Introduction
Intracranial arterial wall abnormal bulge is called
intracranial aneurysm, which often causes subarachnoid hemorrhage
and leads to a series of neurological symptoms, with high
mortality. Epidemiological investigations show that the incidence
of intracranial aneurysms has an increasing trend year by year
(1). Surgical intervention and the
development of interventional materials have made endovascular and
surgical procedures the preferred treatments for patients with
intracranial aneurysms, and the use of imaging techniques has led
to a significant increase in the safety of endovascular treatment
(2). At present, there is scarce
research on the difference of therapeutic effects between
craniotomy and endovascular embolization for patients with
intracranial aneurysms. Li et al (3) found that serum MMP-2 is closely related
to the onset of intracranial aneurysm, and serum MMP-2 expression
in rats with intracranial aneurysm was significantly increased.
MMP-2 is a member of the MMP family which can
degrade the extracellular matrix and regulate the remodeling of the
vascular wall (4). Brinjikji et
al (5) found that in patients
with abdominal aortic aneurysm and animal model group, MMP-2 mRNA
and protein expression was significantly increased, suggesting that
MMP-2 is closely correlated with the formation of aneurysm. Fujii
et al (6) found that
adenosine 5′-monophosphate (AMP)-dependent protein kinase (AMPK)
can regulate the transcription of MMP-2 in vivo and affect
the expression of MMP-2.
In recent years, a number of studies have shown that
the increased levels of proinflammatory cytokines (IL-6 and TNF-α)
as well as the increased levels of reactive oxygen species (ROS)
caused by inflammatory infiltration are important factors in the
formation of multiple hemangiomas. At the same time, caspase3 as a
protease present in the cytoplasm can mediate cell apoptosis by
degrading the polymeric polymerase PARP, thereby affecting the
occurrence and development of hemangiomas (7,8). At
present, there is no study on the effect of endovascular
embolization and craniotomy on the expression of MMP-2, MMP-9 and
caspase3 in the serum of patients with intracranial aneurysms. This
study investigated the difference of serum MMP-2, MMP-9 and
caspase3 expression in patients with intracranial aneurysm treated
by endovascular embolization and craniotomy, and explored the
possible mechanism.
Patients and methods
Research object
A total of 79 patients with intracranial aneurysms
treated in the Second Affiliated Hospital of Soochow University
(Suzhou, China) from September 2011 to August 2014 were enrolled in
this study. All the patients selected were diagnosed as
intracranial aneurysms by experts and imaging examination, and
confirmed that there was no local or distant metastasis. All
patients were eligible for endovascular embolization and craniotomy
for treatment. Patients were divided into the intervention group
(n=41, vascular intervention embolization) and craniotomy group
(n=38, craniotomy) based on treatment methods. The intervention
group consisted of 23 males and 18 females, aged 65.2±19.7 years.
The craniotomy group consisted of 21 males and 17 females, with an
average age of 64.6±21.9 years. Inclusion criteria: diagnosed as
intracranial tumors by the whole brain digital angiography and head
CT examination and diagnosed by experts as intracranial tumors.
Exclusion criteria: severe wasting disease, not eligible for the
conditions of surgery, long-term chronic inflammation, severe liver
and kidney dysfunction, traumatic brain injury and cerebral
infarction. All patients underwent the same treatment and nursing
program after surgery. All patients signed informed consent and all
clinical and pathological data during the hospital stay were
retained. Forty cases of normal volunteers with normal physical
examination were selected as the control group, and all of them
signed informed consent. This study was approved by the Ethics
Committee of The Second Affiliated Hospital of Soochow University
(Suzhou, China) and The Second Clinical Medical School of Inner
Mongolia University for Nationalities (Hulunbuir, China).
Surgical methods
All the patients were administered midazolam 0.1
mg/kg (Sichuan Baili Pharmaceutical Co., Ltd., Sichuan, China) 30
min before the anesthesia and then performed with intravenous
injection of fentanyl 3 µg/kg. Patients in the intervention group
were treated with endovascular embolization: The patient was
punctured on the right femoral artery after anesthesia and inserted
with the microcatheter. One end of the catheter was placed in the
aneurysm of the cerebral artery for embolization (according to the
size of the tumor, a corresponding spring circle can be added), and
the operation condition was determined through angiography. When
the aneurysm cavity disappeared, and no significant blood could be
detected, the microcatheter was extubated, and the puncture point
was wrapped. After the craniotomy, low molecular weight heparin
5000 units were injected twice a day for a total of 5 days.
The patients in the craniotomy group were treated
with craniotomy: After anesthesia, the position of intracranial
aneurysms was determined according to brain DSA and cranial CT. The
meninges were incised after the surgical route was determined, and
the aneurysm was closed under the microscope (Olympus, Tokyo,
Japan). The arteries near the intracranial aneurysm were blocked.
When the aneurysm was closed by a blocking clip, the blocking clip
should be released and the blood supply was restored. After
craniotomy, the success rate of operation was evaluated in both
groups. Venous blood (5 ml) of the selected patients and volunteers
in the control group were drawn, and the serum was separated and
stored at −80°C for future use.
Observation of survival and adverse
reactions
Postoperative complications were observed in both
groups of patients. The incidence of cerebral hemorrhage, aneurysm
rupture, thrombus shedding, infection and other adverse reactions
were closely monitored. Then the two groups of patients were
followed up for 3 years. The patient survival rate was recorded,
and the two treatment methods for the treatment of intracranial
tumors were evaluated.
Reactive oxygen species (ROS) and
inflammatory cytokine content detection
The serum levels of related factors in each group
were measured by ELISA kit (Wuhan Boster Biological Technology Co.,
Ltd., Wuhan, China) using ROS, IL-6, TNF-α and IL-10 ELISA kit,
respectively. The operation was performed strictly according to the
instructions of the ELISA kit. The standards of ROS, IL-6, TNF-α
and IL-10 were diluted to the standard concentration for the
production of standard curve, and working fluid was prepared in
advance. The samples, antibodies, enzyme, color solution and
termination solution were added in strict accordance with the steps
in the instructions, and each sample was repeated three times.
After terminating the reaction, the sample was placed on a
microplate reader (Bio Rad, Hercules, CA, USA), and the optical
density (OD) value of each sample was measured at 450 nm.
CurveExpert1.4 software was used to draw a standard curve. The
concentration of the sample in each well was calculated, and then
ROS, IL-6, TNF-α and IL-10 concentration of the original sample was
measured in pg/ml as a unit.
qPCR detection of gene expression
levels
The serum stored at −80°C was thawed. TRIzol
(Millipore) (1 ml) was added per 100 mg and allowed to stand for 5
min in an ice bath to completely lyse the cells. After adding 200
µl of chloroform, the mixture was mixed completely, then allowed to
stand for 5 min in an ice bath and centrifuged at 3,000 × g at 4°C
for 10 min. The supernatant was discarded, and 1 ml of freshly
prepared 75% ethanol was added. After centrifugation, 60 µl of DEPC
water was added to dissolve the precipitate. The RNA was obtained,
and the OD value of the corresponding RNA and A260/280
were measured to verify the concentration and purity of the
RNA.
According to the reverse transcription kit
(Millipore), 500 ng of RNA template was added, and 20 µl of the
reaction system was used to obtain the cDNA by reverse
transcription. Reverse transcription conditions were: 37°C for 15
min, and 85°C for 5 sec. The reaction system was amplified by qPCR
using β-actin as internal reference. The reaction conditions were
as follows: 95°C for 5 min, 95°C for 30 sec, 63°C for 50 sec, 72°C
for 60 sec, 30 cycles, and 72°C for 5 min. The primers were
synthesized by Inventec Bio-Technology Co., Ltd., Taipei City,
Taiwan. The sequences are shown in Table
I. The relative expression levels of the target genes were
calculated by 2−∆∆Cq (9),
and expressed as MMP-2/actin, MMP-9/actin and caspase3/actin.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Genes | Sequence |
|---|
| MMP-2 | Sense:
5′-atgacagctgaccactgag-3′ |
|
| Antisense:
5′-atttgttgcccaggaaagtg-3′ |
| MMP-9 | Sense:
5′-ttggtttctgccctagtgagaga-3′ |
|
| Antisense:
5′-aaagatgaacgggaacacacagg-3′ |
| Caspase3 | Sense:
5′-tgtcgatgcagcaaacctca-3′ |
|
| Antisense:
5′-gacttctacaacgatcccctc-3′ |
| β-actin | Sense:
5′-ctggaacggtgaaggtgaca-3′ |
|
| Antisense:
5′-gggacttcctgtaacaatgca-3′ |
Western blot detection of protein
expression
After the serum of each group was obtained, the RIPA
lysate (Biotime Biotechnology Co., Ltd., Shanghai, China) was added
in the proportion of 0.5 ml:1 ml and then homogenized with an
ultrasonic homogenizer (containing 1% phosphatase inhibitor and 1%
protease inhibitor). After incubating for 5 min in an ice bath and
centrifuging at 10,800 × g for 15 min at 4°C, the supernatant was
transferred to a new EP tube, and protein quantification was
carried out by using a BCA Protein assay kit (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Protein samples were
placed in the same concentration of the loading system for boiling
denaturation 15 min, configured 12% SDS polyacrylamide gel and 5%
concentrated gel for electrophoresis. The wet transfer method was
performed to transfer the protein to a PVDF membrane, and closed
for 1 h. The purpose band was cut and incubated with the
corresponding primary rabbit anti-human polyclonal antibodies:
MMP-2 (dilution, 1:1,000; cat. no. ab37150; Abcam, Cambridge, UK),
MMP-9 (dilution, 1:1,000; cat. no. ab73734; Abcam), caspase3
(dilution, 1:1,000; cat. no. ab13847; Abcam), p-AMPK (dilution,
1:1,000; cat. no. ab3760; Abcam), Bcl-2 (dilution, 1:1,000; cat.
no. ab196495; Abcam), Bax (dilution, 1:1,000; cat. no. ab53154;
Abcam) and β-actin (dilution, 1:1,000; cat. no. ab8227; Abcam)
overnight. The membrane was washed three times with TBST, and
incubated with goat anti-rabbit horseradish peroxides enzyme
conjugate secondary polyclonal antibody (dilution, 1:5,000; cat.
no. ab6721; Nanjing Jiancheng Biotechnology Institute, Nanjing,
China) at room temperature for 1 h, then washed three times with
TBST, 5 min each time, adding appropriate ECL light Liquid (liquid
A and liquid B were mixed 1:1) in the dark environment, then
developed and fixed, and then the gray value analysis was performed
by ImageJ software after scanning the band to calculate the
relative expression level of the protein.
Statistical analysis
Data of this study are presented as mean ± standard
deviation. Data were analyzed by SPSS 19.0 software (IBM Corp.,
Armonk, NY, USA). The t-test was used to compare the data between
two groups. Chi-square test was used to compare the enumeration
data. Analysis of variance was used for multiple comparisons and
Dunnetts test was the post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
General information
A total of 78 patients with intracranial aneurysm
were selected, and the patients treated with vascular
interventional embolization were selected as the intervention
group, a total of 41 cases. The patients treated by craniotomy were
selected as the craniotomy group, a total of 38 cases. The patients
were examined within 24 h of admission. In addition, sex, age, body
mass index (BMI) and tumor site were recorded. The general
information of both groups of patients are shown in Table II, and the differences in sex, age,
BMI and tumor site of each group of patients had no statistical
significance (P>0.05).
 | Table II.The general information of patients
(mean ± SD). |
Table II.
The general information of patients
(mean ± SD).
|
|
|
|
| Tumor site |
|---|
|
|
|
|
|
|
|---|
| Groups | Sex (M/F) | Age (year) | BMI
(kg/m2) | Anterior
communicating artery aneurysm (case) | Posterior
communicating artery aneurysm (case) | Middle cerebral
artery aneurysm (case) |
|---|
| Intervention | (23/18) | 65.2±19.7 | 24.8±1.5 | 28 | 5 | 8 |
| Operation | (21/17) | 64.6±21.9 | 24.6±1.6 | 25 | 6 | 7 |
| P-value | 0.396 | 0.528 | 0.652 | 0.089 | 0.352 | 0.287 |
| t value | 0.613 | 0.762 | 0.876 | 0.896 | 0.962 | 0.931 |
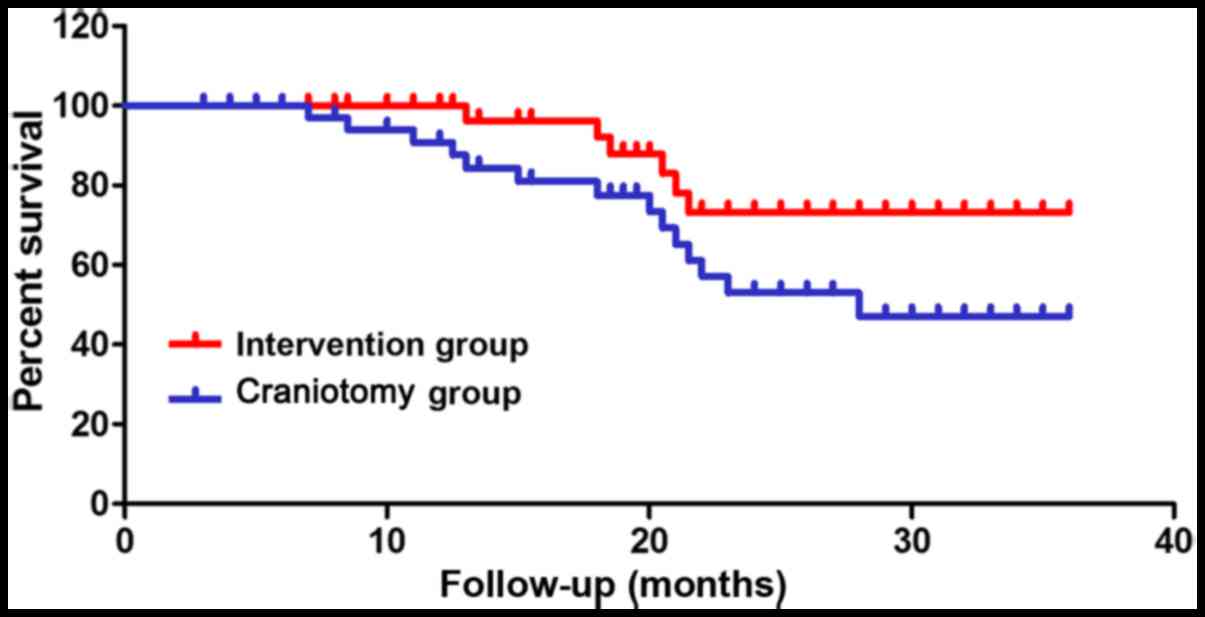
Survival and adverse reaction
rate
The survival rate of both groups of patients and the
incidence of adverse reactions were recorded within 3 years after
operation. The results are shown in Fig.
1 and Table III, and the
3-year survival rate of the intervention group was higher than that
of the craniotomy group. Adverse reactions occurred in 7 patients
in the intervention group, and there were 20 cases of adverse
reactions in the craniotomy group, suggesting the incidence of
adverse reactions in the intervention group was significantly lower
than that in the craniotomy group (P<0.01).
 | Table III.Adverse reaction rate. |
Table III.
Adverse reaction rate.
| Groups | Cerebral hemorrhage
(case) | Aneurysm rupture
(case) | Thrombosis
(case) | Infection (case) | Other (case) | Total |
|---|
| Intervention | 1 | 1 | 1 | 2 | 2 | 7 |
| Operation | 1 | 2 | 4 | 8 | 5 | 20 |
| P-value | >0.05 | >0.05 | <0.05 | <0.01 | <0.05 | <0.01 |
| t value | 0.613 | 0.762 | 0.436 | 0.486 | 0.563 | 0.396 |
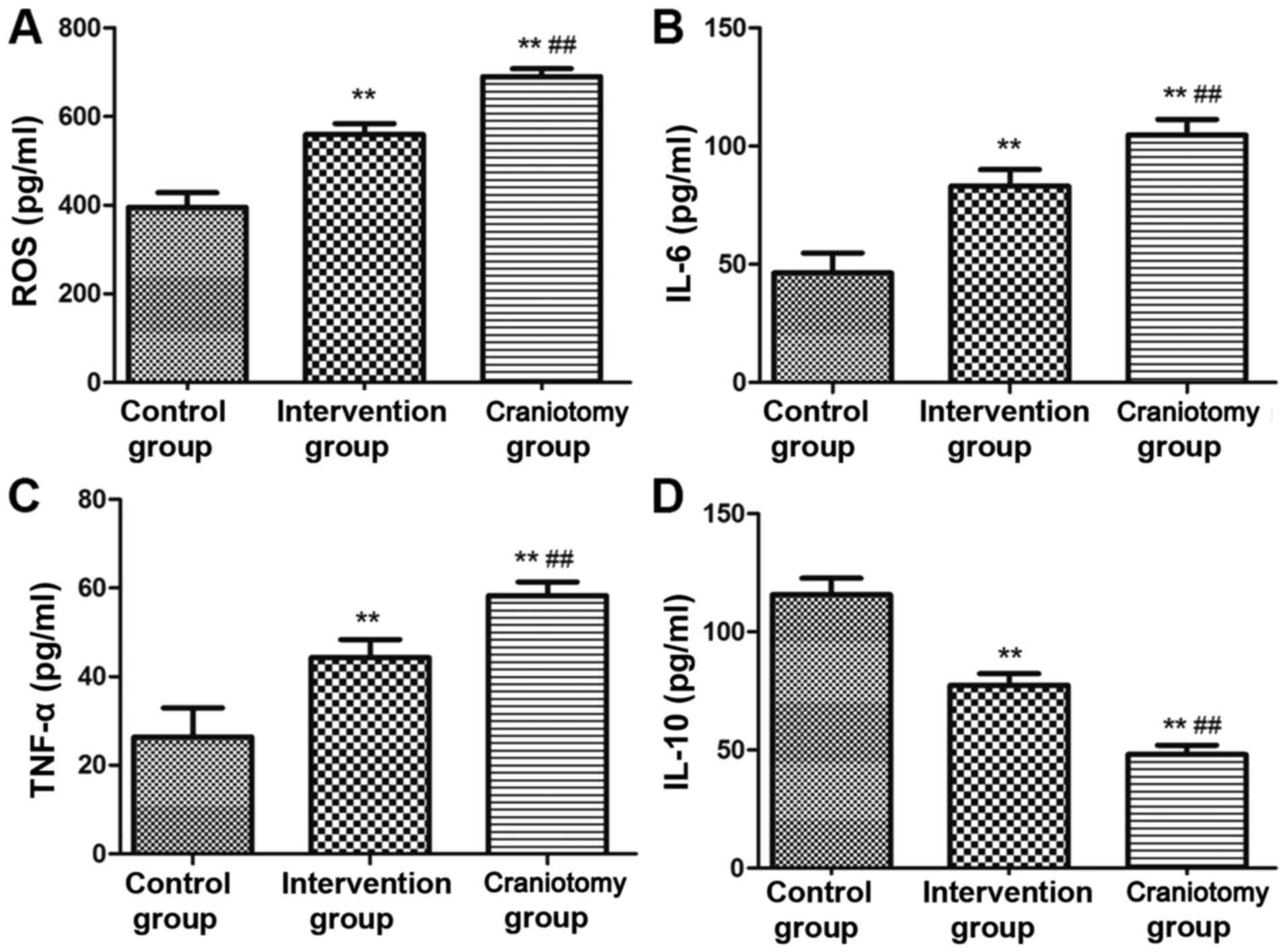
ROS and inflammatory cytokine
content
The serum levels of ROS and inflammatory cytokines
in each group were detected by ELISA kit after surgery. The results
are shown in Fig. 2: the contents of
ROS, IL-6 and TNF-α in the serum of the intervention and craniotomy
groups were significantly higher than that of the control group,
and the level of IL-10 was significantly lower than that of the
control group (P<0.01). The serum levels of ROS, IL-6 and TNF-α
in the intervention group were significantly lower than those in
the craniotomy group, and the content of IL-10 in the serum was
significantly higher than that in the craniotomy group
(P<0.01).
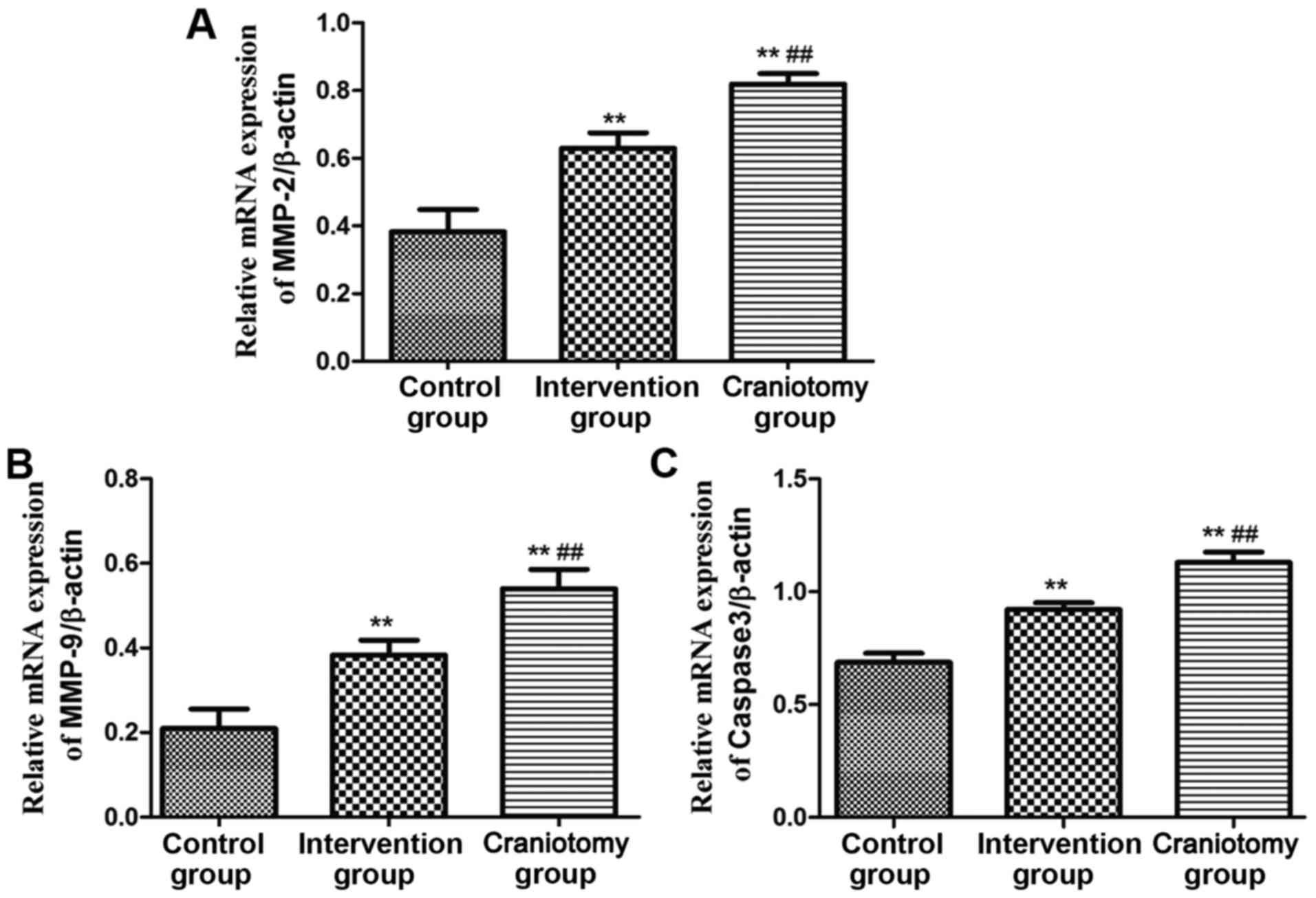
mRNA expression levels
The qPCR was used to detect the mRNA expression of
related genes in the serum of each group. The results are shown in
Fig. 3. The relative expression
levels of MMP-2, MMP-9 and caspase3 mRNA in the serum of
intervention and craniotomy groups were significantly higher than
those of the control group (P<0.01). The relative expression
levels of MP-2, MMP-9 and caspase3 mRNA in the intervention group
were significantly lower than those in the craniotomy group
(P<0.01).
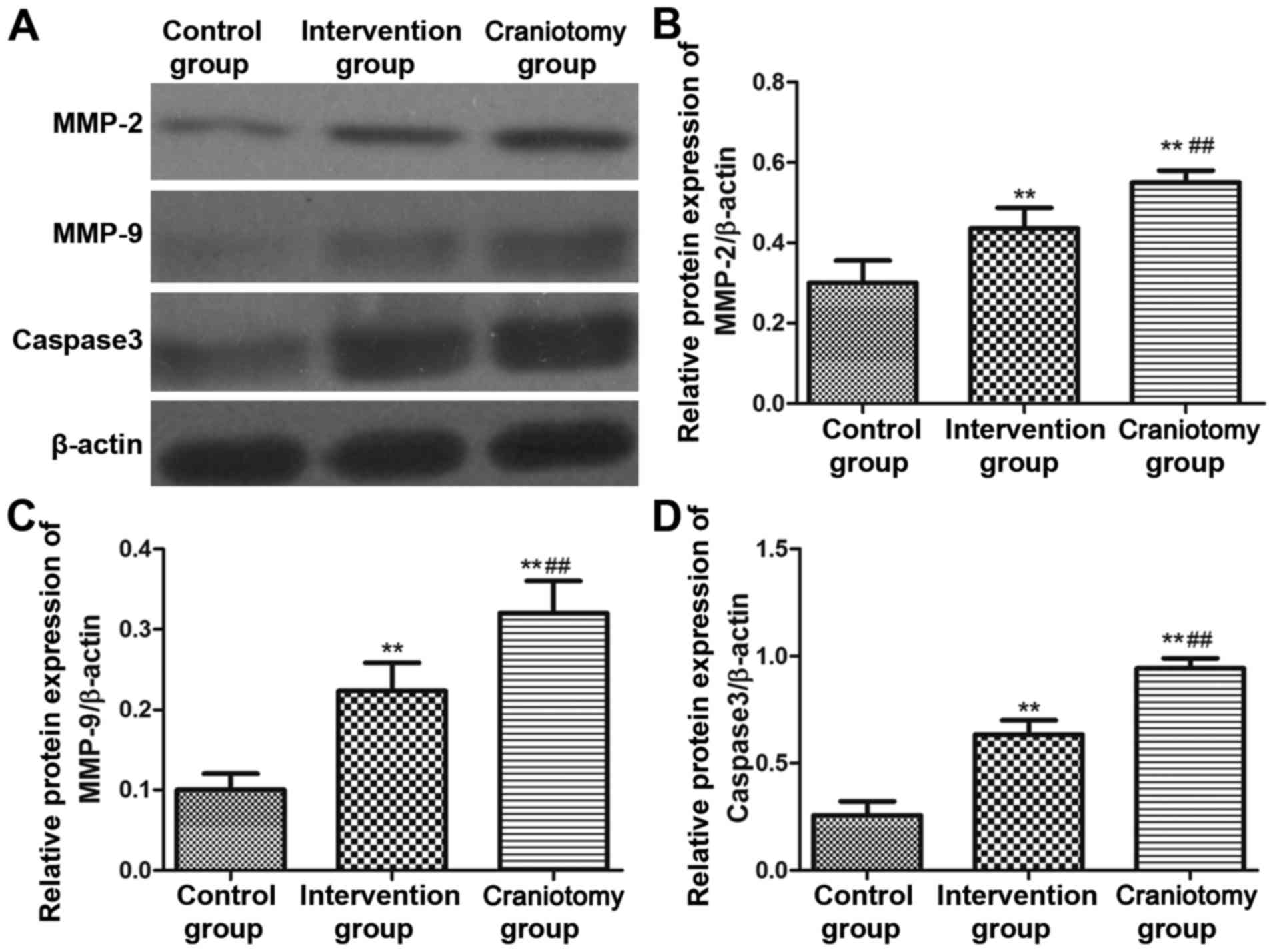
Protein expression level
Western blotting was used to detect the expression
levels of corresponding protein, and the results are shown in
Fig. 4. The results of protein
expression were consistent with the mRNA expression results: the
levels of the serum MMP-2, MMP-9 and caspase3 protein expression in
the intervention and craniotomy groups were significantly increased
(P<0.01). The serum levels of MMP-2, MMP-9 and caspase3 in the
intervention group were significantly lower than those in the
craniotomy group (P<0.01). Further study was made on the
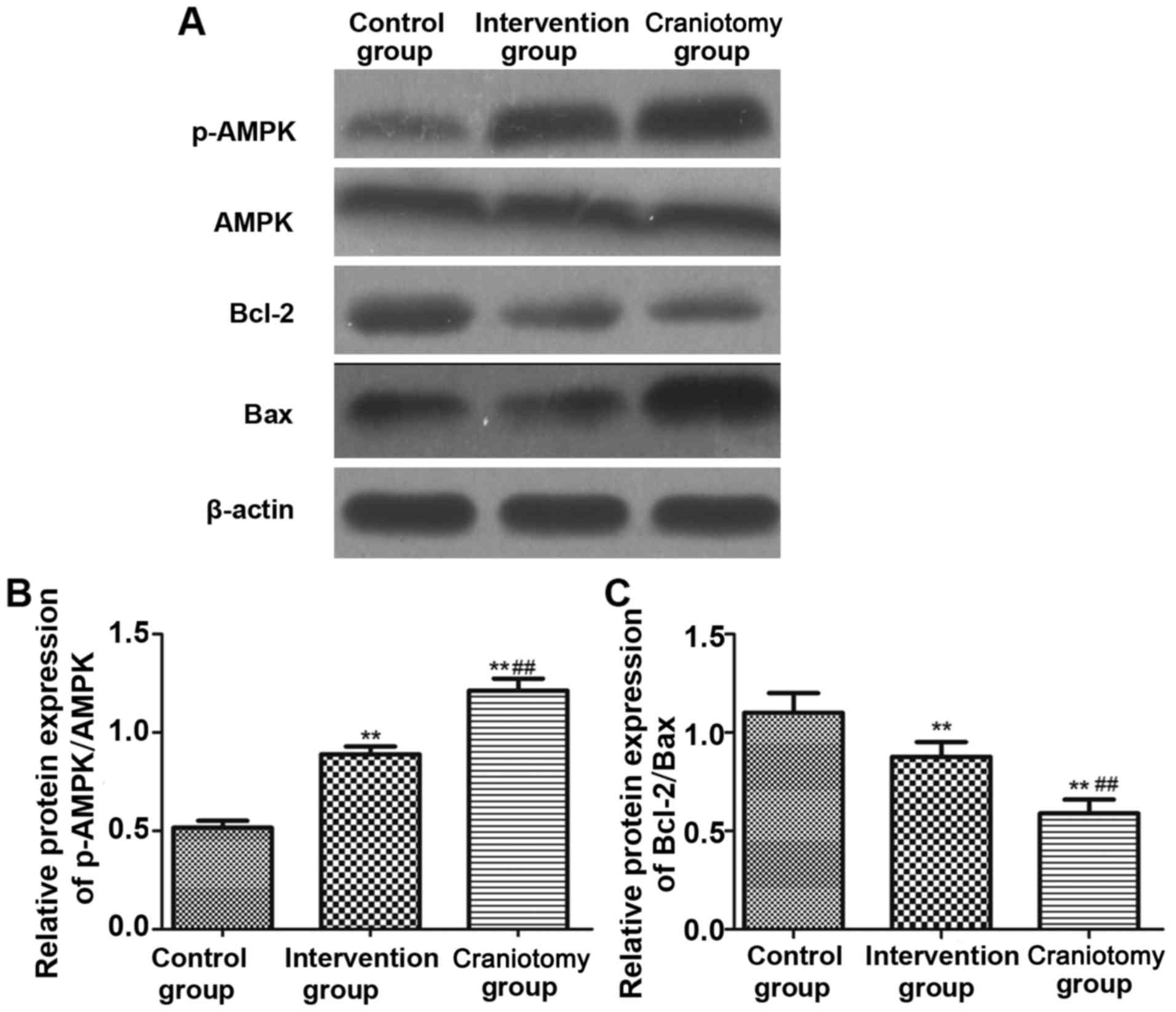
expression level of its upstream protein, and the results (Fig. 5) showed that the expression levels of
p-AMPK and Bcl-2/Bax in the intervention group and surgery groups
were significantly higher than those in the control group
(P<0.01). The serum p-AMPK, Bcl-2/Bax in the intervention group
was significantly lower than those in the craniotomy group
(P<0.01).
Discussion
Intracranial tumor is caused by many factors, and
intracranial atherosclerosis-induced degradation of arterial wall
and the occurrence of inflammatory reaction is the main reason. The
treatment of intracranial hemangiomas by intracranial vascular
embolization increased in the 1970s, and intracranial vascular
embolization and craniotomy are the most common means of treatment
of intracranial aneurysms (10,11). In
this study, by comparing the impact of vascular interventional
embolization and craniotomy on patients with intracranial
aneurysms, the results showed that i) vascular interventional
embolization can effectively increase the 3-year survival rate of
patients with intracranial tumors, and significantly reduce
postoperative adverse reactions; ii) vascular embolization can
effectively reduce the levels of intravascular ROS and
proinflammatory cytokines, and increase the level of inhibitors,
which can effectively control the intracranial inflammatory
response; iii) vascular embolization can effectively reduce the
serum levels of MMP-2, MMP-9 and caspase3 mRNA and protein
expression, and effectively inhibit the expression of induced genes
and proteins in intracranial tumors; and iv) the inhibitory effect
of vascular interventional embolization on the expression of MMP-2,
MMP-9 and caspase3 may be associated with inhibition in patients
with the AMPK expression, and reduce the expression of apoptotic
protein Bcl-2/Bax.
The most common adverse reactions after intracranial
tumor treatment are aneurysm intraoperative and postoperative
rupture and thrombosis, with a high mortality rate if the rescue is
not timely (12). Kanematsu et
al (13) found that among 152
cases of aneurysms with rupture after the treatment, mortality rate
was 8%, and disability rate was 12%, and incidence of serious
central nervous system injury was 21%. Improper operation during
the blocking of intracranial surgery for intracranial tumors is
very easy to cause rupture of aneurysm, and blocked intracranial
aneurysm artery recanalization will occur after craniotomy
(14). Wada et al (15) analyzed 398 patients who underwent
intracranial aneurysm artery surgery with Mate analysis, and the
postoperative recanalization rate was 28.9%. Incidence of trauma
caused by intracranial surgery in patients was significantly higher
than that caused by endovascular embolization, leading to
significantly increased incidence of adverse reactions, seriously
affecting the patient's postoperative survival rate and quality of
life.
Atherosclerosis and other cardiomyopathy cause a
large number of apoptosis, and apoptosis of cells leads to the
reduction of vascular endothelial cells, causing the remodeling of
the vessel wall, leading to the formation of aneurysm (16). It has been found that the signal
transduction pathway can activate caspase family members, and the
expression level of caspase3, which is apoptosis effector, and is
significantly increased, leading to a significant increase in the
expression of TNF-α (16). This
study found that the formation of aneurysms leads to elevated
p-AMPk in patients. Increased p-AMPk level affects the release of
mitochondria and regulates cytochrome c by decreasing the
expression level of Bcl-2, thereby regulating cell apoptosis,
promoting the expression of MMP-2, MMP-9, and caspase3 and
resulting in blood vessel wall reconstruction. Compared with
craniotomy, interventional embolization can effectively reduce the
expression level of p-AMPk, promote the expression of Bcl-2/Bax,
and reduce the release of pro-inflammatory and apoptotic proteins,
thus improving aneurysm. Oh et al (17) found a significant increase in the
level of apoptosis in tumor tissue of patients with aortic
aneurysm, which in turn led to increased release of inflammatory
cytokines. The formation of aneurysms activates the Bcl-2 protein,
which regulates apoptosis through mitochondrial regulation of
cytochrome c release, leading to remodeling of the vessel
wall.
Interleukin-10 (IL-10) is an important inhibitory
cytokine with immune-regulatory function and various biological
activity. It could be produced by many kinds of cells, including
CD4+ Th cells, CD8+ Th cells, dendritic
cells, macrophages and regulatory T cells. IL-10 is an effective
anti-inflammatory substance, which can inhibit the production of
interleukin-2, leukotriene and other inflammatory factors.
Therefore, IL-10 has a strong anti-inflammatory effect. The data
showed that the contents of ROS, IL-6 and TNF-α in the intervention
and craniotomy groups were significantly higher than that in the
control group, and the level of IL-10 was significantly lower than
that in the control group (P<0.01). The serum levels of ROS,
IL-6 and TNF-α in the intervention group were significantly lower
than those in the craniotomy group, and the content of IL-10 in
serum was significantly higher than that in the craniotomy group
(P<0.01). The results also demonstrated that IL-10 is an
important inhibitory cytokine, with strong anti-inflammatory
effect. Ma et al (18) found
that ADR-treated rat cardiomyocytes significantly increased
apoptotic cells, and the pro-apoptotic protein caspase3 activity
and expression were significantly increased, and the anti-apoptotic
protein Bcl-2 expression was significantly reduced.
AMPK can mediate the energy metabolism of cells, and
activate the corresponding ATPase production, thereby increasing
the level of ATP, maintaining energy supply (19). The phosphorylation level is increased
when AMPK is activated, and then involved in the regulation of a
variety of physiological signals. Jiang et al (20) found that pravastatin can increase
AMPK phosphorylation in patients with abdominal aortic aneurysm and
increase in vivo MMP-2 expression. ROS regulates the balance
of oxidation and antioxidation in vivo. Liaw et al
(21) found that the level of ROS in
cardiomyocytes of myocardial infarction rats was significantly
increased and the cells were in an oxidative stress state, leading
to cell injury.
In summary, compared with craniotomy, endovascular
embolization can effectively increase the patient's survival rate,
reduce adverse reactions, reduce the level of intravascular MMP-2,
MMP-9 and caspase3, and the mechanism may be associated with the
impact on ROS levels, which in turn affect the expression of AMPK
and Bcl2/Bax.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, YH and YD conceived and designed the study. LZ,
YH and BY were responsible for the collection and analysis of the
patient data. YH and SL interpreted the data and drafted the
manuscript. LZ and YH revised the manuscript critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Second Affiliated Hospital of Soochow University (Suzhou,
China) and The Second Clinical Medical School of Inner Mongolia
University for Nationalities (Hulunbuir, China). Signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peña-Silva RA, Chalouhi N, Wegman-Points
L, Ali M, Mitchell I, Pierce GL, Chu Y, Ballas ZK, Heistad D and
Hasan D: Novel role for endogenous hepatocyte growth factor in the
pathogenesis of intracranial aneurysms. Hypertension. 25:587–593.
2015. View Article : Google Scholar
|
|
2
|
Jeong HW, Seo JH, Kim ST, Jung CK and Suh
SI: Clinical practice guideline for the management of intracranial
aneurysms. Neurointervention. 9:63–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li B, Li F, Chi L, Zhang L and Zhu S: The
expression of SPARC in human intracranial aneurysms and its
relationship with MMP-2/-9. PLoS One. 8:e584902013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mandelbaum M, Kolega J, Dolan JM, Siddiqui
AH and Meng H: A critical role for proinflammatory behavior of
smooth muscle cells in hemodynamic initiation of intracranial
aneurysm. PLoS One. 8:e743572013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brinjikji W, Shahi V, Cloft HJ, Lanzino G,
Kallmes DF and Kadirvel R: Could statin use be associated with
reduced recurrence rates following coiling in ruptured intracranial
aneurysms? AJNR Am J Neuroradiol. 36:2104–2107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujii M, Sherchan P, Soejima Y, Hasegawa
Y, Flores J, Doycheva D and Zhang JH: Cannabinoid receptor type 2
agonist attenuates apoptosis by activation of phosphorylated
CREB-Bcl-2 pathway after subarachnoid hemorrhage in rats. Exp
Neurol. 261:396–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Wang W, Mai H, Zhang X, Wang J, Gao
Y, Wang Y, Deng G, Gao L, Zhou S, et al: Methazolamide improves
neurological behavior by inhibition of neuron apoptosis in
subarachnoid hemorrhage mice. Sci Rep. 6:350552016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anai S, Hide T, Takezaki T, Kuroda J,
Shinojima N, Makino K, Nakamura H, Yano S and Kuratsu J: Antitumor
effect of fibrin glue containing temozolomide against malignant
glioma. Cancer Sci. 105:583–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao J, Lin H, Summers R, Yang M, Cousins
BG and Tsui J: Current treatment strategies for intracranial
aneurysms: An overview. Angiology. 69:17–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hitchcock E and Gibson WT: A review of the
genetics of intracranial berry aneurysms and implications for
genetic counseling. J Genet Couns. 26:21–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turjman AS, Turjman F and Edelman ER: Role
of fluid dynamics and inflammation in intracranial aneurysm
formation. Circulation. 129:373–382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanematsu Y, Kanematsu M, Kurihara C, Tada
Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y,
et al: Critical roles of macrophages in the formation of
intracranial aneurysm. Stroke. 42:173–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia ZJ, Hong B, Chen DM, Huang QH, Yang
ZG, Yin C, Deng XQ and Liu JM: China's growing contribution to
global intracranial aneurysm research (1991–2012): A bibliometric
study. PLoS One. 9:e915942014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wada K, Makino H, Shimada K, Shikata F,
Kuwabara A and Hashimoto T: Translational research using a mouse
model of intracranial aneurysm. Transl Stroke Res. 5:248–251. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kolega J, Gao L, Mandelbaum M, Mocco J,
Siddiqui AH, Natarajan SK and Meng H: Cellular and molecular
responses of the basilar terminus to hemodynamics during
intracranial aneurysm initiation in a rabbit model. J Vasc Res.
48:429–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh SY, Kwon JT, Park YS, Nam TK, Park SW
and Hwang SN: Clinical features of acute subdural hematomas caused
by ruptured intracranial aneurysms. J Korean Neurosurg Soc.
50:6–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma J, Wang Z, Liu C, Shen H, Chen Z, Yin
J, Zuo G, Duan X, Li H and Chen G: Pramipexole-induced hypothermia
reduces early brain injury via PI3K/AKT/GSK3β pathway in
subarachnoid hemorrhage rats. Sci Rep. 6:238172016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo ZZ, Cao QA, Li ZZ, Liu LP, Zhang Z,
Zhu YJ, Chu G and Dai QY: SP600125 attenuates nicotine-related
aortic aneurysm formation by inhibiting matrix metalloproteinase
production and CC chemokine-mediated macrophage migration.
Mediators Inflamm. 2016:91424252016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Y, Zhang M, He H, Chen J, Zeng H, Li
J and Duan R: MicroRNA/mRNA profiling and regulatory network of
intracranial aneurysm. BMC Med Genomics. 6:36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liaw N, Fox JM, Siddiqui AH, Meng H and
Kolega J: Endothelial nitric oxide synthase and superoxide mediate
hemodynamic initiation of intracranial aneurysms. PLoS One.
9:e1017212014. View Article : Google Scholar : PubMed/NCBI
|