Introduction
Osteoarthritis (OS) is a common disease in
orthopedics with a high incidence in the middle-aged and elderly
individuals (1). Pathologically, it
is mainly characterized by loss of cartilage and degenerative
changes (2). Cartilage tissue has a
poor self-repair ability due to insufficient blood supply and lack
of lymphatic vessel and nerve tissue distribution. The main
symptoms of OS are pain and limited mobility, which affect a
patient's normal life and place a heavy burden on families and
society. Artificial joint replacement is a relatively effective
treatment method of OS (3). To a
certain degree, it eases patient's pain, but this surgery yields
many short-term and long-term complications such as severe trauma,
high costs, potential infection, loosening and sinking of joint
implant. Cartilage transplantation, periosteal transplantation, and
chondrocyte transplantation have not achieved satisfactory
therapeutic effects, and there are many shortcomings such as
limited donor source, immune rejection, and potential implant
degradation. Therefore, their clinical application has remained
limited. It is imperative to find a more simple and effective
treatment.
Mesenchymal stem cells (MSCs), which are derived
from the mesoderm, possess multi-directional differentiation
potential (4). Under different
induction conditions, MSCs can differentiate into osteoblasts
(5), chondroblasts (6), and adipocytes (7). MSCs have been widely used in tissue
engineering and regenerative medicine (8,9). A
previous study has demonstrated that intra-articular injection of
MSCs can repair cartilage defects in OS patients (10). Adipose-derived MSCs (ADMSCs) have
gradually become a new generation of cells for tissue engineering
because they possess the advantages of abundant donor source and
being easily accessible over bone marrow-derived MSCs (BMMSCs) as
well as no concerns about the ethical issue present in use of
embryonic stem cells or harvesting difficulty like that encountered
in obtaining BMMSCs. MSCs differentiation into articular
chondrocytes is the key to treatment of cartilage defects in OS.
Chondrogenic differentiation of MSCs contributes to repair of
cartilage defects.
Bone morphogenetic proteins (BMPs), belonging to
transforming growth factor-β (TGF-β) superfamily, is named because
of their ability to induce the formation of bone and cartilage.
More than 20 BMPs have been known, in which 15 BMPs are human BMPs
(11). BMP2, BMP4 and BMP7 exhibit a
strong ability to induce osteogenesis, and BMP2 and BMP7 have been
widely used in the clinic (12–14).
However, BMP2 and BMP7 induced non-fusion spine occasionally occur
in the clinic (15,16). BMP9, also known as growth
differentiation factor 2 (GDF-2), is the protein with the strongest
ability to induce chondrogenic differentiation among the BMPs
family (17). BMP9 can be obtained
from the liver of mice (18). In
addition to inducing chondrogenic differentiation, BMP9 also has
the ability to induce and maintain cholinergic differentiation of
embryonic neurons (19), inhibit the
production of hepatic glucose, promote the metabolism of fatty
acids (20), stimulate hepcidin-1
expression, and thereby regulate iron homeostasis in vivo
(21). BMP9 exhibits a variety of
biological functions and has been widely concerned because of its
induction of chondrogenic differentiation. However, the mechanism
by which BMP9 induces chondrogenic differentiation remains poorly
understood. Whether BMP9 can be used as a cytokine for bone
regeneration remains to be a hot issue.
Many signaling pathways are involved in the process
of chondrogenic differentiation of ADMSCs, such as the Notch
signaling pathway, Wnt signaling pathway, and TGF-β signaling
pathway (22–24). The Notch signaling pathway is closely
related to ADMSCs differentiation and organ formation and it is a
key regulator of cell fate. There is evidence that during
embryogenesis, the Notch signaling pathway is essential for the
development of limb cartilage and bone (25). The Notch signaling pathway is also
involved in the chondrogenict differentiation of MSCs in
vitro (26). But the precise
mechanism remains unclear. The majority of previous studies mainly
investigated the change of the Notch signaling pathway during
chondrogenic differentiation (27).
There are no studies on actively regulating the Notch signaling
pathway for chondrogenic differentiation of MSCs. At the same time,
the mutual effects of BMP9 and the Notch signaling pathway in the
chondrogenic differentiation of ADMSCs are unclear. In this study,
we investigated the mutual effects of BMP9 and the Notch signaling
pathway during the chondrogenic differentiation of ADMSCs through
regulating BMP9 expression and the Notch signaling pathway. We also
established mouse models of OS to investigate the role of BMP9 and
the Notch signaling pathway in the repair of cartilage in OS
affected knee joint using ADMSCs.
Materials and methods
Animals and cells
Female Balb/c mice, weighing 23–25 g, aged 8 weeks,
were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). Mouse were raised a normal
diet and water, raising conditions: ambient temperature of 20–26°C,
relative humidity of 40–70%, alternating day and night time of
12/12 h. Mouse ADMSCs were purchased from Cyagen Biological
Technology Co., Ltd. (Taicang, China; MUBMD-01001). Animal
experiments were performed in Department of Laboratory Animals,
General Hospital of Shenyang Military Region (Shenyang, China;
license no. SYXK2015002). The experiments were approved by Animal
Ethics Committee of General Hospital of Shenyang Military Region
(no. 2015049).
Groups
Mice were randomly divided into five groups, with
eight mice in each group: Sham, OS, OS + ADMSCs (MSCs), OS + ADMSCs
+ BMP9 (BMP9), and OS + LY411575 + ADMSCs + BMP9 (LY) groups. In
the sham group, mice were not subjected to any procedure. In the OS
group, OS was induced. In the MSCs group, OS was induced, and
ADMSCs were injected into the articular cavity. In the BMP9 group,
OS was induced, and BMP9 overexpressing ADMSCs were injected into
the articular cavity. In the LY group, OS was induced, and LY411575
(1.5 µmol/l) (28,29) was injected into the articular cavity
to inhibit the Notch signaling pathway, and BMP9 overexpressing
ADMSCs were also injected.
In the cell experiment, ADMSCs were divided into
four groups: Control group (only ADMSCs), induced group (induced
chondrogenic differentiation of ADMSCs), BMP9 group (chondrogenic
induced ADMSCs overexpress BMP9), and LY411575 (LY) group (the
Notch signaling pathway was inhibited by LY411575 (ab142164; Abcam,
Cambridge, UK) at a final concentration of 1 nM (30), and chondrogenic induced ADMSCs
overexpressed BMP9).
Chondrogenic induction of ADMSCs
ADMSCs in the logarithmic growth phase were
digested. Cell suspension at a density of 1×105/l was
seeded into a 6-well plate. Chondrogenic medium DMEM (C11885500BT;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10 ng/ml TGF-β1, 50 nM vitamin C, 6.25 mg/l
insulin, and 10% fetal bovine serum (SH30068.03; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) was added and refreshed
every 2 days. After 3 days of culture, cells were collected. Type
II collagen and aggrecan expression in the chondrogenic induced
ADMSCs was detected by PCR and western blot assay.
BMP9-transfected ADMSCs
ADMSCs in the logarithmic growth phase at a final
concentration of 1×105/l were cultured with DMEM
supplemented with pVSV-G-BMP9 (GenePharma Co., Ltd., Shanghai,
China) and Polybrene (5 µg/ml). After 12 h, culture medium was
refreshed and cells were cultured for 2 more days. Cells were
observed under fluorescence microscope. BMP9-transfected ADMSCs
were identified by real-time fluorescence quantitative PCR
method.
Establishment of mouse models of
OS
Mouse models of OS were established by transection
of the knee anterior cruciate ligament (31). Precisely, after anesthesia, an
anesthesia ventilataor was used. The flow rate of isoflurane was
adjusted. A medial patellar incision was made and the skin and
articular capsule were cut open. The patella was laterally
retracted. The knee was flexed as far as possible to expose the
anterior cruciate ligament and the anterior horn of the medial
meniscus. The bilateral anterior cruciate ligament was transected.
The anterior drawer experiment was performed to confirm that the
bilateral anterior cruciate ligament was completely cut off.
Caution should be made to protect articular cartilage surface. The
articular space was flushed with normal saline. Articular capsule
and skin were sutured layer by layer.
Intra-articular injection of
ADMSCs
ADMSCs were digested and prepared into cell
suspension (at a cell density of 1×107/ml) using
chondrogenic medium DMEM (C11885500BT; Gibco; Thermo Fisher
Scientific, Inc.). Four weeks after OS induction, ADMSCs were
injected into the articular capsule once a week. Precisely, after
anesthesia, a 2 mm-long lateral incision was made on the lower
limb. The knee was touched by stretching the skin. Cell suspension
was injected into the articular cavity via the midpoint of the
medial edge of the ligament. 10 µl ADMSCs per articular cavity was
injected. After needle withdrawal, the entry site was slightly
pressurized to prevent the overflow of cell suspension. Skin
incision was sutured.
H&E staining
Four weeks after intra-articular injection of
ADMSCs, knee joint was disarticulated and fixed with formalin. The
harvested tissue sample was de-calcified, dehydrated and embedded
with paraffin. Sample tissues were de-waxed, rehydrated, stained
with hematoxylin for 5 min, washed with PBS, differentiated with
hydrochloric acid ethanol for 3 sec, stained with eosin for 3 min,
dehydrated, cleared, mounted with neutral resin, and finally
observed under the optical microscope (NE950; Leica Microsystems,
Inc., Buffalo Grove, IL, USA).
Toluidine blue staining
Tissue sections (5 µm) were de-waxed by xylene,
rehydrated, and stained with 0.1% toluidine blue for 10 min at room
temperature, washed with PBS three times, differentiated with
glacial acetic acid, dehydrated in ethanol gradients, cleared with
clear liquid, dried, and mounted with neutral gum.
Western blot analysis
Total protein was extracted from ADMSCs and knee
tissue samples in different groups. Protein concentration was
determined using a BCA protein assay kit (23227; Thermo Fisher
Scientific, Inc.). Protein samples were subjected to SDS-PAGE and
then transferred to a PVDF membrane. After addition of type II
collagen (ab34712), aggrecan (ab3778), notch1 (ab52627), Jagged1
(ab7771), and GAPDH (ab181602; all Abcam) protein samples were
incubated at 4°C overnight and washed with PBS. After addition of
secondary antibody (goat anti-rabbit IgG/HRP antibody; 1:2,000;
Bioss, Beijing, China), protein samples were incubated at 37°C for
2 h. Protein bands were visualized using an ECL chemiluminescence
detection kit (32109; Thermo Fisher Scientific, Inc.) and a gel
imaging system (ChemiDoc MP; Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Absorbance analysis was performed using Image J software
(Image J 1.8.0; National Institutes of Health, Bethesda, MD,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Primers were designed according to the sequences of
BMP9, type II collagen, aggrecan reported in Genbank, and were
synthesized in Shanghai Biomedical Biotechnology Co., Ltd. Total
RNA was isolated with TRIzol reagent (15596018), and reversely
transcribed into cDNA (4387406; both Invitrogen; Thermo Fisher
Scientific, Inc.). Real-time PCR kit (RR820A; Takara Biotechnology
Co., Ltd., Dalian, China) was used for the detection. The relative
gene expression data was analyzed with the 2−ΔΔCq method
(32). The primers used for
real-time PCR were listed as follows: BMP9 forward,
GCTGCAGAACTGGGAACA and reverse, AACAAGCATCCCCTGGGG; Collagen II
forward, TGCTGGCCCAACTGGCAA and reverse, ATTGTTGGTCTGCCTGGT;
Aggrecan forward, CCAGTGAGGACCTGGTAGTG and reverse,
CAGGCCTGCATGCACACCG; Notch1 forward, AAGAGGCTTGAGATGCTCC and
reverse, TGCCTCAGCACACCGTGT; Jagged1 forward, TAACACCTTCAATCTCAAG
and reverse, ATGACACTATTCAACCTGA; GAPDH forward,
GAATCGATCCATACTTATC and reverse, CCTTGAAGATATGGGCAC.
Statistical analysis
All data were statistically analyzed using SPSS v
19.0 software (IBM Corp., Armonk, NY, USA). A Kolmogorov-Smirnov
(K-S) test was used to determine whether data were normally
distributed. If data were normally distributed, the data were
presented as mean ± SEM. One-way ANOVA followed by a
Student-Newman-Keuls test was used to test for differences among
more than two groups. A level of P<0.05 was considered to
indicate a statistically significant difference.
Results
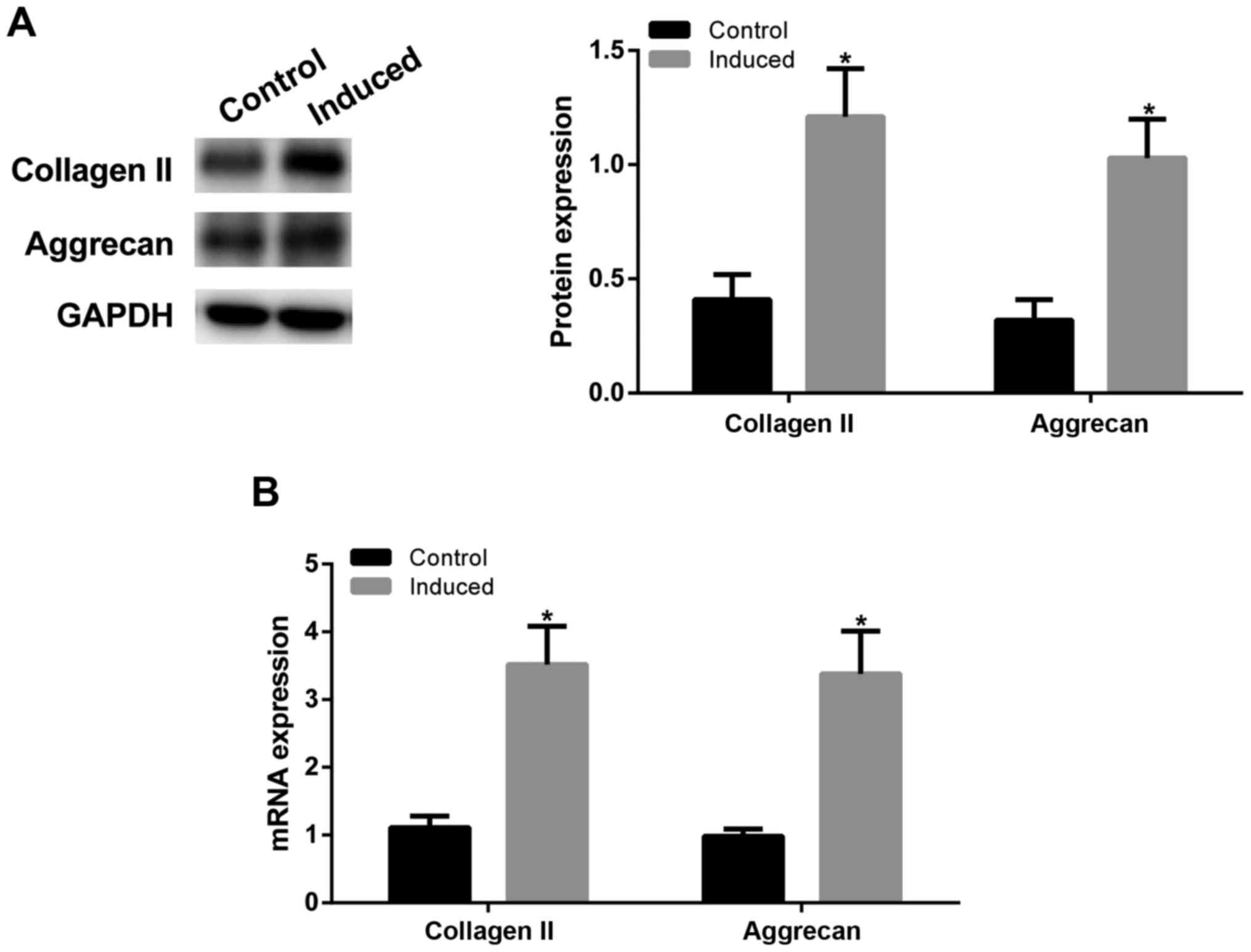
Chondrogenic induction of ADMSCs
To determine the differentiation of ADMSCs into
chondrocytes, as confirmed by western blot assay (Fig. 1A) and RT-qPCR (Fig. 1B), after chondrogenic induction of
ADMSCs, type II collagen and aggrecan expression levels were
significantly increased in induced group (P<0.05 vs. control
group), indicating that ADMSCs can be induced toward chondrogenic
differentiation.
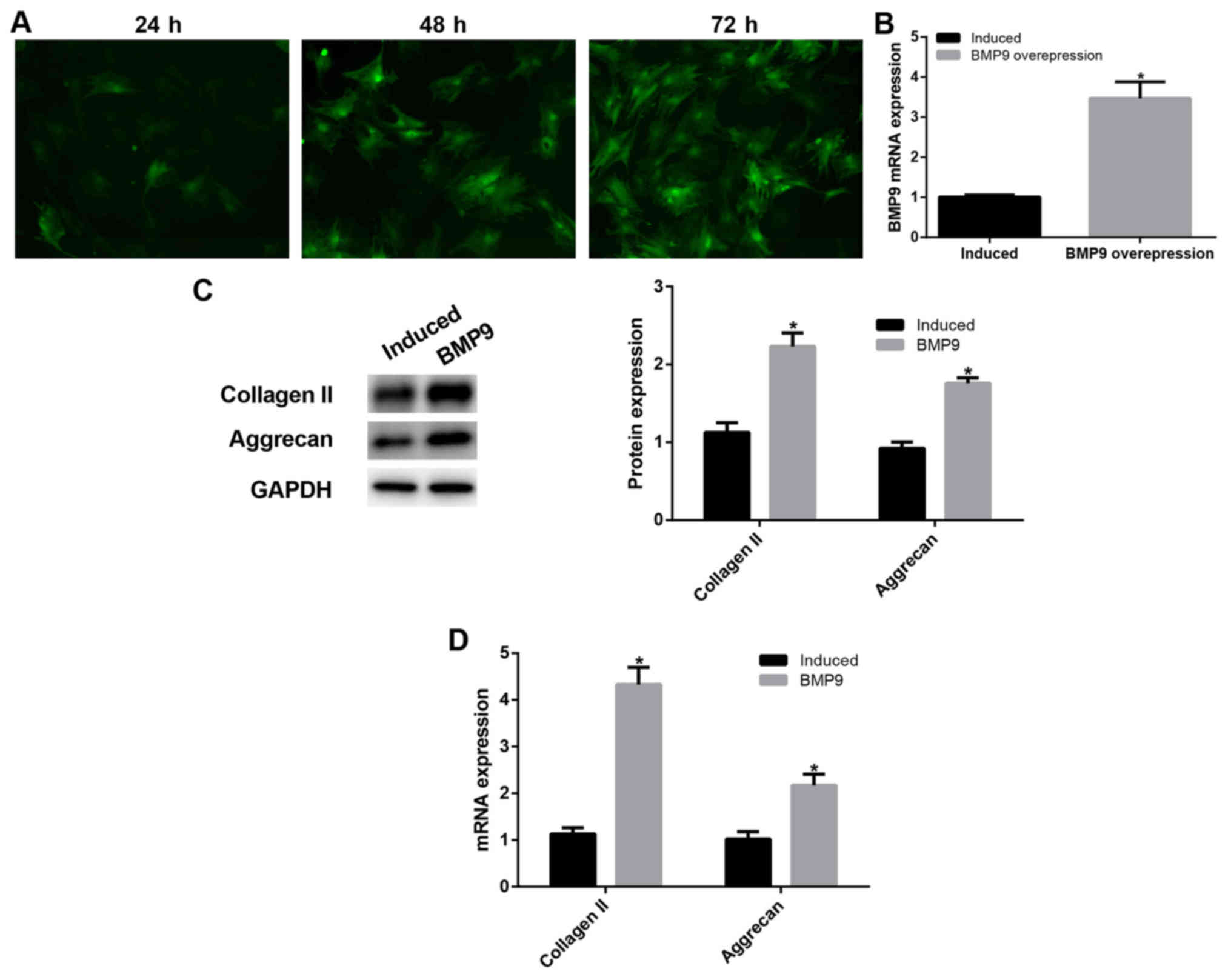
BMP9 promoted chondrogenic
differentiation of ADMSCs
To determine the effect of BMP9, we performed BMP9
overexpression. At 72 h after pVSV-G-BMP9 lentivirus-transfected
ADMSCs were directed toward chondrogenic differentiation, RT-qPCR
was performed to detect intracellular BMP9 expression. After BMP9
transfection, intracellular BMP9 expression was significantly
increased (P<0.05; Fig. 2A and
B), this result proved that our transfection was successful. We
further detected the expression of type II collagen and aggrecan
using western blot assay (Fig. 2C)
and RT-qPCR (Fig. 2D). The results
showed that type II collagen and aggrecan expression was
significantly increased in BMP9-transfected ADMSCs than that in the
chondrogenic induced ADMSCs (P<0.05). These results suggest that
BMP9 promoted chondrogenic differentiation of ADMSCs possibly
through the Notch signaling pathway.
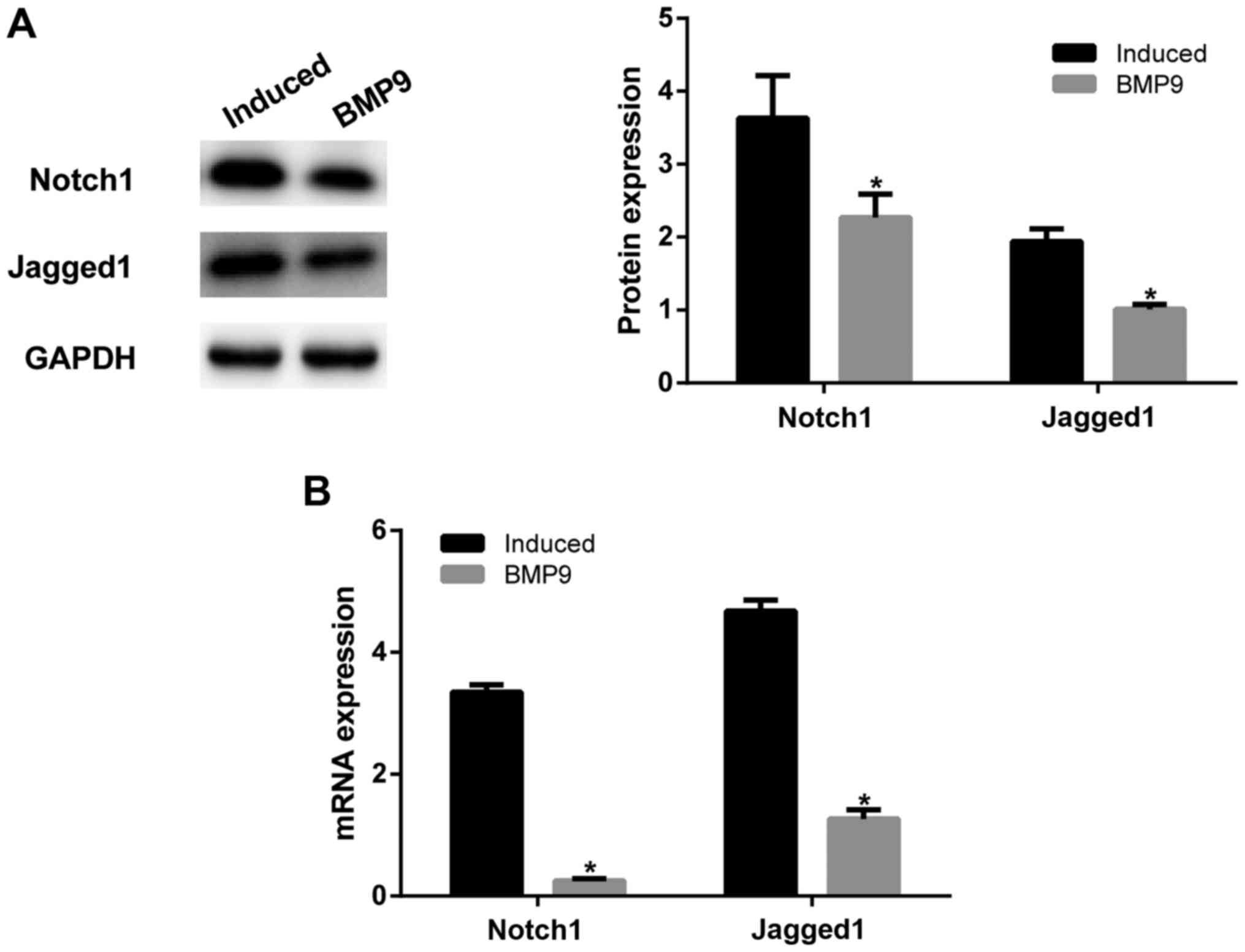
BMP9 regulated the Notch1/Jagged1
signaling pathway to promote the chondrogenic differentiation of
ADMSCs
In order to further explore the mechanism of BMP9,
western blot assay was performed to detect the expression of
Notch1/Jagged1 signaling pathway-related proteins Notch1 and
Jagged1. After BMP9 was overexpressed, Notch1 and Jagged1
expression was significantly decreased than that in the
chondrogenic induced ADMSCs (P<0.05; Fig. 3A). This was consistent with RT-qPCR
findings (Fig. 3B). These results
confirm that BMP9 regulated chondrogenic differentiation of ADMSCs
through the Notch1/Jagged1 signaling pathway.
ADMSCs promoted cartilage repair in OS
affected joints in mice
H&E staining results (Fig. 4) showed that in the OS group,
articular chondrocytes were poorly arranged, and their number was
smaller than that in sham group, and cartilage was thinner than
that in the control group. Toluidine blue staining (Fig. 4B) showed that in the OS group,
proteoglycan was unevenly distributed. After intra-articular
injection of BMP9 overexpressing ADMSCs, the number of chondrocytes
in the articular cavity was increased, and cartilage was thickened.
In order to further explore the role of BMP9 in mice, western blot
assay (Fig. 4C) was performed to
detect intra-articular expression of type II collagen and aggrecan
protein. The results showed that after BMP9 overexpression, type II
collagen and aggrecan expression were significantly increased. When
the Notch signaling pathway in the ADMSCs was inhibited, type II
collagen and aggrecan expression were significantly decreased. This
was consistent with RT-qPCR findings (Fig. 4D). These results demonstrate that
BMP9 overexpressing ADMSCs can promote the healing of OS in
mice.
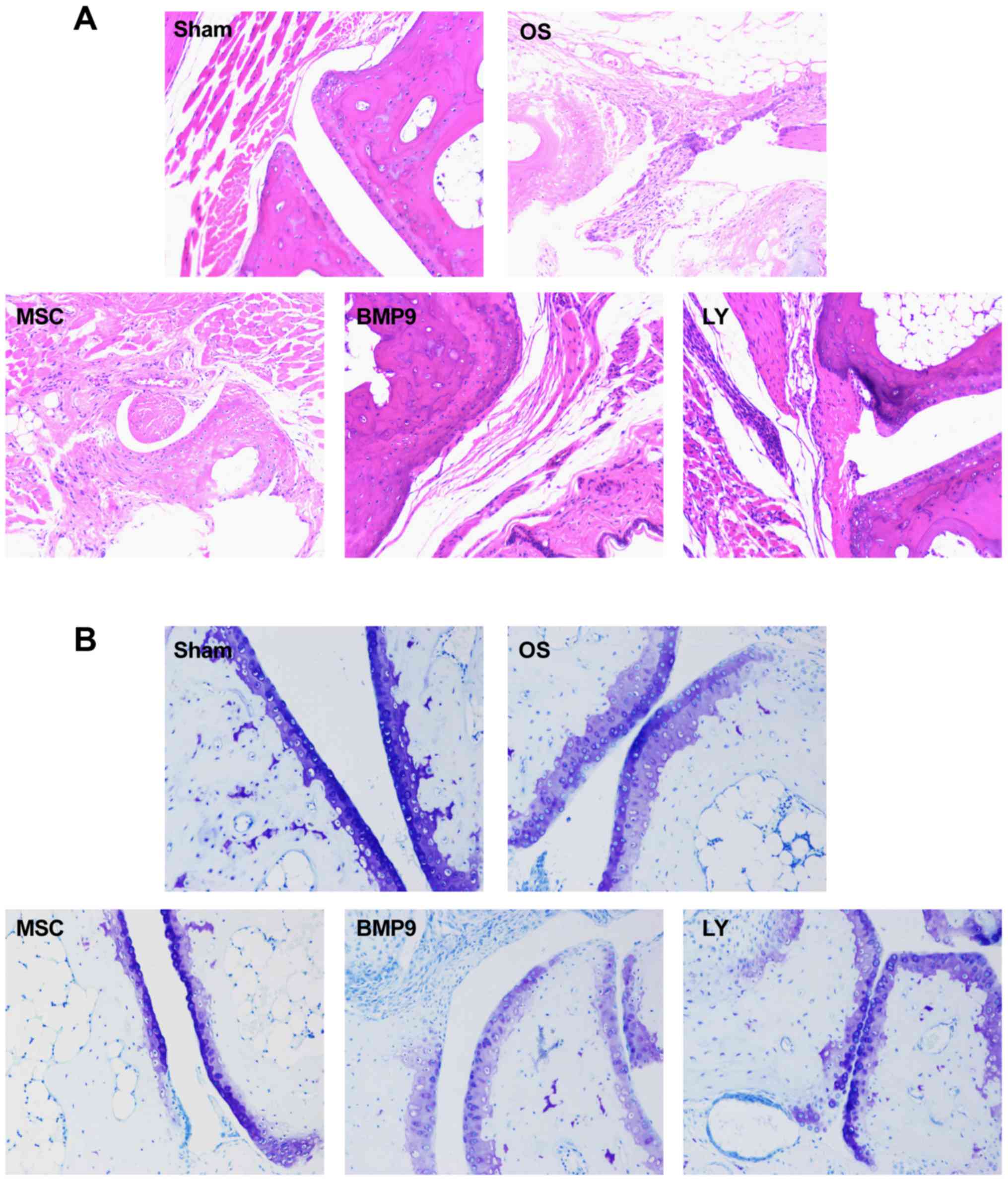 | Figure 4.ADMSCs promoted cartilage repair in
OS affected joints in mice. After OS model was established, ADMSCs
were injected into joint cavity, and the changes of joint were
observed by H&E staining and toluidine blue staining. Western
blot assay and RT-qPCR were used to detect the collagen II and
Aggrecan expression. (A) H&E staining (magnification, ×400).
(B) Toluidine blue staining (magnification, ×400). (C) Western blot
assay. (D) RT-qPCR detection. Compared with sham group, *P<0.05;
Compared with OS group, #P<0.05; Compared with MSC
group, $P<0.05; Compared with BMP9 group,
@P<0.05. BMP9, bone morphogenetic protein-9; OS,
osteoarthritis; MSC, mesenchymal stem cells; ADMSC, adipose-derived
MSC. |
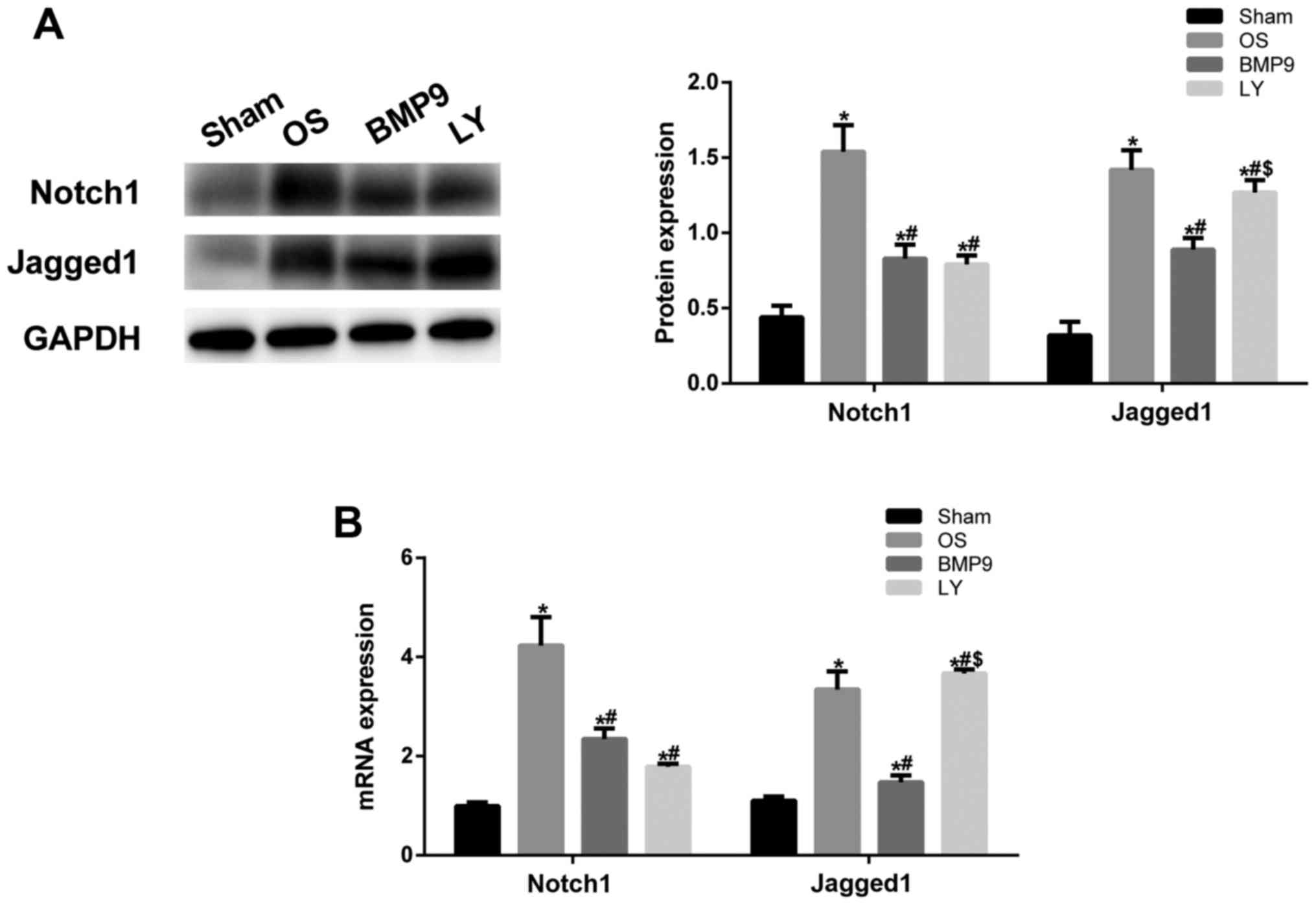
BMP9 regulated the Notch/Jagged1
signaling pathway in ADMSCs to promote OS healing in mice
To further validate whether BMP9 overexpressing
ADMSCs function effect via the Notch1/Jagged1 signaling pathway,
western blot assay was performed to detect intra-articular
expression of notch1 and Jagged1. In the BMP9 group,
intra-articular expression of Notch1 and Jagged1 was significantly
higher than that in the OS group (P<0.05) (Fig. 5A). When the Notch signaling pathway
was inhibited, type II collagen and aggrecan expression was
decreased (Fig. 4C and D) and
Jagged1 expression was also significantly reduced (P<0.05 vs.
BMP9 group). This was supported by RT-qPCR findings (Fig. 5B). These results confirmed that BMP9
overexpressing ADMSCs promote OS healing in mice through the
Notch/Jagged1 signaling pathway.
Discussion
Stem cells are mainly obtained from skeletal muscle
satellite cells, embryonic stem cells, and bone marrow MSCs
(BMMSCs). It is difficult to obtain skeletal muscle satellite cells
because of its lower level. Embryonic stem cells have immunological
rejections and ethical issues. BMMSCs also have the shortcomings of
difficult harvesting and that patients are not willing to
accept.
Zuk et al (33) were the first to harvest ADMSCs with
multi-directional differentiation potential from adipose tissue
suspension during human liposuction and induced them to
differentiate into adipocytes, chondroblasts, osteoblasts, and
neural progenitors. ADMSCs are easily accepted because of easily
accessible rich resource, being able to rapidly proliferate in any
kind of serum, no immunological considerations during autografting,
and no ethical issues. At present, there are no specific surface
markers of ADMSCs. Gronthos and Zannettino (34) cultured cells of fat tissue aspirates,
systematically studied cell surface markers, and found that ADMSCs
were spindle-shaped, had abundant cytoplasm and nucleoli, and grew
in a parallel or spiral-like manner. These cells have surface
markers, including CD59, CD105, CD106, CD146 and CD165, which are
similar to BMMSCs (35). But STRO-1
antigen was not detected on ADMSCs.
Festy et al (36) found that ADMSCs are similar to fully
differentiated adipocyte surface markers, and the surface markers
are not different between subcutaneous ADMSCs and omental ADMSCs.
In the experiments, ADMSCs were isolated. Cell surface markers
CD13, CD44 and CD59 on ADMSCs were detected. Flow cytometry showed
that CD13, CD44, and CD59 were positive, indicating that the
harvested ADMSCs are highly purified. ADMSCs, as a kind of
multi-potential stem cells, share the features with stem cells,
that is to say, ADMSCs theoretically have the ability to infinitely
proliferate.
BMP9, as a less studied BMP, has a very strong
potential for chondrogenesis. There is evidence that BMP9 together
with the Notch signaling pathway plays an important role in
embryonic development, cell proliferation and differentiation, and
the occurrence of diseases (37). In
the early study, BMP9 and the Notch signaling were found to have
synergistic effects in the early stage of osteogenesis (38). However, the precise mechanism remains
poorly understood. This is the problem that needs to be studied in
this paper.
In this study, we overexpressed BMP9 in ADMSCs,
observed change in cell masses under the inverted microscope, and
detected cartilage type II collagen and aggrecan expression.
Results showed that upregulating BMP9 expression can further induce
chondrogenic differentiation of ADMSCs. To further clarify the
mutual effects of the Notch signaling pathway and BMP9 in the
chondrogenic differentiation of ADMSCs, we used LY411575 to inhibit
the Notch signaling pathway. Inhibiting the Notch signaling pathway
using LY411575 can inhibit the chondrogenic differentiation of
ADMSCs, confirming that the Notch signaling pathway can inhibit the
chondrogenic differentiation of ADMSCs.
After intra-articular injection of ADMSCs, a larger
degree of cartilage repair was found in the MSCs group than in the
control group. After intra-articular injection of BMP9
overexpressing ADMSCs, type II collagen and aggrecan protein
expression in the cartilage of OS affected knee joint was further
detected by immunohistochemical staining to further confirm the
mechanism underlying BMP9-overexpressing ADMSCs. BMP9
overexpressing ADMSCs were injected into the articular cavity to
inhibit the Notch signaling pathway. Results showed that ADMSCs
promoted OS healing in mice through the Notch1/Jagged1 signaling
pathway.
Taken together, ADMSCs express multiple stem cell
surface markers and can be induced to differentiate into
chondrocytes, confirming that ADMSCs exhibit multi-directional
differentiation potential. Upregulating BMP9 protein can promote
the chondrogenic differentiation of ADMSCs. Inhibition of the Notch
signaling pathway can inhibit the chondrogenic differentiation of
ADMSCs. Intra-articular injection of ADMSCs contributes to
cartilage repair OS affected knee joint in mice, and the repair is
achieved via the Notch1/Jagged1 signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Liaoning Province Science and Technology Issues in China (grant no.
2013225089).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XinL and XiaL conceived and designed the study,
acquired data, interpreted the results and drafted the manuscript.
XiaL also contributed to acquisition of funding support. MD, YW and
SL performed the experiments. XinL and XiaL analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experiments were approved by Animal Ethics
Committee of General Hospital of Shenyang Military Region, China
(no. 2015049).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BMP9
|
bone morphogenetic protein-9
|
|
OS
|
osteoarthritis
|
|
MSCs
|
mesenchymal stem cells
|
|
ADMSCs
|
adipose-derived mesenchymal stem
cells
|
|
BMMSCs
|
bone marrow mesenchymal stem cells
|
|
TGF-β
|
transforming growth factor-β
|
|
GDF-2
|
growth differentiation factor 2
|
Reference
|
1
|
Kobayashi T, Takagishi K, Shitara H,
Ichinose T, Shimoyama D, Yamamoto A, Osawa T and Tajika T:
Prevalence of and risk factors for shoulder osteoarthritis in
Japanese middle-aged and elderly populations. J Shoulder Elbow
Surg. 23:613–619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nuki G: Osteoarthritis: A problem of joint
failure. Z Rheumatol. 58:142–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding S and Zheng K: Artificial total hip
arthroplasty with collum femoris preserving for treating hip joint.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 24:1–4. 2010.(In
Chinese). PubMed/NCBI
|
|
4
|
Komaki M, Iwasaki K and Morita I: Bone and
stem cells. Mesenchymal stem cells and bone regeneration. Clin
Calcium. 24:565–573. 2014.PubMed/NCBI
|
|
5
|
Endo I and Mastumoto T: Bone and stem
cells. Regulatory mechanism of mesenchymal stem cell
differentiation to osteoblasts. Clin Calcium. 24:555–564.
2014.PubMed/NCBI
|
|
6
|
Nishimura R, Nakamura E, Kida J, Yagi H
and Hata K: Bone and stem cells. Regulation of chondrocyte
differentiation from mesenchymal stem cells. Clin Calcium.
24:509–516. 2014.PubMed/NCBI
|
|
7
|
Hamam D, Ali D, Vishnubalaji R, Hamam R,
Al-Nbaheen M, Chen L, Kassem M, Aldahmash A and Alajez NM:
microRNA-320/RUNX2 axis regulates adipocytic differentiation of
human mesenchymal (skeletal) stem cells. Cell Death Dis.
5:e14992014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanz AR, Carrión FS and Chaparro AP:
Mesenchymal stem cells from the oral cavity and their potential
value in tissue engineering. Periodontol 2000. 67:251–267. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JS, Suryaprakash S, Lao YH and Leong
KW: Engineering mesenchymal stem cells for regenerative medicine
and drug delivery. Methods. 84:3–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Yuan M, Guo QY, Lu SB and Peng J:
Mesenchymal stem cells for treating articular cartilage defects and
osteoarthritis. Cell Transplant. 24:1661–1678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wozney JM: Overview of bone morphogenetic
proteins. Spine (Phila Pa 1976). 27 16 Suppl 1:S2–S8. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Guo J, Wu G and Zhou Y: Effects
of heterodimeric bone morphogenetic protein-2/7 on osteogenesis of
human adipose-derived stem cells. Cell Prolif. 48:650–660. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duan Z, Zheng Q, Guo X, Yuan Q and Chen S:
Experimental research on ectopic osteogenesis of BMP2-derived
peptide P24 combined with PLGA copolymers. J Huazhong Univ Sci
Technolog Med Sci. 27:179–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi YJ, Lee JY, Park JH, Park JB, Suh JS,
Choi YS, Lee SJ, Chung CP and Park YJ: The identification of a
heparin binding domain peptide from bone morphogenetic protein-4
and its role on osteogenesis. Biomaterials. 31:7226–7238. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Werle S, AbuNahleh K and Boehm H: Bone
morphogenetic protein 7 and autologous bone graft in revision
surgery for non-union after lumbar interbody fusion. Arch Orthop
Trauma Surg. 136:1041–1049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mladenov KV, Kunkel P and Stuecker R: The
use of recombinant human BMP-2 as a salvage procedure in the
pediatric spine: A report on 3 cases. Eur Spine J. 19 Suppl
2:S135–S139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang N, Song WX, Luo J, Luo X, Chen J,
Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, et al: BMP-9-induced
osteogenic differentiation of mesenchymal progenitors requires
functional canonical Wnt/beta-catenin signalling. J Cell Mol Med.
13:2448–2464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Breitkopf-Heinlein K, Meyer C, König C,
Gaitantzi H, Addante A, Thomas M, Wiercinska E, Cai C, Li Q, Wan F,
et al: BMP-9 interferes with liver regeneration and promotes liver
fibrosis. Gut. 66:939–954. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
López-Coviella I, Berse B, Krauss R, Thies
RS and Blusztajn JK: Induction and maintenance of the neuronal
cholinergic phenotype in the central nervous system by BMP-9.
Science. 289:313–316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo Y, Li L, Xu X, Wu T, Yang M, Zhang C,
Mou H, Zhou T, Jia Y, Cai C, et al: Decreased circulating BMP-9
levels in patients with Type 2 diabetes is a signature of insulin
resistance. Clin Sci (Lond). 131:239–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Truksa J, Peng H, Lee P and Beutler E:
Different regulatory elements are required for response of hepcidin
to interleukin-6 and bone morphogenetic proteins 4 and 9. Br J
Haematol. 139:138–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fortini C, Cesselli D, Beltrami AP,
Bergamin N, Caragnano A, Moretti L, Cecaro F, Aquila G, Rizzo P,
Riberti C, et al: Alteration of Notch signaling and functionality
of adipose tissue derived mesenchymal stem cells in heart failure.
Int J Cardiol. 174:119–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang J, Wei Y, Lv C, Peng S, Zhao S and
Hua J: CD61 promotes the differentiation of canine ADMSCs into
PGC-like cells through modulation of TGF-β signaling. Sci Rep.
7:438512017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui X, He Z, Liang Z, Chen Z, Wang H and
Zhang J: Exosomes from adipose-derived mesenchymal stem cells
protect the myocardium against ischemia/reperfusion injury through
Wnt/β-catenin signaling pathway. J Cardiovasc Pharmacol.
70:225–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hosaka Y, Saito T, Sugita S, Hikata T,
Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI
and Kawaguchi H: Notch signaling in chondrocytes modulates
endochondral ossification and osteoarthritis development. Proc Natl
Acad Sci USA. 110:1875–1880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen AX, Hoffman MD, Chen CS, Shubin AD,
Reynolds DS and Benoit DS: Disruption of cell-cell contact-mediated
Notch signaling via hydrogel encapsulation reduces mesenchymal stem
cell chondrogenic potential: Winner of the society for biomaterials
student award in the undergraduate category, charlotte, NC, April
15 to 18, 2015. J Biomed Mater Res A. 103:1291–1302. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matthews BG, Grcevic D, Wang L, Hagiwara
Y, Roguljic H, Joshi P, Shin DG, Adams DJ and Kalajzic I: Analysis
of αSMA-labeled progenitor cell commitment identifies Notch
signaling as an important pathway in fracture healing. J Bone Miner
Res. 29:1283–1294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kramer J, Schwanbeck R, Pagel H, Cakiroglu
F, Rohwedel J and Just U: Inhibition of Notch signaling ameliorates
acute kidney failure and downregulates platelet-derived growth
factor receptor β in the mouse model. Cells Tissues Organs.
201:109–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ortiz-Martínez F, Gutiérrez-Aviñó FJ,
Sanmartín E, Pomares-Navarro E, Villalba-Riquelme C,
García-Martínez A, Lerma E and Peiró G: Association of Notch
pathway down-regulation with triple negative/basal-like breast
carcinomas and high tumor-infiltrating FOXP3+ tregs. Exp Mol
Pathol. 100:460–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Chang H, Peng X, Gu Y, Yi L, Zhang
Q, Zhu J and Mi M: 3,6-dihydroxyflavone suppresses the
epithelial-mesenchymal transition in breast cancer cells by
inhibiting the Notch signaling pathway. Sci Rep. 6:288582016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lorenz J and Grässel S: Experimental
osteoarthritis models in mice. Methods Mol Biol. 1194:401–419.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gronthos S and Zannettino AC: Methods for
the purification and characterization of human adipose-derived stem
cells. Methods Mol Biol. 702:109–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gronthos S, Franklin DM, Leddy HA, Robey
PG, Storms RW and Gimble JM: Surface protein characterization of
human adipose tissue-derived stromal cells. J Cell Physiol.
189:54–63. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Festy F, Hoareau L, Bes-Houtmann S, Péquin
AM, Gonthier MP, Munstun A, Hoarau JJ, Césari M and Roche R:
Surface protein expression between human adipose tissue-derived
stromal cells and mature adipocytes. Histochem Cell Biol.
124:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu P, Man Y, Wang Y and Bao Y: Mechanism
of BMP9 promotes growth of osteosarcoma mediated by the Notch
signaling pathway. Oncol Lett. 11:1367–1370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liao J, Wei Q, Zou Y, Fan J, Song D, Cui
J, Zhang W, Zhu Y, Ma C, Hu X, et al: Notch signaling augments
BMP9-induced bone formation by promoting the
osteogenesis-angiogenesis coupling process in mesenchymal stem
cells (MSCs). Cell Physiol Biochem. 41:1905–1923. 2017. View Article : Google Scholar : PubMed/NCBI
|