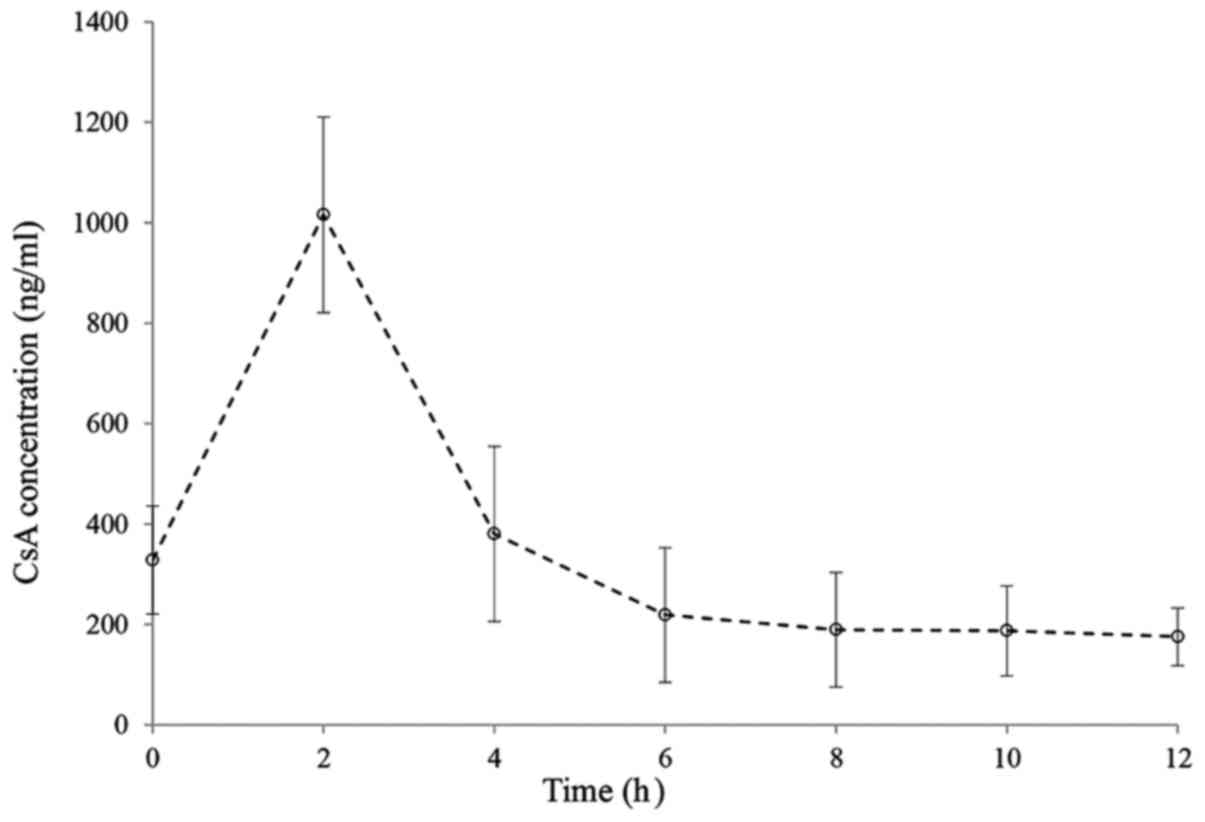
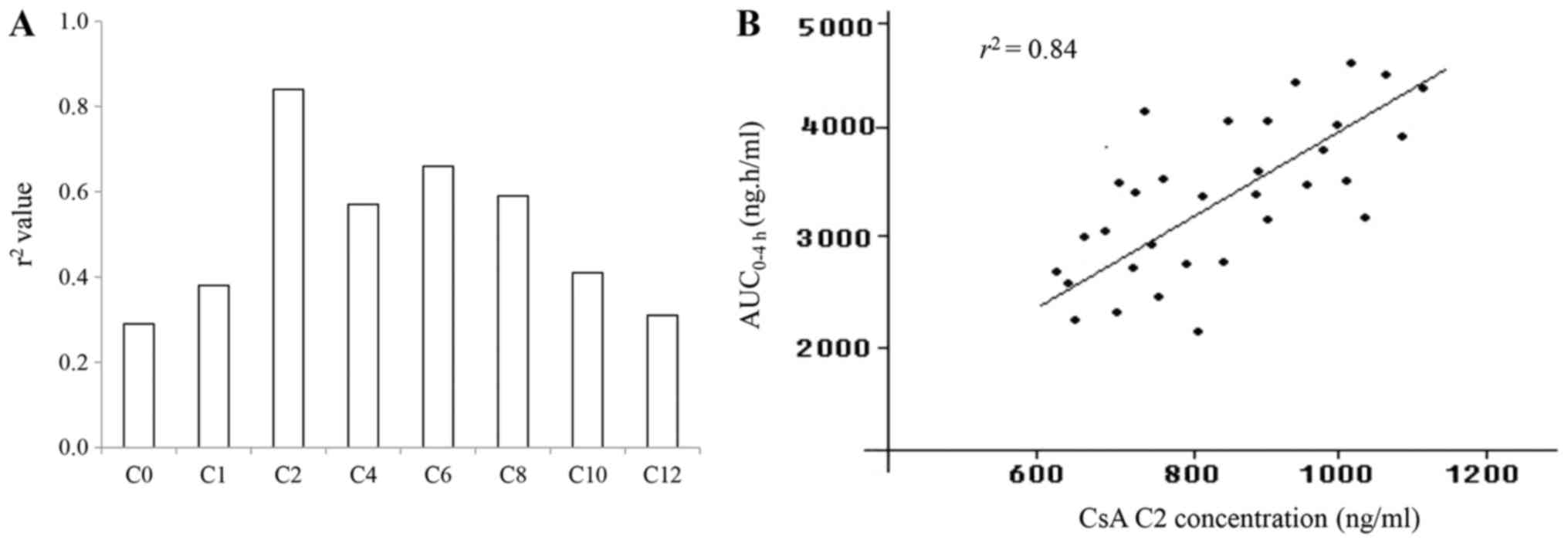
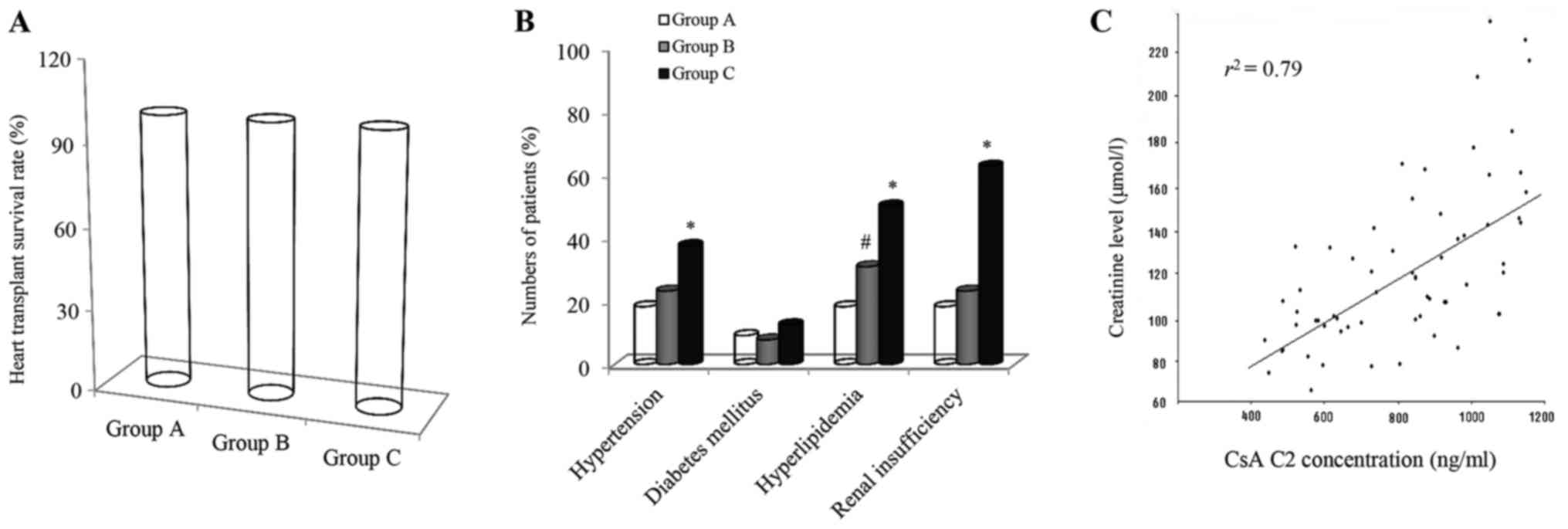
|
1
|
Burchill LJ: Heart transplantation in
adult congenital heart disease. Heart. 102:1871–1877. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korewicki J: Cardiac transplantation is
still the method of choice in the treatment of patients with severe
heart failure. Cardiol J. 16:493–499. 2009.PubMed/NCBI
|
|
3
|
Patel JK, Kittleson M and Kobashigawa JA:
Cardiac allograft rejection. Surgeon. 9:160–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel JK and Kobashigawa JA: Improving
survival during heart transplantation: Diagnosis of
antibody-mediated rejection and techniques for the prevention of
graft injury. Future Cardiol. 8:623–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ippoliti G, Rinaldi M, Pellegrini C and
Viganò M: Incidence of cancer after immunosuppressive treatment for
heart transplantation. Crit Rev Oncol Hematol. 56:101–113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Söderlund C and Rådegran G:
Immunosuppressive therapies after heart transplantation-the balance
between under- and over-immunosuppression. Transplant Rev
(Orlando). 29:181–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banner NR and Yacoub MH: Cyclosporine in
thoracic organ transplantation. Transplant Proc. 36 2
Suppl:302S–308S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haverich A and Gorler H: Experience with
cyclosporine: From revolution to evolution of immunosuppressive
protocols in thoracic organ transplantation. Transplant Proc. 36 2
Suppl:314S–317S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cantarovich M, Barkun J, Giannetti N,
Cecere R, Besner JG and Tchervenkov J: History of C2 monitoring in
heart and liver transplant patients treated with cyclosporine
microemulsion. Transplant Proc. 36 2 Suppl:442S–447S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oellerich M and Armstrong VW: Two-hour
cyclosporine concentration determination: An appropriate tool to
monitor neoral therapy? Ther Drug Monit. 24:40–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levy GA: C2 monitoring strategy for
optimising cyclosporin immunosuppression from the Neoral
formulation. BioDrugs. 15:279–290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delgado DH, Rao V, Hamel J, Miriuka S,
Cusimano RJ and Ross HJ: Monitoring of cyclosporine 2-hour
post-dose levels in heart transplantation: Improvement in clinical
outcomes. J Heart Lung Transplant. 24:1343–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balram C, Sivathasan C, Cheung YB, Tan SB
and Tan YS: A limited sampling strategy for the estimation of
12-hour Neoral systemic drug exposure in heart transplant
recipients. J Heart Lung Transplant. 21:1016–1021. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scheff JD, Almon RR, Dubois DC, Jusko WJ
and Androulakis IP: Assessment of pharmacologic area under the
curve when baselines are variable. Pharm Res. 28:1081–1089. 2001.
View Article : Google Scholar
|
|
15
|
Banner NR, David OJ, Leaver N, Davis J,
Breen J, Johnston A and Yacoub MH: Pharmacokinetics of oral
cyclosporine (Neoral) in heart transplant recipients during the
immediate period after surgery. Transpl Int. 15:649–654. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schulz E, Jabs A, Gori T, Hink U, Sotiriou
E, Tschöpe C, Schultheiss HP, Münzel T and Wenzel P: Feasibility
and safety of left ventricular endomyocardial biopsy via
transradial access: Technique and initial experience. Catheter
Cardiovasc Interv. 86:761–765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stewart S, Winters GL, Fishbein MC,
Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A,
Berry GJ, Burke MM, et al: Revision of the 1990 working formulation
for the standardization of nomenclature in the diagnosis of heart
rejection. J Heart Lung Transplant. 24:1710–1720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morris RG: Cyclosporin therapeutic drug
monitoring-an established service revisited. Clin Biochem Rev.
24:33–46. 2003.PubMed/NCBI
|
|
19
|
Jorga A, Holt DW and Johnston A:
Therapeutic drug monitoring of cyclosporine. Transplant Proc. 36 2
Suppl:396S–403S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Midtvedt K: Therapeutic drug monitoring of
cyclosporine. Transplant Proc. 36 2 Suppl:430S–433S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frassetto LA, Tan-Tam CC, Barin B, Browne
M, Wolfe AR, Stock PG, Roland M and Benet LZ: Best single time
point correlations with AUC for cyclosporine and tacrolimus in
HIV-infected kidney and liver transplant recipients.
Transplantation. 97:702–707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mardigyan V, Giannetti N, Cecere R, Besner
JG and Cantarovich M: Best single time points to predict the
area-under-the-curve in long-term heart transplant patients taking
mycophenolate mofetil in combination with cyclosporine or
tacrolimus. J Heart Lung Transplant. 24:1614–1618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monchaud C and Marquet P: Pharmacokinetic
optimization of immunosuppressive therapy in thoracic
transplantation: Part I. Clin Pharmacokinet. 48:419–462. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathias HC, Ozalp F, Will MB, Borland W,
Payne C, Kerr M, Lockhart J and Murday A: A randomized, controlled
trial of C0- Vs C2-guided therapeutic drug monitoring of
cyclosporine in stable heart transplant patients. J Heart Lung
Transplant. 24:2137–2143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knight SR and Morris PJ: The clinical
benefits of cyclosporine C2-level monitoring: A systematic review.
Transplantation. 83:1525–1535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Narula AS, Murthy M, Patrulu K and Saxena
VK: Routine Cyclosporine concentration-C2 level monitoring. Is it
helpful during the early post transplant period? Med J Armed Forces
India. 60:326–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zakliczynski M, Krynicka A, Szewczyk M,
Wojarski J and Zembala M: Limited utility of cyclosporine C2
monitoring in heart transplant recipients receiving ketoconazole.
Transplant Proc. 35:2333–2334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blanche C, Blanche DA, Kearney B, Sandhu
M, Czer LS, Kamlot A, Hickey A and Trento A: Heart transplantation
in patients seventy years of age and older: A comparative analysis
of outcome. J Thorac Cardiovasc Surg. 121:532–541. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stehlik J, Edwards LB, Kucheryavaya AY,
Benden C, Christie JD, Dipchand AI, Dobbels F, Kirk R, Rahmel AO
and Hertz MI: International Society of Heart and Lung
Transplantation: The registry of the international society for
heart and lung transplantation: 29th official adult heart
transplant report-2012. J Heart Lung Transplant. 31:1052–1064.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Colvin-Adams M, Smith JM, Heubner BM,
Skeans MA, Edwards LB, Waller C, Snyder JJ, Israni AK and Kasiske
BL: OPTN/SRTR 2011 annual data report: Heart. Am J Transplant. 13
Suppl 1:S119–S148. 2013. View Article : Google Scholar
|
|
31
|
Wang SS, Chou NK, Chi NH, Huang SC, Wu IH,
Wang CH, Yu HY, Chen YS, Tsao CI, Ko WJ and Shun CT: Can
cyclosporine blood level be reduced to half after heart
transplantation? Transplant Proc. 42:930–933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boffini M, Sansone F, Patanè F, Bonato R,
Ribezzo M, Iacovino C, Comoglio C and Rinaldi M: Does everolimus
associated with a low dose of cyclosporine in long-term cardiac
transplant recipients improve renal function? Initial experience.
Transplant Proc. 41:1349–1352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Damiano S, Ciarcia R, Montagnaro S,
Pagnini U, Garofano T, Capasso G, Florio S and Giordano A:
Prevention of nephrotoxicity induced by cyclosporine-A: Role of
antioxidants. J Cell Biochem. 116:364–369. 2015. View Article : Google Scholar : PubMed/NCBI
|