Introduction
The formation of endochondral bone is a complex
developmental process. This process is initiated by the
differentiation and subsequent proliferation of mesenchymal stem
cells to chondroblasts. After leaving the cell cycle for terminal
differentiation, the chondrocytes become pre-hypertrophic and
finally hypertrophic. Cytokines, hormones and transcription factors
may all affect this process known as chrondrogenesis (1–3).
Sulfate conjugation reactions are catalyzed via
sulfotransferase enzymes via 3′-phosphoadenosine 5′-phosphosulfate
(PAPS). PAPS serves as a high-energy sulfate donor and is
synthesized from adenosine trisphosphate and inorganic sulfate.
This synthesis uses two isoforms of PAPS synthetase (PAPSS): PAPSS1
and PAPSS2 (4–7). Availability of PAPS is a prerequisite
for the sulfation of biological molecules, including proteoglycans
that function as key extracellular matrix components. This
highlights the importance of sulfation in bone development and
growth, which in turn depends on the integrity of the extracellular
matrix. Due to the requirement for PAPS in sulfation, the
biosynthesis of PAPS may influence the rate of sulfation reactions
in cells.
Previously, a large Pakistani pedigree was reported
to have a homozygous mutation (S475X) resulting in
spondyloepimetaphyseal dysplasia (SEMD), Pakistani type. The
symptoms include enlarged knee joints, short stature, short, bowed
lower limbs, as well as kyphoscoliosis and brachydactyly
(characterized by complex shortening of the digits) (8–11). SEMD
of the Omani type is caused by deficient chondroitin
6-O-sulfotransferase activity due to an abnormal PAPSS2 gene that
impairs PAPS biosynthesis (4,12). A
deficiency of PAPSS2 activity results in long bone shortening and
bowing, as well as knee arthritis and degenerative joint disease.
Mutant mice lacking PAPSS2 activity have been proposed as a model
to study PAPSS2 deficiency-associated arthrosis, due to the
features of premature and degenerative joint disease and other
similarities to human SEMD (9). Of
note, postnatal skeletal development is specifically affected in
this PAPSS2 mutant mouse model. Although the skeleton appears to be
normal in newborn brachymorphic mice, the columnar and hypertrophic
zones of the epiphyseal growth plates are small, which is
consistent with reduced growth (11,13).
In previous studies by our group, microarray
analysis provided evidence for the involvement of PAPSS2 genes in
patients suffering from endemic knee osteoarthritis and Kashin-Beck
disease, which manifests as shortened long bones and enlarged
joints in the knees and fingers; in addition, it was demonstrated
that PAPSS2 influenced osteoblast differentiation via the Smad
signaling pathway (14). These
observations suggest that PAPSS2 participates in fibrillogenesis
and/or matrix calcification/mineralization. However, the precise
mechanisms through which PAPSS2 influences cartilage development
and formation are still largely elusive. To investigate cell
signaling mechanisms involving PAPSS2, biochemical and molecular
studies on the structural and functional characteristics of this
enzyme are required. The present study examined the role of PAPSS2
in the extracellular signal-regulated kinase pathway that controls
chondrocyte differentiation to better understand how PAPSS2
influences chondrogenesis.
Materials and methods
Ethics statement
The present study was approved by the Animal
Experimental Ethics Committee of Xi'an Jiaotong University (Xi'an,
China).
Cell line and culture
Experiments were performed using the murine teratoma
cell line ATDC5 (American Type Culture Collection, Manassas, VA,
USA). They are chondrogenic cells with processes analogous to
chondrocyte differentiation (15,16). The
cells were cultured on Dulbecco's modified Eagle's medium with
nutrient mixture F-12 (DMEM/F-12; Life Sciences; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) as well as 100 U/ml
penicillin and 100 mg/ml streptomycin. Cell culture was performed
at 37°C in a humidified atmosphere with 5% CO2. The
seeding density was 1×104 cells/cm2 and cells
were passaged every 5–7 days, but for no more than 20 passages. For
pre-chondrocytic ATDC5-cell differentiation, the cells were induced
via a chondrogenic growth medium containing 100 mg/ml ascorbic acid
(17,18). To induce chondrocytic
differentiation, cells were seeded on 6-well plates and incubated
for 2 weeks in DMEM/F-12 supplemented with 1,600 nM human
biosynthetic insulin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) (18).
PAPSS2 small hairpin (sh)RNA
lentivirus packaging, retrovirus vector, assessment of viral titers
and cell transfection
A small hairpin RNA (shRNA) lentiviral packaging
system (Open Biosystems, Lafayette, CO, USA) was used to modify
PAPSS2 gene expression in accordance with the manufacturer's
instructions (14). The human
embryonic kidney cell line (293T) was obtained from the American
Type Culture Collection (Manassas, VA, USA) and transfected with
expression constructs (pLenti-P2A or pLenti-P2B) or constructs
containing a scrambled shRNA sequence and associated packaging.
PAPSS2 shRNA sequences are presented in Table I. Five individual pLB-PAPSS2 shRNA
(P-S) vectors (each, 500 ng/µl) and a control pLB-scramble shRNA
(PLB) vector (Open Biosystems) were co-transfected with the
packaging plasmids (500 ng/µl). Retroviral vector pBMP-PAPSS2 was
constructed by inserting a full-length 3.64 kb PAPSS2 cDNA (access
no. NM_011864) into the EcoRI and NotI site of pBMN-I-GFP (Addgene)
and packaging was performed following the protocol from the Dr
Garry Nolan Laboratory, Stanford University (Stanford, CA, USA)
(14). Briefly, retrovirus vectors
pBMN-I-GFP (control vector) and pBMN-PAPSS2 were separately
transfected into the Phoenix-eco tropic packaging cells using the
CaCl2 precipitation method. A total of 2–3 days later,
the viral supernatant was harvested, and lentiviral titers were
assessed in 293T cells with serial dilutions of the lentivirus in
the presence of 4 µg/ml polybrene (Sigma-Aldrich; Merck KGaA).
Following the transfection, the cells were placed in a 32°C
humidified incubator for 48 h (32°C aids in stabilizing the virus).
The media containing infectious virus was harvested and filtered
through a 0.45 mm filter for the titration assay and infecting
ATDC5 cells and following 24 h, virus containing media was removed
and replaced with fresh complete medium. Following further 48 h,
GFP and PAPSS2 protein expression were confirmed by observation of
GFP+ cells, immunostaining, and western blot analysis. The
retroviruses carrying pBMP-PAPSS2 and pBMN-I-GFP were then used to
infect 70–80% subconfluent ATDC5 cells in the presence of 6 µg/ml
polybrene (Sigma-Aldrich; Merck KGaA) and viral supernatant for 24
h. After induction with chondrogenic medium for various durations
according to the experimental protocol, the cells were analyzed. On
days 0, 2, 4, 6, 8 and 10, they were then detached from 6-well
plates by trypsinization for counting with a cell counting
chamber.
 | Table I.Short hairpin RNA sequences used with
3′-phosphoadenosine 5′-phosphosulfate synthetase 2. |
Table I.
Short hairpin RNA sequences used with
3′-phosphoadenosine 5′-phosphosulfate synthetase 2.
|
|
| Primers
(5′-3′) |
|---|
|
|
|
|
|---|
| Target | Sequence
(5′-3′) | Sense | Antisense |
|---|
| TRCN0000353201 |
CCGGGCGTGGAAAGTGTTGACAGATCTCGAGATCTGTCAACACTTTCCACGCTTTTTG |
GCGTGGAAAGTGTTGACAGAT |
ATCTGTCAACACTTTCCACGC |
| TRCN0000280504 |
CCGGCCATCATGTGAGCAGGAACAACTCGAGTTGTTCCTGCTCACATGATGGTTTTTG |
CCATCATGTGAGCAGGAACAA |
TTGTTCCTGCTCACATGATGG |
| TRCN0000280502 |
CCGGGCAGGAGAGATTAAAGGGTTTCTCGAGAAACCCTTT
AATCTCTCCTGCTTTTTG |
GCAGGAGAGATTAAAGGGTTT |
AAACCCTTTAATCTCTCCTGC |
| TRCN0000280566 |
CCGGGCTCTATTACAGGACCCTGAACTCGAGTTCAGGGTCCTGTAATAGAGCTTTTT |
CTCTATTACAGGACCCTGAA |
TTCAGGGTCCTGTAATAGAGC |
| TRCN0000024944 |
CCGGGCTTTGGAAGAGTACCTTGTACTCGAGTACAAGGTACTCTTCCAAAGCTTTTT |
GCTTTGGAAGAGTACCTTGTA |
TACAAGGTACTCTTCCAAAGC |
Construction of overexpression vectors
and production of recombinant retrovirus
The retroviral vector pBMN-PAPSS2 carried a
full-length 3.64-kb PAPSS2 complementary (c)DNA (GenBank accession
no. NM_011864). This construct was inserted into the EcoRI
and NotI restriction sites of the pBMN-I-green fluorescent
protein (GFP) expression plasmid (Addgene, Cambridge, MA, USA).
This downstream insertion and viral packaging was performed based
on Stanford/Nolan lab protocols (14). In brief, the retroviral pBMN-I-GFP
(control) as well as pBMN-PAPSS2 vectors were transfected
separately into Phoenix ecotropic packaging cells via calcium
chloride precipitation. At 48 h after transfection, the cells were
cultured at 32°C for 48 h to stabilize the virus. The media with
infectious viral particles were collected and filtered through a
0.45-µm filter to complete titration assays and transfected into
ATDC5 cells. The expression of GFP and PAPSS2 was measured by
fluorescence detection, immunostaining and western blot analysis.
The pBMN-PAPSS2 and pBMN-I-GFP were used to transfect ATDC5 cells
in subconfluent culture (80% subconfluency) with 6 µg/ml polybrene.
Cell proliferation was assessed in DMEM/F12 in 6-well plates. Cells
were induced under conditions to either overexpress or silence the
PAPSS2 gene (depending on the differentiation RNA interference
medium used). On days 0, 2, 4, 6, 8 and 10, they were then detached
from 6-well plates by trypsinization for counting with a cell
counting chamber.
RT-qPCR
Tissues from 14-day-old c57BL/6J female mice
(weight, 30±3 g; Experiment Center, Xi'an Jiaotong University),
including lung, spleen, kidney, liver, muscle, heart, calvaria,
brain and bone, were collected and homogenized prior to RNA
extraction (5 min; 2,000 × g; 4°C). The total RNA was extracted
using TRIzol and the RNA concentration was determined according to
spectrophotometry, followed by RT-qPCR (both Invitrogen; Thermo
Fisher Scientific, Inc.). An RNeasy Mini kit (Qiagen, Valencia, CA,
USA) was used to extract RNA from growth plate chondrocyte
cultures. The mRNA levels of aggrecan, Wnt4, β-catenin, SRY-box
(SOX)-9, collagen type II (COL2) and collagen type X (COLX) were
then determined as described previously (19). Total RNA (1 µg) was reverse
transcribed into the cDNA using an Omniscript RT kit (Qiagen). The
resulting cDNA was used as a template at 1:100 dilution to measure
the relative mRNA levels via real-time qPCR on an ABI Prism 7300
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). qPCR was based on the SYBR® Green
detection method and was performed using the following primers:
PAPSS2 forward, 5′-TGTAAAACGACGGCCAGT-3′ and reverse,
5′-CAGGAAACAGCTATGACC-3′; COLX forward,
5′-AGTGCTGTCATTGATCTCATGGA-3′ and reverse,
5′-TCAGAGGAATAGAGACCATTGGATT-3′; SOX9 forward,
5′-AGGAAGCTGGCAGACCAGTA-3′ and reverse, 5′-TCCACGAAGGGTCTCTTCTC-3′;
COL2A1 forward, 5′-CTACGGTGTCAGGGCCAG-3′ and reverse,
5′-GCAAGA-TGAGGGCTTCCATA-3′; Wnt4 forward, 5′-AACCGGCGCTGGAACTG-3′
and reverse, 5′-GGTCCCTTGTGTCACCACCTT-3′; β-catenin forward,
5′-TTTATGAGTGGGAGCAAGGC-3′ and reverse, 5′-TGCCCTCATCTAGTGTCTCA-3′;
β-actin forward, 5′-CACCCTGTGCTGCTCACCGAGGCC-3′ and reverse,
5′-CCACACAGATGACTTGAGCTCAGG-3′. Reactions were performed on an ABI
The PRISM 7500 sequence detection system with SYBR®
GREEN PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used according to the manufacturer's instructions. The
PCR conditions were 94°C for 1 min, followed by 95°C for 30 sec and
58°C for 40 sec for a total of 35 cycles. All of the reactions were
run in triplicate and normalized to the housekeeping gene β-actin.
The relative mRNA expression in each group was calculated using the
quantitative cycle method (20).
Western blot analysis
Cells were lysed with Nonidet P (NP)-40 buffer (1%
NP-40, 0.15 M NaCl, 50 mM Tris; pH 8.0) containing protease
inhibitors (Sigma-Aldrich; Merck KGaA). A bicinchoninic acid assay
was used for protein quantitation (Pierce; Thermo Fisher
Scientific, Inc.). Samples were denatured in SDS buffer and 50 µg
protein was loaded per lane and separated by electrophoresis on SDS
gels with 8–10% polyacrylamide. The proteins were transferred onto
polyvinylidene difluoride membranes. These were then incubated with
the following antibodies: Anti-PAPSS2 (cat. no. ab37611; 1:100)
anti-COL2 (cat. no. ab185430; 2 µg/ml) and anti-COLX (cat. no.
ab58632; 2 µg/ml), and anti-β-actin (cat. no. AC-40; 1:2,000; all
Abcam, Cambridge, MA, USA) for 12 h at 4°C. Subsequently, membranes
were washed and incubated with horseradish peroxidase
(HRP)-conjugated secondary anti-mouse [m-IgGκ binding protein
horseradish peroxidase (HRP) conjugated; cat. no. sc-516102] or
anti-rabbit (mouse anti-rabbit HRP-IgG; cat. no. sc-2357)
antibodies (each 1:5,000; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at room temperature for 2 h. Proteins were visualized
using enhanced chemiluminescence (Advansta, Inc., Menlo Park, CA,
USA) and the blots were imaged and quantified with the Fluor-S
Multi-Imager system and Multi-Analyst software version 1.1 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Immunocytochemical and
immunohistochemical staining
For immunocytochemical staining, ATDC5 cells
(1×104 cells/well) were seeded in 24-well plates
containing poly-L-lysine-coated 7×7 mm2 coverslips.
These were induced with chondrogenic media. At 14 days after
seeding, the cells were fixed with 4% paraformaldehyde in PBS for
20 min and rinsed with PBS at 4°C. The avidin-biotin-peroxidase
complex method was used for immunocytochemistry. In brief, cells
were permeabilized with 0.2% Triton X-100 for 15 min at 4°C to
facilitate uptake of antibodies for labeling of intracellular
antigens. Prior to exposure to primary antibodies, unspecific
binding was blocked with 10% FBS in PBS for 1 h at 37°C, followed
by washing with PBS. Cells were incubated overnight with the
primary antibody (PAPSS2; as above) at 4°C, washed and incubated
with HRP-conjugated secondary antibodies (anti-mouse as above) for
1 h at room temperature, prior to visualization using
chemiluminescence (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
according to the manufacturer's instructions. Cells were washed
with PBS. A 0.3% (v/v) 3,3′ diaminobenzidine solution in 0.1% (v/v)
hydrogen peroxide was used for visualization via peroxidase
reaction. DAB/peroxide was used for visualization of the secondary
antibody. For microscopic analysis, the cells were counterstained
with hematoxylin, washed with distilled water and successively
dehydrated in ethanol (70, 95 and 99.9%) as well as xylene. Images
of the cells were captured using a Nikon light microscope (Nikon,
Tokyo, Japan). The negative control, which was prepared without
incubation of cells with primary antibody, verified the specificity
and reliability of the secondary antibody. Cells with visible
yellow staining were considered positively stained.
For immunohistochemical staining, cartilage slices
were fixed with 4% paraformaldehyde for 24 h at 4°C following the
removal of the tissue and decalcified at room temperature for 10
min in 3% ethylenediaminetetraocetic acid. Samples were dehydrated
in a series of alcohol, cleared in xylene, and embedded in paraffin
wax at room temperature. Paraffin sections (6–8 µm) were cut,
mounted on slides, pretreated with 2% poly-L-lysine at 4°C for 10
min and stored at room temperature until used. Deparaffinized
cartilage sections were incubated with testicular hyaluronidase (2
mg/ml in PBS, pH 5; cat. no. E0037; Shanghai Baoman Biotechnology
Co., Ltd., Shanghai, China) for 30 min at room temperature. Samples
were incubated with primary antibodies (as aforementioned)
overnight at 4°C and visualized using alkaline phosphatase-labeled
secondary antibodies (1:100) obtained form and using the
Histostain™-SAP kit (cat. no. SAP-9100; OriGene Technologies, Inc.,
Rockville, MD, USA). Visualization was performed for 30 min at room
temperature using 3-hydroxy-2-naphthoic acid 2,4-dimethylanilide
(1%). Finally, nuclei were counterstained with hematoxylin for 2
min at room temperature. Sections were examined and counted using a
light microscope for cytoplasmic and pericellular staining. Four to
six randomly selected fields in each zone were counted at a
magnification of ×400.
Alcian blue staining
Alcian blue staining of ATDC5 cells was performed as
previously described (21).
Following fixing in 4% (w/v) parafomraldehyde in PBS for 30 min,
samples were stained with Alcian blue (Sigma-Aldrich; Merck KGaA)
for 5 min, followed by dehydration with 95% ethanol. Images were
then captured under a microscope (Nikon TE2000-S; Nikon) and
analyzed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Statistical analyses
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used for one-way analysis of variance, followed by
Tukey's honest significant differences test for post-hoc analysis.
For comparison between two groups, statistical significance was
assessed using Student's t-test. All experiments were performed at
least in triplicate. Values are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of PAPSS2 mRNA in cartilage
and role in medium-induced cell differentiation
PAPSS2 mRNA expression was measured in various
tissues from mice via RT-qPCR. The expression levels were elevated
in calvaria, bone, liver and lung, and moderately in the heart. The
expression in calvaria and long bone was significantly higher than
in the other tissues as indicated in the figure (Fig. 1A). The PAPSS2 mRNA expression was low
in the muscle, spleen, kidney and brain (Fig. 1A). The housekeeping gene murine
β-actin was present at constant levels across all tissues. This
highlights the reliability of the chosen RT-qPCR method in
measuring PAPSS2 mRNA. These results are consistent with those of a
previous study (4). PAPSS2 mRNA
expression was higher in mouse chondrocytes than in other tissues
(4). PAPSS2 was also detected in
cartilage tissue following immunohistochemical staining for PAPSS2
(Fig. 1A-C), and in the murine ATDC5
cell line at the protein level by western blot analysis (Fig. 1D and E). These results indicate
marked PAPSS2 mRNA expression in mouse cartilage, as well as
protein expression in mouse chondrocytes exposed to chondrogenic
media. The in vitro results indicate an important role for
PAPSS2 in chondrocyte differentiation. To analyze the molecular
functions of PAPSS2 in the proliferation and differentiation of
chondrocytes, groups of PAPSS2-overexpressing (PO) and -silenced
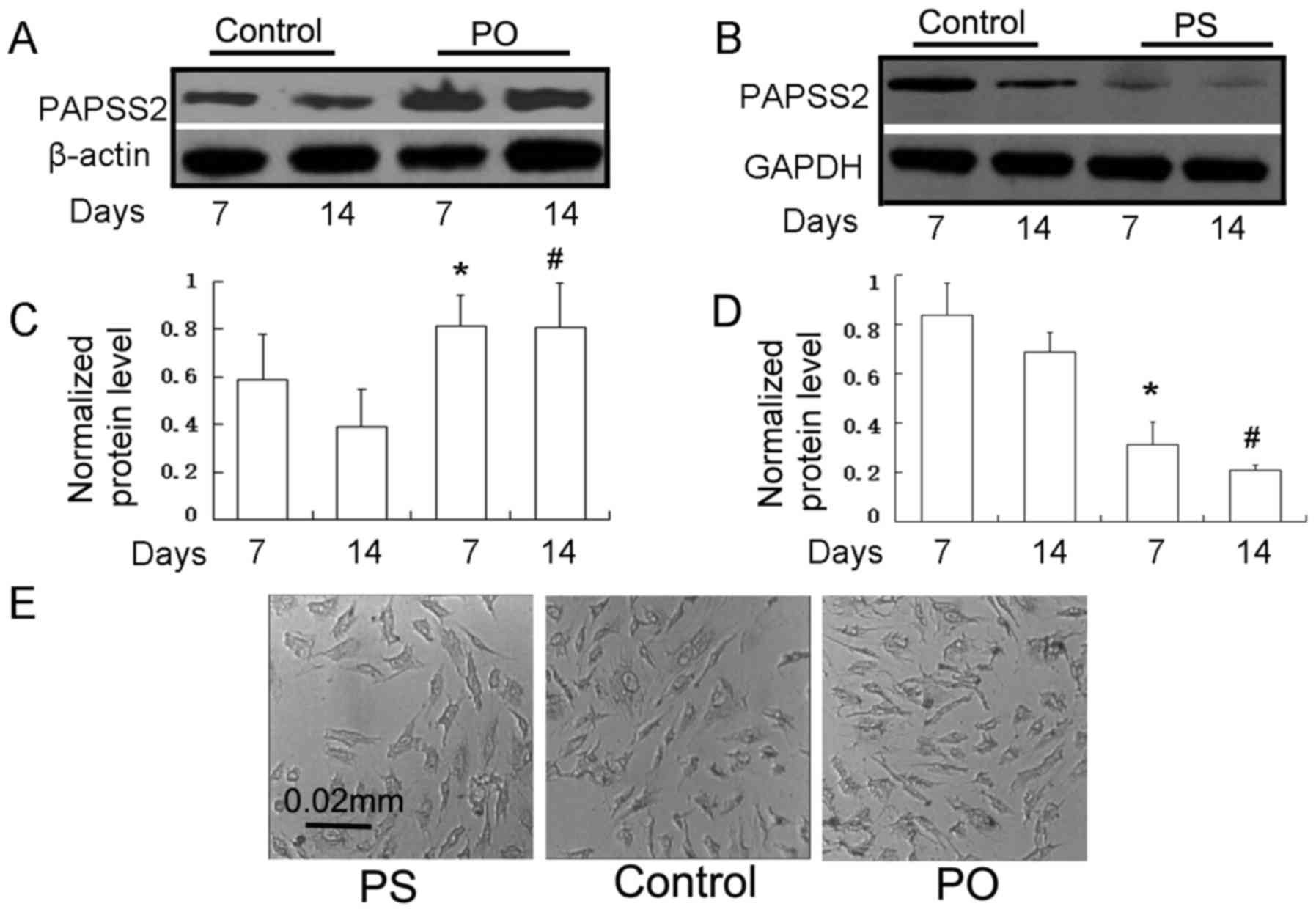
(PS) ATDC5 cells were established (Fig.
2A-D). The silencing efficiency of the RNAi technique was
assessed by RT-qPCR (data not shown) and western blot analysis
following transfection with siRNA specific to PAPSS2 and PAPSS2
mRNA. The protein expression of PAPSS2 was almost silenced in these
stably transfected cells relative to that in the controls
(transfected with empty vector), at 7 and 14 days
post-transfection. Compared with the parental cells, the PAPSS2
protein had decreased expression in knockdown cells when assessed
at 7 days (Fig. 2B and D). At 14
days of differentiation, no morphological alterations were observed
in the cells of the PS and PO groups when compared with control
ATDC5 cells (Fig. 2E). There were no
morphological changes after chondrogenic differentiation compared
with baseline (e.g. day 14 vs. 0).
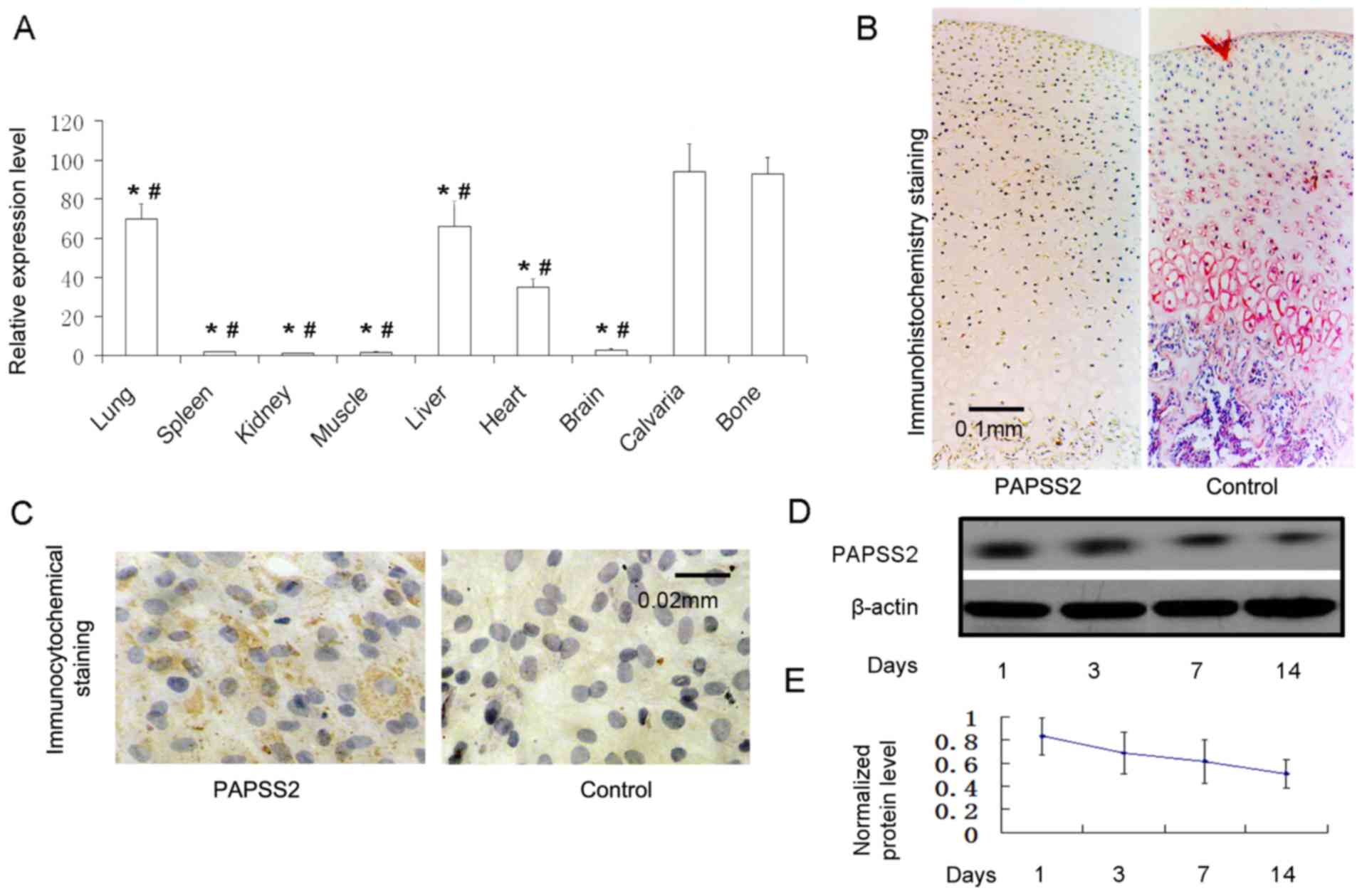 | Figure 1.PAPSS2 is present in chondrocytes,
bone and osteoblasts. (A) Reverse transcription-quantitative
polymerase chain reaction analysis of PAPSS2 mRNA expression in
14-day-old mouse tissues. The total RNA relative to β-actin
expression from calvaria, long bone, brain, heart, liver, muscle,
spleen, kidney and lung. (B) Immunohistochemical localization of
PAPSS2 cartilage of the knee in adult mice (magnification, ×100;
scale bar, 0.1 mm). (C) Immunocytochemical staining for PAPSS2 in
monolayer chondrocytes (ATDC5 cells at the 12th day; magnification,
×400; scale bar, 0.02 mm). Western blot analysis of PAPSS2 protein
expression in ATDC5 cells treated with culture medium for 0, 3, 7
and 14 days. (D) Representative western blot image and (E)
quantified expression levels normalized to β-actin, indicating that
the results are consistent with those in B. Values are expressed as
the mean ± standard deviation (n=6). *P<0.0001 vs. calvaria;
#P<0.0001 vs. long bone. PAPSS2, 3′-phosphoadenosine
5′-phosphosulfate synthetase 2. |
PAPSS2 promotes aggrecan activity and
chondrocyte matrix production in chondrogenic terminal
differentiation events
During development, chondrocytes undergo a
hypertrophic phase followed by terminal differentiation and
mineralization. To highlight the importance of PAPSS2 in
chondrocyte differentiation, a lentivirus-mediated RNA interference
(RNAi) technique was applied to silence the PAPSS2 gene in ATDC5
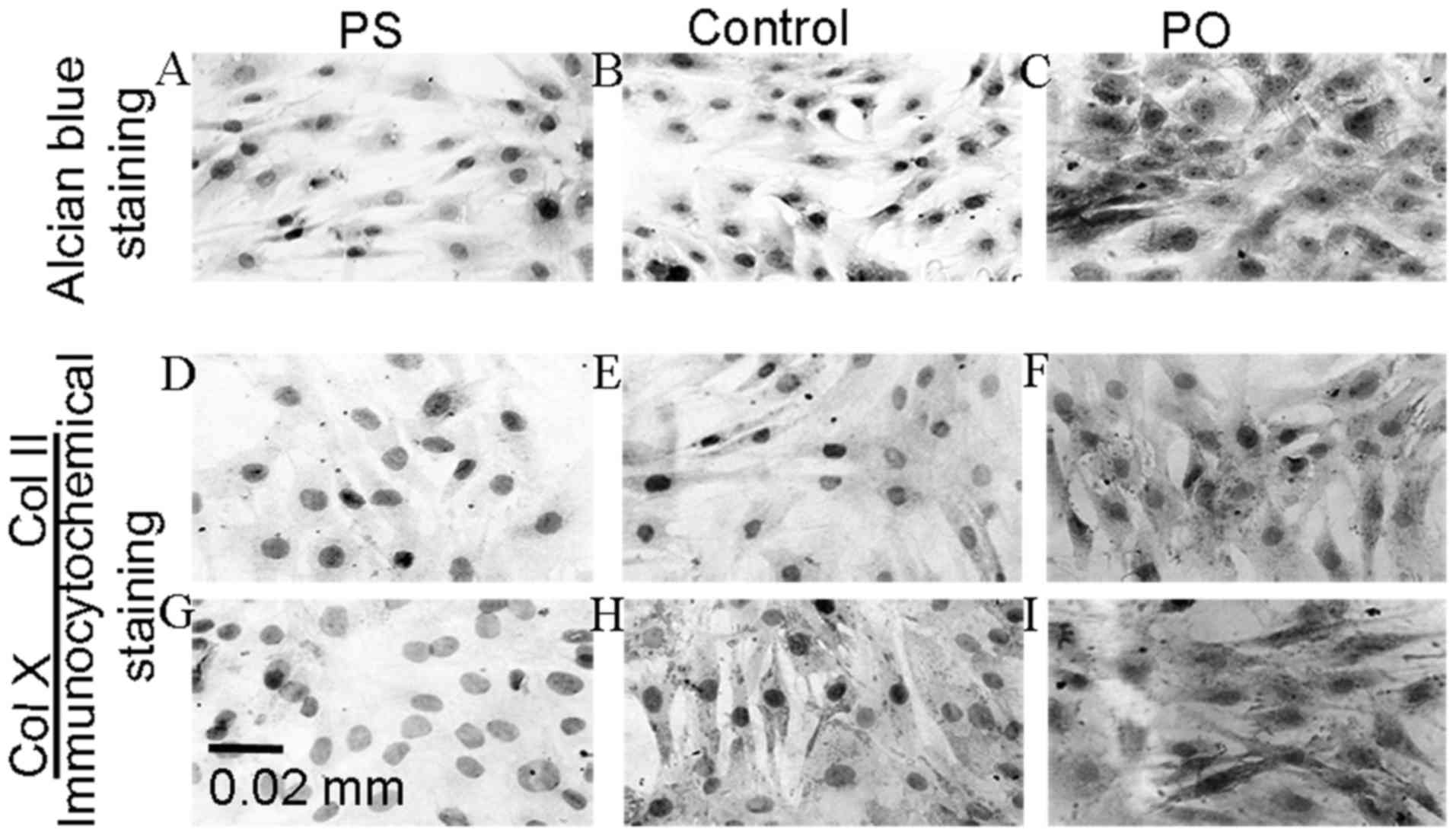
cells exposed to chondrogenic media. To analyze the effect of
PAPSS2 overexpression and knockdown on aggrecan expression, Alcian
blue staining assays were performed after induction of ATDC5 cells
in chondrogenic media containing the vector pBMN-PAPSS2 for 14 days
(Fig. 3A). Following the silencing
of the PAPSS2 gene, extracellular matrix differentiation was
significantly reduced as determined by alcian blue staining and the
levels of ColII and COlX were decreased as determined by
immunocytochemical staining. To evaluate the effect of PAPSS2
overexpression on differentiation, the ATDC5 cells were induced for
7 days in media either containing retroviral constructs to induce
PAPSS2 overexpression or control (empty) constructs. shRNA was used
to modify PAPSS2 gene expression. PAPSS2 overexpression resulted in
increased levels of ColII and COlX as determined by
immunocytochemical staining. Furthermore, extracellular matrix
differentiation was significantly increased, as determined by
alcian blue staining (Fig. 3A). Of
note, PAPSS2 overexpression was associated with marked increases in
aggrecan activity and chondrocyte matrix production (Fig. 3A). Furthermore, positive
immunostaining for COL2 and COLX was evaluated in the PS and PO
groups, revealing that the percentage of the chondrocytes positive
for COLX and COL2 in the PS group was significantly lower than that
in the control group (P<0.01; Table
II) (Fig. 3). The percentage of
positive staining for COLX and COL2 in the PO group was
significantly higher than that in the controls (P<0.001).
 | Table II.Percentage of positive
immunocytochemical staining of chondrocytes of the PS, PO and
control groups for types II and X collagen at 14 days of
induction/transfection. |
Table II.
Percentage of positive
immunocytochemical staining of chondrocytes of the PS, PO and
control groups for types II and X collagen at 14 days of
induction/transfection.
| Group | Positive stain for
Col II (%) | Positive stain for
Col X (%) |
|---|
| PS |
22.3±6.9a |
0.0±0.0b |
| Control | 55.0±7.6 | 44.3±6.7 |
| PO |
82.4±8.0a |
84.7±6.6b |
Effect of ectopic PAPSS2 gene
expression on the time course of cell proliferation
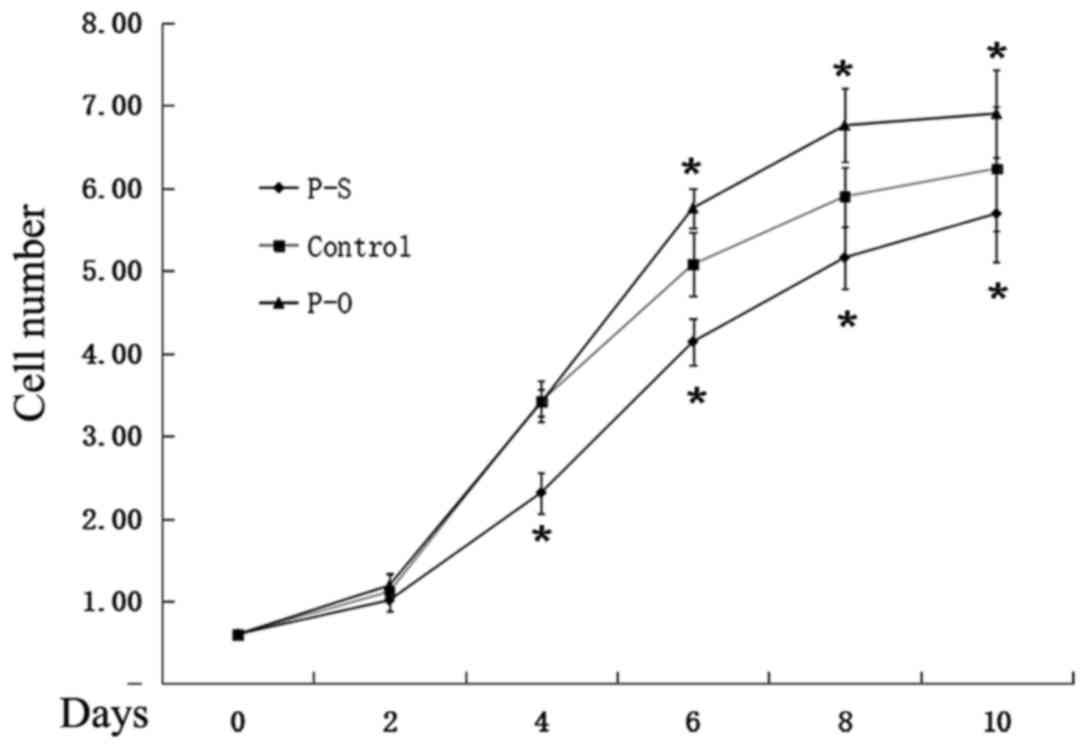
The ATDC5 cells upon reaching 80% subconfluency were
grown in chondrogenic media and simultaneously transfected with
retroviral pBMN-PAPSS2 vector to induce PAPSS2 overexpression. A
lentivirus-mediated RNAi was applied to silence the PAPSS2 gene in
ATDC5 cells exposed to chondrogenic media. The effects of these
treatments to either overexpress or silence the PAPSS2 gene during
ATDC5 proliferation and differentiation were assessed. Cell counts
were assessed on days 0, 1, 2, 4, 7 and 10. At the selected
time-points, the cells were trypsinized and counted. The cells
maintained in medium to overexpress PAPSS2 proliferated at a
significantly higher rate compared with the cells in the control
group, while proliferation was significantly decreased for cells
treated with medium to silence the PAPSS2 gene (Fig. 4).
Effect of ectopic PAPSS2 gene
expression on chondrocyte-specific markers and modulation of the
Wnt/β-catenin pathway in the transfected ATDC5 cells
Chondrogenic induction was initiated in ATDC5 cells
using chondrogenic media containing retroviral vector pBMN-PAPSS2
(to promote PAPSS2 overexpression) and an empty lentivirus vector
(to knockdown PAPSS2 expression) to study the effect of ectopic
PAPSS2 gene expression on known chondrogenic markers and collagen
proteins. Following exposure to media either containing retroviral
constructs to induce PAPSS2 overexpression or control (empty)
constructs, the PAPSS2 protein levels were evaluated by western
blot analysis. As expected, the levels of various chondrogenic
markers were significantly higher in cells incubated in
chondrogenic media containing constructs to induce PAPSS2
overexpression vector compared with that in the control group.
RT-qPCR analysis indicated significant increases in the expression
of the chondrocyte marker genes COLX, COL2, Wnt4, and SOX9 in cells
overexpressing PAPSS2 (Fig. 5A-D).
However, the levels of various chondrogenic markers were
significantly lower in cells of the PAPSS2 silence vector group
compared with that in the control group. RT-qPCR analysis indicated
significant decreases in the expression of the chondrocyte marker
genes COLX, COL2, Wnt4, and SOX9 in cells silenced PAPSS2 (Fig. 5A-D). To further investigate whether
PAPSS2 affects Wnt/β-catenin signaling expression activation,
β-catenin and Wnt4 levels were observed by RT-qPCR. As presented in
Fig. 5, there was a significant
increase in Wnt4 and β-catenin in the PAPSS2 silence group compared
with the control, and a decrease in the PAPSS2 overexpression group
compared with the control. These results clearly indicated that
both silencing and overexpression of PAPSS2 affected Wnt/β-catenin
signaling activation. The effect of PAPSS2 overexpression on COL2
and COLX confirmed the effect observed at the protein level by
immunocytochemistry (Fig. 3). In
addition, quantification of alcian blue staining after induction of
ATDC5 cells in chondrogenic media containing the retroviral vector
pBMN-PAPSS2 for 14 days from Fig. 3A
indicated that PAPSS2 overexpression was associated with marked
increases in aggrecan activity and chondrocyte matrix production
(Fig. 5).
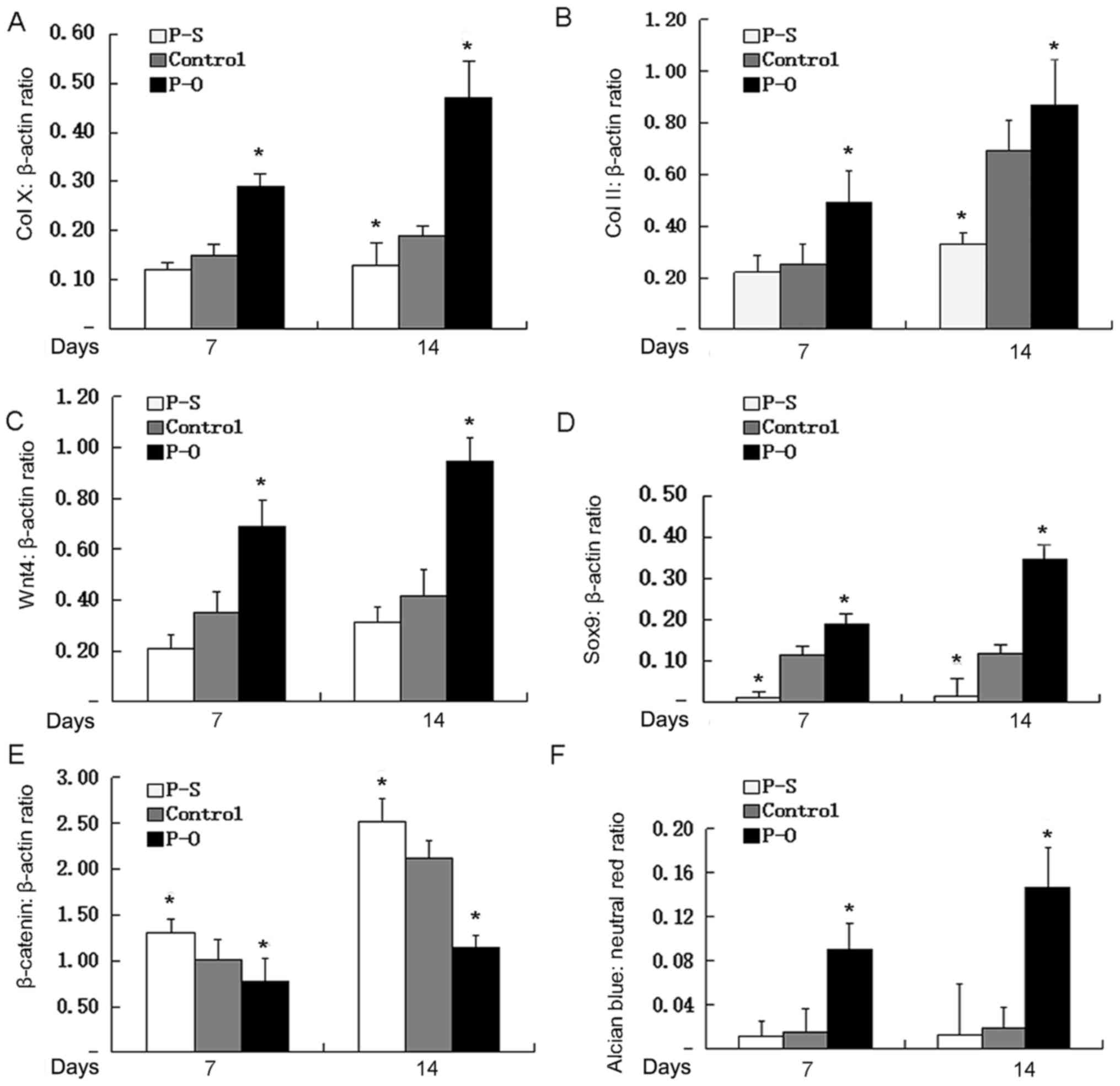 | Figure 5.Effect of ectopic PAPSS2 gene
expression on chondrogenic genes. Upon reaching 80% subconfluency,
ATDC5 cells were incubated in differentiation media containing
either lentiviral plasmid expressing PAPSS2 small hairpin RNA for
knockdown or pBMN-PAPSS2 vector for overexpression of PAPSS2 for 7
or 14 days. The expression levels of (A) COLX, (B) COL2, (C) Vnt4,
(D) SOX9 and (E) β-catenin relative to
β-actin expression were determined by reverse
transcription-quantitative polymerase chain reaction analysis. (F)
Cells were subjected to Alcian blue and neutral red staining,
followed by dye extraction for absorbance measurements. The ratio
of Alcian blue to neutral red was determined. Values are expressed
as the mean ± standard deviation (n=3). *P<0.05 vs. control. PO,
PAPSS overexpression group; PS, PAPSS suppression group; PAPSS2,
3′-phosphoadenosine 5′-phosphosulfate synthetase 2; COL, collagen;
SOX, SRY-box. |
Discussion
PAPSS2 is mostly expressed during the formation of
bone elements in the mouse embryo as well as in the cartilage of
the newborn mouse (11,22,23).
Consistent with these previous studies, the results of the present
study indicated that PAPSS2 is highly expressed in the calvaria and
bone tissues of 14-day-old c57BL/6J mice. A notable amount of
PAPSS2 was observed in the liver lung and heart, however it was not
significant. A minimal amount of PAPSS2 was observed in the brain,
muscle, kidneys, spleen. Cartilage is well known to be avascular,
alymphatic and aneural, and chondrocytes are its only cell type.
Consequently, cartilage tissue has limited innate capacity for
self-regeneration (e.g., after damage from physical injury or
degenerative disease). This makes it vulnerable to changing
environmental conditions. In addition to physical trauma,
autoimmune reactions may lead to dysfunction of cartilage and
predispose to severe joint conditions, including osteoarthritis
(OA) and rheumatoid arthritis. To achieve a thorough understanding
of the factors that regulate cartilage and bone formation, studies
are required to identify key signaling pathways and molecules that
control chondrogenesis. Chondrogenesis is a key stage in cartilage
and bone formation and is the process through which mesenchymal
cells differentiate into chondrocytes. In recent decades, interest
in the development of novel tissue engineering strategies for
cartilage repair has increased, as reflected by the large number of
studies performed in this field (24–26).
To improve treatment of cartilage-associated
diseases, the potential utility of strategies to regenerate
functional cartilage tissue via the induction of chondrogenesis is
becoming increasingly important. The chondrogenic ATDC5 cell line
is derived from mouse teratocarcinoma cells and enters a sequential
differentiation process analogous to that in chondrocytes. This
makes them useful for in vitro studies of cell behavior
during chondrogenesis. It also provides a useful model for studies
addressing the role of signaling pathways in skeletal development
and for the characterization of interactions of chondrocytes with
novel synthetic materials. To date, >200 studies based on the
ATDC5 cell line have generated a wealth of data (27–32).
Mutations that inactivate the PAPSS2 gene are
associated with severe inherited developmental skeletal disorders,
including Kashin-Beck disease, SEMD in humans, and brachymorphism
in mice (14,22,33).
Under-sulfation due to the inhibition of PAPS synthetase controls
extracellular matrix (ECM) expression during chondrogenesis
(34–37). PAPSS2 deficiency produces
osteochondrodysplasias. These are genetically heterogeneous
disorders that damage normal skeletal growth, linear growth, as
well as cartilage and bone health. The physical presentation
includes a short limb stature, cleft palate, generalized dysplasia
and hitchhiker's thumb. Inbred mice display a distinct form of SEMD
that is inherited through a recessive pattern attributed to a
PAPSS2 polymorphism in 10q23-24. Diastrophic dysplasia is an
autosomal recessive osteochondrodysplasia (38–40). It
is caused by reduced levels of intracellular sulfate levels, which
lead to under-sulfation of proteoglycans. Sequence polymorphisms in
the PAPSS2 gene in OA patients were investigated by Oostdijk et
al (22). OA is a
musculoskeletal disorder featuring degeneration of articular
cartilage. Single nucleotide polymorphism analysis in certain
Japanese populations with OA affecting the knee has also provided
evidence for involvement of the gene in OA pathogenesis; these
investigations revealed differences in the distribution of two
PAPSS2 isoforms that were only apparent in OA-affected tissues
(10,11).
ATDC5 pre-chondrocytes undergo differentiation into
chondrocytes that produce ECM components. The ATDC5 cell line is
frequently used to study particular genes in chondrocyte
differentiation. In the present study, overexpression of PAPSS2 or
knockdown via shRNA suggested an important role for PAPSS2 in
initiating differentiation of chondrocytes. After silencing of the
PAPSS2 gene, the levels of multiple markers associated with ATDC5
cell differentiation were significantly reduced. PAPSS2
overexpression caused the levels of ColII, COlX, Sox9 and Wint4 to
be increased. According to the present immunocytochemistry results,
the percentages of chondrocytes positive for COL2 and COLX were
significantly higher in the PAPSS2 overexpression group than in the
controls. Conversely, the percentages of chondrocytes positive for
COL2 and COLX were significantly lower in PAPSS2 knockdown cells
compared with those in the control cells.
For chondrogenesis to proceed effectively,
differentiation requires specification and maintenance of lineage
decisions through activation of stage-specific markers. Genes
belonging to the SOX family, which includes >30 members, has a
central role in regulating chondrogenesis. The SOX9 gene is
expressed in mesenchymal precursors and developing chondrocytes
until the pre-hypertrophic stage, but not in subsequent lineages.
SOX9 is required for chondrocyte specification and early
differentiation (41–43). It maintains growth plate chondrocyte
proliferation, delays pre-hypertrophy and facilitates subsequent
hypertrophy prior to terminal maturation and apoptosis. In
addition, SOX9 directly activates all major cartilage-specific ECM
genes expressed by early-stage chondrocytes and is involved in the
chondrocyte differentiation pathway at multiple stages. SOX9 has
been demonstrated to induce COL2A1 expression by activating a 48-bp
enhancer element residing in the first intron of this gene. It
promotes the differentiation of mesenchymal stem cells into
chondrocytes (44). SOX9
inactivation in the growth plate resulted in dwarfism due to
shortening of columnar and hypertrophic zones and in advanced
ossification due to premature pre-hypertrophy and matrix
mineralization. These effects are typical of campomelic dysplasia,
a severe genetic disorder that affects the development of the
skeleton, and are consistent with the notion that this disease
arises due to growth plate and primordial cartilage defects.
The Wnt/β-catenin signaling pathway has an important
role in regulating the growth and differentiation of chondrocyte
cells (45–47). To study the possible role of PAPSS2
in the regulation of the Wnt4 expression as part of the Wnt
pathway, ATDC5 cells were treated with differentiation medium
containing PAPSS2 overexpression or shRNA vector. The results
indicated that PAPSS2 treatment inhibits Wnt/β-catenin activity and
chondrocyte differentiation by upregulating Wnt4 and decreasing
β-catenin mRNA levels in ATDC5 cells. These results suggest that
PASS2 suppresses chondrocyte dedifferentiation locally by
modulating Wnt/β-catenin signaling activity. It may interact with
parathyroid hormone-related peptide in a negative feedback loop,
and Wnt/β-catenin regulates chondrocyte differentiation and
initiation of the hypertrophic phase. Wnt/β-catenin in chondrocytes
is regulated by several factors (47,48). The
present study indicated that PAPSS2 regulates Wnt/β-catenin
signaling expression during the transition of chondrocytes from
proliferation to hypertrophic differentiation. The effects of
PAPSS2 on Wnt/β-catenin signaling pathways were further examined
and silencing as well as overexpression of PAPSS2 affected this
signaling pathway. It is suggested that PAPSS2 is an upstream
regulator of Wnt/β-catenin and its chondrogenic properties may be
mediated through mechanisms that need further investigation.
The results of the present study rule out the
possibility that PAPSS2 modulates osteopontin activity (either
directly or indirectly) or chondrogenic genes via multiple
mechanisms, including the Wnt/β-catenin signaling pathway. Cells
maintained in medium to overexpress PAPSS2 proliferated at a
significantly higher rate compared with the cells in the control
group, while proliferation was significantly decreased for cells
treated with medium to silence the PAPSS2 gene. PAPSS2
overexpression caused the levels of ColII, COlX, Sox9 and Wint4 to
be increased. After silencing of the PAPSS2 gene, the levels of
multiple markers ColII, COlX, Sox9 and Wint4 associated with ATDC5
cell differentiation were significantly reduced. The present study
supports an essential role for PAPSS2 in chondrocyte
differentiation via inducing early signaling events. More detailed
studies are required to assess other genes identified to be
affected by PAPSS2 knockdown, including transcription factors,
chondrocyte differentiation enzymes, proteins associated with bone
morphogenesis as well as ECM proteins, and their potential
mechanisms regarding PAPSS2.
The present results indicate that PAPSS2 induces the
chondrogenic differentiation of ATDC5 cells by crosstalk with Wnt
signaling. PAPSS2 promotes ATDC5 differentiation by upregulating
the production of collagenous matrix components. Wnt/β-catenin
pathway activation after matrix formation leads to chondrocyte
differentiation. Future studies will assess the key markers in the
underlying pathways of bone formation or metabolic processes at the
gene and protein levels, including the role of PAPSS2 in the
detailed regulation of collagen assembly and its functional
properties in Wnt/β-catenin signaling pathways during bone and
cartilage formation. Transcription and growth factors that regulate
expression at the gene and protein level, accumulation and
degradation of PAPSS2 will be assessed. Finally, the role of PAPSS2
in controlling the activity of other signaling pathways will also
be addressed.
Acknowledgements
The authors thank Dr Li Fang and Dr Cao Rui for
their critical reading of the manuscript and Dr. He Xiaoning for
his technical assistance with fluorescence microscopy and image
analysis.
Funding
This project was funded by the Natural Science
Foundation of China (grant no. 81001225), the Fundamental Research
Funds for the Central Universities (grant no. xjj2016107), the
International Co-operative Plan of Shaanxi (grant no.
S2016YFKW0013), the International Co-operative Fund of Xian
Jiaotong University (grant no. 08143004) and the Integrative
Medicine Research and Innovation Team of Degenerative Bone Disease
Prevention of Shaanxi Traditional Chinese Medicine College (grant
no. 2013KCT-26).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and XG conceived and designed the experiments. LF
performed the experiments. YH analyzed the data. JH and PY helped
perform the experiments. WW and LF prepared the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Experimental Ethics Committee of Xi'an Jiaotong University.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PAPSS2
|
3′-phosphoadenosine 5′-phosphosulfate
synthetase 2
|
|
RNAi
|
RNA interference
|
|
SEMD
|
spondyloepimetaphyseal dysplasia
|
|
shRNA
|
small hairpin RNA
|
|
GFP
|
green fluorescent protein
|
|
COL2
|
collagen type II
|
|
COLX
|
collagen type X
|
References
|
1
|
Stupina TA and Shchudlo MM: A method for
making preparations from nondecalcified articular cartilage with
sublying subchondral bone for multipurpose studies. Bull Exp Biol
Med. 157:401–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mobasheri A, Kalamegam G, Musumeci G and
Batt ME: Chondrocyte and mesenchymal stem cell-based therapies for
cartilage repair in osteoarthritis and related orthopaedic
conditions. Maturitas. 78:188–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Acharya C, Yik JH, Kishore A, Van Dinh V,
Di Cesare PE and Haudenschild DR: Cartilage oligomeric matrix
protein and its binding partners in the cartilage extracellular
matrix: Interaction, regulation and role in chondrogenesis. Matrix
Biol. 37:102–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang F, Guo X, Wang W, Wu S, Ma W and Yan
H: Expression profile analysis of mycotoxin-related genes in
cartilage with endemic osteochondropathy Kashin-Beck Disease. BMC
Musculoskelet Disord. 13:1302012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu ZH, Freimuth RR, Eckloff B, Wieben E
and Weinshilboum RM: Human 3′-phosphoadenosine 5′-phosphosulfate
synthetase 2 (PAPSS2) pharmacogenetics: Gene resequencing, genetic
polymorphisms and functional characterization of variant allozymes.
Pharmacogenetics. 12:11–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu ZH, Thomae BA, Eckloff BW, Wieben ED
and Weinshilboum RM; Pharmacogenetics of human 3′-phosphoadenosine
5′-phosphosulfate synthetase 1 (PAPSS1), . gene resequencing,
sequence variation, and functional genomics. Biochem Pharmacol.
65:1787–1796. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peitzmeier SM, Reisner SL, Harigopal P and
Potter J: Female-to-male patients have high prevalence of
unsatisfactory Paps compared to non-transgender females:
Implications for cervical cancer screening. J Gen Intern Med.
29:778–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu ZH, Otterness DM, Freimuth RR, Carlini
EJ, Wood TC, Mitchell S, Moon E, Kim UJ, Xu JP, Siciliano MJ and
Weinshilboum RM: Human 3′-phosphoadenosine 5′-phosphosulfate
synthetase 1 (PAPSS1) and PAPSS2: Gene cloning, characterization
and chromosomal localization. Biochem Biophys Res Commun.
268:437–444. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ford-Hutchinson AF, Ali Z, Seerattan RA,
Cooper DM, Hallgrimsson B, Salo PT and Jirik FR: Degenerative knee
joint disease in mice lacking 3′-phosphoadenosine 5′-phosphosulfate
synthetase 2 (Papss2) activity: A putative model of human PAPSS2
deficiency-associated arthrosis. Osteoarthritis Cartilage.
13:418–425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramaswamy G, Sohn P, Eberhardt A and Serra
R: Altered responsiveness to TGF-β results in reduced Papss2
expression and alterations in the biomechanical properties of mouse
articular cartilage. Arthritis Res Ther. 14:R492012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stelzer C, Brimmer A, Hermanns P, Zabel B
and Dietz UH: Expression profile of Papss2 (3′-phosphoadenosine
5′-phosphosulfate synthase 2) during cartilage formation and
skeletal development in the mouse embryo. Dev Dyn. 236:1313–1318.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiele H, Sakano M, Kitagawa H, Sugahara
K, Rajab A, Höhne W, Ritter H, Leschik G, Nürnberg P and Mundlos S:
Loss of chondroitin 6-O-sulfotransferase-1 function results in
severe human chondrodysplasia with progressive spinal involvement.
Proc Natl Acad Sci USA. 101:10155–10160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alnouti Y and Klaassen CD: Tissue
distribution and ontogeny of sulfotransferase enzymes in mice.
Toxicol Sci. 93:242–255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang W, Li F, Wang K, Cheng B and Guo X:
PAPSS2 promotes alkaline phosphates activity and mineralization of
osteoblastic MC3T3-E1 cells by crosstalk and Smads signal pathways.
PLoS One. 7:e434752012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng F, He A, Zhang Z, Zhang Z, Lin Z,
Yang Z, Long Y, Wu G, Kang Y and Liao W: Chondrogenic
differentiation of ATDC5 and hMSCs could be induced by a novel
scaffold-tricalcium phosphate-collagen-hyaluronan without any
exogenous growth factors in vitro. J Biomed Mater Res A.
102:2725–2735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao Y and Wang Y: ATDC5: An excellent in
vitro model cell line for skeletal development. J Cell Biochem.
114:1223–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Newton PT, Staines KA, Spevak L, Boskey
AL, Teixeira CC, Macrae VE, Canfield AE and Farquharson C:
Chondrogenic ATDC5 cells: An optimised model for rapid and
physiological matrix mineralisation. Int J Mol Med. 30:1187–1193.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Temu TM, Wu KY, Gruppuso PA and
Phornphutkul C: The mechanism of ascorbic acid-induced
differentiation of ATDC5 chondrogenic cells. Am J Physiol
Endocrinol Metab. 299:E325–E334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang WZ, Guo X, Duan C, Ma WJ, Zhang YG,
Xu P, Gao ZQ, Wang ZF, Yan H, Zhang YF, et al: Comparative analysis
of gene expression profiles between the normal human cartilage and
the one with endemic osteoarthritis. Osteoarthritis Cartilage.
17:83–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Young AD, Phipps DE and Astroff AB:
Large-scale double-staining of rat fetal skeletons using Alizarin
Red S and alcian blue. Teratology. 61:273–276. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oostdijk W, Idkowiak J, Mueller JW, House
PJ, Taylor AE, O'Reilly MW, Hughes BA, de Vries MC, Kant SG, Santen
GW, et al: PAPSS2 deficiency causes androgen excess via impaired
DHEA sulfation-in vitro and in vivo studies in a family harboring
two novel PAPSS2 mutations. J Clin Endocrinol Metab. 100:E672–E680.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ikeda T, Mabuchi A, Fukuda A, Hiraoka H,
Kawakami A, Yamamoto S, Machida H, Takatori Y, Kawaguchi H,
Nakamura K and Ikegawa S: Identification of sequence polymorphisms
in two sulfation-related genes, PAPSS2 and SLC26A2, and an
association analysis with knee osteoarthritis. J Hum Genet.
46:538–543. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang CH, Chen CC, Liao CH, Lin FH, Hsu YM
and Fang HW: Human acellular cartilage matrix powders as a
biological scaffold for cartilage tissue engineering with
synovium-derived mesenchymal stem cells. J Biomed Mater Res A.
102:2248–2257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Assche D, Staes F, Van Caspel D,
Vanlauwe J, Bellemans J, Saris DB and Luyten FP: Autologous
chondrocyte implantation versus microfracture for knee cartilage
injury: A prospective randomized trial, with 2-year follow-up. Knee
Surg Sports Traumatol Arthrosc. 18:486–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edwards PK, Ebert JR, Janes GC, Wood D,
Fallon M and Ackland T: Arthroscopic versus open matrix-induced
autologous chondrocyte implantation: Results and implications for
rehabilitation. J Sport Rehabil. 23:203–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Temu TM, Phornphutkul C and Gruppuso P:
Characterization of chondrocyte differentiation of ATDC5 cell line
induced by Ascorbic acid. FASEB J. 24:2010.
|
|
28
|
Takayama Y and Mizumachi K: Inhibitory
effect of lactoferrin on hypertrophic differentiation of ATDC5
mouse chondroprogenitor cells. Biometals. 23:477–484. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kwon HJ, Yasuda K, Ohmiya Y, Honma K, Chen
YM and Gong JP: In vitro differentiation of chondrogenic ATDC5
cells is enhanced by culturing on synthetic hydrogels with various
charge densities. Acta Biomater. 6:494–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Itoh R, Miura S, Takimoto A, Kondo S, Sano
H and Hiraki Y: Stimulatory actions of lysophosphatidic acid on
mouse ATDC5 chondroprogenitor cells. J Bone Miner Metab.
28:659–671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Challa TD, Rais Y and Ornan EM: Effect of
adiponectin on ATDC5 proliferation, differentiation and signaling
pathways. Mol Cell Endocrinol. 323:282–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakatani S, Mano H, Im R, Shimizu J and
Wada M: Glucosamine regulates differentiation of a chondrogenic
cell line, ATDC5. Biol Pharm Bull. 30:433–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cortes M, Singh B, Kurima K and Schwartz
N: PAPS synthetase gene expression relates directly to the murine
Brachymorphism (Bm) phenotype. FASEB J. 17:A1303. 2003.
|
|
34
|
Al Attia HM: HLA tissue typing in patients
with PAPS (Hughes syndrome) evolving into SLE. Lupus. 11:399–400.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shimizu C, Lee YC, Fuda H and Strott CA:
Promoter analysis of human PAPS synthase (PAPSS) 1 and 2. FASEB J.
15:A882. 2001.
|
|
36
|
Gomez JA, Martin H, Amigo MC, Aguirre MA,
Cuadrado MJ, Khamashta MA and Hughes GRV: Long-term follow-up in 90
patients with primary antophospholipid syndrome (PAPS). Do they
develop lupus? Arthritis Rheum-Us. 44:S1462001.
|
|
37
|
Venkatachalam KV: Human
3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase:
biochemistry, molecular biology and genetic deficiency. IUBMB Life.
55:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weber M, deBandt M, Hayem G, Roux S, Kahn
MF and Meyer O: Familial study of primary (PAPS) and secondary
(SAPS) syndrome. Arthritis Rheum-Us. 40:16241997.
|
|
39
|
Krakow D, Sebald ET, Pogue R, Rimoin LP,
King L and Cohn DH: Analysis of clones from a human cartilage cDNA
library provides insight into chondrocyte gene expression and
identifies novel candidate genes for the osteochondrodysplasias.
Mol Genet Metab. 79:34–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Krakow D, Sebald ET, King LM and Cohn DH:
Cartilage expressed sequence tags provide insight into chondrocyte
gene expression and identify novel candidate genes for the
osteochondrodysplasias. Am J Hum Genet. 67:370. 2000.
|
|
41
|
Zhu Z, Yu A, Hou M, Xie X and Li P:
Effects of Sox9 gene therapy on the healing of bone-tendon
junction: An experimental study. Indian J Orthop. 48:88–95. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Raspopovic J, Marcon L, Russo L and Sharpe
J: Modeling digits. Digit patterning is controlled by a
Bmp-Sox9-Wnt turing network modulated by morphogen gradients.
Science. 345:566–570. 2014.
|
|
43
|
Raspaglio G, Petrillo M, Martinelli E,
Puma DDL, Mariani M, De Donato M, Filippetti F, Mozzetti S, Prislei
S, Zannoni GF, et al: Sox9 and Hif-2α regulate TUBB3 gene
expression and affect ovarian cancer aggressiveness. Gene.
542:173–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang H, Zhao X, Zhang Z, Chen W and Zhang
X: An immunohistochemistry study of Sox9, Runx2, and Osterix
expression in the mandibular cartilages of newborn mouse. Biomed
Res Int. 2013:2653802013.PubMed/NCBI
|
|
45
|
Yano F, Kugimiya F, Ohba S, Ikeda T,
Chikuda H, Ogasawara T, Ogata N, Takato T, Nakamura K, Kawaguchi H
and Chung UI: The canonical Wnt signaling pathway promotes
chondrocyte differentiation in a Sox9-dependent manner. Biochem
Biophys Res Commun. 333:1300–1308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yates KE, Shortkroff S and Reish RG: Wnt
influence on chondrocyte differentiation and cartilage function.
DNA Cell Biol. 24:446–457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao Y, Liu S, Huang J, Guo W, Chen J,
Zhang L, Zhao B, Peng J, Wang A, Wang Y, et al: The ECM-cell
interaction of cartilage extracellular matrix on chondrocytes.
Biomed Res Int. 2014:6484592014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dao DY, Jonason JH, Zhang YC, Hsu W, Chen
D, Hilton MJ and O'Keefe RJ: Cartilage-specific β-catenin signaling
regulates chondrocyte maturation, generation of ossification
centers, and perichondrial bone formation during skeletal
development. J Bone Miner Res. 27:1680–1694. 2012. View Article : Google Scholar : PubMed/NCBI
|