Introduction
Lung cancer is one of the most malignant tumors in
the world, for which there is still no effective treatment
(1). In China, the morbidity
(20.48%) and mortality (40.71%) of lung cancer rank as the first
among all malignant tumors, representing a great threat to public
health (2). Studies have
demonstrated that non-small cell lung cancer (NSCLC), as the main
clinical pathological type, accounts for ~90% of lung cancer cases
(3–5). Due to the difficulty for the early
diagnosis of NSCLC, distant metastasis occurs in ~40% of patients
at the first diagnosis, whereas 50% of patients in the treatment
process suffer from tumor metastasis (6). It has been demonstrated that, when the
patients of NSCLC without distant metastasis receive appropriate
treatment, the five-year survival rate may be 50–70%, whereas the
survival rate for the patients with metastasis would be <5%
(7). A growing body of evidence has
demonstrated that tumor recurrence and metastasis represent key
factors affecting the efficacy of the clinical treatment for NSCLC
(8,9). However, the underlying molecular
mechanism has yet to be elucidated.
Recurrence and metastasis of NSCLC are closely
associated with the imbalanced expression between oncogenes and
tumor suppressor genes (10). When
oncogenes are obviously activated and tumor suppressor genes are
inactivated, NSCLC cells exhibit severe malignancy, which would
gain the characteristics of metastasis, drug resistance, and
self-renewal capacity (11,12). Accordingly, in recent years, there
has been intensive investigation focusing on the expression
regulation of oncogenes and tumor suppressor genes in NSCLC, and
there have been important breakthroughs (13). It has been demonstrated that
epidermal growth factor receptor and Kras gene mutations can be
used as predictors for the disease prognosis and the responses to
molecular targeting therapies (14,15).
Furthermore, patients with BRAF mutations are more suitable for the
target therapy with tyrosinase inhibitors (16). Furthermore, the expression of
oncogene Met has important clinical value in assessing the
prognosis of patients with NSCLC (17). However, at present, there is little
knowledge regarding the oncogenes and tumor suppressor genes in
NSCLC. Therefore, it is of great clinical importance to investigate
the associated regulatory mechanisms for these oncogenes and tumor
suppressor genes.
microRNAs (miRNAs or miRs), with the length of 18–22
nucleotides, are important regulatory factors for gene
post-transcription, which bind to the mRNA 3′-untranslated region
(UTR) to inhibit the mRNA translation (18). Studies have indicated the abnormal
expression spectrum of the miR molecules in tumor tissues,
including a variety of miRNAs with tumor promoting or suppressing
functions, which are associated with the regulation of tumor cell
proliferation, invasion and metastasis, angiogenesis, self-renewal
capacity, drug resistance, and apoptotic processes (19,20).
miR-520b is a tumor-related miRNA molecule discovered in recent
years, which serves the role as tumor suppressor by targeting
different genes associated with the regulation of tumor
proliferation, metastasis, tumor stemness, drug resistance,
autophagy and immune escape. miR-520b has represented a potential
target for clinical nucleic acid therapy (21). However, at present, the expression
and role of miR-520b in NSCLC remains unclear. In the present
study, the association between miR-520b and NSCLC pathogenesis was
investigated at both the tissue and cellular levels.
Materials and methods
Tissue sample collection
NSCLC tissue samples and adjacent healthy tissue
samples were collected from 52 patients with NSCLC, 29 males and 23
females, with a mean age of 46.7 years old (ranging from 24–76
years), who were diagnosed in Shandong Chest Hospital (Jinan,
China) from January 2015-December 2016. Following resection, these
tissue samples were washed with pre-cold saline and stored in
liquid nitrogen. According to the pathological type, there were 35
cases of adenocarcinoma, 13 cases of squamous cell carcinoma and 4
cases of adenosquamous carcinoma. Based on the lymph node
metastasis status, these cases were divided into the no metastasis
(N0) group, the lymph node metastasis (N1) group (n=39) and the
non-lymph node metastasis (N2) group (n=13). TNM staging (22) indicated 21 cases of stage I, 18 cases
of stage II, and 13 cases of stage III. None of these patients had
received any radiotherapy and/or chemotherapy, or other anti-tumor
drug treatment, prior to surgical resection. Prior written and
informed consent were obtained from every patient, and the study
was approved by the Ethics Review Board of Shandong Chest Hospital
(Jinan, China).
Cell lines and cell culture
NSCLC A549 and Calu-3 cell lines (Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China)
were cultured in RPMI-1640 complete medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C and 5% CO2.
Culture medium was changed every other day. Cells were passaged
when 80–90% confluence was reached, and cells from passages 3–6
were used for investigation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Expression levels of miR-520b in the tissues and
transfected cells were detected via RT-qPCR. A total of 100 mg
fresh tissue was frozen with liquid nitrogen and ground into
powder. Total RNA was extracted from the ground tissues, and A549
and Calu-3 cell lines using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.). The cDNA template was obtained through reverse
transcription with the miScript II RT kit (Qiagen GmbH, Hilden,
Germany), according to the manufacturer's protocol. qPCR was
performed with the miScript SYBR®-Green PCR Kit (Qiagen
GmbH) on the ABI Step one plus system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The reaction system consisted of 10 µl
RTq-PCR-Mix, 0.5 µl primer each, 2 µl cDNA and 7 µl
ddH2O. The forward primer sequence for miR-520b was
5′-AAAGTGCTTCCTTTTAGAGGG-3′, and a general primer was used for
reverse primer (U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; provided within the kit). Reaction
conditions were as follows: Initial denaturation at 95°C for 10
min, followed by 40 cycles of denaturation at 95°C for 1 min, and
annealing and elongation at 60°C for 30 sec. The target gene
expression was determined with the 2−ΔΔCt method
(23).
Cell transfection
For cell transaction, 2×105 cells were
seeded onto the 24-well plates. These cells were cultured with 500
µl antibiotic-free RPMI 1640 medium containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), in a 37°C, 5%
CO2 incubator, overnight. Cell transfection was
performed when 70% confluence was reached. Briefly, 1.5 µl miR-520b
mimics (5′-AAAGTGCTTCCTTTTAGAGGG-3′; 20 pmol/µl; Hanheng
Biotechnology Company, Shanghai, China) and 1 µl Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) were added to an Eppendorf tube
containing 50 µl OptiMemi medium (Thermo Fisher Scientific, Inc.).
The normal control (NC) group was transfected with 20 pmol/µl of a
nonsense miRNA sequence (5′-UCACAACCUCCUAGAAAGAGUAGA-3′; Guangzhou
RiboBio Co., Ltd., Guangzhou, China). Following incubation at room
temperature for 5 min, the solution was mixed together, which,
following another 20 min, was used to incubate the cells at 37°C
for 6 h. Following culture with RPMI 1640 medium containing 10% FBS
for 48 h in a 37°C, 5% CO2 incubator, these cells were
collected and prepared for the following experiments. For the
transfection with Rab22A lentivirus, Lv-GFP-Rab22A lentivirus
(Hanheng Biotechnology Company) was added to incubate the adhered
cells at 60% confluence (at the rate of multiplicity of
infection=20) at 37°C for 12 h. Subsequently, the culture medium
was replaced with RPMI 1640 medium containing 10% FBS.
Cell counting kit-8 (CCK-8) assay
Cell proliferation was assessed with the CCK-8 assay
(Beyotime Institute of Biotechnology, Haimen, China). Cells were
seeded onto 96-well plates at a density of 2×103
cells/well. A total of 20 µl CCK-8 (5 g/l) was added into the wells
for incubation times of 0, 24, 48 and 72 h, at 37°C. On the last
day, 150 µl CCK-8 was added to the cells at 37°C for 2 h. Then the
absorption at 490 nm (OD490 nm) was measured for each
well, and cell proliferation curves were plotted accordingly.
Transwell chamber assay
Cell invasion and migration were assessed with the
Transwell chamber assay (Corning Incorporated, Corning, NY, USA).
Matrigel matrix was stored in a 4°C refrigerator overnight.
Following dilution with serum-free RPMI 1640 medium at a ratio of
1:3, the matrix was evenly smeared onto the upper Transwell
chamber, which was subsequently incubated at 37°C for 60 min.
Subsequently, 1×105 cells were seeded into the upper
chamber with 200 µl serum-free medium, whereas the lower chamber
was seeded with 500 µl RPMI 1640 medium containing 10% FBS.
Following incubation at 37°C for 24 h, the cells were fixed with 4%
formaldehyde at room temperature for 10 min. The cells were stained
using the ABC solution from the kit for 2 min at room temperature.
Following washing, cell invasion was observed under light
microscope and 5 fields were randomly selected under high
magnification, and the cells infiltrating through the membrane were
counted, based on which the cell invasion and migration abilities
were assessed.
Flow cytometry
At 48 h following transfection, cells were digested
with trypsin and rinsed twice with pre-cold PBS. Cell cycle was
detected by flow cytometry, using a Cell Cycle Assay kit (BD
Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's instructions. Cells (2×106 cells/ml) were
incubated with 200 µl solution A at room temperature for 10 min,
and then incubated with 150 µl solution B at room temperature for
10 min, followed by incubation at room temperature with 120 µl
solution C in the dark for 10 min. Fluorescence was detected using
a flow cytometer, and the results were analyzed with Modfit
software (version 3.2; Verity Software House, Inc., Topsham, ME,
USA).
Western blot analysis
Cells were lysed with RIPA lysis buffer (containing
1% phenylmethane sulfonyl fluoride) on ice for 40 min. Following
centrifugation at 4°C at 10,000 × g for 10 min, the supernatant was
collected. Following protein concentration determination using the
BCA method (Beyotime Institute of Biotechnology), 10 µl protein
samples were subjected to 10% SDS-PAGE, and electronically
transferred onto a polyvinylidene difluoride membrane. Following
blocking with 50 g/l non-fat milk at room temperature for 1 h, the
membrane was incubated with rabbit anti-human anti-Rab22A
polyclonal primary antibody (1:1,000; ab99205; Abcam, Cambridge,
UK), rabbit anti-human anti-E-cadherin primary antibody (1:1,000;
Beyotime Institute of Biotechnology), rabbit anti-human
anti-vimentin primary antibody (1:1,000; Beyotime Institute of
Biotechnology), and mouse anti-human anti-GAPDH primary antibody
(1:4,000; Abcam), respectively, at 4°C overnight. Following washing
with TBS-Tween-20 three times, the membrane was incubated with
horseradish peroxidase-conjugated goat anti-mouse (1:4,000; A0208)
or goat anti-rabbit (1:2,000; A0216; both Beyotime Institute of
Biotechnology) secondary antibodies at room temperature for 1 h.
Colorization was performed using BeyoECL Plus (Beyotime Institute
of Biotechnology).
Dual-luciferase reporter assay
According to bioinformatics prediction (Targetscan
7.1; targetscan.org/vert_72/), the
wild-type and mutant seed regions of Rab22A 3′-UTR for miR-520b
were added with Spe-1 and HindIII restriction sites, respectively,
which were then cloned into the pMIR-REPORT luciferase reporter
plasmid (Thermo Fisher Scientific, Inc.). A total of 0.5 µg plasmid
containing wild-type or mutant 3′-UTR DNA sequence was transfected
into HEK293T cells (Type Culture Collection of the Chinese Academy
of Sciences) with Lipofectamine 2000® (Thermo Fisher
Scientific, Inc.), together with the miR-520b mimics. Following 24
h, the cells were collected and lysed with a
Dual-Luciferase® Reporter Assay system (Promega
Corporation, Madison, WI, USA), and the luciferase was detected
with a GloMax 20/20 luminometer. Renilla was used as an
internal reference.
Statistical analysis
Data were expressed as mean ± standard deviation.
Statistical analysis was performed with SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Student's t-test was used for comparison
between two groups. One-way analysis of variance was used for
comparisons between multiple groups, followed by the
Student-Newman-Keuls method as a post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
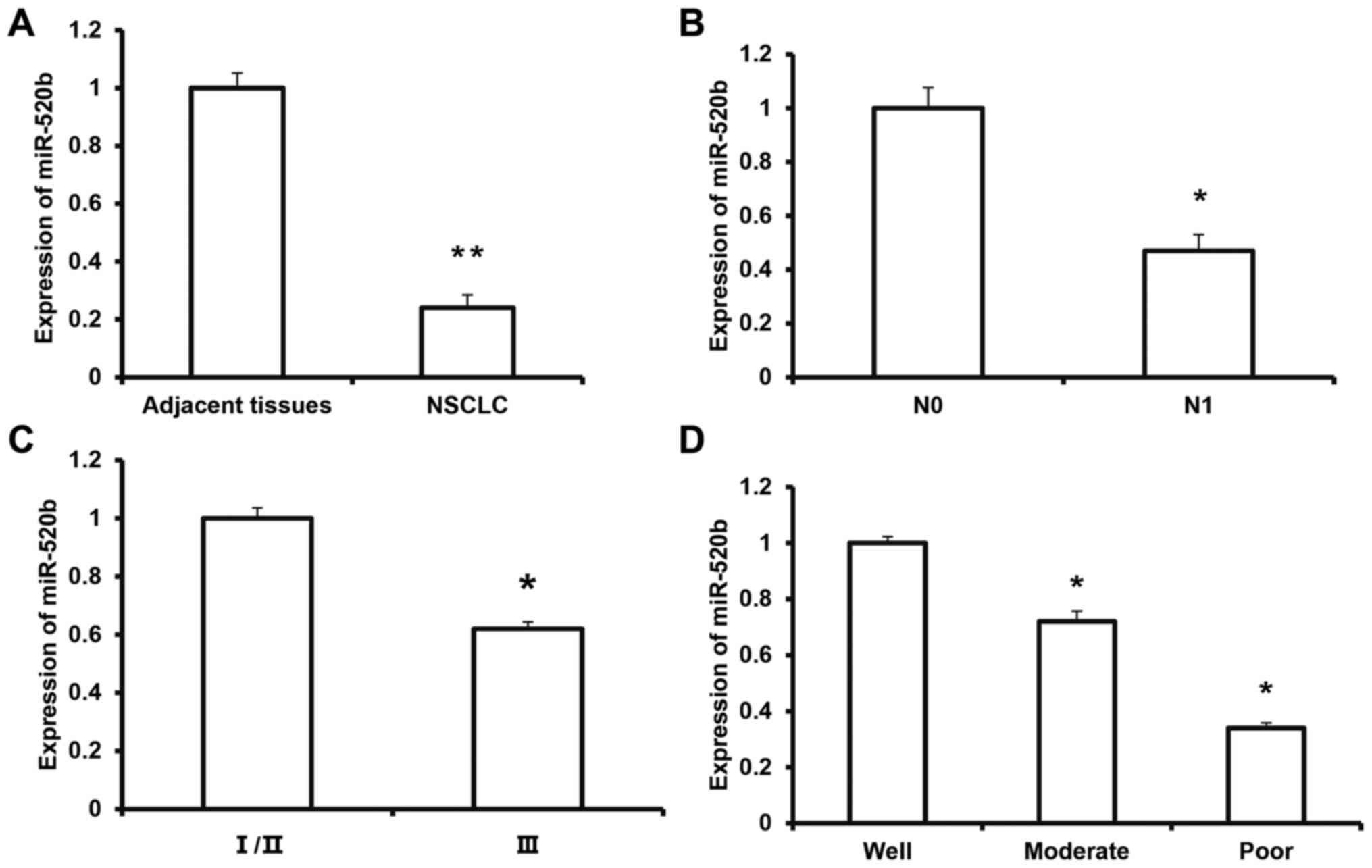
Expression of miR-520b in NSCLC
tissue
To investigate the expression of miR-520b in NSCLC
tissue and its clinical significance, RT-qPCR was performed. The
results demonstrated that the expression level of miR-520b was
significantly decreased in NSCLC tissue (0.24±0.04) compared with
adjacent tissue (P<0.01; Fig.
1A). Furthermore, the expression level of miR-520b in the
melanoma tissue from the N1 group (0.47±0.06) was significantly
lower than that in the N0 group (P<0.05) (Fig. 1B). According to the TNM staging, the
expression level of miR-520b in the tumors at stage III (0.62±0.07)
was significantly lower than that of the tumors at stages I/II
(P<0.05) (Fig. 1C). The
expression levels of miR-520b for the moderate- and
poor-differentiation groups were significantly lower than the
well-differentiation group (P<0.05; Fig. 1D). These results suggest that the
expression of miR-520b is downregulated in NSCLC tissue, which may
be closely associated with tumor invasion and metastasis, and
disease pathogenesis.
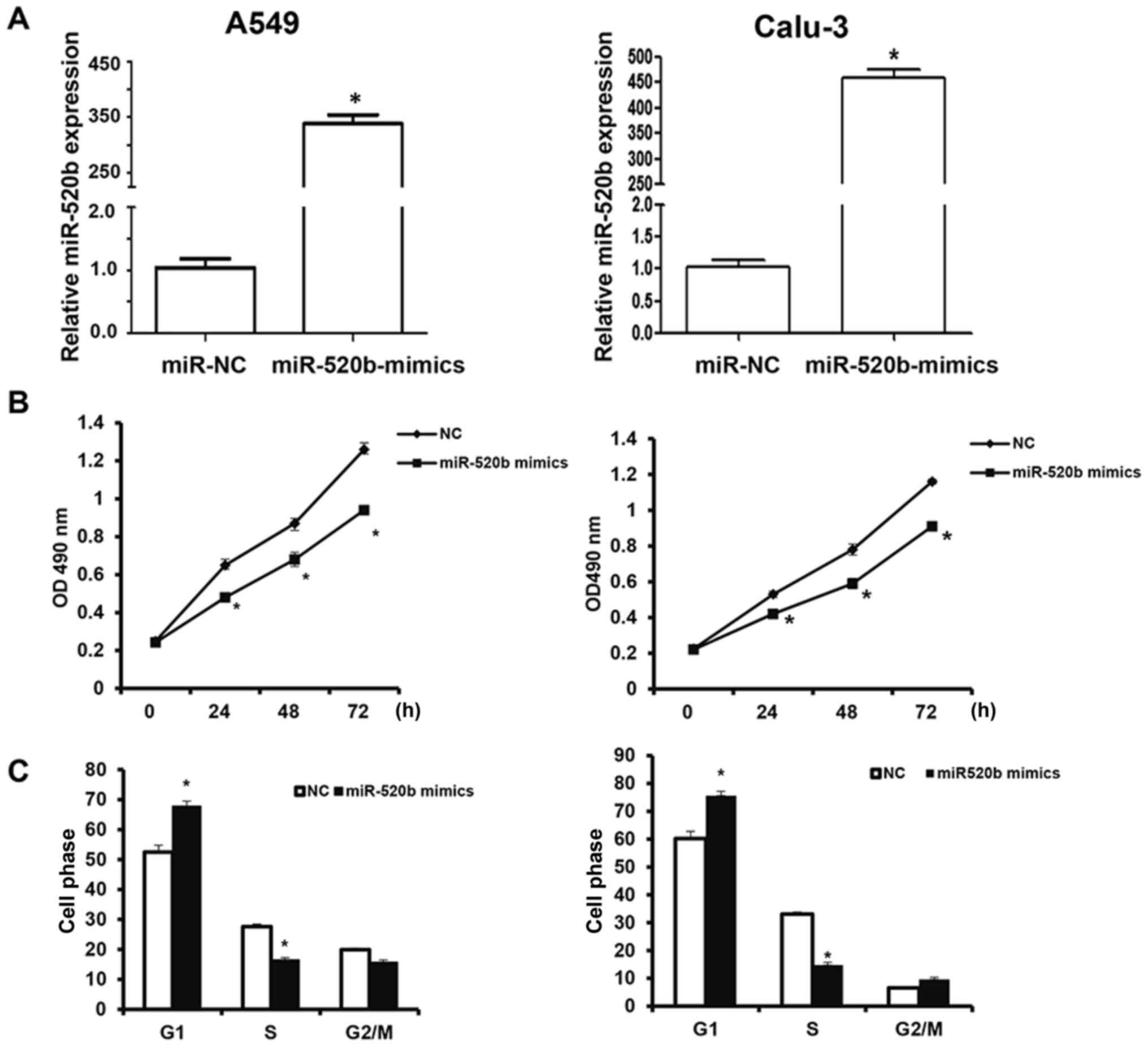
Effects of miR-520b on proliferation
of NSCLC cells
To investigate the effects of miR-520b on the
proliferation of NSCLC cells, the CCK-8 assay was performed. The
results revealed that following transfection, the miR-520b
expression levels were significantly elevated in the A549 and
Calu-3 cells (Fig. 2A). The findings
demonstrated that, following transfection with miR-520b mimics, the
OD490 nm values of A549 and Calu-3 cells at 24, 48, and
72 h post-transfection were significantly lower than the NC group
(all P<0.05; Fig. 2B). Cell cycle
stage was detected by flow cytometry. The results demonstrated
that, the number of miR-520b-transfected cells in the G1
phase was significantly elevated, whereas the miR-520b-transfected
cells in the S phase was significantly reduced, indicating
G1/S phase arrest of these cells following miR-520b
transfection (Fig. 2C). These
results suggest that miR-520b inhibited the proliferation of NSCLC
cells in vitro, the over-expression of which may be one of
the reasons for the recurrence and metastasis of NSCLC.
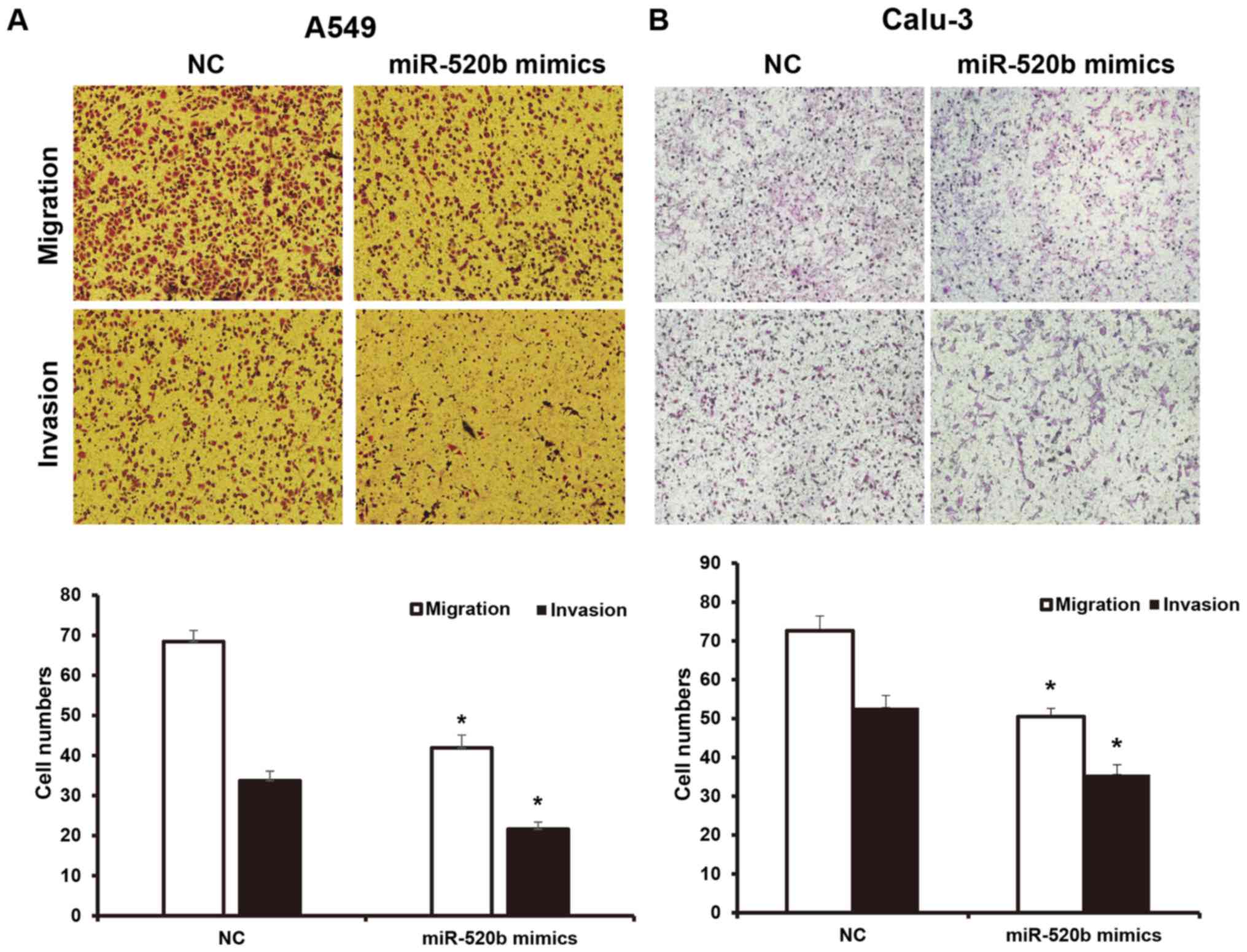
Effects of miR-520b on invasion and
migration abilities of NSCLC cells
To investigate the invasion and migration abilities
of NSCLC cells, a Transwell chamber assay was performed. The
results demonstrated that, following transfection with miR-520b,
the migration ability of NSCLC cells was significantly inhibited.
The numbers of A549 and Calu-3 cells crossing the chamber membrane
were significantly lower than the NC groups (41.9±3.2 vs. 68.3±2.8,
and 50.5±2.1 vs. 72.6±3.8, respectively; P<0.05). Results from
the invasion test demonstrated that the number of transfected A549
cells crossing the Transwell chamber membrane (21.6±1.8) was
significantly lower than the NC group (33.7±2.4). Furthermore, the
number of the transfected Calu-3 cells crossing the Transwell
chamber membrane (35.6±2.5) was significantly lower than the NC
group (52.8±3.1; P<0.05; Fig. 3A and
B). These results suggest that miR-520b may inhibit the
invasion and metastasis abilities of NSCLC cells.
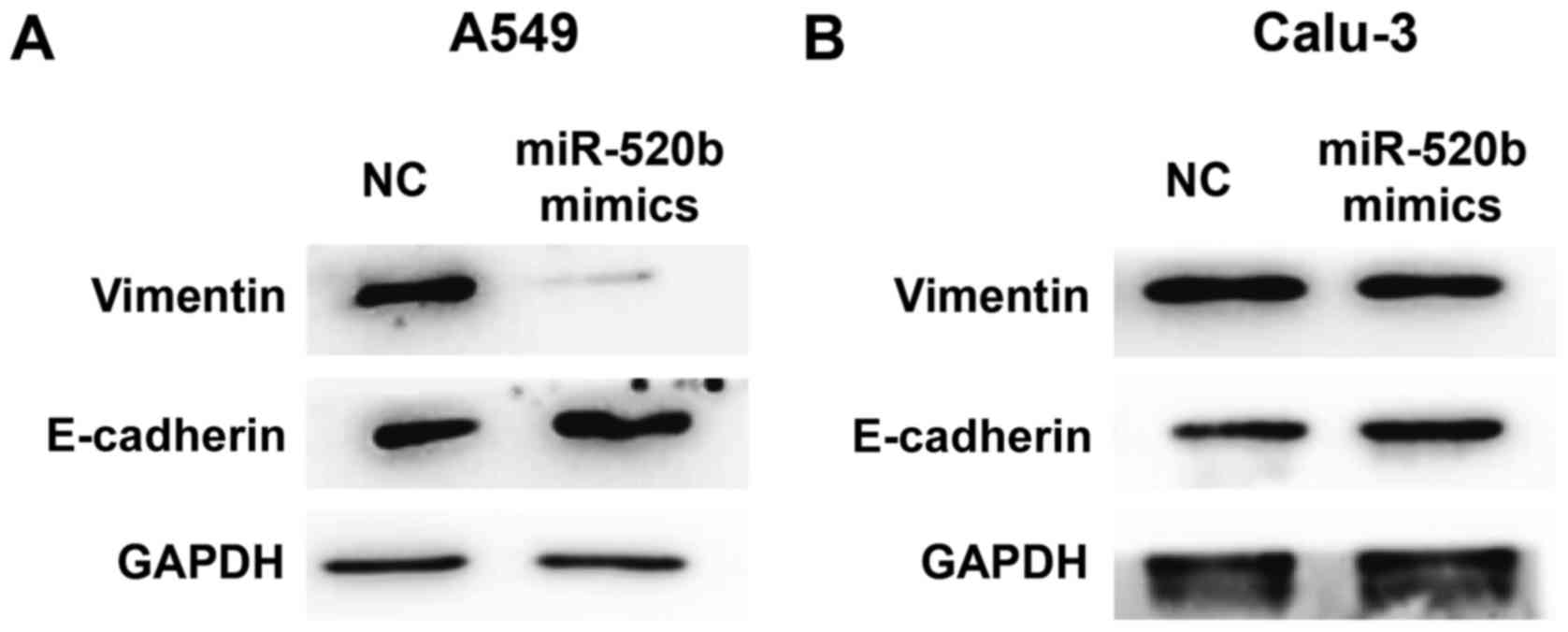
Effects of miR-520b on epithelial
mesenchymal transformation (EMT) of NSCLC
To investigate the effects of miR-520b on EMT of
NSCLC, expression levels of E-cadherin (an EMT marker) were
detected by western blot analysis. The results demonstrated that,
following transfection with miR-520 mimics, the expression levels
of E-cadherin were markedly elevated, whereas the expression levels
of vimentin were markedly declined in the A549 cells (Fig. 4A). Similar results and expression
trends were observed in Calu-3 cells although the changes were
minimal (Fig. 4B). These results
suggest that miR-520b may inhibit the EMT of NSCLC.
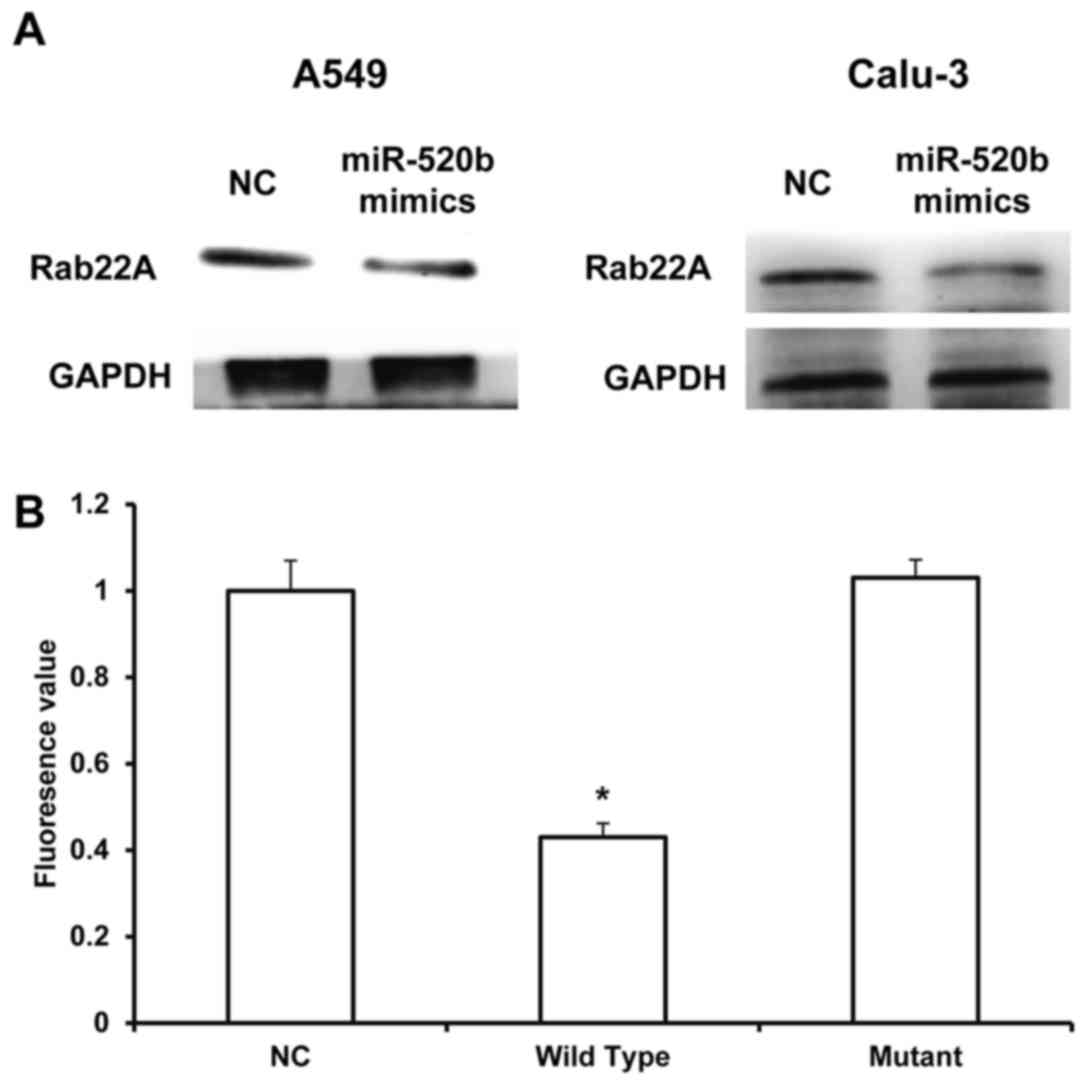
Rab22A is a direct target for
miR-520b
miRNAs target the 3′-UTR of mRNAs to exert
biological functions. Therefore, the target for miR-520b was
investigated to further explore its underlying mechanism. Through
the mRNA target gene prediction, using Targetscan7.1 software
(targetscan.org), the tumor suppressor gene Rab22A
was recognized as the potential target gene for miR-520b. The
results from western blot analysis demonstrated that the expression
levels of Rab22A in the NSCLC cells transfected with miR-520b
mimics were markedly downregulated. Dual luciferase reporter assay
results demonstrated that, the fluorescence value in the wild-type
group was significantly lower than the control group (P<0.05),
whereas no significant differences were observed between the mutant
and NC groups (Fig. 5A and B). These
results suggested that miR-520b could directly regulate the
expression of Rab22A.
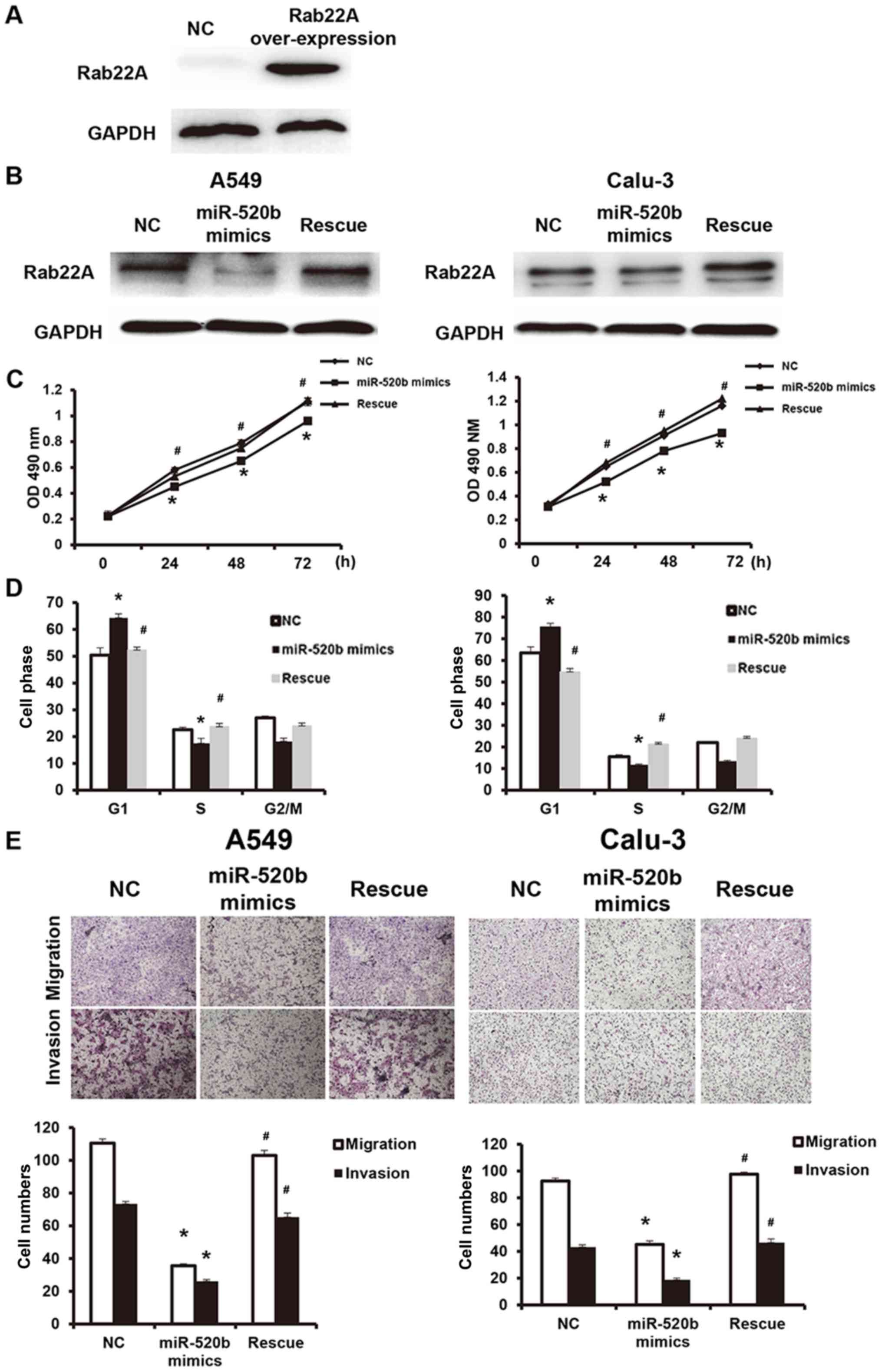
miR-520b regulates proliferation and
metastasis of NSCLC through targeting on Rab22A
To further investigate the effects of miR-520b
regulating Rab22A on NSCLC cell functions, these cells were
transfected with Rab22A-over-expressed lentivirus (western blot
analysis confirmed the successful overexpression of Rab22A in the
transfected cells, as presented in Fig.
6A), and the reversal effects of Rab22A on the miR-520b
mimics-transfected cell functions were analyzed. In addition,
compared with the miR-520b mimics group, Rab22A expression was
significantly elevated in the reversal group (Fig. 6B). The results demonstrated that,
compared with miR-520b mimics, the OD490 nm values at
24, 48 and 72 h in the reversal group were significantly elevated
(P<0.05; Fig. 6C). Cell cycle
analysis demonstrated that, compared with the miR-520b mimics
group, the cell number in the G1 phase was significantly
higher, whereas the cell number in the S phase was significantly
lower, in the reversal group, indicating accelerated
G1/S phase conversion (Fig.
6D). Results from Transwell chamber assay demonstrated that the
cell numbers of migration and invasion in the Rab22A reversal group
were both significantly higher than the miR-520b mimics group,
indicating enhanced invasion and metastasis abilities (Fig. 6E). These results suggest that Rab22A
could reverse the miR-520b-induced inhibiting effects on
proliferation and metastasis of NSCLC.
Discussion
Tumor recurrence and metastasis represent key
factors limiting the clinical treatment efficacy of NSCLC, the
underlying mechanism of which remains to be elucidated (7). A number of previous studies have
demonstrated that the recurrence and metastasis of NSCLC are
closely associated with abnormal gene expression, methylation, gene
mutation, drug resistance and immunosuppression (24,25).
miRNA molecules are associated with almost all pathophysiological
biological processes, which provides rationale for their use in
early diagnosis and target therapy for tumors (26). A number of miRNAs are associated with
the development of NSCLC (27). In
the present study, it was demonstrated that miR-520b expression was
significantly downregulated in NSCLC tissue, which was negatively
correlated with lymph node metastasis and TNM staging. Cellular and
molecular experiments confirmed that miR-520b targeted Rab22A to
regulate the proliferation and metastasis of NSCLC.
miRNAs are a class of post-transcriptional
regulators that have attracted research attention in recent years,
which are associated with the regulation of development in various
diseases (28). Studies have
demonstrated that various miRNAs serve important roles as oncogenes
or tumor suppressor genes in tumor development, and that their
abnormal expression may induce intracellular oncogene/tumor
suppressor gene imbalance, thereby promoting cell carcinogenesis,
and tumor recurrence and metastasis (29). It has been indicated that miR-26a
inhibits the proliferation, invasion and metastasis of malignant
melanoma by targeting microphthalmia-associtated transcription
factor genes (30). Furthermore,
miR-125b promotes the sensitivity of nasopharyngeal carcinoma to
radiotherapy via regulating the A20/nuclear factor (NF)-κB
signaling pathway (31). Ren et
al (32) have recently
demonstrated that miR-210-3p promotes EMT and bone metastasis of
pancreatic cancer through the regulation of NF-κB signaling
pathway. In addition, due to the disturbed expression profiles of
mRNA in both tumor tissues and cells, the same miRNA may target
different genes to exert biological functions in different tumors
(33). miR-503 has been demonstrated
to target and regulate the CyclinD1 gene in esophageal squamous
cell carcinoma (34), and targets B
cell lymphoma-2 in the hepatocellular carcinoma (35). miR-520b is a newly discovered
tumor-associated miRNA molecule, which acts as a tumor suppressor
in a variety of tumors. It has been demonstrated that miR-520b
inhibits the stemness of head and neck tumors via targeting cluster
of differentiation (CD)44 expression (36). Furthermore, miR-520b may target
defective in cullin neddylation 1 domain containing 1 expression in
colon cancer and inhibit tumor development (37).
The present results demonstrated that the expression
of miR-520b was significantly downregulated in NSCLC tissues, which
was negatively associated with lymph node metastasis and TNM
staging. Results from in vitro experiments demonstrated that
the upregulation of miR-520b inhibited the proliferation and
metastasis of NSCLC A549 and Calu-3 cells. These results indicate
that miR-520b may serve a role as a tumor suppressor in the
development of NSCLC. Further in-depth studies are required to
investigate the association between miR-520b and disease outcomes.
Rab22A is a small molecule GTP binding protein, or small molecule
GTPase, which belongs to the Ras superfamily (38,39).
Rab22A is generally activated following binding to GTP in transit
vesicles. GTP is converted to GDP following energy dissipation, and
Rab22A is also inactive (39). A
large number of studies have demonstrated that Rab22A exerts
oncogene function in the development of various tumors (40). Su et al (41) have recently demonstrated that Rab22A
is highly expressed in melanoma and promotes tumor proliferation
and metastasis (41). Furthermore,
Zhou et al (42) have
suggested that Rab22A promotes the metastasis of lung cancer cells
by regulating the recycling of CD147. In the present study,
bioinformatics prediction demonstrated that miR-520b may be
associated with the regulation of Rab22A expression. The results
from western blot analysis demonstrated that upregulation of
miR-520b could downregulate the expression of Rab22A. Conversely,
dual luciferase reporter assay confirmed that Rab22A was the target
gene for miR-520b. In order to further verify that miR-520b exerted
biological functions though Rab22A, the expression of Rab22A was
reversed, which inhibited the tumor suppressing function of
miR-520b in NSCLC. These findings suggest that miR-520b exerts
biological functions through regulating Rab22A. Although these
results demonstrated that Rab22A was the target of miR-520b, the
underlying mechanisms for cell proliferation, invasion and
migration are yet to be elucidated.
In conclusion, the present results demonstrated that
miR-520b served a role as a tumor suppressor gene in the
development of NSCLC. The downregulated expression of miR-520b in
lung cancer tissue was closely associated with tumor development.
miR-520b targeted Rab22A to exert the tumor suppressing
function.
Acknowledgements
The authors are grateful to Dr Hua Hu, Thoracic
Hospital of Shandong Province (Jinan, China) for providing
assistance with project support and manuscript preparation.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and SY designed the study, performed the
experiments, collected and analyzed the data, and wrote the
manuscript.
Ethics approval and consent to
participate
Prior written and informed consent was obtained from
every patient and the study present was approved by the Ethics
Review Board of Shandong Chest Hospital.
Patient consent for publication
Prior written and informed consent was obtained from
every patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gu Y, Lv H, Zhao J, Li Q, Mu G, Li J,
Wuyang J, Lou G, Wang R, Zhang Y and Huang X: Influence of the
number and interval of treatment cycles on cytokine-induced killer
cells and their adjuvant therapeutic effects in advanced
non-small-cell lung cancer (NSCLC). Int Immunopharmacol.
50:263–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lamb YN and Scott LJ: Osimertinib: A
review in T790M-positive advanced non-small cell lung cancer.
Target Oncol. 12:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Powell AC, Mirhadi AJ, Loy BA, Happe LE,
Long JW, Kren EM and Gupta AK: Presentation at computed tomography
(CT) scan of the thorax and first year diagnostic and treatment
utilization among patients diagnosed with lung cancer. PLoS One.
12:e01813192017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He J, Yu L, Wang CM and Zhou XF: MiR-1275
promotes non-small cell lung cancer cell proliferation and
metastasis by regulating LZTS3 expression. Eur Rev Med Pharmacol
Sci. 22:2680–2687. 2018.PubMed/NCBI
|
|
5
|
Chae YK, Arya A, Iams W, Cruz MR, Chandra
S, Choi J and Giles F: Current landscape and future of dual
anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons
learned from clinical trials with melanoma and non-small cell lung
cancer (NSCLC). J Immunother Cancer. 6:392018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Proto C, Russo Lo G, Corrao G, Ganzinelli
M, Facchinetti F, Minari R, Tiseo M and Garassino MC: Treatment in
EGFR-mutated non-small cell lung cancer: How to block the receptor
and overcome resistance mechanisms. Tumori. 103:325–337. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Botsa EI, Thanou IL, Papatheodoropoulou AT
and Thanos LI: Thermal ablation in the management of adrenal
metastasis originating from Non-small cell lung cancer: A 5-year
Single-center experience. Chin Med J (Engl). 130:2027–2032. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang G, Zhang D, Wu J, Zhang F, Zhu Z,
Chen K, Zhang N, Jin J, Feng J, Lin N, et al: Low serum levels of
Pre-surgical total cholesterol are associated with unfavorable
overall survival in patients with operable Non-small cell lung
cancer. Clin Lab. 64:321–327. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Camidge DR, Kim DW, Tiseo M, Langer CJ,
Ahn MJ, Shaw AT, Huber RM, Hochmair MJ, Lee DH, Bazhenova LA, et
al: Exploratory analysis of brigatinib activity in patients with
anaplastic lymphoma Kinase-positive Non-small-cell lung cancer and
brain metastases in two clinical trials. J Clin Oncol:
JCO2017775841. 2018. View Article : Google Scholar
|
|
10
|
Xu Y, Zhang F, Pan X, Wang G, Zhu L, Zhang
J, Wen D and Lu S: Xenograft tumors derived from malignant pleural
effusion of the patients with non-small-cell lung cancer as models
to explore drug resistance. Cancer Commun (Lond). 38:192018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, Yu Y, Pei Y, Cao C, Ding C, Wang
D, Sun L and Niu G: A potential prognostic biomarker SPC24 promotes
tumorigenesis and metastasis in lung cancer. Oncotarget.
8:65469–65480. 2017.PubMed/NCBI
|
|
12
|
Ali A, Kim SH, Kim MJ, Choi MY, Kang SS,
Cho GJ, Kim YS, Choi JY and Choi WS: O-GlcNAcylation of NF-κB
promotes lung metastasis of cervical cancer cells via upregulation
of CXCR4 expression. Mol Cells. 40:476–484. 2017.PubMed/NCBI
|
|
13
|
Casaluce F, Sgambato A, Maione P, Sacco
PC, Santabarbara G and Gridelli C: Selumetinib for the treatment of
non-small cell lung cancer. Expert Opin Investig Drugs. 26:973–984.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shlomi D, Abud M, Liran O, Bar J, Gai-Mor
N, Ilouze M, Onn A, Ben-Nun A, Haick H and Peled N: Detection of
lung cancer and EGFR mutation by electronic nose system. J Thorac
Oncol. 12:1544–1551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee WY, Chen PC, Wu WS, Wu HC, Lan CH,
Huang YH, Cheng CH, Chen KC and Lin CW: Panobinostat sensitizes
KRAS-mutant non-small-cell lung cancer to gefitinib by targeting
TAZ. Int J Cancer. 141:1921–1931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma W, Brodie S, Agersborg S, Funari VA and
Albitar M: Significant improvement in detecting BRAF, KRAS, and
EGFR mutations using Next-generation sequencing as compared with
FDA-cleared kits. Mol Diagn Ther. 21:571–579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh PK and Silakari O: Novel EGFR
(T790M)-cMET dual inhibitors: Putative therapeutic agents for
non-small-cell lung cancer. Future Med Chem. 9:469–483. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Tang M, Ou L, Hou M, Feng T, Huang
YE, Jin Y, Zhang H and Zuo G: Biological analysis of cancer
specific microRNAs on function modeling in osteosarcoma. Sci Rep.
7:53822017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Zhang B, Zheng W, Kang M, Chen Q,
Qin W, Li C, Zhang Y, Shao Y and Wu Y: Exosomes derived from
pancreatic cancer cells induce insulin resistance in C2C12 myotube
cells through the PI3K/Akt/FoxO1 pathway. Sci Rep. 7:53842017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farooqi AA, Shu CW, Huang HW, Wang HR,
Chang YT, Fayyaz S, Yuan SF, Tang JY and Chang HW: TRAIL, Wnt,
sonic hedgehog, TGFβ, and miRNA signalings are potential targets
for oral cancer therapy. Int J Mol Sci. 18:pii: E15232017.
View Article : Google Scholar
|
|
21
|
Li S, Zhang H, Ning T, Wang X, Liu R, Yang
H, Han Y, Deng T, Zhou L, Zhang L, et al: MiR-520b/e regulates
proliferation and migration by simultaneously targeting EGFR in
gastric cancer. Cell Physiol Biochem. 40:1303–1315. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yasukawa M, Kawaguchi T, Kawai N, Tojo T
and Taniguchi S: Prognostic significance of mode of nodal
involvement in pulmonary pN1 squamous cell carcinoma. Kyobu Geka.
71:163–168. 2018.(In Japanese). PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nia ES, Garland LL, Eshghi N, Nia BB,
Avery RJ and Kuo PH: Incidence of brain metastases on follow-up
18F-FDG PET/CT scans of non-small cell lung cancer
patients: Should we include the brain? J Nucl Med Technol.
45:193–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang Y, Xu X, Wang T, Li Y, You W, Fu J,
Liu Y, Jin S, Ji Q, Zhao W, et al: The EGFR/miR-338-3p/EYA2 axis
controls breast tumor growth and lung metastasis. Cell Death Dis.
8:e29282017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H,
Kong J, Ding K, Shen HM, Wu H, et al: Long non-coding RNA linc00673
regulated non-small cell lung cancer proliferation, migration,
invasion and epithelial mesenchymal transition by sponging
miR-150-5p. Mol Cancer. 16:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuwen DL, Sheng BB, Liu J, Wenyu W and Shu
YQ: MiR-146a-5p level in serum exosomes predicts therapeutic effect
of cisplatin in non-small cell lung cancer. Eur Rev Med Pharmacol
Sci. 21:2650–2658. 2017.PubMed/NCBI
|
|
28
|
Lu Y, Tang L, Zhang Q, Zhang Z and Wei W:
MicroRNA-613 inhibits the progression of gastric cancer by
targeting CDK9. Artif Cells Nanomed Biotechnol. 46:980–984. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moles R: MicroRNAs-based therapy: A novel
and promising strategy for cancer treatment. Microrna. 6:102–109.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian H, Yang C and Yang Y: MicroRNA-26a
inhibits the growth and invasiveness of malignant melanoma and
directly targets on MITF gene. Cell Death Discov. 3:170282017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng Z, Qu JQ, Yi HM, Ye X, Huang W, Xiao
T, Li JY, Wang YY, Feng J, Zhu JF, et al: MiR-125b regulates
proliferation and apoptosis of nasopharyngeal carcinoma by
targeting A20/NF-kappaB signaling pathway. Cell Death Dis.
8:e28552017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ren D, Yang Q, Dai Y, Guo W, Du H, Song L
and Peng X: Oncogenic miR-210-3p promotes prostate cancer cell EMT
and bone metastasis via NF-κB signaling pathway. Mol Cancer.
16:1172017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Muralimanoharan S, Guo C, Myatt L and
Maloyan A: Sexual dimorphism in miR-210 expression and
mitochondrial dysfunction in the placenta with maternal obesity.
Int J Obes (Lond). 39:1274–1281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang L, Zhao Z, Zheng L, Xue L, Zhan Q
and Song Y: Downregulation of miR-503 promotes ESCC cell
proliferation, migration, and invasion by targeting cyclin D1.
Genomics Proteomics Bioinformatics. 15:208–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang X, Yin J, Xiang Q, Xie H, Yu J, Gan R
and Lei X: MiR-503 sensitizes human hepatocellular carcinoma cells
to cisplatin by targeting bcl-2. Zhong Nan Da Xue Xue Bao Yi Xue
Ban. 42:605–610. 2017.(In Chinese). PubMed/NCBI
|
|
36
|
Lu YC, Cheng AJ, Lee LY, You GR, Li YL,
Chen HY and Chang JT: MiR-520b as a novel molecular target for
suppressing stemness phenotype of head-neck cancer by inhibiting
CD44. Sci Rep. 7:20422017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao J, Li G, Zhou J, Wang S, Liu D, Shu
G, Zhou J and Ren F: MicroRNA-520b functions as a tumor suppressor
in colorectal cancer by inhibiting DCUN1D1. Oncol Res. May
4–2017.(Epub ahead of print). View Article : Google Scholar
|
|
38
|
Xiong F, Liu K, Zhang F, Sha K, Wang X,
Guo X and Huang N: MiR-204 inhibits the proliferation and invasion
of renal cell carcinoma by inhibiting RAB22A expression. Oncol Rep.
35:3000–3008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Z, He M, Wang K, Sun G, Tang L and Xu
Z: Tumor suppressive microRNA-193b promotes breast cancer
progression via targeting DNAJC13 and RAB22A. Int J Clin Exp
Pathol. 7:7563–7570. 2014.PubMed/NCBI
|
|
40
|
Wang T, Gilkes DM, Takano N, Xiang L, Luo
W, Bishop CJ, Chaturvedi P, Green JJ and Semenza GL:
Hypoxia-inducible factors and RAB22A mediate formation of
microvesicles that stimulate breast cancer invasion and metastasis.
Proc Natl Acad Sci USA. 111:E3234–E3242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Su F, Chen Y, Zhu S, Li F, Zhao S, Wu L,
Chen X and Su J: RAB22A overexpression promotes the tumor growth of
melanoma. Oncotarget. 7:71744–71753. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou Y, Wu B, Li JH, Nan G, Jiang JL and
Chen ZN: Rab22a enhances CD147 recycling and is required for lung
cancer cell migration and invasion. Exp Cell Res. 357:9–16. 2017.
View Article : Google Scholar : PubMed/NCBI
|