therapy, 3 cases whose visual acuity improved did not receive
operation, 2 cases refused operation for personal reasons, 24 cases
whose visual acuity did not change after drug therapy received
surgical treatment. In terms of surgical treatments, optic canal
decompression can directly clean bone chips and haematomas, relieve
optic nerve compression due to ischaemia, and decrease or minimize
secondary nerve injuries, thus providing direct treatment (16). Consequently, we performed surgical
treatment for most of the patients. This choice is theoretically
supported by one relevant previous study: Gupta et al
(11) recommend performing surgery
as soon as possible for children with TON and believe that nasal
endoscopic optic nerve decompression has many advantages, such as
less trauma, clear vision and fewer complications.
Regarding the timing of surgical treatment, 24–72 h
after glucocorticoid pulse therapy has been recommended as the
optimal time for surgery (17). At
present, there is no uniform diagnostic or treatment standard;
consequently, the duration of the injury time and preoperative
treatment of the patients in our study differed, and most of the
surgeries were performed 3 days after injury. However, we suggest
performing optic canal decompression as early as possible.
Nonetheless, 1 child underwent surgery on the 21st day after injury
and experienced a visual acuity improvement from 0.04 to 0.1, and
another child showed a visual acuity improvement from hand movement
to 0.5 with only pharmacological treatment. Thus, even if the
optimal surgical window is missed, efforts should be made to
optimize the prognosis, and assessment and treatment should be
individualized for different patients.
When performing surgery in children, it is difficult
to distinguish the optic canal and the internal carotid artery
because the sinus anatomy is different in children than in adults;
sometimes the bone wall of optic nerve is thicker, so the thermal
damage caused by diamond drill can not be ignored. Furthermore, the
surgery space is limited because the nasal cavity and sinus are
relatively small and poorly gasificated. Consequently, the relevant
structures should be carefully observed with targeted CT before
surgery to explore the position of the optic canal, the presence of
optic nerve protrusion and the position of the optic nerve, which
should be further confirmed by pressing the eyeball and observing
the relative motility of the presumed optic nerve. The distance,
relative position and fracture should also be noted. To minimize
secondary injury to the optic canal, fine manipulation was
conducted; additionally, during bone thinning with a diamond drill,
the area should be washed with chilled saline, and the bones cannot
be peeled until they are sufficiently thinned. Sponges soaked in
saline rather than NE should be applied in cases of para-optic
canal bleeding to prevent convulsion of the retinal artery and
further impairment of visual acuity. As branches of the retinal
artery are mainly distributed in the ventral or ventrolateral side
of the optic nerve, the hydrocele should be dissected from the
optic nerve above to minimize the artery injury (18).
The prognosis of TON in children is relatively poor
and may affect their lives during both childhood and adulthood. As
traumatic injury is the main cause of TON, it is important to
minimize accidents by reinforcing safety education, controlling
traffic and forbidding children from driving without supervision.
In the meantime, children should wear safety gear when they are
passengers in vehicles. In cases of accidents and craniofacial
injury, visual function examinations should be performed for early
diagnosis, and TON should be treated to improve the prognosis.
Acknowledgements
Not applicable.
Funding
This study has been funded with the ‘National
Natural Science Foundation of China: Regulation mechanism of
histone demethylation enzyme Jarid1b on repair and regeneration of
optic nerve injury (NSFC81770978)’.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
NL performed the surgery, designed and drafted the
study. MC collected the clinical data, analyzed the data and
drafted the study. YJ collected the clinical data, analyzed the
data and assisted in the modification of the manuscript. JZ helped
with the data analysis. All authors agreed to be accountable for
the content of the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Affiliated Hospital of Qingdao University (Qingdao, China).
Informed consents were freely given by the parents of the children
before the study, and the parents of the patients provided consent
for the publication of their images.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al-Qurainy IA, Stassen LF, Dutton GN, Moos
KF and El-Attar A: The characteristics of midfacial fractures and
the association with ocular injury: A prospective study. Br J Oral
Maxillofac Surg. 29:291–301. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Landers MB III, Bradbury MJ and Sydnor CF:
Retinal vascular changes in retrograde optic atrophy. Am J
Ophthalmol. 86:177–181. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McClenaghan FC, Ezra DG and Holmes SB:
Mechanisms and management of vision loss following orbital and
facial trauma. Curr Opin Ophthalmol. 22:426–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nau HE, Gerhard L, Foerster M, Nahser HC,
Reinhardt V and Joka T: Optic nerve trauma: Clinical,
electrophysiological and histological remarks. Acta Neurochir
(Wien). 89:16–27. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kountakis SE, Maillard AA, El-Harazi SM,
Longhini L and Urso RG: Endoscopic optic nerve decompression for
traumatic blindness. Otolaryngol Head Neck Surg. 123:34–37. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cirovic S, Bhola RM, Hose DR, Howard IC,
Lawford PV, Marr JE and Parsons MA: Computer modelling study of the
mechanism of optic nerve injury in blunt trauma. Br J Ophthalmol.
90:778–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steinsapir KD and Goldberg RA: Traumatic
optic neuropathy: An evolving understanding. Am J Ophthalmol.
151:928–933.e2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu-Wai-Man P: Traumatic optic neuropathy -
clinical features and management issues. Taiwan J Ophthalmol.
5:3–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldenberg-Cohen N, Miller NR and Repka
MX: Traumatic optic neuropathy in children and adolescents. J
AAPOS. 8:20–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ford RL, Lee V, Xing W and Bunce C: A
2-year prospective surveillance of pediatric traumatic optic
neuropathy in the United Kingdom. J AAPOS. 16:413–417. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta AK, Gupta AK, Gupta A and Malhotra
SK: Traumatic optic neuropathy in pediatric population: Early
intervention or delayed intervention? Int J Pediatr
Otorhinolaryngol. 71:559–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Andersen MR and Gade E: Traumatic optic
neuropathy after fall with a bamboo stick. Ugeskr Laeger.
176:V051303302014.(In Danish). PubMed/NCBI
|
|
13
|
Entezari M, Esmaeili M and Yaseri M: A
pilot study of the effect of intravenous erythropoetin on
improvement of visual function in patients with recent indirect
traumatic optic neuropathy. Graefes Arch Clin Exp Ophthalmol.
252:1309–1313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Connick P, Kolappan M, Crawley C, Webber
DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ,
et al: Autologous mesenchymal stem cells for the treatment of
secondary progressive multiple sclerosis: An open-label phase 2a
proof-of-concept study. Lancet Neurol. 11:150–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu-Wai-Man P and Griffiths PG: Steroids
for traumatic optic neuropathy. Cochrane Database Syst Rev.
6:CD006032. 2013.PubMed/NCBI
|
|
16
|
Vaitheeswaran K, Kaur P and Garg S:
Minimal invasive transcaruncular optic canal decompression for
traumatic optic neuropathy. Orbit. 33:456–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spoor TC, Hartel WC, Lensink DB and
Wilkinson MJ: Treatment of traumatic optic neuropathy with
corticosteroids - correction. Am J Ophthalmol. 111:5261991.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sandu K, Monnier P and Pasche P:
Anatomical landmarks for transnasal endoscopic skull base surgery.
Eur Arch Otorhinolaryngol. 269:171–178. 2012. View Article : Google Scholar : PubMed/NCBI
|