Introduction
Angiogenesis is defined as the formation of new
blood vessels from the pre-existing vascular system through
endothelial proliferation and migration due to abnormal expression
of several pro-angiogenic factors, including vascular endothelial
growth factor (VEGF), fibroblast growth factor (FGF), their
receptors and genes of associated signaling pathways (e.g.,
phosphoinositide-3 kinase/Akt, mitogen-activated protein kinase and
Notch) (1–4). Accumulating evidence suggests that
angiogenesis is an essential process for the initiation and
development of numerous diseases, including cancer (5), psoriasis (6), rheumatoid arthritis (7), retinopathy (8) and endometriosis (9). Therefore, targeted inhibition of
angiogenesis may potentially be beneficial for the treatment of the
above diseases, which has been demonstrated in clinical trials on
VEGF inhibitors (e.g. dovitinib, sunitinib, sorafenib or
bevacizumab) (10,11). However, high proportional rates of
adverse events caused by the currently available drugs limits their
wide application and acceptability (10,11). The
aforementioned experimental results indicate the requirement of
more effective and safe angiogenesis inhibitors.
Natural products (NPs) are biologically active
secondary metabolites produced by plants, animals and
microorganisms. NPs have long served as an important source for
drug discovery due to their potentially low toxicity. It is
estimated that >2/3 of Food and Drug Administration-approved
drugs are NPs or their derivatives (12). Thus, screening of NPs has been
proposed as an important approach to obtain effective and safe
angiogenesis inhibitors. Although previous studies have reported
the anti-angiogenic effects of certain NPs, including genistein
(13), camptothecin (14), kaempferol (15), ferulic acid (16) and quercetin (17), studies that identify anti-angiogenic
NPs by screening of a drug library remain rare.
The goal of the present study was to screen NPs with
anti-angiogenic activity from the Natural Products Collection of
MicroSource Discovery Systems (http://www.msdiscovery.com) (18) using the zebrafish embryo in
vivo model. This model allows for direct visualization of the
vascular system via endothelium-specific enhanced green fluorescent
protein (EGFP) expression in the Tg(fli1a: EGFP)y1
zebrafish line, which ensures rapid evaluation of the responses of
live embryos to drugs (19). In the
present study, mundoserone was identified to have potent
anti-angiogenic activity in zebrafish embryos and this effect was
dose-dependent. The present results may provide a theoretical basis
for the future clinical use of this compound to treat diseases
associated with excessive angiogenesis.
Materials and methods
Zebrafish care and maintenance
The zebrafish line Tg(fli1a: EGFP)y1 with
transgenic endothelial cells expressing EGFP was provided by the
Shanghai Research Center for Model Organisms (Shanghai, China). The
zebrafish were maintained in a constant flow water system at a
temperature of 28.5°C under a 14-h light/10-h dark cycle. All
protocols were in accordance with the guidelines of the American
Association for Accreditation of Laboratory Animal Care (20) and approved by the Institutional
Animal Care and Use Committee of Shanghai Ninth People's Hospital
(Shanghai, China).
Embryo collection and drug
treatment
Zebrafish embryos were generated by natural
pair-wise mating (5–6 pairs for the generation of 200–300 embryos)
and raised at 28.5°C in deionized water. At 24 h post-fertilization
(hpf), embryos were distributed into 12-well plates (30
embryos/well) in 0.2% Instant Ocean Salt (Aquarium Systems, Inc.,
Mentor, OH, USA) in deionized water and treated with 5 µM PTK787 [a
VEGF receptor (VEGFR) antagonist for generation of a positive
control group; Selleck Chemicals, Houston, TX, USA; dissolved in
0.4% v/v dimethyl sulfoxide (DMSO)], 0.1% DMSO (negative control)
and 10 µM natural product (Natural Products Collection,
Microsource, Gaylordsville, CT, USA) for 24 h to screen
anti-angiogenic drugs (Fig. 1), of
which mundoserone was selected. Subsequently, other embryos were
added to 12-well plates (30 embryos per well) and exposed to 2.5, 5
and 10 µM of mundoserone for 24 h to observe the
concentration-dependent anti-angiogenic effects of mundoserone. All
of the experiments were repeated three times.
Angiogenesis assessment
After 48 hpf, 10 embryos in each well were
anesthetized with 0.016% MS-222 (tricaine methanesulfonate;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and the morphology
of the intersegmental vessels (ISVs), which normally connect the
dorsal longitudinal anastomotic vessels (DLAVs) to the dorsal aorta
(DA), was visually assessed using a Nikon SMZ 1500 Fluorescence
microscope (Nikon Corp., Tokyo, Japan) equipped with a digital
camera. Quantitative image analyses were performed using image
based morphometric analysis software (NIS-Elements D3.1; Nikon
Corp.) and Adobe Photoshop 7.0 software (Adobe Systems, Inc., San
Jose, CA, USA). A total of 10 embryos per group were evaluated by
two blinded observers in 3 independent experiments. Drug effects
were calculated according to the following formula: %
Inhibition=(1-ISVconcentration of
compound/ISVvehicle) ×100%.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The effects of mundoserone on the expression of
angiogenesis-associated genes were determined by RT-qPCR. Total RNA
was extracted from 30 embryos that had been treated with DMSO or 10
µM mundoserone for 24 h using TRIzol reagent (cat. no. 15596026;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA
was reverse transcribed using the PrimeScript RT reagent kit with
gDNA Eraser (cat. no. RR047A; Takara Bio, Inc., Otsu, Japan).
Quantification of the gene expression was performed in triplicate
using Bio-Rad iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with detection on the Realplex system
(Eppendorf, Hamburg, Germany). Gene expression was relatively
quantified based on the comparative threshold cycle method
(2−ΔΔCq) (21) using
β-actin as an endogenous control gene. Primer sequences are listed
in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| DLL4 |
AGGCCTGGCACTCACCTTACTC |
CACCCCAGCCCTCTTTACAGTT |
| NOTCH1A |
GCCGCAGATGCAGGGCAATGAAGT |
GAGGGCAGGCAGGGCTGGTAGAGG |
| HEY2 |
CGGCTTCCGGGAGTGTCTGACT |
TCCCCACGGTCGGTATGGTTTA |
| EFNB2A |
TTGGGGCCTGGAGTTCTTCAGA |
TCTTGGGCGTGGCTAATGTGCT |
| SLIT2 |
GAGCGACTGGACCTGAATG |
GTAGATCCTGAAATGCCCCTC |
| SLIT3 |
GCGAGTGTTTCCAAGACCTG |
GATTTCATTGTCGTTCAGCCG |
| ROBO1 |
AGGAGTCACATACAGGCTAGAG |
GTCTGAGATCTGCTGGGAAATG |
| ROBO2 |
GAGGTGTGGATGTGGACTATG |
CTACAATCCGAGGTGGAGAATC |
| ROBO4 |
GAGATCAGTCCCAAACCACAG |
CCCACAGATATAGCCCAACG |
| FGFR2 |
CCCCGACAACCGCACGCTCGTA |
TAGCCGCCCATGCGATCCTCCTGT |
| FGFR3 |
TCCCCGTATCCAGGTATCC |
TCTGAACGTGGGTCTTTGTG |
| COX2 |
CCTTCCGGCCATCATTCTTATT |
CCGCAGATTTCAGAGCATTGTC |
| PTP-RB |
TTGGGCAGCATGCGGAATACTGAG |
TTACCAGGCTGCCATGAAACATCC |
| VEGFAA |
CTCCATCTGTCTGCTGTAAAGG |
GGGATACTCCTGGATGATGTCTA |
| VEGFR-2 |
CCTGAGACCATCTTTGACCG |
GTTCCCTCTTTAAGTCGCCTG |
| VEGFR-1 |
GTATTTGAACAGCACGGGTTTAG |
CGGCTTCTTGATATGCGTTTG |
| PIK3R2 |
CCCGGAAACTGCTCCCCCTAATCT |
AGCGGGAGGAGTCGGCTCTTGTT |
| β-actin |
TCCGGTATGTGCAAAGCCGG |
CCACATCTGCTGGAAGGTGG |
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analysis and graphical representation of the
data were performed using GraphPad Prism software (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA). Statistical
significance of differences in the number of ISVs among the
negative control and positive control groups as well as those
treated with different doses of mundoserone was assessed using
analysis of variance followed by Tukey's post-hoc test for multiple
comparisons. The difference in gene expression between the control
and mundoserone groups was analyzed using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
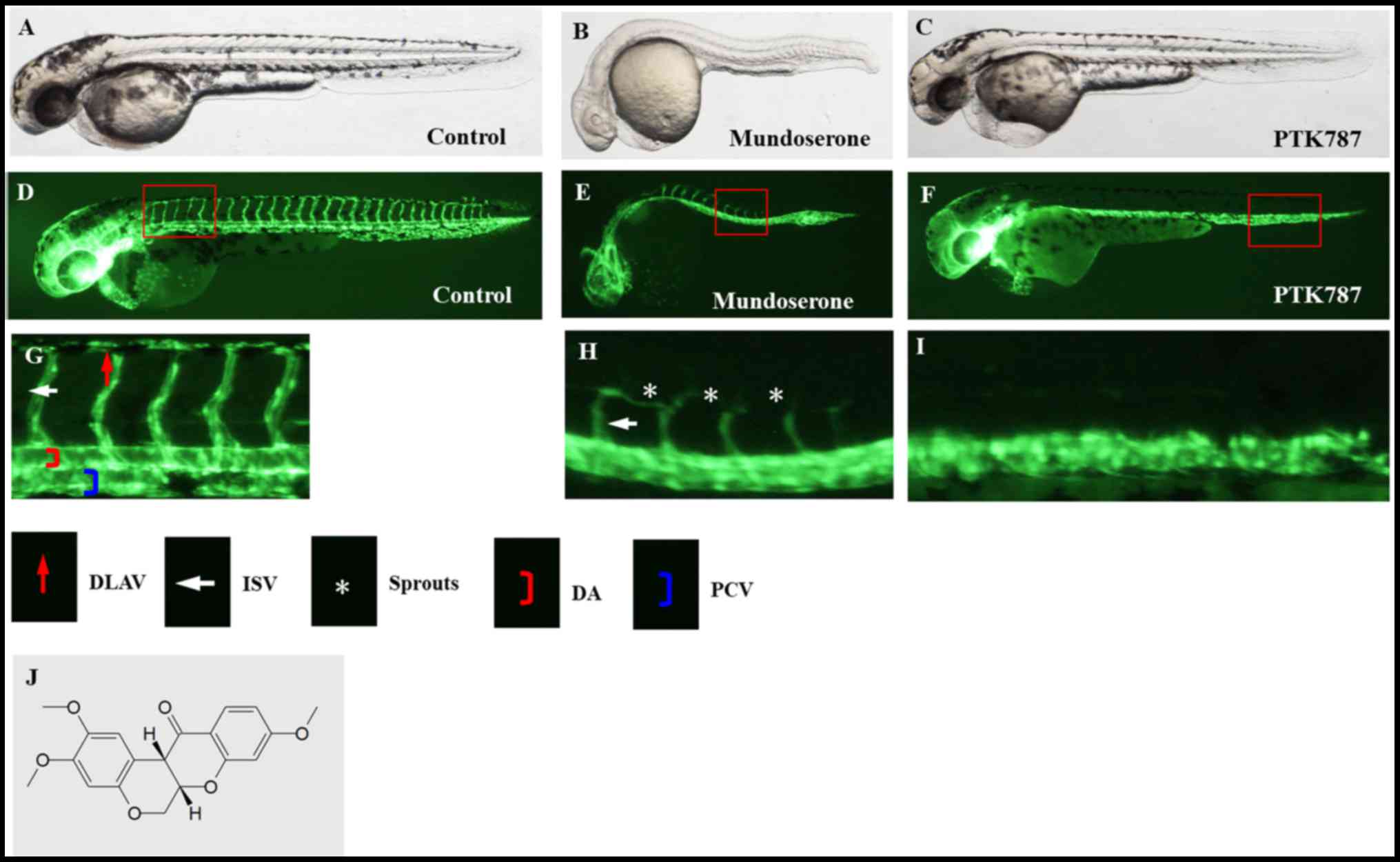
Mundoserone inhibits the formation of
the ISVs in zebrafish
Transgenic zebrafish with fluorescent blood vessels
were used to screen angiogenic inhibitors from 240 compounds
commercially available from the Microsource NP library. Of the
compounds in the library, mundoserone, was identified to have
anti-angiogenic activity, for which it has not been previously
reported, to the best of our knowledge. Exposure of zebrafish to
mundoserone inhibited the development of the ISVs and DLAV in
comparison with the vehicle and positive control (PTK787) (Fig. 1).
Furthermore, the dose-response effect of mundoserone
on the ISVs of zebrafish was also investigated. As presented in
Fig. 2, the ISVs were generally
intact, but appeared thinner in zebrafish treated with 2.5 µM
mundoserone for 24 h. In addition to becoming thinner, certain ISVs
were incompletely formed in zebrafish treated with 5 µM mundoserone
for 24 h. Of note, most of the ISVs in zebrafish were incomplete
and DLAV development was inhibited by 10 µM mundoserone at 48 hpf.
These results suggested that the anti-angiogenic effect of
mundoserone was dose-dependent, which was further indicated by the
quantitative results: The number of ISVs remaining in zebrafish
following exposure to 5 (21.4±0.5) or 10 µM (7.3±1.2) mundoserone
was significantly lower compared with the negative control
(27.6±0.5). Similarly, the ISV inhibition ratio was higher in
zebrafish following exposure to 10 µM mundoserone (73.6±1.3%)
compared with the control (0±0.6%), 2.5 (0±0.6%) or 5 µM
(22.5±0.6%) mundoserone groups.
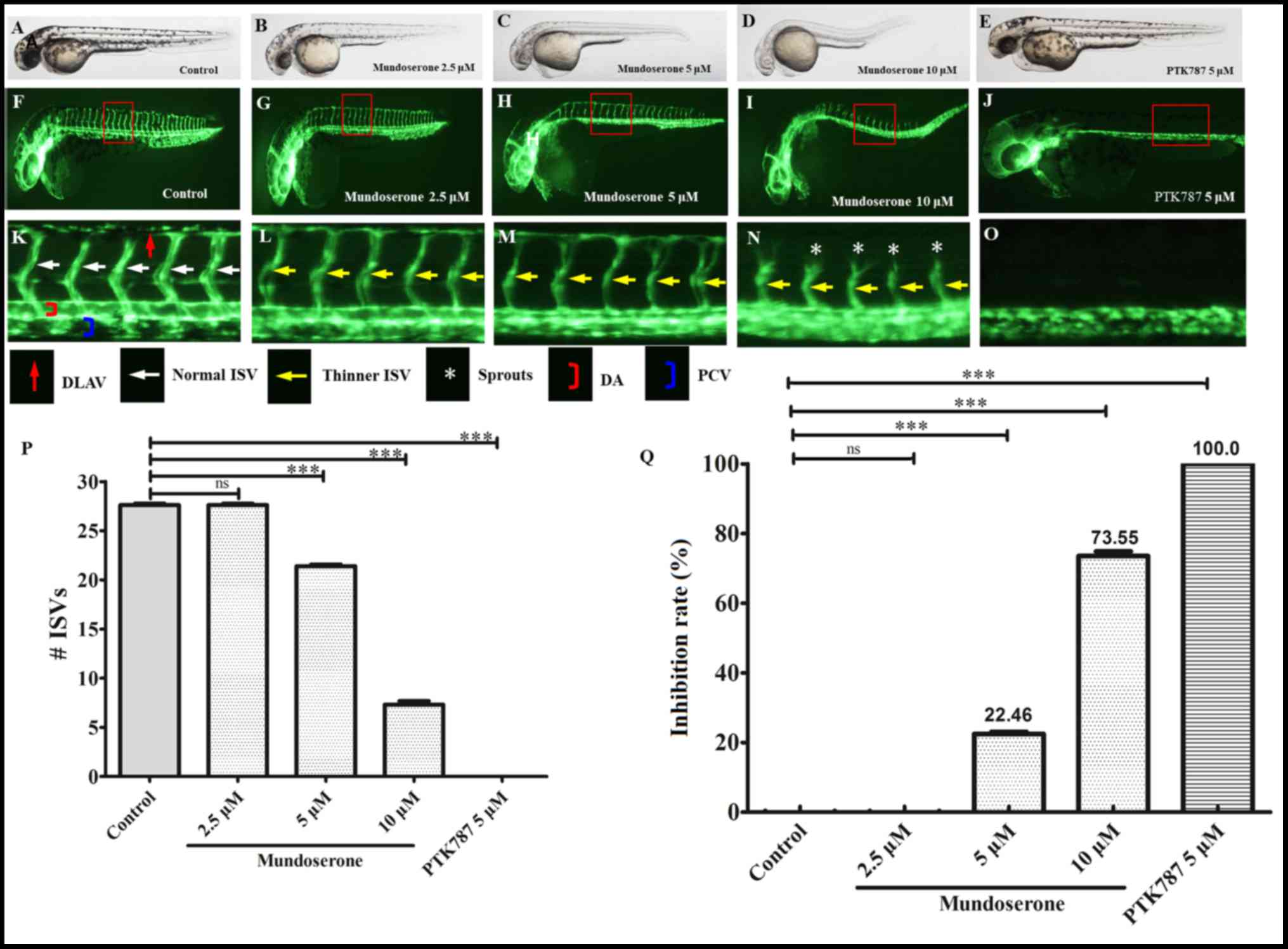 | Figure 2.Mundoserone inhibits the trunk
angiogenesis of zebrafish in a dose-dependent manner.
Representative (A-E) bright field and (F-J) fluorescent images of
zebrafish embryos at 24 h post-fertilization treated with 0.1%
dimethyl sulfoxide (control), mundoserone (2.5, 5 or 10 µM) or 5 µM
PTK787 (positive control) for 24 h (magnification, ×40). (K-O)
Magnification of images F-J (magnification, ×112.5). Compared with
the control, embryos exposed to mundoserone exhibited a lower
number of incomplete ISVs and only occasional sprouts (asterisks)
of the DA were observed. Quantification of (P) the number of
complete ISVs and (Q) inhibition rate in mundoserone-treated
embryos. Values are expressed as the mean ± standard error of the
mean (n=10). ***P<0.001. ns, not significant; DLAV, dorsal
longitudinal anastomotic vessels; ISVs, intersegmental vessels; DA,
dorsal aorta; PCV, posterior cardinal vein. |
Mundoserone exerts anti-angiogenesis
effects via the downregulation of slit guidance ligand 3
(SLIT3)/roundabout guidance receptor (ROBO)1 and FGF receptor
(FGFR)/protein tyrosine phosphatase, receptor type B (PTP-RB), and
the upregulation of NOTCH1A
To investigate the potential mechanisms of the
anti-angiogenesis effects of mundoserone, RT-qPCR was performed to
analyze the expression of genes involved in angiogenesis-associated
signaling pathways in embryos treated with 10 µM mundoserone. The
results indicated that mundoserone significantly reduced the
expression of SLIT3, ROBO1, ROBO2, FGFR2, FGFR3 and PTP-RB, but
increased the expression of NOTCH1A. No significant difference was
observed in the expression of cyclooxygenase 2, SLIT2, ROBO4,
deltalike ligand 4 (DLL4), hes-related family basic
helix-loop-helix transcription factor with YRPW motif 2 and ephrin
B2A between the mundoserone treatment and negative control groups
(Fig. 3). As an unexpected result,
mundoserone promoted the expression of VEGFAA, VEGFR-2 and
VEGFR-1.
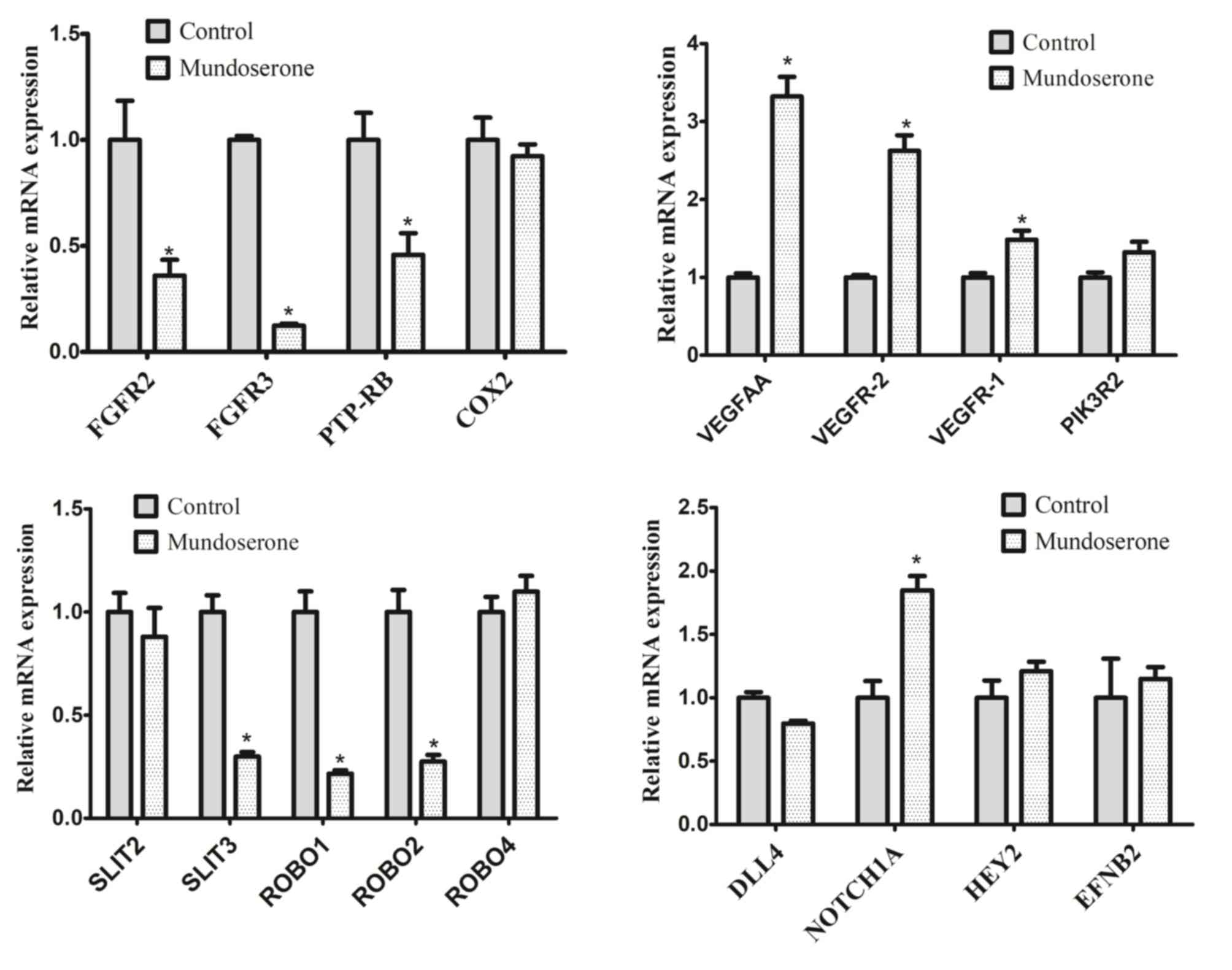 | Figure 3.Expression of angiogenesis-associated
genes in zebrafish embryos after treatment with 10 µM mundoserone.
Values are expressed as the mean ± standard error of the mean
(n=4). *P<0.05 vs. Control. FGFR, fibroblast growth factor
receptor; SLIT3, slit guidance ligand 3; ROBO, roundabout guidance
receptor; PTP-RB, protein tyrosine phosphatase, receptor type B;
DLL, deltalike ligand 4; COX, cyclooxygenase; VEGFR, vascular
endothelial growth factor receptor; PIK3R2,
phosphoinositide-3-kinase regulatory subunit 2; HEY2, hes related
family bHLH transcription factor with YRPW motif 2; EFNB2, ephrin
B2. |
Discussion
To the best of our knowledge, the present study was
the first to identify mundoserone as a potential anti-angiogenic
drug, which dose-dependently suppressed the formation of ISVs in a
zebrafish embryo model. This inhibitory effect may be exerted by
blocking of the SLIT/ROBO and FGFR pathways, as well as activation
of NOTCH1A signaling.
Although studies investigating the role of
mundoserone are rare (22),
mundoserone is a structural analog of rotenone, and extensive
studies have demonstrated that rotenone inhibits cell proliferation
and migration. For instance, Srivastava and Panda (23) reported that rotenone inhibited the
proliferation of HeLa and MCF-7 cells by perturbing microtubule
assembly dynamics, with half maximal inhibitory concentrations of
0.2±0.1 and 0.4±0.1 µM, respectively. Ishido and Suzuki (24) indicated that exposure to rotenone
inhibited the migration, decreased the proliferation and increased
the apoptotic rate of mesencephalic neural stem cells in a
dose-dependent manner. In addition, administration of rotenone for
6 h was reported to result in a significant dissipation of the
mitochondrial membrane potential in microvascular endothelial
cells, indicating the disruption of the mitochondrial respiratory
chain and induction of apoptosis (25). These results imply that application
of mundoserone or rotenone may possibly prevent excessive
angiogenesis, and this hypothesis was also demonstrated in the
present study, as a 73.55% ISV inhibition ratio was achieved with
mundoserone.
Although VEGFs and FGF are considered as
indispensable angiogenic factors for angiogenesis (1–4), the
results of the present study suggested that mundoserone did not
exert any anti-angiogenic activity by inhibiting VEGFs and VEGFRs,
but only FGFR2 and −3. This may be a potential reason of the
failure of blockage of angiogenesis by only using VEGF inhibitors
in certain clinical patients. VEGF-independent angiogenic pathways
should be considered when resistance to VEGF-targeted therapies is
encountered (16). Furthermore,
in vitro studies demonstrated that suppression of FGF
signaling reduced the expression levels of PTP non-receptor type 11
(PTPN11) and then disrupted the PTPN11/VE-cadherin interaction,
leading to the loss of endothelial junction integrity (26) and terminating endothelial cell
interface elongation, ultimately preventing angiogenesis (27). As expected, PTP-RB expression was
also lower after mundoserone treatment, indirectly inferring that
mundoserone inhibits angiogenesis via the FGFR2/FGFR3/PTP-RB
interaction.
In addition to VEGFs and FGF, SLIT/ROBO signaling
has also been reported to have a significant role in angiogenesis
(28,29). SLIT2 stimulation induced the
expression of ROBO1 in lymphatic endothelial cells and mediation of
the migration and tube formation, which was reversed by using the
SLIT/ROBO-specific antibodies (30).
Further in vivo experiments confirmed that overexpression of
SLIT2 significantly enhanced tumor lymphangiogenesis and
subsequently promoted mesenteric lymph node metastasis of
pancreatic islet tumors (30). The
mRNA and protein levels of SLIT3, ROBO1 and ROBB4 were reported to
be significantly increased in human umbilical vein endothelial
cells after hypoxia, which is a common cause for preeclampsia
(31). SLIT2 was demonstrated to
induce ocular neovascular diseases by promoting sprouting
angiogenesis in retinas through ROBO1 and ROBO2 (32). Thus, blockade of SLIT/ROBO signaling
may be therapeutically exploited to inhibit angiogenesis. As
anticipated, mundoserone significantly reduced the expression of
SLIT3, as well as ROBO1 and −2 in zebrafish embryos in the present
study.
Although the role is controversial, studies have
suggested that activation of the Notch signaling pathway may be a
potential approach of inhibiting angiogenesis and preventing tumor
metastasis (33,34). For instance, Banerjee et al
(33) reported that inhibition of
NOTCH via a soluble NOTCH1 decoy, which acts as antagonists of DLL
and protein jagged ligands, caused a marked increase in liver
metastasis of neuroblastoma and breast cancer. This result was also
confirmed in transgenic mice with heterozygous loss of NOTCH1
(33). A study by Lee et al
(34) further illustrated that NOTCH
activation suppresses retinal angiogenesis by reducing the
expression of transcription factor sex-determining region Y box 17,
which may be inhibited by DLL4 blockade. By constructing a
transgenic mouse model with Cre-conditional expression of the
constitutively active intracellular domain of NOTCH1, Liu et
al (35) demonstrated that NOTCH
signaling may retard basic FGF-induced angiogenesis and ovarian
follicle development. In line with these studies, the present
results also suggested that mundoserone treatment increased the
expression of NOTCH1A and thus inhibited angiogenesis in
zebrafish.
Of note, the present study had certain limitations.
First, the study only preliminarily screened mundoserone as a
natural product with anti-angiogenic activity using a zebrafish
embryo model. The efficiency of mundoserone in inhibiting
angiogenesis in humans and the optimal concentration require
further validation in a series of mammalian models (i.e., mice,
monkey) and phase-III clinical trials. Second, further in
vitro studies are necessary to confirm the effects of
mundoserone on the proliferation and migration of microvascular
endothelial cells and the expression of associated pathway proteins
(SLIT, ROBO1, FGFR, PTP-RB and NOTCH1A) (15,16).
Third, the safety of mundoserone should be further evaluated by
in vivo and in vitro experiments in the future
because growth retardation of the embryos was observed with
mundoserone treatment. Fourth, the VEGF-independent, but
FGF-dependent mechanisms of mundoserone should also be validated.
In addition, the unexpected upregulation of VEGFAA, VEGF-1 and
VEGF-2 may also be a result of the stress response, which
counteracts the effects of mundoserone on angiogenesis. Thus,
mundoserone and other anti-VEGF drugs should be combined for
anti-angiogenesis treatment (36).
Fifth, other angiogenesis-associated mechanisms should be examined
in the future, including matrix metalloproteinases (37,38).
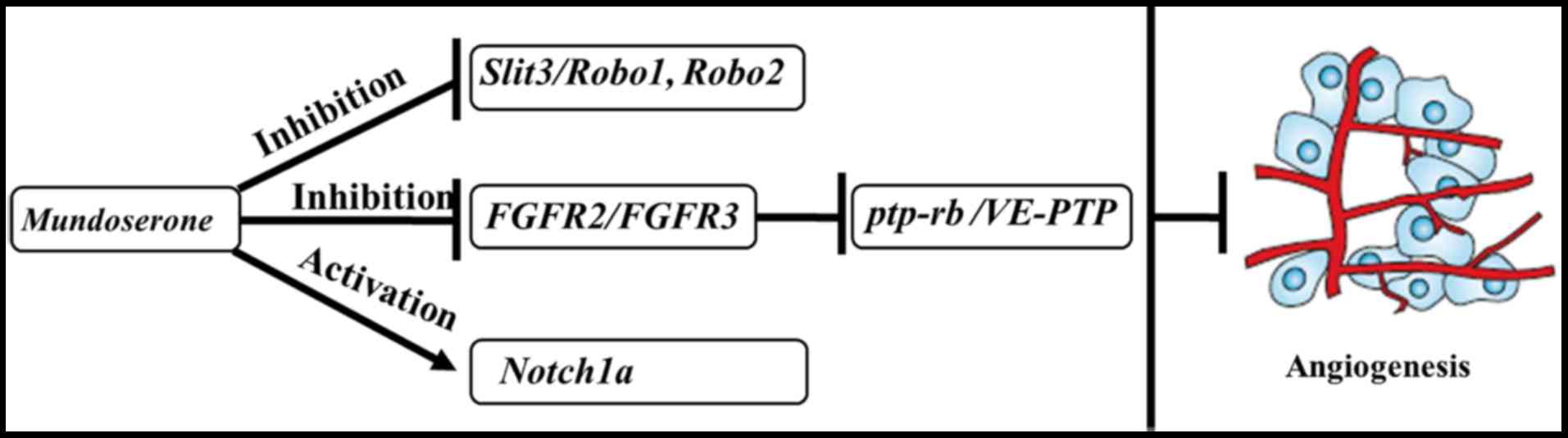
In conclusion, the present study identified a novel
compound, mundoserone, which may be an effective angiogenic
inhibitor, which acts via downregulation of the SLIT/ROBO1 and
FGFR/PTP-RB pathways, as well as upregulation of NOTCH1A signaling
(Fig. 4). Further in vitro
studies are necessary to confirm the role of mundoserone on the
proliferation and migration of microvascular endothelial cells via
the above signaling pathways.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81500331) and the Science
and Technology Commission of Shanghai (grant nos. 17140902600 and
14JC1404400).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KC, CW and YF designed the study and interpreted the
data. JG, ZH and YW collected the data. LG and HZ performed the
statistical analyses. KC, JG and ZH prepared the figures. All
authors wrote the manuscript. All authors read and approved the
final version of the manuscript.
Ethical approval and consent to
participate
This study was approved by the Institutional Animal
Care and Use Committee of Shanghai Ninth People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ISVs
|
intersegmental vessels
|
|
VEGF
|
vascular endothelial growth factor
|
|
FGF
|
fibroblast growth factor
|
|
NPs
|
natural products
|
|
EGFP
|
enhanced green fluorescent protein
|
|
hpf
|
hours post-fertilization
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Zhao J, Zhang ZR, Zhao N, Ma BA and Fan
QY: VEGF silencing inhibits human osteosarcoma angiogenesis and
promotes cell apoptosis via PI3K/AKT signaling pathway. Int J Clin
Exp Med. 8:12411–12417. 2015.PubMed/NCBI
|
|
2
|
Duan J, Hu H, Feng L, Yang X and Sun Z:
Silica nanoparticles inhibit macrophage activity and angiogenesis
via VEGFR2-mediated MAPK signaling pathway in zebrafish embryos.
Chemosphere. 183:483–490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim BS, Park JY, Kang HJ, Kim HJ and Lee
J: Fucoidan/FGF-2 induces angiogenesis through JNK- and
p38-mediated activation of AKT/MMP-2 signalling. Biochem Biophys
Res Commun. 450:1333–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hasan SS, Tsaryk R, Lange M, Wisniewski L,
Moore JC, Lawson ND, Wojciechowska K, Schnittler H and Siekmann AF:
Endothelial Notch signalling limits angiogenesis via control of
artery formation. Nat Cell Biol. 19:928–940. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu H, Zhang Y, Peña MM, Pirisi L and Creek
KE: Six1 promotes colorectal cancer growth and metastasis by
stimulating angiogenesis and recruiting tumor-associated
macrophages. Carcinogenesis. 38:281–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malecic N and Young HS: Excessive
angiogenesis associated with psoriasis as a cause for
cardiovascular ischaemia. Exp Dermatol. 26:299–304. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Y, Yu SS, Zong M, Fan SS, Lu TB, Gong
RH, Sun LS and Fan LY: Glucose-6-phosphate isomerase (G6PI)
mediates hypoxia-induced angiogenesis in rheumatoid arthritis. Sci
Rep. 7:402742017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abu E, l-Asrar AM, Struyf S, Mohammad G,
Gouwy M, Rytinx P, Siddiquei MM, Hernández C, Alam K, Mousa A, De
Hertogh G, et al: Osteoprotegerin is a new regulator of
inflammation and angiogenesis in proliferative diabetic
retinopathy. Invest Ophthalmol Vis Sci. 58:3189–3201. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rakhila H, Al-Akoum M, Bergeron ME,
Leboeuf M, Lemyre M, Akoum A and Pouliot M: Promotion of
angiogenesis and proliferation cytokines patterns in peritoneal
fluid from women with endometriosis. J Reprod Immunol. 116:1–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
OH WK, McDermott D, Porta C, Levy A,
Elaidi R, Scotte F, Hawkins R, Castellano D, Bellmunt J, Rha SY, et
al: Angiogenesis inhibitor therapies for advanced renal cell
carcinoma: Toxicity and treatment patterns in clinical practice
from a global medical chart review. Int J Oncol. 44:5–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motzer RJ, Porta C, Vogelzang NJ,
Sternberg CN, Szczylik C, Zolnierek J, Kollmannsberger C, Rha SY,
Bjarnason GA, Melichar B, et al: Dovitinib versus sorafenib for
third-line targeted treatment of patients with metastatic renal
cell carcinoma: An open-label, randomised phase 3 trial. Lancet
Oncol. 15:286–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patridge E, Gareiss P, Kinch MS and Hoyer
D: An analysis of FDA-approved drugs: Natural products and their
derivatives. Drug Discov Today. 21:204–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rathinasamy VS, Paneerselvan N and
Ragunathan M: Effect of genistein on regenerative angiogenesis
using zebrafish as model organism. Biomed Prev Nutri. 4:469–474.
2014. View Article : Google Scholar
|
|
14
|
Song CX, Song SL, Liang H and Liu X:
Effect of camptothecin on the embryonic development and
angiogenesis of zebrafish embryos. Adv Mater Res. 750–752:1–1475.
2013.
|
|
15
|
Liang F, Han Y, Gao H, Xin S, Chen S, Wang
N, Wei Q, Zhong H, Lin S, Yao X and Li S: Kaempferol identified by
zebrafish assay and fine fractionations strategy from dysosma
versipellis inhibits angiogenesis through VEGF and FGF pathways.
Sci Rep. 5:144682015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang GW, Jiang JS and Lu WQ: Ferulic acid
exerts anti-angiogenic and anti-tumor activity by targeting
fibroblast growth factor receptor 1-mediated angiogenesis. Int J
Mol Sci. 16:24011–24031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao D, Qin C, Fan X, Li Y and Gu B:
Inhibitory effects of quercetin on angiogenesis in larval zebrafish
and human umbilical vein endothelial cells. Eur J Pharmacol.
723:360–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pantel J, Williams SY, Mi D, Sebag J,
Corbin JD, Weaver CD and Cone RD: Development of a high throughput
screen for allosteric modulators of melanocortin-4 receptor
signaling using a real time cAMP assay. Eur J Pharmacol.
660:139–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cross LM, Cook MA, Lin S, Chen JN and
Rubinstein AL: Rapid analysis of angiogenesis drugs in a live
fluorescent zebrafish assay. Arterioscler Thromb Vasc Biol.
23:911–912. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pakes SP: Adequate veterinary care as
viewed by the American Association for Accreditation of Laboratory
Animal Care. J Am Vet Med Assoc. 168:519–521. 1976.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yeh JR, Munson KM, Elagib KE, Goldfarb AN,
Sweetser DA and Peterson RT: Discovering chemical modifiers of
oncogene-regulated hematopoietic differentiation. Nat Chem Biol.
5:236–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Srivastava P and Panda D: Rotenone
inhibits mammalian cell proliferation by inhibiting microtubule
assembly through tubulin binding. FEBS J. 274:4788–4801. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishido M and Suzuki J: Inhibition by
rotenone of mesencephalic neural stem-cell migration in a
neurosphere assay in vitro. Toxicol In Vitro. 24:552–557. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Yao K, Fan Y, He P, Wang X, Hu W
and Chen Z: Carnosine protects brain microvascular endothelial
cells against rotenone-induced oxidative stress injury through
histamine H1 and H2 receptors in vitro. Clin
Exp Pharmacol Physiol. 39:1019–1025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hatanaka K, Lanahan AA, Murakami M and
Simons M: Fibroblast growth factor signaling potentiates
VE-cadherin stability at adherens junctions by regulating SHP2.
PLoS One. 7:e376002012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sauteur L, Krudewig A, Herwig L,
Ehrenfeuchter N, Lenard A, Affolter M and Belting HG:
Cdh5/VE-cadherin promotes endothelial cell interface elongation via
cortical actin polymerization during angiogenic sprouting. Cell
Rep. 9:504–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang B, Xiao Y, Ding BB, Zhang N, Yuan Xb,
Gui L, Qian KX, Duan S, Chen Z, Rao Y and Geng JG: Induction of
tumor angiogenesis by Slit-Robo signaling and inhibition of cancer
growth by blocking Robo activity. Cancer Cell. 4:19–29. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujiwara M, Ghazizadeh M and Kawanami O:
Potential role of the Slit/Robo signal pathway in angiogenesis.
Vasc Med. 11:115–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang XM, Han HX, Sui F, Dai YM, Chen M and
Geng JG: Slit-Robo signaling mediates lymphangiogenesis and
promotes tumor lymphatic metastasis. Biochem Biophys Res Commun.
396:571–577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liao WX, Laurent LC, Agent S, Hodges J and
Chen DB: Human placental expression of SLIT/ROBO signaling cues:
Effects of preeclampsia and hypoxia. Biol Reprod. 86:1112012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rama N, Dubrac A, Mathivet T, Chárthaigh
Ní RA, Genet G, Cristofaro B, Pibouin-Fragner L, Ma L, Eichmann A
and Chédotal A: Slit2 signaling through Robo1 and Robo2 is required
for retinal neovascularization. Nat Med. 21:483–491. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Banerjee D, Hernandez SL, Garcia A,
Kangsamaksin T, Sbiroli E, Andrews J, Forrester LA, Wei N,
Kadenhechiweshe A, Shawber CJ, et al: Notch suppresses angiogenesis
and progression of hepatic metastases. Cancer Res. 75:1592–1602.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee SH, Lee S, Yang H, Song S, Kim K,
Saunders TL, Yoon JK, Koh GY and Kim I: Notch pathway targets
proangiogenic regulator Sox17 to restrict angiogenesis. Circ Res.
115:215–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu J, Deutsch U, Jeong J and Lobe CG:
Constitutive notch signaling in adult transgenic mice inhibits
bFGF-induced angiogenesis and blocks ovarian follicle development.
Genesis. 52:809–816. 2015. View Article : Google Scholar
|
|
36
|
Butler CT, Reynolds AL, Tosetto M, Dillon
ET, Guiry PJ, Cagney G, O'Sullivan J and Kennedy BN: A quininib
analogue and cysteinyl leukotriene receptor antagonist inhibits
vascular endothelial growth factor (VEGF)-independent Angiogenesis
and exerts an additive antiangiogenic response with bevacizumab. J
Biol Chem. 292:3552–3567. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Webb AH, Gao BT, Goldsmith ZK, Irvine AS,
Saleh N, Lee RP, Lendermon JB, Bheemreddy R, Zhang Q, Brennan RC,
et al: Inhibition of MMP-2 and MMP-9 decreases cellular migration,
and angiogenesis in in vitro models of retinoblastoma. BMC Cancer.
17:4342017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Chen D, Li B, He J, Duan D, Shao D
and Nie M: Fe-MIL-101 exhibits selective cytotoxicity and
inhibition of angiogenesis in ovarian cancer cells via
downregulation of MMP. Sci Rep. 26:261262016. View Article : Google Scholar
|