Introduction
Chronic obstructive pulmonary disease (COPD) is a
progressive disease characterized by reduced expiratory airflow and
dyspnea on exertion. Gradually progressing breathlessness limits
daily activities and is a major reason for medical treatment in
patients with COPD. COPD has significant systemic effects, such as
weight loss and nutritional abnormalities, which affect exercise
capacity, health-related quality of life (HRQOL), and survival
(1–3). Potential mechanisms of weight loss are
energy imbalance, disuse atrophy, hypoxemia, systemic inflammation,
and hormone insufficiency (3).
Pulmonary rehabilitation (PR) is a useful
non-pharmacological therapy that improves exercise capacity,
dyspnea, and HRQOL in patients with COPD (4). Recent studies report that the
combination of low-intensity exercise and nutritional support
(5–7), or the combination of exercise training
and ghrelin administration (8) may
benefit malnourished patients with COPD. However, poor responders
to nutritional therapy were older, and had lower appetites and more
systemic inflammation (9).
A Japanese herbal medicine, Hochuekkito (i.e.,
TJ-41), is commonly used to treat chronic diseases or weakness
after an illness. It improves the HRQOL and immune status in
elderly individuals who complain of general fatigue or appetite
loss due to chronic wasting disease (10,11). In
animal models of lung injury and viral infection, TJ-41 suppressed
the production of inflammatory cytokines (12–14).
Tatsumi et al (15) also
reported that the administration of TJ-41 improved nutritional
status, decreased the level of cytokines, and decreased the number
of acute exacerbations in patients with COPD. However, to date, the
additive effect of TJ-41 on PR in patients with COPD has not been
well researched.
We hypothesized that the addition of TJ-41 treatment
to PR may benefit malnourished patients with COPD, with respect to
their exercise capacity, dyspnea, and HRQOL. In the present study,
we evaluated the efficacy and safety of TJ-41 in malnourished
patients with COPD who received PR.
Materials and methods
Study design
The study was a 12-week, open-label, randomized,
parallel-group comparative study of TJ-41 administration during PR.
It was conducted at six clinical hospitals from September 2014 to
March 2016. The study was conducted in accordance with the
Declaration of Helsinki guidelines and was approved by the Ethics
Committees of Hiroshima University Hospital (Hiroshima, Japan),
Higashi-Hiroshima Medical Center (Higashi-Hiroshima, Japan),
Hiroshima City Medical Association-administered Hiroshima City Aki
Hospital (Hiroshima, Japan), Mihara Medical Associations Hospital
(Mihara, Japan), Miyoshi Central Hospital (Miyoshi, Japan), and
Tadanoumi Hospital (Takehara, Japan). All participants provided
written informed consent. This study was registered in the
University Hospital Medical Information Network of Japan
(http://www.umin.ac.jp/ctr/; number,
UMIN000015092).
Patients
The inclusion criteria for the current study were as
follows: i) moderate to severe COPD; defined by forced expiratory
volume in 1 sec/forced vital capacity (FEV1/FVC) <70% and FEV1%
predicted to be >30% and <80% [i.e., stages II and III, based
on the classification of the Global Initiative for Chronic
Obstructive Lung Disease (GOLD)] (16); ii) predicted percent ideal body
weight (%IBW) less than 100%; iii) clinically stable disease and
able to participate in PR for 12 weeks; iv) 40 years or older; and
(5) smoking history of more than 10
pack-years.
Patients were excluded if they had any of the
following: i) undergone PR within 24 weeks preceding the study; ii)
a diagnosis of other pulmonary diseases or alpha1-antitrypsin
deficiency; iii) diagnosis of an acute exacerbation within 4 weeks
preceding the study; iv) received pulmonary transplantation; v)
received herbal medicine for any problem within 4 weeks preceding
the study; vi) had newly received bronchodilators or inhaled or
systemic corticosteroids within 2 weeks before the study; vii) a
diagnosis of other severe diseases, such as malignant tumors,
autoimmune disease, liver disease, renal disease, heart disease,
hematologic disease, and metabolic disease; viii) engaged in
another clinical trial within 4 weeks preceding the study; ix)
definitely or possibly pregnant; or x) the physician judged the
patient as unable to participate in the present study.
Study protocol
Randomization for all patients was accomplished
manually by using a central registration system. Patients who met
the eligibility criteria were enrolled and randomly assigned to
receive PR with Group A [i.e., treated with TJ-41 powder (2.5 g;
TJ-41, Extract Granules for Ethical Use, Tsumura Co., Tokyo Japan),
which was taken orally three times per day before each meal or
between meals] or Group B (i.e., untreated with TJ-41 powder).
Randomization to Group A or Group B occurred at a 1:1 ratio, with
stratification by the clinical stage of COPD. Within 4 weeks after
enrollment and randomization, visits to the study institutes were
scheduled on the first day of the administration of the study drug
and PR, and every 2 weeks thereafter, throughout the 12-week
treatment period.
Throughout the study period, patients were permitted
to use a procaterol or salbutamol metered-dose inhaler, as needed,
as well as stable doses of theophylline, long-acting
β2-agonist, long-acting muscarinic antagonist, and
inhaled and oral corticosteroids. To control acute COPD
exacerbations, investigators could administer any additional
medication, such as antibiotics and systemic corticosteroids, as
necessary.
In the present study, PR involved a home-based
low-intensity exercise, as previously reported (6,7).
Exercise training included upper and lower limb exercises,
respiratory muscle stretching calisthenics, and level walking for
at least 20 min. Breathing retraining consisted of pursed-lip
breathing, diaphragmatic breathing, and slow deep breathing in the
supine and sitting positions. Patients were strongly instructed to
practice this program daily at home and were supervised by
respiratory therapists every 2 weeks. Relaxation and stair climbing
exercises were also performed under the supervision of the
respiratory therapists. The completion rate of PR was also checked
when the patients visited the hospital.
Test drug
TJ-41 was obtained from the Ibaraki Plant of Tsumura
and Co. (Tokyo, Japan). TJ-41 contains spray-dried hot water
extracts of a mixture of the following medicinal plants: Astragali
radix (16.7%), Atractylodis lanceae rhizome (16.7%), Ginseng radix
(16.7%), Angelicase radix (12.5%), Bupleuri radix (8.3%), Zizyphi
fructus (8.3%), Aurantii nobilis pericarpium (8.3%), Glycyrrhizae
radix (6.3%), Cimicifugae rhizome (4.2%), and Zingiberis rhizoma
(2.0%).
Outcome measurements
The primary outcome was a change in the 6-min walk
distance (6MWD). Secondary outcomes were a change in body weight,
%IBW, body mass index (BMI), fat-free mass (FFM), pulmonary
function, peripheral muscle strength, blood tests, modified Medical
Research Council (mMRC) dyspnea scale score, visual analog scale
(VAS) score for dyspnea, VAS score for fatigue, COPD assessment
test (CAT) score, and the number of acute exacerbations.
The 6MWD was measured based on the ATS/ERS criteria
(17,18). Body height was measured to the
nearest 0.5 cm, and weight was measured to the nearest 0.1 kg,
using a weight and body composition meter (Omron, Kyoto, Japan).
The FFM was estimated using a bioelectrical impedance analysis. The
BMI was calculated as the weight in kilograms divided by the square
of the height value in meters. Pulmonary function parameters were
measured based on the guidelines of ATS/ERS criteria (16,19). For
the muscle force parameters, peripheral muscle strength was
measured by using the maximal voluntary handgrip maneuver. Dyspnea
was assessed using the mMRC dyspnea scale, which is a questionnaire
consisting of five statements about an individual's perceived
breathlessness (16,20). The severity of dyspnea in the
patients were subjectively evaluated using the VAS, which consisted
of a 10-cm horizontal line, indicating a score of 0 (i.e., no
dyspnea) on the left end and 10 (i.e., extremely strong) on the
right. In addition, the severity of fatigue was also assessed using
a VAS. Each patient's HRQOL was evaluated using the CAT, which is a
questionnaire used to assess the impact of COPD on the patient's
health status (21,22). The CAT has eight questions that
assess cough, phlegm production, chest tightness, breathlessness,
activity limitation, confidence, sleep, and energy. In blood tests,
serum albumin level, hemoglobin level, white blood cell count, and
lymphocyte counts (which are markers representing the nutritional
or immunological status of patients) were measured. An acute
exacerbation was defined as a complex of respiratory symptoms
(i.e., new onset or an increase in at least one of the following
symptoms: cough, sputum production, dyspnea, wheeze, and chest
discomfort) lasting at least 3 days. These were generally treated
with antibiotics and/or oral steroids (16,23).
The 6MWD, pulmonary function, mMRC dyspnea scale
score, and blood tests were measured at baseline and 12 weeks after
treatment. Body composition parameters, peripheral muscle strength,
VAS score for dyspnea, and VAS score for fatigue were measured at
baseline, and subsequently, every 4 weeks.
Safety measurements
Adverse events were assessed throughout the
12−week study period. Physical examinations and blood
tests, such as liver function tests (e.g., levels of alanine
aminotransferase, aspartate aminotransferase, alkaline phosphatase,
γ-glutamyl transpeptidase, and total bilirubin) and the potassium
level were measured as markers representing adverse events. These
markers were measured at baseline, and subsequently, every 4
weeks.
Statistical analysis
Results are presented as the mean ± standard
deviation. Partially missing data of the clinical evaluation were
carried forward using the principle of the Last Observation Carried
Forward. Data between the groups were compared using Fisher's exact
tests and Mann-Whitney U tests. Data at baseline and 12 weeks were
compared using a Wilcoxon signed-ranks test. Statistical analyses
were performed using IBM SPSS version 21 software for Windows (IBM
Japan, Tokyo, Japan). Statistical significance was set at
P<0.05.
Results
Patients' characteristics
A total of 35 patients were randomized in the
current study; 2 patients (1 patient at stage II and 1 patient at
stage III) in Group B were excluded from the analysis because of
withdrawal. Thus, 33 patients were finally enrolled in this study.
A total of 18 patients (7 patients at stage II and 11 patients at
stage III) received TJ-41 (i.e., Group A), while 15 patients (6
patients at stage II and 9 patients at stage III) did not receive
TJ-41 (i.e., Group B).
At baseline, there were no significant differences
in age, BMI, and medical treatment between the groups (Table I). In addition, no significant
differences were observed in FFM, FEV1% predicted, 6MWD, mMRC
dyspnea score, VAS score for fatigue, total CAT score, and serum
albumin and hemoglobin levels between the groups at baseline.
 | Table I.Baseline characteristics of patients
with chronic obstructive pulmonary disease. |
Table I.
Baseline characteristics of patients
with chronic obstructive pulmonary disease.
| Variables | Group A (n=18) | Group B (n=15) |
|---|
| Age (years) | 75.3±6.1 | 74.7±7.1 |
| Sex |
|
|
|
Male | 16 (88.9) | 14 (93.3) |
|
Female | 2 (11.1) | 1 (6.7) |
| Height (cm) | 161.8±6.1 | 159.0±7.9 |
| Body weight
(kg) | 49.5±7.7 | 50.0±4.8 |
| Body mass
index | 18.9±2.4 | 19.8±1.4 |
| GOLD stage |
|
|
| Stage
II | 7 (38.9) | 6 (40.0) |
| Stage
III | 11 (61.1) | 9 (60.0) |
| Medical
treatment |
|
|
| Use of
LABA | 14 (77.8) | 12 (80.0) |
| Use of
LAMA | 14 (77.8) | 12 (80.0) |
| Use of
ICS | 7 (38.9) | 8 (53.3) |
| Use of
theophylline | 2 (11.1) | 1 (6.7) |
Outcomes
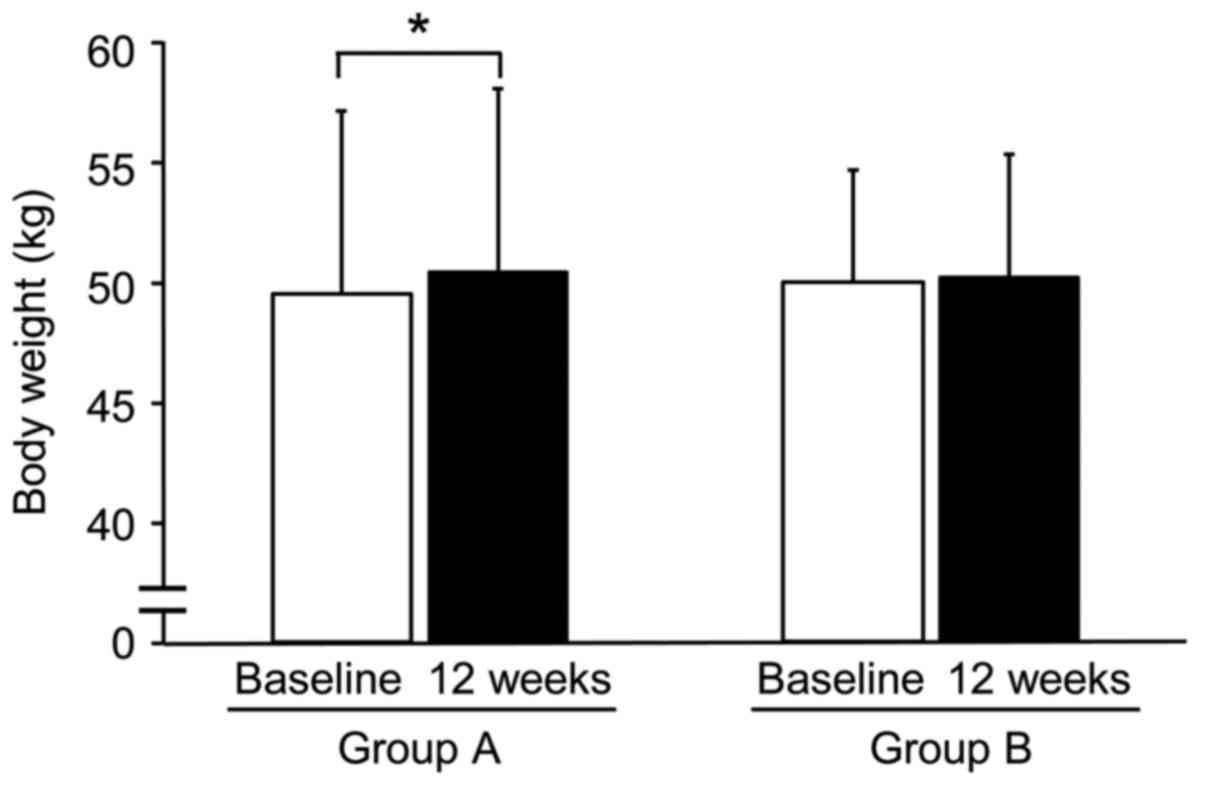
The body weight (Fig.
1) and %IBW were significantly increased after 12 weeks of
treatment in Group A, but not in Group B. There were no significant
differences in either group in the other parameters of body
composition, between baseline and after the 12-week treatment. The
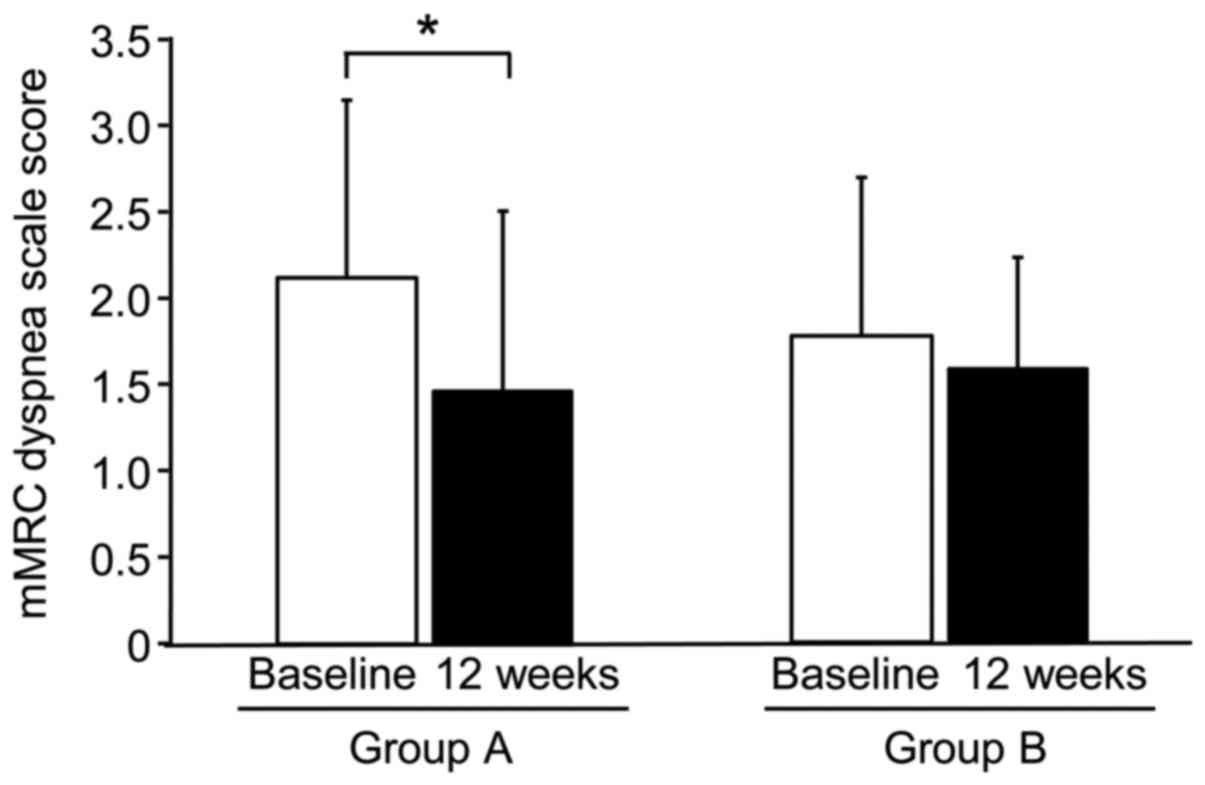
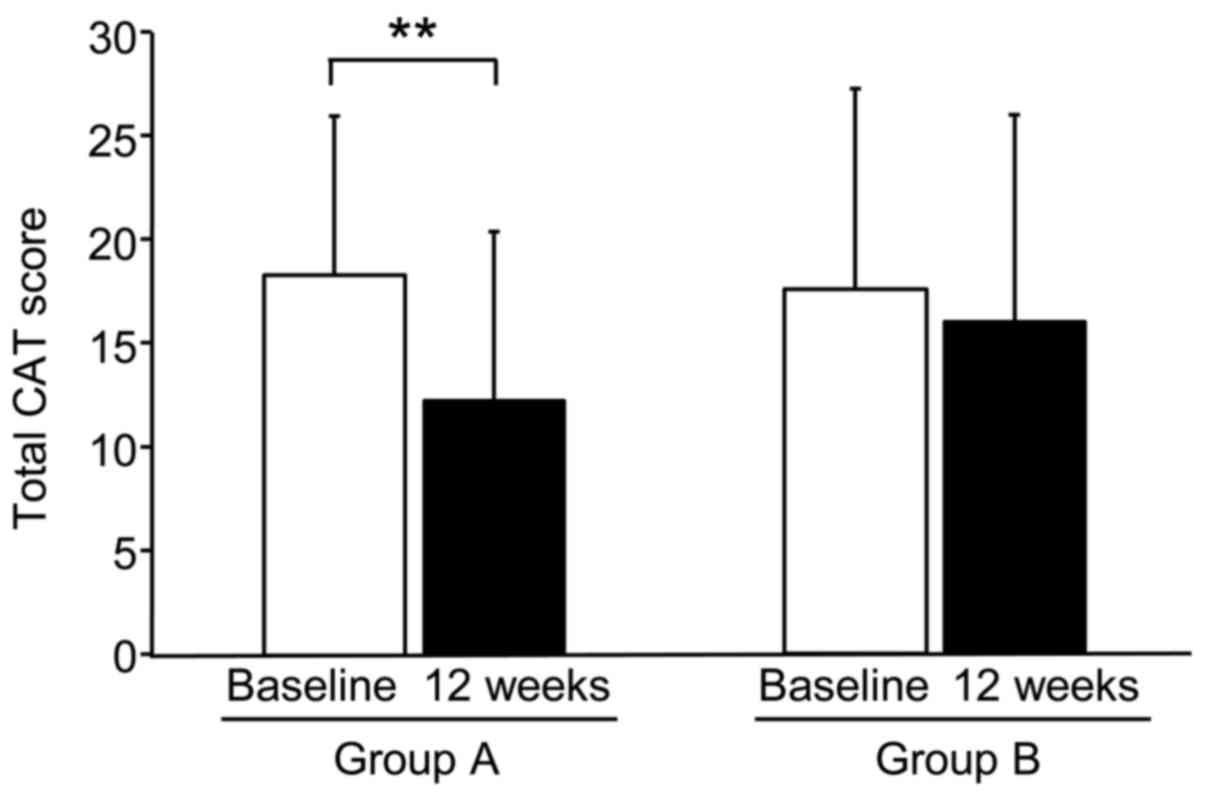
mMRC dyspnea scores (Fig. 2), VAS
scores for dyspnea, VAS scores for fatigue, and total CAT scores
(Fig. 3) were significantly
decreased after 12 weeks of treatment in Group A, but not in Group
B. In particular, the CAT scores of 7 of 8 individuals in Group A
were significantly decreased after 12 weeks of treatment. No
significant differences were noted in the 6MWD, peripheral muscle
strength, and blood markers between baseline and 12 weeks after
treatment in either group. The values of FEV1 in Group B were
increased after 12 weeks of treatment; however, the predicted
values of FEV1 were not increased. Table II shows changes in the parameters of
body composition, pulmonary function, muscle force, respiratory
symptoms, HRQOL, and blood markers.
 | Table II.Changes in body composition, muscle
force, health-related quality of life and blood tests. |
Table II.
Changes in body composition, muscle
force, health-related quality of life and blood tests.
|
| Group A (n=18) | Group B (n=15) |
|---|
|
|
|
|
|---|
| Variable | Baseline | 12 weeks | Baseline | 12 weeks |
|---|
| Body weight
(kg) | 49.5±7.7 |
50.1±8.1b | 50.0±4.8 | 50.1±5.3 |
| Body mass
index | 18.9±2.4 | 19.1±2.5 | 19.8±1.4 | 19.8±1.7 |
| Ideal body weight
(% predicted) | 85.0±10.7 |
86.8±11.2b | 89.9±6.6 | 90.2±7.7 |
| Fat free mass
(kg) | 38.9±5.2 | 39.1±5.4 | 38.5±5.3 | 38.9±5.3 |
| FVC (l) | 2.3±0.8 | 2.3±0.7 | 2.6±0.7 | 2.7±0.6 |
| FEV1 (l) | 1.1±0.5 | 1.0±0.5 | 1.1±0.5 |
1.2±0.5b |
| FEV1 (%
predicted) | 46.9±18.4 | 44.9±18.6 | 49.5±15.8 | 51.3±16.2 |
| FEV1/FVC (%) | 47.1±14.4 | 44.3±11.2 | 42.4±7.9 | 44.4±9.0 |
| 6MWD (m) | 335.3±102.1 | 341.8±103.8 | 363.7±98.7 | 359.0±125.1 |
| Handgrip strength
(kg) | 28.4±6.5 | 28.2±6.1 | 27.7±8.0 | 28.7±8.0 |
| mMRC dyspnea scale
score | 2.1±1.0 |
1.5±1.0b | 1.8±0.9 | 1.6±0.6 |
| VAS score for
dyspnea |
5.4±2.4a |
3.7±2.4b | 4.0±1.7 | 4.3±3.1 |
| VAS score for
fatigue | 4.8±2.4 |
3.5±2.3b | 4.3±1.8 | 4.4±2.3 |
| Total CAT
score | 18.4±7.4 |
12.2±8.1c | 17.8±9.4 | 16.2±9.9 |
| Score
of cough | 2.2±1.5 |
1.1±1.5c | 1.4±1.5 | 1.8±1.6 |
| Score
of production of phlegm | 1.6±1.6 |
1.0±1.4b | 2.1±1.9 | 2.2±1.7 |
| Score
of chest tightness | 2.6±1.5 |
1.8±1.3b | 2.6±1.2 | 2.1±1.5 |
| Score
of breathlessness | 3.8±1.2 |
2.9±1.3b | 3.6±1.3 | 3.4±1.3 |
| Score
of activity limitation | 1.6±1.5 | 1.2±1.3 | 1.9±1.7 | 1.5±1.7 |
| Score
of confidence | 1.8±1.7 |
1.2±1.3b | 1.9±1.9 | 1.4±1.7 |
| Score
of sleep | 1.9±1.5 |
1.0±1.2b | 1.7±1.5 | 1.9±1.4 |
| Score
of energy | 2.8±1.4 |
1.9±1.4b | 2.6±1.5 | 2.0±1.3 |
| Serum albumin
(g/dl) | 4.3±0.2 | 4.2±0.3 | 4.1±0.3 | 4.0±0.3 |
| Hemoglobin
(g/dl) | 13.7±1.6 | 13.6±1.8 | 13.9±1.3 | 13.8±1.1 |
| Lymphocyte counts
(/µl) | 1794.0±517.4 | 1809.9±827.0 | 1793.0±671.0 | 1724.6±716.0 |
Adverse Events
No patient experienced TJ-41 treatment-related
adverse events, such as liver dysfunction and hypokalemia. One
patient in Group A died due to dissection of an abdominal aneurysm.
One patient in Group B developed pneumonia and required
hospitalization. Two patients in Group B experienced acute
exacerbations of COPD, but no patients in Group A had such an
experience.
Discussion
In the present study, adding TJ-41 treatment to PR
significantly increased a patient's body weight, and significantly
decreased the mMRC dyspnea scores, VAS scores for dyspnea, VAS
scores for fatigue, and total CAT scores of patients with COPD.
TJ-41 combined with PR may benefit malnourished patients with COPD,
with respect to dyspnea and HRQOL.
The addition of TJ-41 treatment to PR may have
beneficial effects on dyspnea scores and HRQOL scores in
malnourished COPD patients. A previous meta-analysis (24) demonstrated the efficacy of the
Chinese herbal medicine, Bu-Zhong-Yi-Qi-Tang (BZYQT), in patients
with stable COPD. Many studies have examined the utility of drugs
similar to TJ-41 in this population, but the benefits of TJ-41 in
patients with stable COPD remain largely unexplored. The
investigators in two studies reported that BZYQT improved CAT
scores (24). Tatsumi et al
(15) reported that after 6 months
of treatment, the symptoms score on the St. George's Respiratory
Questionnaire decreased in patients with COPD receiving TJ-41, but
did not decrease in the control group. This finding indicated that
TJ-41 improved HRQOL in patients with COPD. Furthermore, the
beneficial effects of TJ-41 on cancer-related fatigue and HRQOL in
patients with cancer have also been reported (25). In the present study, the mMRC dyspnea
scores, VAS scores for dyspnea, VAS scores for fatigue, and total
CAT scores significantly decreased after 3 months of treatment in
Group A, but not in Group B. The scores of individual CAT
parameters, including sleep and pulmonary symptoms, significantly
decreased in Group A.
TJ-41 improved systemic inflammation and nutritional
status. It suppressed the production of inflammatory cytokines in
animal models of lung injury and viral infection (12–14). In
patients with COPD, Tatsumi et al (15) reported decreased serum levels of
inflammatory proteins, such as C-reactive protein, tumor necrosis
factor alpha, and interleukin-6, and increased serum levels of
prealbumin, a nutritional marker, after 6 months of TJ-41
treatment; however, these levels remained unchanged in the control
group. They also showed that body weight increased from baseline to
the end of the trial in the TJ-41 group, but remained unchanged in
the control group. Compared to the control group, patients with
Mycobacterium avium complex disease showed increased body
weight and serum albumin levels when treated with TJ-41 (26). In the present study, body weight and
%IBW in Group A were significantly increased after 3 months of
treatment, but not in Group B. However, there was no significant
change in serum albumin levels in either group.
The reason for the improvement in the dyspnea scores
and HRQOL scores after TJ-41 administration in patients with COPD
is unclear. Improvements in systemic inflammation and the
nutritional status may have ameliorated dyspnea scores and HRQOL
scores in patients with COPD. Improvements in the scores of
dyspnea, cough, and breathlessness could be attributed to the
decrease of phlegm production, based on the anti-inflammatory
effects of TJ-41. HRQOL seems to have consequently increased due to
the improvement of these scores. In addition, Katsura et al
(27) reported that low body weight
in patients with COPD is associated with worsening dyspnea and a
deterioration in generic and disease-specific HRQOL. Further, the
antidepressive effect of TJ-41 may improve the dyspnea score and
HRQOL score in patients with COPD. It was reported that large
numbers of patients with COPD had clinically significant levels of
depression and/or anxiety (28,29).
Tohda and Mingmalairak (30)
reported that TJ-41 had an antidepressive effect in a mouse model
of depression with learned helplessness behavior. Further study is
needed to clarify the antidepressive effect of TJ-41 in patients
with COPD.
Although several studies (24) demonstrated the efficacy of BZYQT on
the 6MWD in stable patients with COPD, the addition of TJ-41
treatment to PR did not improve exercise capacity in patients with
COPD in the current study. The reason for this discrepancy is
unclear. In our study, TJ-41 increased body weight, but not FFM or
muscle strength in patients with COPD, which may have contributed
to our discrepant findings. It was reported that the reduction in
FFM is a better predictor of exercise performance compared to the
BMI (31,32). In addition, patients with a low FFM
often have muscular weakness, especially in the lower limbs
(33,34). Further study is needed to clarify the
effects of TJ-41 on exercise capacity in patients with COPD.
Several limitations in the current study must be
noted. First, it only included a small number of patients. We
screened approximately double the number of enrolled malnourished
patients with COPD, but many patients were excluded due to several
reasons, such as taking herbal medicines, having a diagnosis of
severe diseases or orthopedic diseases, and refusal to participate
in the study. Second, our study was an open label study without the
administration of a placebo. It was very difficult to produce a
placebo for an herbal medicine that contains many active
ingredients. In addition, this is the first pilot study to evaluate
the effect of an herbal medicine combined with PR in patients with
COPD. Thus, we designed it as an open-label, randomized,
parallel-group comparative study. Larger randomized
placebo-controlled studies are required to validate the findings of
this study.
Our study demonstrated that the addition of TJ-41
treatment to PR improved body weight, dyspnea scores, and HRQOL
scores in patients with COPD. No adverse events were observed after
12 weeks of TJ-41 treatment. TJ-41 combined with PR may be a new
therapeutic strategy to treat patients with COPD.
Acknowledgements
The abstract was presented at the European
Respiratory Society International Congress 2017, September 9–13
2017 in Milan, and published as abstract no. PA3712 in European
Respiratory Journal 50 (suppl 61), 2017.
Funding
The present study was supported by the Fundamental
Budget for Education and Research and the pharmaceutical company
Tsumura and Co., which provided the TJ-41.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HH, KS and NH designed the study and drafted the
manuscript. HH and KS participated in the statistical design and
analyses. HH, IM, KaK, KO, YA, KeK and TO recruited patients in the
hospitals. KS, KA, TS, TD, MW and HY engaged in PR. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics committees of
Hiroshima University Hospital, Higashi-Hiroshima Medical Center,
Hiroshima City Medical Association-administered Hiroshima City Aki
Hospital, Mihara Medical Associations Hospital, Miyoshi Central
Hospital, and Tadanoumi Hospital. All participants provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The present study received partial financial support
from the pharmaceutical company Tsumura and Co. However, the
company had no control over the interpretation, writing, or
publication of the present study. The authors have no competing
interests directly relevant to the content of this article.
Glossary
Abbreviations
Abbreviations:
|
6MWD
|
6-min walk distance
|
|
BZYQT
|
Bu-Zhong-Yi-Qi-Tang
|
|
CAT
|
chronic obstructive pulmonary disease
assessment test
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
FEV1
|
forced expiratory volume in 1 sec
|
|
FFM
|
fat-free mass
|
|
FVC
|
forced vital capacity
|
|
GOLD
|
Global Initiative for Chronic
Obstructive Lung Disease
|
|
HPLC
|
high-performance liquid
chromatography
|
|
HRQOL
|
health-related quality of life
|
|
IBW
|
ideal body weight
|
|
mMRC
|
modified Medical Research Council
|
|
PR
|
pulmonary rehabilitation
|
|
VAS
|
visual analog scale
|
References
|
1
|
Schols A: Nutritional modulation as part
of the integrated management of chronic obstructive pulmonary
disease. Proc Nutr Soc. 62:783–791. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao C, Wang R, Wang J, Bunjhoo H, Xu Y and
Xiong W: Body mass index and mortality in chronic obstructive
pulmonary disease: A meta-analysis. PLoS One. 7:e438922012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wagner PD: Possible mechanisms underlying
the development of cachexia in COPD. Eur Respir J. 31:492–501.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCarthy B, Casey D, Devane D, Murphy K,
Murphy E and Lacasse Y: Pulmonary rehabilitation for chronic
obstructive pulmonary disease. Cochrane Database Syst Rev.
CD003793:2015.
|
|
5
|
Ferreira IM, Brooks D, White J and
Goldstein R: Nutritional supplementation for stable chronic
obstructive pulmonary disease. Cochrane Database Syst Rev.
12:CD0009982012.PubMed/NCBI
|
|
6
|
Sugawara K, Takahashi H, Kasai C, Kiyokawa
N, Watanabe T, Fujii S, Kashiwagura T, Honma M, Satake M and Shioya
T: Effects of nutritional supplementation combined with
low-intensity exercise in malnourished patients with COPD. Respir
Med. 104:1883–1889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugawara K, Takahashi H, Kashiwagura T,
Yamada K, Yanagida S, Homma M, Dairiki K, Sasaki H, Kawagoshi A,
Satake M and Shioya T: Effect of anti-inflammatory supplementation
with whey peptide and exercise therapy in patients with COPD.
Respir Med. 106:1526–1534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miki K, Maekura R, Nagaya N, Nakazato M,
Kimura H, Murakami S, Ohnishi S, Hiraga T, Miki M, Kitada S, et al:
Ghrelin treatment of cachectic patients with chronic obstructive
pulmonary disease: A multicenter, randomized, double-blind,
placebo-controlled trial. PLoS One. 7:e357082012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Creutzberg EC, Schols AM, Weling-Scheepers
CA, Buurman WA and Wouters EF: Characterization of nonresponse to
high caloric oral nutritional therapy in depleted patients with
chronic obstructive pulmonary disease. Am J Respir Crit Care Med.
161:745–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuroiwa A, Liou S, Yan H, Eshita A, Naitoh
S and Nagayama A: Effect of a traditional Japanese herbal medicine,
hochu-ekki-to (Bu-Zhong-Yi-Qi Tang), on immunity in elderly
persons. Int Immunopharmacol. 4:317–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Satoh N, Sakai S, Kogure T, Tahara E,
Origasa H, Shimada Y, Kohoda K, Okubo T and Terasawa K: A
randomized double blind placebo-controlled clinical trial of
Hochuekkito, a traditional herbal medicine, in the treatment of
elderly patients with weakness N of one and responder restricted
design. Phytomedicine. 12:549–554. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mori K, Kido T, Daikuhara H, Sakakibara I,
Sakata T, Shimizu K, Amagaya S, Sasaki H and Komatsu Y: Effect of
Hochu-ekki-to (TJ-41), a Japanese herbal medicine, on the survival
of mice infected with influenza virus. Antiviral Res. 44:103–111.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tajima S, Bando M, Yamasawa H, Ohno S,
Moriyama H, Takada T, Suzuki E, Gejyo F and Sugiyama Y: Preventive
effect of Hochu-ekki-to on lipopolysaccharide-induced acute lung
injury in BALB/c mice. Lung. 184:318–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tajima S, Bando M, Yamasawa H, Ohno S,
Moriyama H, Terada M, Takada T, Suzuki E, Gejyo F and Sugiyama Y:
Preventive effect of hochu-ekki-to, a Japanese herbal medicine, on
bleomycin-induced lung injury in mice. Respirology. 12:814–822.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tatsumi K, Shinozuka N, Nakayama K, Sekiya
N, Kuriyama T and Fukuchi Y: Hochuekkito improves systemic
inflammation and nutritional status in elderly patients with
chronic obstructive pulmonary disease. J Am Geriatr Soc.
57:169–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Global initiative for chronic obstructive
lung disease: Global strategy for the diagnosis, management, and
prevention of chronic obstructive pulmonary disease. 2018 Report.
http://www.goldcopd.orgJuly
12–2018
|
|
17
|
ATS Committee on Proficiency Standards for
Clinical Pulmonary Function Laboratories, . ATS statement:
Guidelines for the six-minute walk test. Am J Respir Crit Care Med.
166:111–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holland AE, Spruit MA, Troosters T, Puhan
MA, Pepin V, Saey D, McCormack MC, Carlin BW, Sciurba FC, Pitta F,
et al: An official European Respiratory Society/American Thoracic
Society technical standard: Field walking tests in chronic
respiratory disease. Eur Respir J. 44:1428–1446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller MR, Hankinson J, Brusasco V, Burgos
F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP,
Gustafsson P, et al: Standardisation of spirometry. Eur Respir J.
26:319–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Celli BR and MacNee W; ATS/ERS Task Force,
. Standards for the diagnosis and treatment of patients with COPD:
A summary of the ATS/ERS position paper. Eur Respir J. 23:932–946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones PW, Harding G, Berry P, Wiklund I,
Chen WH and Kline Leidy N: Development and first validation of the
COPD Assessment Test. Eur Respir J. 34:648–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glaxo Smith Kline group of companies. COPD
Asessment Test 2016. http://www.catestonline.orgJuly 12–2018
|
|
23
|
Vogelmeier C, Hederer B, Glaab T, Schmidt
H, Rutten-van Mölken MP, Beeh KM, Rabe KF and Fabbri LM; POET-COPD
investigators, . Tiotropium versus salmeterol for the prevention of
exacerbations of COPD. N Engl J Med. 364:1093–1103. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Shergis JL, Wu L, Yu X, Zeng Q, Xu
Y, Guo X, Zhang AL, Xue CC and Lin L: A systematic review and
meta-analysis of the herbal formula Buzhong Yiqi Tang for stable
chronic obstructive pulmonary disease. Complement Ther Med.
29:94–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong JS, Ryu BH, Kim JS, Park JW, Choi WC
and Yoon SW: Bojungikki-tang for cancer-related fatigue: A pilot
randomized clinical trial. Integr Cancer Ther. 9:331–338. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Enomoto Y, Hagiwara E, Komatsu S,
Nishihira R, Baba T, Kitamura H, Sekine A, Nakazawa A and Ogura T:
Pilot quasi-randomized controlled study of herbal medicine
Hochuekkito as an adjunct to conventional treatment for progressed
pulmonary Mycobacterium avium complex disease. PLoS One.
9:e1044112014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katsura H, Yamada K and Kida K: Both
generic and disease specific health-related quality of life are
deteriorated in patients with underweight COPD. Respir Med.
99:624–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kunik ME, Roundy K, Veazey C, Souchek J,
Richardson P, Wray NP and Stanley MA: Surprisingly high prevalence
of anxiety and depression in chronic breathing disorders. Chest.
127:1205–1211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanania NA, Müllerova H, Locantore NW,
Vestbo J, Watkins ML, Wouters EF, Rennard SI and Sharafkhaneh A;
Evaluation of COPD Longitudinally to Identify Predictive Surrogate
Endpoints (ECLIPSE) study investigators, . Evaluation of COPD
Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE)
study investigators. Determinants of depression in the ECLIPSE
chronic obstructive pulmonary disease cohort. Am J Respir Crit Care
Med. 183:604–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tohda M and Mingmalairak S: Evidence of
antidepressive effects of a Wakan-yaku, Hochuekkito, in depression
model mice with learned-helplessness behavior. Evid Based
Complement Alternat Med. 2013:3190732013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baarends EM, Schols AM, Mostert R and
Wouters EF: Peak exercise response in relation to tissue depletion
in patients with chronic obstructive pulmonary disease. Eur Respir
J. 10:2807–2813. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sabino PG, Silva BM and Brunetto AF:
Nutritional status is related to fat-free mass, exercise capacity
and inspiratory strength in severe chronic obstructive pulmonary
disease patients. Clinics (Sao Paulo). 65:599–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Franssen FM, Broekhuizen R, Janssen PP,
Wouters EF and Schols AM: Limb muscle dysfunction in COPD: Effects
of muscle wasting and exercise training. Med Sci Sports Exerc.
37:2–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Teopompi E, Tzani P, Aiello M, Ramponi S,
Andrani F, Marangio E, Clini E and Chetta A: Fat-free mass
depletion is associated with poor exercise capacity irrespective of
dynamic hyperinflation in COPD patients. Respir Care. 59:718–725.
2014. View Article : Google Scholar : PubMed/NCBI
|