Introduction
Atrial septal aneurysm (ASA) is a rare cardiac
malformation characterized by an integral or localized protrusion
of the septum into the right atrium (RA), left atrium (LA) or both.
The reported incidence varies between 0.2 and 10.0% (1–3),
depending on diverse diagnostic criteria [maximum vertical distance
(MVD) of protrusion, >5 mm in children and 6–15 mm in adults]
(2–4)
and the assessment methods involved, including autopsy,
transthoracic echocardiography (TTE) or transesophageal
echocardiography (TEE). ASA is routinely evaluated by
echocardiography (4,5).
For over a decade, electrocardiography (ECG)-gated
multidetector computed tomography (MDCT) has gained popularity as a
noninvasive imaging method in the evaluation of heart disease
(3). With the advent of 4-detector
computed tomography (CT) and further advancements in 16- and
64-detector CT, the MDCT imaging has rapidly improved, which
contributes to significantly reduced gantry rotation durations and
elevated image resolutions. Dual source CT (DSCT) coronary
angiography has emerged as an important novel modality for the
non-invasive assessment of heart disease. DSCT imaging is
characterized by higher temporal resolution (83 msec for DSCT and
75 msec for 128-section DSCT) with simultaneous data acquisition by
two sets of X-ray tubes and detectors, which facilitates better
evaluation of lesions and without the need to control heart rate in
most situations (6). With the
growing population of patients with suspected coronary artery
disease undergoing CT coronary angiography with DSCT, increased
comorbidity findings arise in CT coronary angiography images.
However, misdiagnosis of comorbidities, such as ASA, remains common
among radiologists. Furthermore, only a limited number of studies
regarding the CT features of ASA are available in the literature
(3,7,8).
Therefore, in the present study, the aim was to
evaluate the incidence rate of ASA in a large cohort of patients
with suspected coronary artery disease and to address the
morphologic characteristics of ASA by DSCT imaging.
Materials and methods
Study population
A total of 8,626 patients (4,284 men and 4,342
women) with suspected coronary artery disease who underwent DSCT
coronary angiography between December 2012 and October 2014 at the
Affiliated Hospital of Xuzhou Medical College (Xuzhou, China) were
enrolled into the present study. The entry criteria for inclusion
were as follows: i) No allergy to iodine-containing contrast
medium; ii) sufficient renal function; iii) hemodynamic stability;
and iv) no pregnancy in women. The ASA patients were classified by
sex (male or female) and the direction of protrusion to give four
groups: Male right atrium (RA), male left atrium (LA), female RA
and female LA groups.
The Ethics Committee of the Affiliated Hospital of
Xuzhou Medical College approved the study protocol, and written
informed consent was obtained from all patients.
Scanning protocol and image
reconstruction
The patients were placed in supine position medial
to the DSCT scanner (Somatom Definition; Siemens Healthineers,
Erlangen, Germany) to ensure that the heart was covered by the
smaller field of view of the second tube detector array. A bolus of
80 ml nonionic iodinated contrast agent (350 mg I/ml; GE Healthcare
Co. Ltd., Shanghai, China) was injected at a velocity of 5 ml/sec,
followed by an injection of 50 ml normal saline at the same
velocity into an antecubital vein via an 18-gauge cannula. Image
acquisition commenced 5 sec after the attainment of a predefined
threshold of attenuation in the region of interest, which was
allocated in the ascending aorta (signal attenuation threshold, 100
HU). The entire volume of the heart was covered during one breath
holding within 15–20 sec, and ECG pulsing was recorded.
Retrospective gating ensured that the data reconstruction was
synchronous with the electrocardiographic signals. The parameters
for the data acquisition in a craniocaudal direction are as
follows: Rotation time of 0.33 sec, tube voltage of 120 kVp,
effective tube current of 420 mAs, adapted pitch values of
0.20–0.43 (according to the heart rates of patients), slice
thickness of 0.75 mm, a reconstruction increment of 0.4 mm, a
medium soft-tissue convolution kernel (B26f) and an image matrix of
512×512 pixels.
Imaging analysis and measurements
ASA was diagnosed in patients with an MVD of
protrusion from the atrial septum plane of >10 mm and a diameter
of the aneurysm base of >15 mm (9). Data sets were evaluated with a 3-D
workstation (Syngo MMWP VE30A; Siemens Healthineers, Erlangen,
Germany), and analyzed by multiplanar reconstruction (MPR). The MVD
and diameters of the aneurysm base were measured in the MPR. The
following features were evaluated in the images: MVD of protrusion,
diameter of the aneurysm base, direction of the maximal protrusion
(into the right or left atrium, or even protruding with bilaterally
equidistance during one cardiac cycle), other atrial
septum-associated morphological abnormalities, as well as thrombus
attachment to the ASA.
Statistical analysis
Statistical analysis was performed with the SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). The
quantitative variables are presented as the means ± standard
deviation. Values across groups were compared using the
independent-sample t-test, while differences in qualitative
variables were assessed with the χ2. P<0.05 was
considered to indicate differences that were statistically
significant.
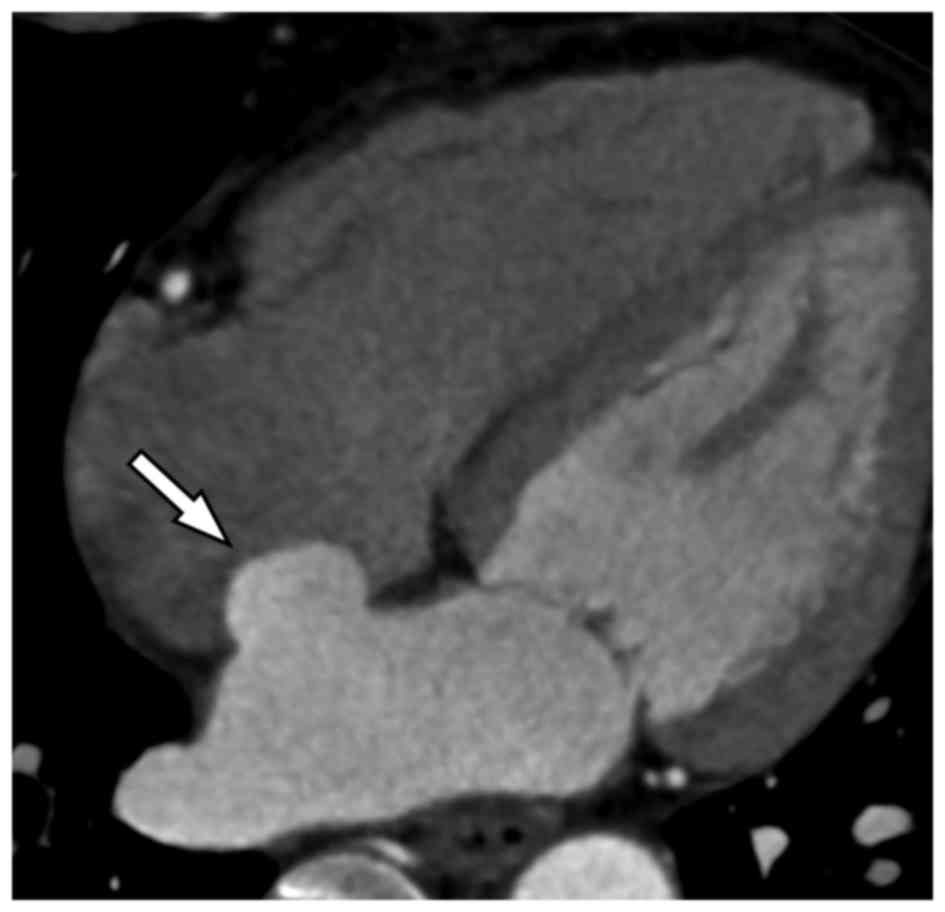
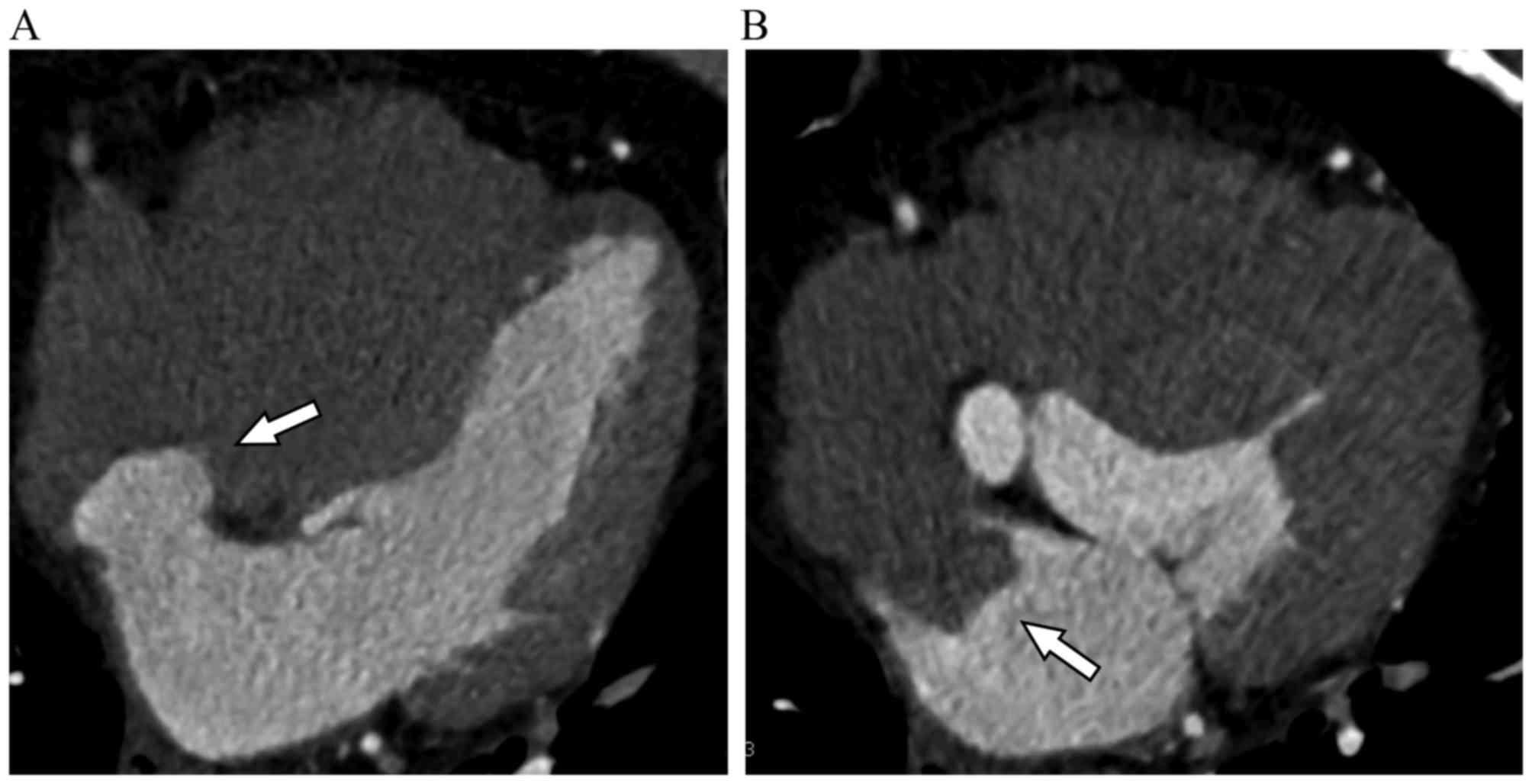
Results
Clinical characteristics of patients
with ASA
The clinical characterization of the ASA population
is summarized in Table I and
Figs. 1–3. Of the 8,626 subjects enrolled into the
present study, 51 (0.6%) patients, including 23 males (0.5%) and 28
females (0.6%), were confirmed to suffer from ASA. Patients with
ASA had a mean age of 62±10 years, a median age of 64 years and an
age range of 34–79 years. Atrial septal aneurysms bulged towards
the right atrium in 33 patients and towards the left atrium in 17
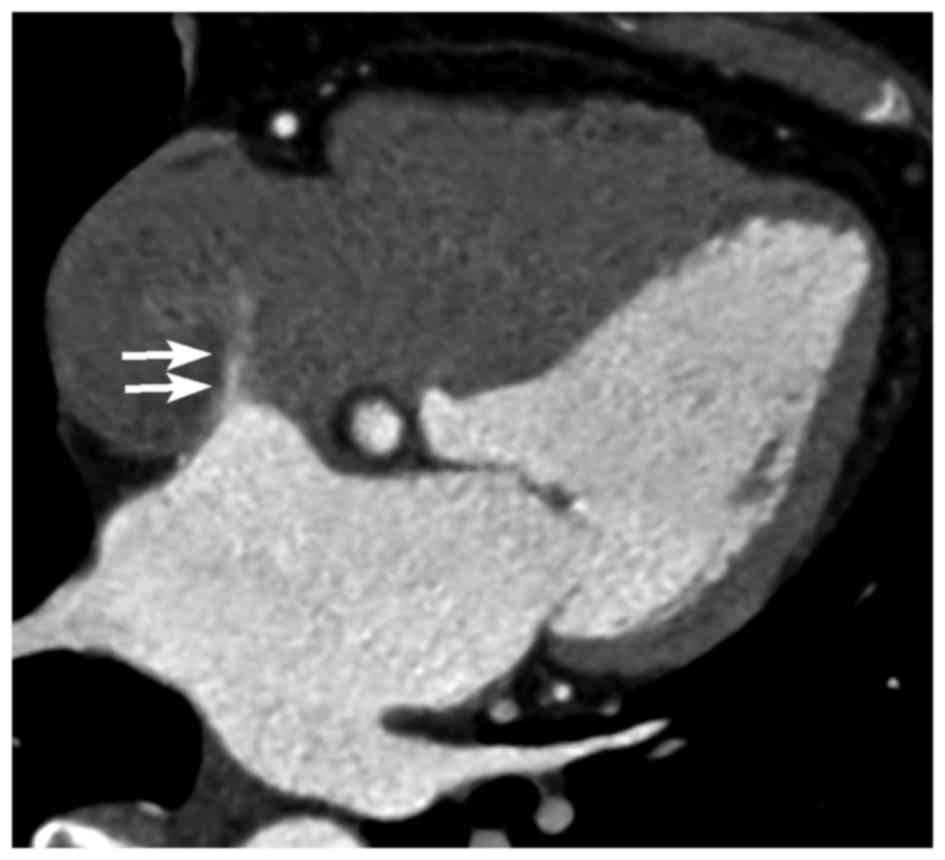
patients. Interatrial shunting through the foramen in the wall of
the aneurysm was identified in 1 patient (Fig. 4). In addition, ASA was concomitant
with atrial fibrillation in 4 patients, and there was no thrombosis
on the surface of the ASA.
 | Table I.Clinical characteristics of patients
with ASA. |
Table I.
Clinical characteristics of patients
with ASA.
| Characteristics | Males | Females |
|---|
| No. of ASA patients
(n) | 23 | 28 |
| Age
(years)a | 60±12 | 64±8 |
| Direction of the
maximal protrusions |
|
|
| RA | 10 | 23 |
| LA | 12 | 5 |
|
Bilateral | 1 | 0 |
| Comorbidities |
|
|
| PFO | 0 | 1 |
| AF | 2 | 2 |
|
Intra-atrial thrombi | 0 | 0 |
Comparison of the RA and LA
groups
One ASA patient was excluded from the present study
due to bilaterally equidistant protrusions during one cardiac
cycle. The characteristics of the RA and LA groups are summarized
in Table II. No significant
differences were observed between males and females with respect to
the age and incidence rate in ASA patients (t=−1.37, P>0.05;
χ2=0.43, P>0.05). By contrast, patients in the LA
group had a significantly higher age compared with those in the RA
group (t=−2.20, P<0.05). Furthermore, a significantly greater
number of female patients were included in the RA group compared
with the LA group (P<0.05).
 | Table II.Characteristics of patients with ASA
predominantly protruding into the right or left atrium. |
Table II.
Characteristics of patients with ASA
predominantly protruding into the right or left atrium.
|
| Direction of MVD |
|---|
|
|
|
|---|
| Characteristics | RA | LA |
|---|
| No. of patients
(n) | 33 | 17 |
| Age
(years) | 60±11 | 66±7 |
| Diameter of the base
of |
|
|
| ASA (mm) |
|
|
|
Males | 25.39±10.14 | 19.90±4.45 |
|
Females | 25.78±7.82 | 17.50±4.59 |
| MVD of protrusion
(mm) |
|
|
|
Males | 11.70±2.12 | 10.98±2.01 |
|
Females | 11.51±2.00 | 11.66±1.57 |
Comparison of male and female
groups
In the male groups, the differences in the ASA base
diameter and the MVD of protrusion between the RA and LA group were
not statistically significant. In the female groups, the difference
in the MVD between the RA and LA were also non-significant, whereas
the diameter of the ASA base in the RA group was significantly
higher compared with the LA group (t=2.27, P<0.05).
Discussion
The etiology of ASA remains controversial, with
certain aneurysms considered to be a primary or congenital
malformation, or secondary to the elevation of interatrial pressure
gradients, which bulges the septum into the lower pressure side
(3,10). Thus, ASA may be putatively
attributable to an abnormal structure of the interatrial septum or
modifications of the normal interatrial pressure gradients, or
both. In addition, the septal bulging may be static or dynamic
(4,5), bulging into the right atrium, the left
atrium or both atria.
The reported prevalence of ASA in human adults
varies widely among studies due to distinctions of imaging method
selections and absence of a ‘gold standard’ for the definition of
true ASA. The diagnostic criteria used in different studies vary,
with an aneurysm protruding >10 mm beyond the plane of the
atrial septum (4,9,11,12), or
an aneurysm with an MVD of >7.5 mm (1) or >15 mm (13) commonly required for ASA diagnosis. In
the present study, ASA was defined as a protrusion beyond the plane
of the atrial septum with an MVD of >10 mm. Previously, the
prevalence of ASA has been reported to range between 0.2 and 4%
using TTE, and between 2 and 8% using TEE (9). To the best of our knowledge, only one
previous study (3) has employed MDCT
as the diagnostic device in ASA assessment, with a reported
incidence of 1.33%. In the present study, the ASA incidence rate
was 0.6%, with no marked difference observed between the two
genders. The direction of the MVD into the left atrium was
predominantly observed in elderly patients. It is possible that
these patients are more predisposed to valvular diseases or other
maladies as a result of elevated pressure in the right atrium.
Furthermore, the direction of the MVD into the right atrium was
more frequently observed in female patients.
ASA reportedly occurs as a discrete cardiac
malformation or, more frequently, in concomitance with other
cardiac anomalies, particularly atrial septal defects and patent
foramen ovale (PFO) (3–5). ASA in adults is also associated with
atrial fibrillation, mitral valve prolapse and migraines with aura
(1,4,9,12). In the current study, no thrombosis
was detected on the surface of the ASA, and ASA was comorbid with
PFO in only 1 patient.
Although ASA is usually identified by chance, this
abnormality may lead to severe clinical outcomes. ASA has been
demonstrated to be an independent predictor of an embolic event in
multivariate analyses (5,13), while the prevalence of ASA in
patients with cerebral ischemia was 27.7% and it was the only
potential cardiac source of embolism in patients <45 years of
age (13). However, ASA was not
significantly associated with ischemic stroke unless concomitant
with PFO, particularly in patients aged <55 years (14–16). In
addition, patients suffering from stroke comorbid with PFO and ASA
were at a high risk of recurrent stroke (17).
To date, TEE and TTE are routine approaches used for
the diagnosis of ASA. Owing to its proximity to the atria, TEE is
superior to TTE in the imaging of atrial abnormalities (4). Furthermore, it is difficult to
differentiate between ASA with thrombotic attachment and
motion-induced artifacts, as well as bulging of the fossa ovalis
(4). In the case of suboptimal
echocardiography, however, ECG-gated cardiac MDCT scanning may
provide an alternative modality for such diagnoses. The current
spatial and temporal resolution of 64-MDCT of the heart is
approximately 0.4×0.4×0.4 mm3 voxel and 165 msec, which facilitate
the visualization of the delicate intracardiac structures, such as
septal membranes. Further technological advances of the improved
temporal resolution of DSCT (83 msec) may potentiate ECG-gated
cardiac CT in the evaluation of intracardiac abnormalities
(8). This technique can readily
recognize such clinical entities and minimize the misdiagnosis of
tumors, as well as avoid subsequent investigations. In addition,
echocardiography exempts the patients from ionizing radiation and
iodinated contrast media used in CT scanning, which is particularly
preferable when examining infants and children. Since the imaging
examinations in the present study were intended for suspected
coronary artery disease, all the patients were subjected to CT
coronary angiography and the simultaneous diagnosis of ASA, without
modifications of the doses of ionizing radiation and iodinated
contrast media. With the innovation of DSCT technology in MDCT, the
ionizing radiation and contrast media dosage can be minimized in
conformance with a weight-based low-dose protocol (18). Additionally, DSCT facilitates high
temporal resolution in patients with high heart rates or
arrhythmias without the administration of β-blockers.
Although ASA is a well recognizable cardiac
malformation, this abnormality remains commonly neglected among
radiologists. Despite the popularity of echocardiography for ASA
diagnosis, DSCT imaging, particularly when coupled with ECG gating,
may benefit the precise visualization of the anatomical details of
the atrial septum. Given the association of ASA with intracardiac
shunting and thromboembolic complications, it is advisable for
radiologists to recognize this clinical entity and minimize the
probabilities of misdiagnosis of ASA as a cardiac tumor.
In conclusion, the present study demonstrated that
DSCT may serve as a novel diagnostic technique for the detection of
interatrial septal abnormalities. DSCT enables ASA and associated
heart anomalies to be accurately visualized.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KX, LXX and ZXL conceived and designed the study.
XLZ, QX, LL, CFH, SGH and JJL collected and processed the data. LXX
and ZXL wrote the paper. KX, LXX and ZXL reviewed and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the Affiliated Hospital of Xuzhou Medical College,
Xuzhou, China (approval number xyfylw2012016). All patients
provided written informed consent for participation in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASA
|
atrial septal aneurysm
|
|
MVD
|
maximum vertical distance
|
|
TTE
|
transthoracic echocardiography
|
|
TEE
|
transesophageal echocardiography
|
|
MDCT
|
multidetector computed tomography
|
|
DSCT
|
dual-source computed tomography
|
|
MPR
|
multiplanar reconstruction
|
|
PFO
|
patent foramen ovale
|
References
|
1
|
Janion M and Kurzawski J: Atrial
fibrillation in patients with atrial septal aneurysm. Cardiol J.
14:580–584. 2007.PubMed/NCBI
|
|
2
|
Madan V, Harkness K, Brien EO and
Warburton EA: Lone atrial septal aneurysm and stroke-a case report
and review of the literature. Eur J Intern Med. 16:520–522. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Czekajska-Chehab E, Tomaszewski A,
Tomaszewska M, Siek E, Uhlig S and Drop A: ECG-gated multi-slice
computed tomography in the detection of atrial septal aneurysms.
Folia Morphol (Warsz. ). 67:126–128. 2008.PubMed/NCBI
|
|
4
|
Mugge A, Daniel WG, Angermann C, Spes C,
Khandheria BK, Kronzon I, Freedberg RS, Keren A, Denning K and
Engberding R: Atrial septal aneurysm in adult patients. A
multicenter study using transthoracic and transesophageal
echocardiography. Circulation. 91:2785–2792. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giannopoulos A, Gavras C, Sarioglou S,
Agathagelou F, Kassapoglou I and Athanassiadou F: Atrial septal
aneurysms in childhood: Prevalence, classification, and concurrent
abnormalities. Cardiol Young. 24:453–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Achenbach S and Kondo T: Technical
advances in cardiac CT. Cardiol Clin. 30:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeina AR, Orlov I, Sharif D and Barmeir E:
Detection of atrial septal aneurysm by ECG-gated MDCT. AJR Am J
Roentgenol. 187:W229–W230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dodd JD, Aquino SL, Holmvang G, Cury RC,
Hoffmann U, Brady TJ and Abbara S: Cardiac septal aneurysm
mimicking pseudomass: Appearance on ECG-gated cardiac MRI and MDCT.
AJR Am J Roentgenol. 188:W550–W553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Demir M: The relationship between atrial
septal aneurysm and autonomic dysfunction. Exp Clin Cardiol.
18:104–106. 2013.PubMed/NCBI
|
|
10
|
Oyedeji AT, Okunola O and Sani Umar M:
Atrial septal aneurysm mimicking a cor triatriatum sinister: A case
report and review of the literature. Clin Med Insights Case Rep.
5:143–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Homma S and Sacco RL: Patent foramen ovale
and stroke. Circulation. 112:1063–1072. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carerj S, Narbone MC, Zito C, Serra S,
Coglitore S, Pugliatti P, Luzza F, Arrigo F and Oreto G: Prevalence
of atrial septal aneurysm in patients with migraine: An
echocardiographic study. Headache. 43:725–728. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mattioli AV, Aquilina M, Oldani A,
Longhini C and Mattioli G: Atrial septal aneurysm as a
cardioembolic source in adult patients with stroke and normal
carotid arteries. A multicentre study. Eur Heart J. 22:261–268.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurzawski J, Janion M and Sielski J: Does
the morphology of atrial septal aneurysm influence cerebral
arterial embolus occurrence? Echocardiography. 24:895–900. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overell JR, Bone I and Lees KR:
Interatrial septal abnormalities and stroke: A meta-analysis of
case-control studies. Neurology. 55:1172–1179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Messe SR, Silverman IE, Kizer JR, Homma S,
Zahn C, Gronseth G and Kasner SE; Quality StandardsSubcommittee of
the American Academy of Neurology, : Practice parameter: Recurrent
stroke with patent foramen ovale and atrial septal aneurysm: Report
of the quality standards subcommittee of the american academy of
neurology. Neurology. 62:1042–1050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mas JL, Arquizan C, Lamy C, Zuber M,
Cabanes L, Derumeaux G and Coste J; Patent Foramen Ovale and Atrial
Septal Aneurysm Study Group, : Recurrent cerebrovascular events
associated with patent foramen ovale, atrial septal aneurysm, or
both. N Engl J Med. 345:1740–1746. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koplay M, Cimen D, Sivri M, Güvenc O,
Arslan D, Nayman A and Oran B: Truncus arteriosus: Diagnosis with
dual-source computed tomography angiography and low radiation dose.
World J Radiol. 6:886–889. 2014. View Article : Google Scholar : PubMed/NCBI
|