Introduction
The incidence of esophageal adenocarcinoma (EAC) has
increased 6-fold over the last two decades in the western world
(1). The prognosis of EAC is
extremely poor and the 5-year survival is <20% (2). According to the current understanding,
Barrett's esophagus (BE) is a well-known precancerous lesion that
is caused by long-standing pathologic exposure to gastroduodenal
refluxate (3,4). Histopathologically, BE takes place when
normal squamous cells of esophageal mucosal cells are replaced with
specific columnar cells, with or without intestinal metaplasia
(5). Although the majority of
patients with gastroesophageal reflux disease do not develop BE
(6), it has been reported that
<5% of BE cases progress into EAC (7).
Multiple animal models of BE and EAC have been
developed to investigate the mechanism underlying BE formation.
Pera et al (8) introduced the
first surgical rat model of ECA by inducing chronic
duodenogastroesophageal reflux, with exposure to a known carcinogen
(2,6-dimethylnitrosomorpholine) in 1989. The surgical procedure
used to induce chronic esophageal reflux was an end-to-side
esophagojejunostomy with gastric preservation (8). Since then, several animal models with
different surgical procedures have been developed (9–16). The
construction of these surgical models have included:
Esophagojejunostomy and esophagojejunostomy plus total Gastrectomy
(9,11), esophagogastroplasty, side-to-side and
end-to-side esophagoduodenostomy (10,11) and
esophagoduodenostomy with various degrees of gastrectomy (12).
However, the incidence of BE in these models are low
and their induction is usually time consuming. It is therefore
necessary to identify novel methods to fully elucidate the
mechanism underlying BE formation, and its association with EAC.
The purpose of the present study was to introduce an improved
procedure in establishing a gastroesophageal reflux model with a
high incidence of BE in rats. A number of modifications to the
surgical procedure developed by Zhang et al (17) were made in order to achieve a greater
occurrence of BE within 25 weeks.
Materials and methods
Animals
In total, 50 specific pathogen-free female Sprague
Dawley rats (35 weeks old; weight, 350±10 g) were purchased from
the Laboratory Animal Center of Hubei University of Medicine
(Shiyan, China). All rats were housed in a controlled environment
(temperature, 22±2°C; humidity, 60±5%; air renovations/h: 15; light
cycle/h: 12/12) and had access to water and food. Animal care and
experimental protocols were approved by the Animal Research Ethical
in Hubei University of Medicine. Rats were allowed to acclimatize
for at least 1 week prior to surgery. Rats were randomly divided
into two groups: The model group (n=40) and the sham operation
group (n=10). Rats were fasted for 24 h prior to surgery.
Anesthesia methods
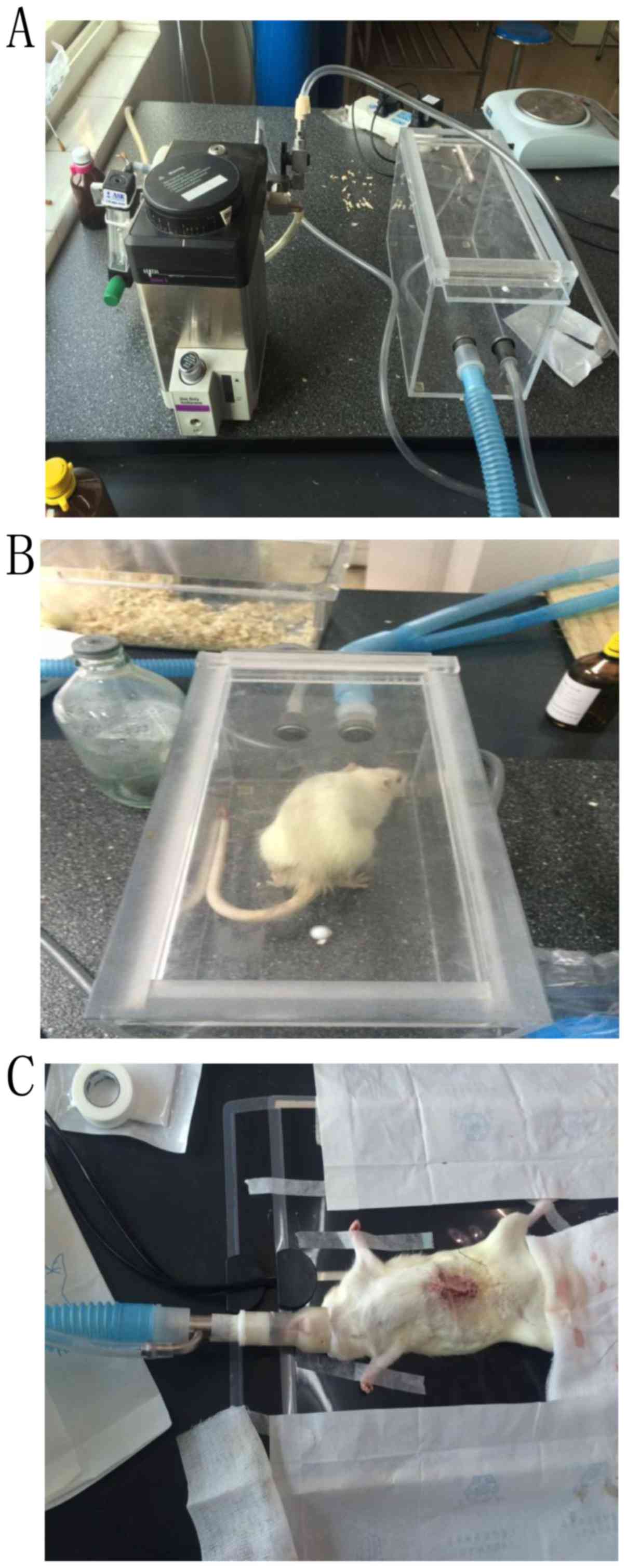
Anesthesia ventilation with isoflurane inhalational
anesthetic (Datex-Ohmeda, Inc., Madison, Wisconsin, USA) was
performed as follows: The ventilator's oxygen pressure was adjusted
to a controlled level at 0.5 MPa. Isoflurane was used for induction
(3%) and maintenance (1.5–3%). Rats were placed into a closed
induction-box and the anesthetic flow was switched to the 5th gear.
Following 1–2 min of exposure, the rats were induced into a coma.
Subsequently the rats were then positioned on the operation table,
the ventilator mask was quickly fixed and the anesthetic flow was
switched to the 1st gear simultaneously. The breathing was slow and
even for rats, owing to the anesthetic effect (Fig. 1).
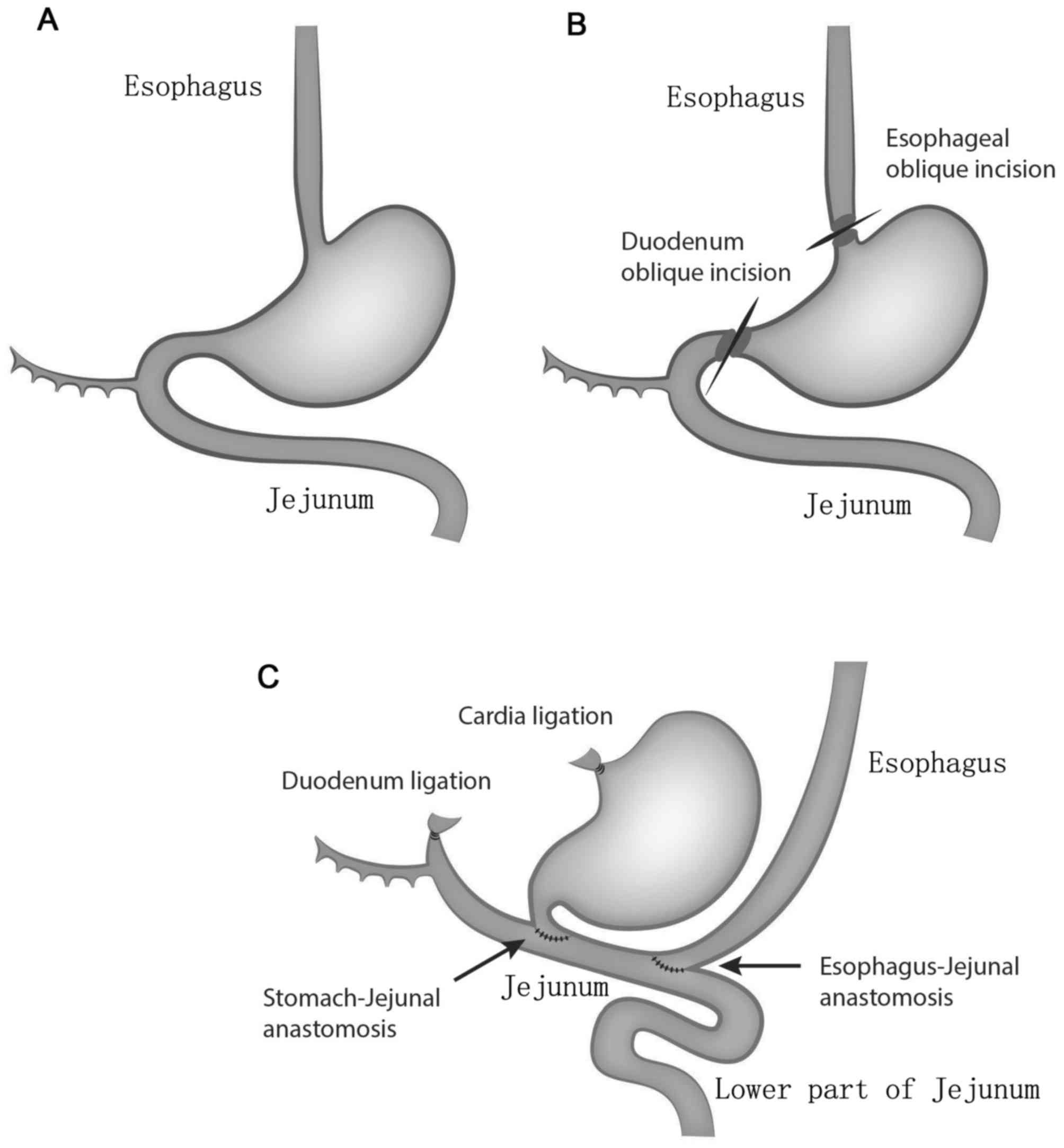
Surgical methods
The surgical procedure was performed under aseptic
conditions. The limbs of rats were fixed in the supine position.
The tongue was retracted from the mouth using a hemostat and fixed
to maintain constant smooth breathing. Once the operative field was
disinfected with 0.5% povidone-iodine and paved with a surgical
drape, an incision into the abdominal cavity was made at the
subxiphoid midline abdominal peritoneum. The incision was roughly 4
cm in length. In the model group, the stomach was exposed and
raised with ophthalmic tweezers. The esophagus was exposed and the
liver-stomach ligament was carefully cut with an ophthalmic
hemostat. The Vagus nerve was carefully protected during this
process. The cardia and esophagus were separately ligated with a
3.0 suture near the cardia; the two ligations were 3 mm apart. The
esophagus and stomach were cut and separated in intervals along the
ligation. The distal esophagus ligature was pulled gently to avoid
esophageal retraction within the thoracic cavity. Subsequently, the
duodenum and pylorus were carefully exposed. The duodenum was
ligated with a 3.0 suture at a distance of 7 mm to the pylorus and
cut at a distance of 5 mm to the pylorus. Following this, the
stomach was flushed twice with iodine water (1:2 ratio of iodine
and saline). Additionally, the jejunum was exposed 3 cm underneath
the Treitz ligament and the pylorus and jejunum were sutured with a
6.0 suture needle on the upper part of jejunum. The gastro-jejunal
anastomotic stoma was 5–6 mm in length. The oblique incision was
prepared in the lower esophagus with a scalpel. An oblique
esophageal and jejunal incision was sutured with a 6.0 suture
needle for the entire layer on the upper jejunum. The
esophageal-jejunal anastomotic stoma was 3–4 mm in length. The
distance between the two anastomotic stoma was 1–1.5 cm. Finally,
the abdominal cavity was flushed with a sterile gauze and closed. A
diagram of the surgical procedure is indicated in Fig. 2. In the sham group, the abdominal
cavity was incised, opened and closed 10 min thereafter. Following
surgery, the anesthesia ventilator was turned off and the
respirator mask was quickly removed. Rats were fasted for 24 h
post-surgery and were supplied with 5% dextrose and 0.9% saline
(1:1) mixture and purified water. The incision was disinfected with
iodine once a day in week 1 following the surgery and the rats were
caged together (4–5 rats per cage) 10 days following this.
General postoperative condition
Following the surgery, physiological indices of
rats, including water intake, food intake, body weight, stool and
mental status were recorded. The rats were sacrificed 16 weeks
post-surgery and 3-cm tissue specimens of the esophagus and 0.4-cm
jejunum specimens surrounding the esophagus-jejunal anastomosis
were collected and cut longitudinally. Gross morphological
observations of the esophagus were made simultaneously. The
specimens were fixed in 10% neutral-buffered formalin for 24 h at
4°C, then processed and embedded in paraffin. Following routine
staining, with hematoxylin (5 min) and eosin (2 min) at room
temperature, histological changes were observed under an optical
microscope (magnification, ×100 and ×200; Olympus Corporation,
Tokyo, Japan).
Histopathological evaluation
Histopathological evaluation was performed according
to Miwa et al (11) and
standards proposed by the American College of Gastroenterology
(18). Inflammation was defined as
the infiltration of inflammatory cells into epithelial tissue,
including neutrophils and lymphocytes, with or without esophageal
tissue edema. Epithelial hyperplasia was defined as the thickness
of the esophageal epithelium increasing more than twice of the
normal thickness. Proliferation of the basal cells was defined as
the thickness of the squamous basal layer increasing to >15%, or
with an organizational cyst. An ulcer was defined as an epithelial
defect, with inflammatory cell infiltration. Squamous epithelial
dysplasia was defined as the esophageal mucosa consisting of
squamous cells and increased mitotic nuclear staining. Furthermore,
BE was defined as the replacement of squamous epithelium by
columnar epithelium, with or without metaplasia to the columnar
epithelium and esophageal adenocarcinoma was defined as mucinous
adenocarcinoma with submucosal tissue infiltration.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
statistical analysis. One-way Analysis of variance followed by the
Dunnett's post hoc test and χ2 tests were used to
compare enumeration data. P<0.05 was considered to indicate a
statistically significant difference.
Results
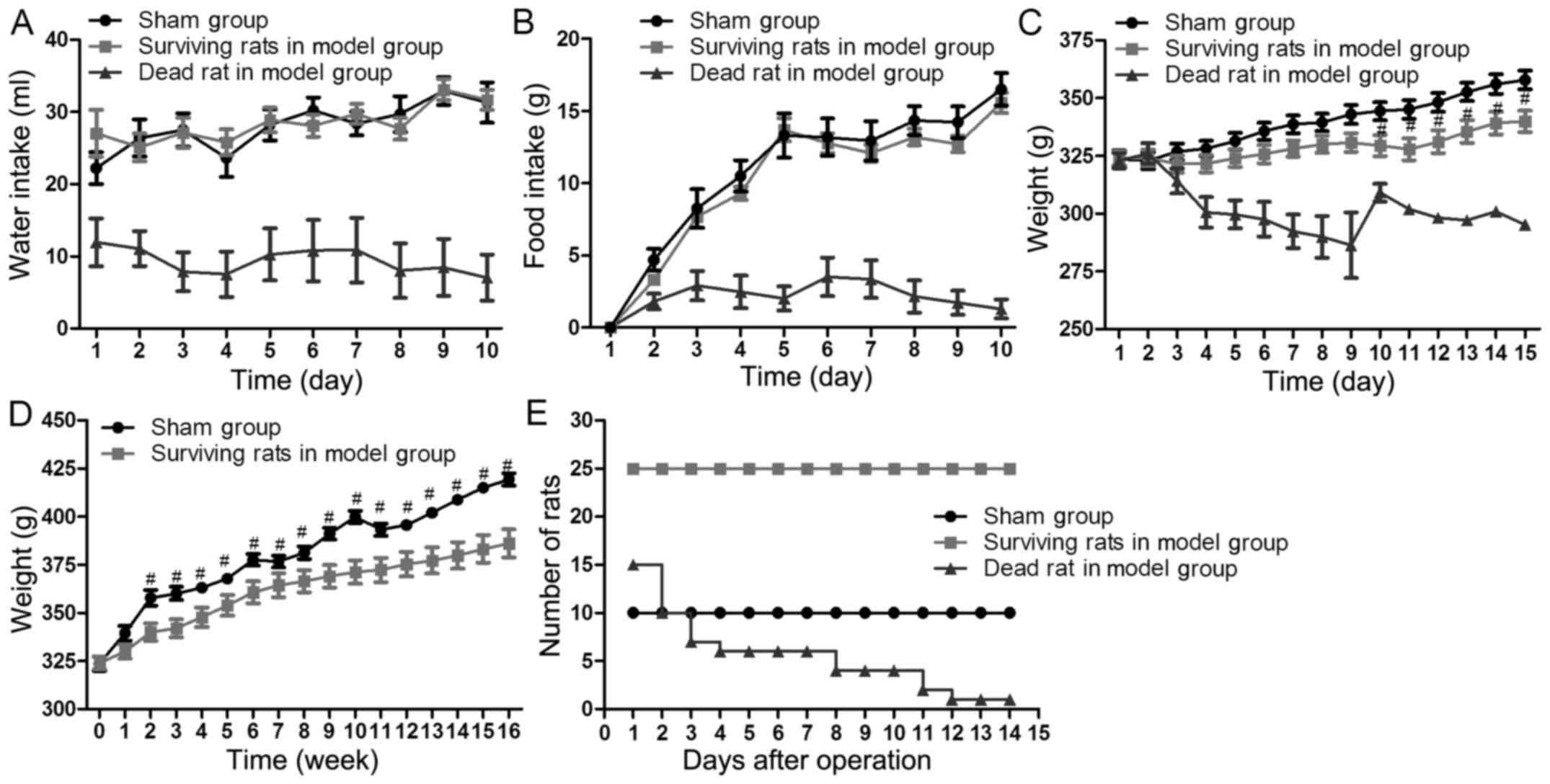
Rat survival
All rats in the sham group survived. A total of 15
rats in model group succumbed to complications; the mortality rate
was 37.5% (15/40). The majority of fatalities occurred in the first
3 days following the surgery and the mortality rate peaked on the
day 3. The causes of fatality were attributed to surgical
complications, including bleeding, obstruction and necrosis
(Table I). The number of rats in
each group at each time point were presented in Fig. 3.
 | Table I.Causes of fatality in rats at
different post-surgical time points. |
Table I.
Causes of fatality in rats at
different post-surgical time points.
| Variable | Day 0–3 | Day 4–7 | Week 2–3 | Week 4–16 |
|---|
| Causes of fatality
(number) | Bleeding in surgery
(1) | Diarrhea (1) | Diarrhea (2) |
|
|
| Mechanical
intestinal | Aspiration | Aspiration |
|
|
| obstruction (1) | pneumonia (1) | pneumonia (2) |
|
|
| Anastomotic
obstruction (4) |
|
|
|
|
| Gastric ischemic
necrosis (1) |
|
|
|
|
| Anastomotic leak
(1) |
|
|
|
|
| Gross hematuria
(1) |
|
|
|
| Total number of
fatalities | 9 | 2 | 4 | 0 |
Drinking and eating results of
rats
Water and food intake of each rat was recorded for
10 consecutive days following the surgery. Rats in the sham group
were able to eat and drink as normal. Rats that drank and ate
little in the model group did not survive. However, the rats that
drank and ate normally in model group survived. There was no
significant difference in drinking (P≥0.325) and eating (P≥0.234)
between surviving rats in the sham group and the model group
(Fig. 3A and B).
Weight
The body weight of each rat was measured every day
for 15 consecutive days post-surgery. From 2 weeks post-surgery to
the end of 16 weeks, the weight of each rat was measured every
week. The weight of the rats in the model group was significantly
decreased 10–14 days post-surgery compared with the sham group
(P≤0.031; Fig. 3C). The weight of
the surviving rats in the model group gradually increased 2–16
weeks post-surgery. However, the weight of surviving rats in the
model group remained significantly lower than that of the sham
group at the end of the 16 weeks (P=0.0194; Fig. 3D).
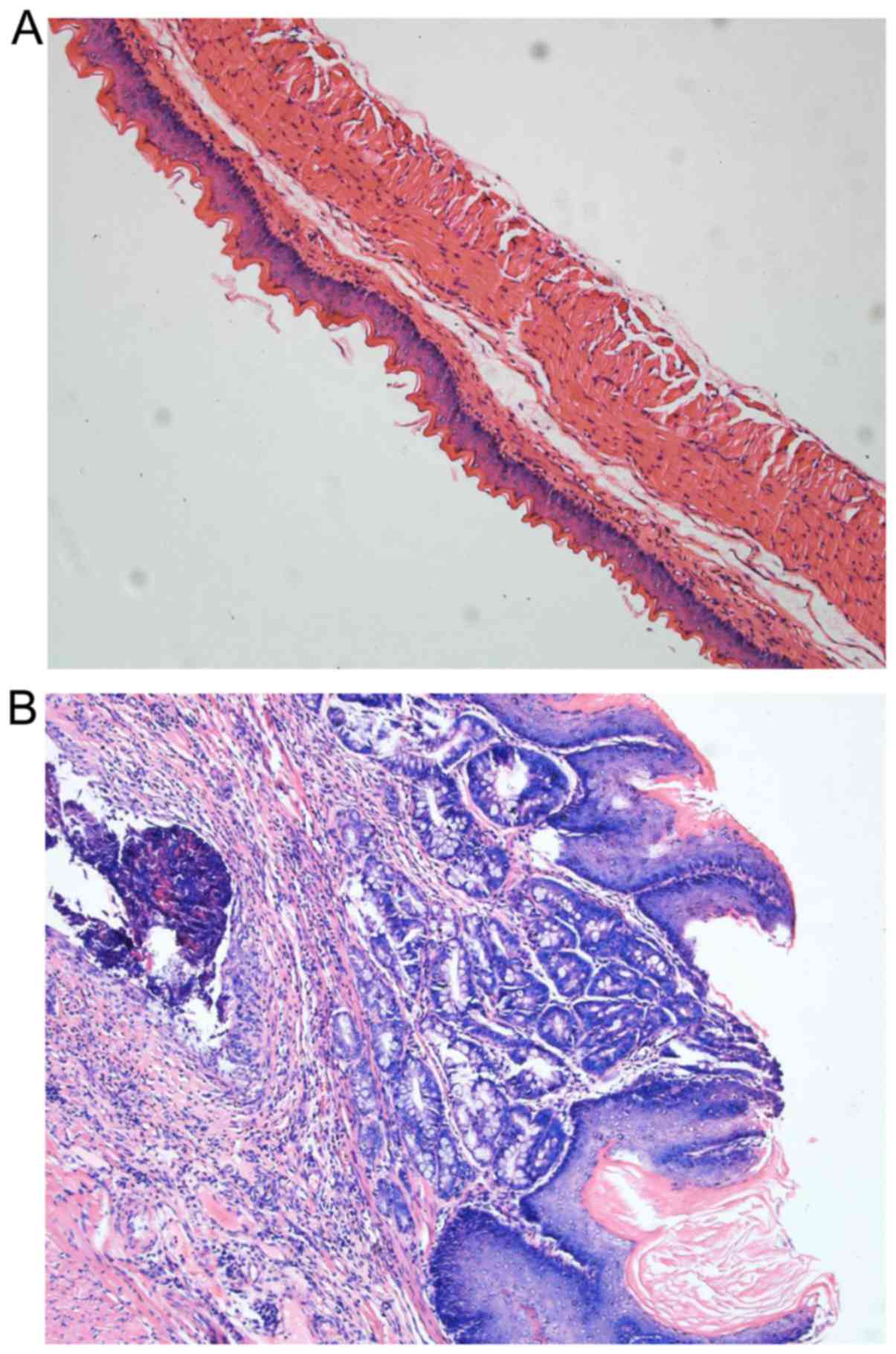
Histological changes
BE and inflammation was identified in all surviving
rats in the model group (100%, 25/25) at the end of the 16 weeks;
the difference between the sham and model group was statistically
significant (P<0.01). Erosions and ulcers were common and had an
incidence of 80% (20/25) in the model group compared with the
control group (P<0.01). Dysplasia of the squamous epithelium was
identified in 40% (10/25) of rats in the model group (P=0.018;
Table II and Fig. 4).
 | Table II.Histological changes in esophageal
tissues. |
Table II.
Histological changes in esophageal
tissues.
| Variable | Sham group, n
(%) | Model group, n
(%) | P-value |
|---|
| Number | 10 | 25 |
|
| Barrett
esophagus | 0 | 25
(100) | P<0.01 |
| Inflammation | 1 (10) | 25
(100) | P<0.01 |
| Erosion or ulcer | 0 | 20 (80) | P<0.01 |
| Dysplasia of squamous
epithelium | 0 | 10 (40) | P=0.018 |
| Esophageal
adenocarcinoma | 0 | 0 |
|
Discussion
Gastroesophageal reflux can lead to esophageal
mucosal damage and inflammation (19). The interaction of acid, bile or the
mixture of bile and acid reflux, are believed to serve important
roles in the development of BE. Acid and duodenogastroesophageal
reflux occurs simultaneously in the majority of reflux episodes
(20). Hence, animal models of BE
are usually induced by mixed reflux. There are three known
mixed-reflux models: Esophagus-duodenum anastomosis (EDA); EJA; and
esophagus-jejuna gastro-jejunal anastomosis (EJGJ). In the EDA
model, end-to-end anastomosis occurs between the lower esophageal
sphincter and the beginning section of the duodenum (1 cm apart
from the pylorus), and the Vagus nerve is protected (21). In the EDA model, gastric secretions,
together with duodenal secretions, are refluxed into the esophagus
through the anastomosis (21). The
EDA model can simulate the reflux state in patients with
gastroesophageal reflux disease (GERD) (21). However, the induction of BE is
difficult under this condition because duodenal fluid reflux is
reduced (12). In the EJA model,
end-to-end anastomosis is made between the lower esophagus and the
beginning section of the jejunum, and the Vagus nerve is protected
(9). In the EJA model, all fluid
from the stomach and duodenum can flow into the lower esophagus.
However, this model cannot simulate the true situation in human BE
because reflux is too vigorous (20). Subsequently, the EJGJ model created
by Zhang et al (17) was
adopted in the present study. In this model, gastric juice, which
is discharged from the stomach into the jejunum, is mixed with
duodenal fluid and then flowed into the lower esophagus due to the
short distance between the two anastomoses (15). The EJGJ model simulated the
pathophysiological state in human GERD in a superior manner
compared with the EDA and EJA models (17). In those models, the Vagus nerve and
pylorus were preserved to avoid duodenal fluid reflux into the
stomach, which did not affect gastric acid secretion and sustained
gastric acid secretion. Modifications to the EJGJ model established
by Zhang et al (17) were
made in the present study. Firstly, the distance (3 cm in length)
between the Tveitz ligament and the gastro-jejunal anastomosis was
shortened. Secondly, the distance (10–15 mm in length) between the
gastro-jejunal anastomosis and esophagus-jejunal anastomosis was
prolonged. It was revealed that the incidence of BE in the
surviving rats reached up to 100%, which was greater than the 47%
(15/32) in the EJGJ model established by Zhang et al
(17). It was also indicated that 16
weeks were required for BE development, which was shorter than the
25 weeks in the EJGJ model (17).
Gastric juice could be mixed well with duodenal fluid because the
distance of gastro-jejunal anastomosis and esophagus-jejunal
anastomosis was prolonged. Thus, the acidity of fluxed fluid most
likely decreased through this improved surgical procedure. Notably,
the decreased acidity of fluxed fluid has been proven to contribute
to the high incidence of BE in a shorter time (22–27).
The reason for the high mortality rate demonstrated
in the present study was due to limited skill of the surgeons in
their surgical techniques. In a subsequent study, it was revealed
that more practice can reduce the mortality rate of surgery (Wen
et al; unpublished data). To increase the esophagus-jejunum
anastomotic lumen aperture and avoid obstruction, parallel
incisions we changed into an oblique incision at the lower section
of the esophagus. Through this modified procedure, only a total of
5 rats succumbed to fatality due to obstruction.
In conclusion, the improved surgical procedure
demonstrated in the present study could enhance the incidence of BE
in rats. Therefore, this model could be used as a reliable animal
model in the basic research of BE.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW designed the current study and performed HE
staining. TL took care of the rats, and recorded and analyzed the
data. HL performed surgery. JT aided the surgical procedure. SL led
the research and provided research funding.
Ethics approval and consent to
participate
Animal care and experimental protocols were approved
by the Animal Research Ethical in Hubei University of Medicine
(Hubei, China).
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Serag HB, Sweet S, Winchester CC and
Dent J: Update on the epidemiology of gastro-oesophageal reflux
disease: A systematic review. Gut. 63:871–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubenstein JH and Shaheen NJ:
Epidemiology, diagnosis, and management of esophageal
adenocarcinoma. Gastroenterology. 149(302–317): e12015.
|
|
3
|
Altorki NK, Oliveria S and Schrump DS:
Epidemiology and molecular biology of Barrett's adenocarcinoma.
Semin Surg Oncol. 13:270–280. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isolauri J, Luostarinen M, Isolauri E,
Reinikainen P, Viljakka M and Keyriläinen O: Natural course of
gastroesophageal reflux disease: 17–22 year follow-up of 60
patients. Am J Gastroenterol. 92:37–41. 1997.PubMed/NCBI
|
|
5
|
Wani S, Rubenstein JH, Vieth M and Bergman
J: Diagnosis and management of low-grade dysplasia in barrett's
esophagus: Expert review from the clinical practice updates
committee of the American gastroenterological association.
Gastroenterology. 151:822–835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phillips WA, Lord RV, Nancarrow DJ, Watson
DI and Whiteman DC: Barrett's esophagus. J Gastroenterol Hepatol.
26:639–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grant KS, DeMeester SR, Kreger V, Oh D,
Hagen JA, Chandrasoma P and DeMeester TR: Effect of Barrett's
esophagus surveillance on esophageal preservation, tumor stage, and
survival with esophageal adenocarcinoma. J Thorac Cardiovasc Surg.
146:31–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pera M, Cardesa A, Bombi JA, Ernst H, Pera
C and Mohr U: Influence of esophagojejunostomy on the induction of
adenocarcinoma of the distal esophagus in Sprague-Dawley rats by
subcutaneous injection of 2,6-dimethylnitrosomorpholine. Cancer
Res. 49:6803–6808. 1989.PubMed/NCBI
|
|
9
|
Fein M, Peters JH, Chandrasoma P, Ireland
AP, Oberg S, Ritter MP, Bremner CG, Hagen JA and DeMeester TR:
Duodenoesophageal reflux induces esophageal adenocarcinoma without
exogenous carcinogen. J Gastrointest Surg. 2:260–268. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Attwood SE, Smyrk TC, DeMeester TR,
Mirvish SS, Stein HJ and Hinder RA: Duodenoesophageal reflux and
the development of esophageal adenocarcinoma in rats. Surgery.
111:503–510. 1992.PubMed/NCBI
|
|
11
|
Miwa K, Sahara H, Segawa M, Kinami S, Sato
T, Miyazaki I and Hattori T: Reflux of duodenal or gastro-duodenal
contents induces esophageal carcinoma in rats. Int J Cancer.
67:269–274. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ireland AP, Peters JH, Smyrk TC, DeMeester
TR, Clark GW, Mirvish SS and Adrian TE: Gastric juice protects
against the development of esophageal adenocarcinoma in the rat.
Ann Surg. 224:358–371. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mirvish SS: Studies on experimental
animals involving surgical procedures and/or nitrosamine treatment
related to the etiology of esophageal adenocarcinoma. Cancer Lett.
117:161–174. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldstein SR, Yang GY, Curtis SK, Reuhl
KR, Liu BC, Mirvish SS, Newmark HL and Yang CS: Development of
esophageal metaplasia and adenocarcinoma in a rat surgical model
without the use of a carcinogen. Carcinogenesis. 18:2265–2270.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishijima K, Miwa K, Miyashita T, Kinami
S, Ninomiya I, Fushida S, Fujimura T and Hattori T: Impact of the
biliary diversion procedure on carcinogenesis in Barrett's
esophagus surgically induced by duodenoesophageal reflux in rats.
Ann Surg. 240:57–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su Y, Chen X, Klein M, Fang M, Wang S,
Yang CS and Goyal RK: Phenotype of columnar-lined esophagus in rats
with esophagogastroduodenal anastomosis: Similarity to human
Barrett's esophagus. Lab Invest. 84:753–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang T, Zhang F, Han Y, Gu Z, Zhou Y,
Cheng Q, Zhu Y, Zhang C and Wang Y: A rat surgical model of
esophageal metaplasia and adenocarcinoma-induced by mixed reflux of
gastric acid and duodenal contents. Dig Dis Sci. 52:3202–3208.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shaheen NJ, Falk GW, Iyer PG and Gerson
LB: American College of Gastroenterology: ACG clinical guideline:
Diagnosis and management of Barrett's esophagus. Am J
Gastroenterol. 111:30–50; quiz 51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Farré R: Pathophysiology of
gastro-esophageal reflux disease: A role for mucosa integrity?
Neurogastroenterol Motil. 25:783–799. 2013.PubMed/NCBI
|
|
20
|
Vaezi MF and Richter JE: Role of acid and
duodenogastroesophageal reflux in gastroesophageal reflux disease.
Gastroenterology. 111:1192–1199. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Theisen J, Peters JH, Fein M, Hughes M,
Hagen JA, Demeester SR, Demeester TR and Laird PW: The mutagenic
potential of duodenoesophageal reflux. Ann Surg. 241:63–68.
2005.PubMed/NCBI
|
|
22
|
Champion G, Richter JE, Vaezi MF, Singh S
and Alexander R: Duodenogastroesophageal reflux: Relationship to pH
and importance in Barrett's esophagus. Gastroenterology.
107:747–754. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marshall RE, Anggiansah A, Owen WA and
Owen WJ: Investigation of gastro-oesophageal reflux in patients
with an intact stomach: Is oesophageal bilirubin monitoring a
useful addition to pH monitoring? Scand J Gastroenterol.
35:904–909. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vaezi MF and Richter JE: Synergism of acid
and duodenogastroesophageal reflux in complicated Barrett's
esophagus. Surgery. 117:699–704. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun D, Wang X, Gai Z, Song X, Jia X and
Tian H: Bile acids but not acidic acids induce Barrett's esophagus.
Int J Clin Exp Pathol. 8:1384–1392. 2015.PubMed/NCBI
|
|
26
|
Cheng P, Li JS, Gong J, Zhang LF and Chen
RZ: Effects of refluxate pH values on duodenogastroesophageal
reflux-induced esophageal adenocarcinoma. World J Gastroenterol.
17:3060–3065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uno K, Iijima K, Hatta W, Koike T, Abe Y,
Asano N, Kusaka G and Shimosegawa T: Direct measurement of
gastroesophageal reflux episodes in patients with squamous cell
carcinoma by 24-h pH-impedance monitoring. Am J Gastroenterol.
106:1923–1929. 2011. View Article : Google Scholar : PubMed/NCBI
|