Introduction
Schizophrenia is a serious psychotic disease which
leads to both physical and psychotic symptoms (1). Treatments for schizophrenia cost US$ 94
million-102 billion worldwide annually (2), with atypical antipsychotic drugs,
including olanzapine, risperidone and aripiprazole, representing
the most commonly administered medicines due to their abilities to
control positive syndromes, including hallucination and delusion,
and negative syndromes, including emotional blunting, emotional
withdrawal and emotional exchange disorder, of schizophrenia
(3). In addition, these drugs reduce
side effects, including extrapyramidal reactions caused by typical
antipsychotic drugs. (3). However,
the responses to these drugs vary markedly between patients, which
makes it difficult for doctors to predict drug effects (4,5).
Paliperidone (PAL) is the metabolite of risperidone
and is a relatively novel atypical antipsychotic drug that is
effective in improving the positive and negative syndrome of
schizophrenia, and can also improve the associated cognitive
impairment (6,7). Similarly to other atypical
antipsychotics, the responses to PAL vary widely; the effective PAL
daily dose typically varies from 3–15 mg, and 13–26% patients
reportedly exhibit side effects, including dyskinesia, in the
extrapyramidal system (8,9). There is therefore a need to identify
the mechanisms underlying the differences in individual responses
to PAL treatment.
A number of factors can affect the outcome of
antipsychotic medication, with one of the most important being the
drug penetration into the brain. Atypical antipsychotic drugs have
to cross the blood-brain barrier (BBB) prior to exerting their
effects, with their concentration within the brain being affected
by the drug molecular weight and lipophilicity, and the presence of
transport proteins in the BBB (10).
There are a number of efflux proteins in the BBB, such as
P-glycoprotein (P-gp), multidrug resistance-associated protein
(MRP), breast cancer resistance protein (BCRP), organic
anion-transporting polypeptides (OATs), and organic anion
transporting polypeptides (OATPs) (11,12). As
PAL exists as a positive ion, MRP, OATs and OATPs, which mainly
efflux negative ions, might not influence its penetration into the
brain (13). Both in vitro
and in vivo studies have demonstrated that P-gp may impede
the penetration of PAL into the brain, with the ATP binding
cassette (ABC) subfamily B member 1 genetic polymorphism possibly
influencing the plasma concentration of PAL (14,15).
BCRP is a member of the ABC superfamily that has been associated
with the phenomenon of multidrug resistance (16). Wang et al (17) identified that PAL can inhibit the
function of BCRP in vitro, but the efflux effect of BCRP on
PAL has not yet been reported.
In the present study the affinity of PAL with BCRP
was investigated in vitro, and the uptake and transport of
PAL in 293 and 293/BCRP cells, porcine renal endothelial cell
(LLC-PK1) and LLC-PK1/BCRP cell monolayers was also
investigated.
Materials and methods
Materials
Human BCRP (Arg482) membranes (5 mg/ml) and a
control membrane preparation for ABC transporters were purchased
from Gentest; Corning Incorporated (Corning, NY, USA). Anti-BCRP
mouse monoclonal antibody (clone BXP21; cat. no. MC-236) and
horseradish peroxidase-labeled goat anti-mouse secondary antibody
(cat. no. 5220-0341) were purchased from Kamiya Biomedical Company
(Tukwila, WA, USA) and SeraCare Life Sciences (Milford, MA, USA),
respectively. β-Actin mouse monoclonal antibody (cat. no. AF0003)
was purchased from Beyotime Institute of Biotechnology (Shanghai,
China). PAL, Ko143, sulfasalazine, and dimethyl sulfoxide were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Fetal bovine serum (FBS), trypsin, Dulbecco's modified Eagle's
medium (DMEM), Ham's F12 nutrient (F12) medium, PBS and TRIzol
reagent were obtained from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Microplates (with 6 and 96 wells) and cell
culture flasks were obtained from Corning Costar; Merck KGaA.
Methanol of high-performance liquid chromatography (HPLC) grade was
obtained from Merck KGaA and the other solvents used were of
analytical grade. The lentiviral vector encoding the BCRP gene was
purchased from Shanghai GeneChem Co., Ltd. (Shanghai, China).
Cell culture and transfection
The porcine renal endothelial cell line LLC-PK1 and
LLC-PK1/BCRP cells were provided by Professor Zeng Su from the
College of Pharmaceutical Sciences, Zhejiang University (Hangzhou,
China). LLC-PK1/BCRP cells are transgenic cells that were
established by using liposome as vehicles to import human BCRP
genes into LLC-PK1 cells (18). Both
types of cell were cultured in F12 medium supplemented with 20%
FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in an
atmosphere of 5% CO2 and 95% relative humidity. Cells
were supplemented with fresh media every 2–3 days. All of the
cultured cells used in subsequent experiments were at passages
5–25.
In addition, 293 cells were seeded in 6-well plates
at a density of 1×104 cells/well and were cultured in
DMEM supplemented with 20% FBS, 100 U/ml penicillin, and 100 µg/ml
streptomycin at 37°C in an atmosphere of 5% CO2 and 95%
relative humidity. Cells were exposed to lentiviral vector encoding
the BCRP gene with a green fluorescence protein (GFP)-tag at a
multiplicity of infection of 50 in the presence of 8 µg/ml
polybrene (Sigma-Aldrich; Merck KGaA) added to enhance the
transduction efficiency. The transfection medium was replaced with
DMEM after 24 h. Cells were observed under a fluorescence
microscope at 4 days following transfection, at which time >90%
of the cells were found to be positive for green fluorescence
protein-tag fluorescence. Both 293 cells and BCRP-transfected 293
(293/BCRP) cells were then cultured in DMEM supplemented with 20%
FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in an
atmosphere of 5% CO2 and 95% relative humidity. Cells
were supplemented with fresh media every 2–3 days. All of the
cultured cells used in the subsequent experiments were at passages
2–5.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analyses
RT-qPCR was used to detect gene expression levels in
293 cells and lentivirus-BCRP-transduced 293 cells at 10 and 20
days following transduction. Total RNA was isolated from cells
using TRIzol reagent (Life Technologies; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RNA samples (0.5
µg) were used for RT using the Prime Script™ 174 RT reagent kit
(Takara, Biotechnology, Dalian, China) according to the
manufacturer's instructions. SYBR Green Realtime PCR Master mix
(Qiagen, Inc., Valencia, CA, USA) was used to detect the expression
level of ABC subfamily G member 2 (ABCG2), with a 20 µl
reaction mixture containing 2 µl synthesized cDNA, 10 µl SYBR
Premix Ex Taq, 0.2 µl each primer and 7.6 µl ddH2O. The
cycling conditions were as follows: Initial denaturation at 95°C
for 30 sec followed by 36 cycles of 95°C for 5 sec and 60°C for 30
sec. The primers used for human ABCG2 were forward,
5′-GTTGTGATGGGCACTCTGAC-3′ and reverse),
5′-CCCTGTTAATCCGTTCGTTT-3′; and for β-actin forward,
5′-CTCTTCCAGCCTTCCTTCCT-3′ and reverse), 5′-AGCACTGTGTTGGCGTACAG-3′
were used for human β-actin (GenBank accession no. NM 01101). Each
sample was analyzed in triplicate. Endogenous β-actin mRNA was used
to determine the relative quantitative expression using the
2−ΔΔCq method (19).
Western blotting
293 and lentivirus-BCRP-transduced 293 cells were
cultured in 6-well plates for harvesting the cells and the cell
monolayer was lysed at 4°C for 30 min in radioimmunoprecipitation
assay buffer with protease inhibitors (Sigma-Aldrich; Merck KGaA)
and the amount of protein was determined by bicinchoninic acid
assay. Proteins (60 µg) were separated on 12% SDS-PAGE gels and
transferred onto a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). The membrane was blocked in
Dulbecco's PBS containing 0.1% Tween-20 and 5% non-fat dry milk
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) for 2 h at room
temperature. The PVDF membranes were then washed three times for 10
min with tris-buffered saline with Tween-20 (TBST) buffer and
incubated overnight at room temperature with a mouse monoclonal
antibody (1:1,000) against human BCRP and β-actin. Following
extensive washing, the membranes were further incubated with
secondary antibody (1:5,000) for 1 h at room temperature. The
membranes were then washed three times with TBST and
immunoreactivity was visualized using an enhanced chemiluminescence
kit and the membranes were exposed to photographic film (Kodak,
Rochester, NY, USA). Protein levels were expressed as the ratio of
the band intensities of BCRP to the endogenous control β-actin.
Each sample was analyzed in triplicate.
Cellular accumulation
Intracellular accumulation experiments were
conducted with 293 and 293/BCRP cells. Cells were cultured in
6-well plates at a density of 2×105 cells/well for 2
days and on the day of the experiment the culture medium was
replaced with DMEM with, containing PAL at varying concentrations
(0.1, 1, 10, 25 and 50 µM) with or without BCRP inhibitor Ko143 (5
µM). Following incubation for 2 h in a humidified atmosphere of 5%
CO2 at 37°C, the cells were washed three times with PBS
and then were lysed by repeated freezing and thawing. The lysed
cells were centrifuged at 16,770 × g at 4°C for 5 min and 150 µl
liquid supernatant was collected for PAL detection by high
performance liquid chromatography/mass spectrometry (HPLC/MS),
whereas 20-µl samples were taken for bicinchoninic acid assays to
determine the protein concentration. Data are presented as the mean
± standard deviation (n=3) following normalization to the total
protein concentration in each well.
Bidirectional transport
experiments
For the bidirectional transport experiments, LLC-PK1
and LLC-PK1/BCRP cells were seeded into Transwell inserts in 6-well
plates at a density of 2×105 cells/well. The cells were
then cultured in F12 medium supplemented with 20% FBS, at 37°C for
7 days, at which time the integrity of the monolayer was evaluated
by measuring the transepithelial electrical resistance (TEER) as
described previously (20) across
the monolayer (EVOM2; World Precision Instruments, Inc., Sarasota,
FL, USA). Monolayers with TEER values exceeding 200
Ω/cm2 were chosen for use in the transport
experiments.
On the day of transport experiments, the culture
medium was replaced with F12 medium containing PAL at varying
concentrations (0.1, 1, 10, 25 and 50 µM) on the upper or lower
side and with blank Hank's balanced salt solution (HBSS; Thermo
Fisher Scientific, Inc.) on the other side. Cells were incubated at
37°C. Six sequential samples (0.2 ml) were collected at different
times (0, 0.5, 1, 2, 3 and 4 h) from both sides of the cell
monolayer. The same volume of testing solution or blank HBSS was
immediately added to replace the samples obtained. Transport
experiments were also performed in the presence of the BCRP
inhibitor (5 µM Ko143) loaded on the same side of the monolayers
with PAL.
The permeability of PAL was estimated by calculating
the apparent permeability coefficient (Papp) as follows:
Papp=dQ/dtxAxC0, where C0 is the
concentration of PAL added to the upper or lower side, A is the
surface area of the monolayer (4.67 cm2 in the present
study) and dQ/dt is the rate at which the drug(s) appeared on the
opposite side.
The efflux ratio was calculated as: Efflux
ratio=Papp(BL→AP)/Papp(AP→BL), where apical
(AP) and basal (BL) are the two sides of the compartment, with the
indicated transport direction. Data are presented as the mean ±
standard deviation (n=3).
HPLC/MS analysis
PAL concentrations following cellular accumulation
and bidirectional transport experiments were determined using
HPLC/MS. An 80-µl aliquot obtained from the transport investigation
and 20 µl internal standard moclobemide (100 ng/ml; Sigma-Aldrich;
Merck KGaA) in the mobile phase were mixed on a high-speed vortex
for 30 sec. Methyl tert-butyl ether (400 µl) was added to the
mixture and vortexed for 5 min. Following centrifugation at 22,160
× g for 10 min at 4°C, the supernatant (300 µl) was transferred to
an 1.5-ml Eppendorf tube and solvent was evaporated to dryness for
40 min at 37°C using a vacuum drying system (RVT4104-230; Thermo
Fisher Scientific, Inc.). The dry residue was reconstituted in 80
µl mobile phase and mixed on a vortex for 5 min. The mixture was
then centrifuged at 22,160 × g for 5 min at 4°C, and a 5-µl aliquot
of the supernatant was injected into an HPLC-MS system for
analysis. The calibration standards ranged from 2–5,000 ng/ml.
PAL concentration was measured using an HPLC system
(2690; Waters Corporation, Milford, MA, USA) and a mass
spectrometer (Micromass; Waters Corporation) equipped with an
electrospray ionization (ESI) ion source. Separation was performed
on an Ultimate XB-C18 column (3 µm; 2.1× 100 mm; Welch Materials,
Inc., Shanghai, China). The mobile phase was water (25 mM ammonium
acetate, 0.06% ammonium) and acetonitrile (68:32) at a flow rate of
0.25 ml/min. The total run time was 3 min and the column and
autosampler were maintained at 40 and 25°C, respectively.
The compounds were ionized in the positive ESI ion
source mode of the mass spectrometer. The detection conditions were
a capillary voltage of 3.00 kV, a cone voltage of 22.00 V, an
extractor voltage of 2.00 V, a source temperature of 120°C, a
desolvation temperature of 350°C and cone and desolvation gas flows
of 50 and 500 l/h, respectively. The selected ion recording mode
was used for quantification, with m/z=427.5 for PAL and m/z=269.5
for moclobemide.
In vitro BCRP affinity for PAL
The in vitro BCRP affinity for PAL was
investigated as described by Boulton et al (21) with residue R482/BCRP membranes
analyzed for both basal and drug-stimulated ATP hydrolysis by
colorimetric detection of inorganic phosphate release. The reaction
was conducted in 96-well plates, and initiated by adding 20 µl
Mg-ATP (12 mM) solution (heated to 37°C) and 20 µl (heated to 37°C
for 5 min) BCRP membrane (2 mg/ml) to the reaction system (20 µl),
which contained 100 µM sodium orthovanadate and PAL or
sulfasalazine (a substrate for ABCG2) at varying concentrations
(0.1, 1, 10, 25, 50 and 100 µM). All of the reagents were made up
in Tris and 2-(N-morpholino) ethanesulfonic acid buffer (pH 6.8).
Following 60 min of incubation at 37°C, the reaction was terminated
by the addition of 30 ml ice-cold 10% sodium dodecylsulfate plus
0.1% antifoam A. The phosphate concentration (Pi) was assayed by
adding 200 µl 35 mM ammonium molybdate in 15 mM zinc acetate: 10%
ascorbic acid (pH 5.0, freshly prepared) at a ratio of 1:4 to each
well and the mixture was incubated for 20 min at 37°C. The
liberation of Pi was detected by the ultraviolet absorption of the
Pi-molybdate complex at 620 nm by a spectrophotometer and the Pi
concentration was calculated from the standard curve. Each sample
was analyzed in triplicate. Data were plotted as drug concentration
vs. Pi release and analyzed by least-squares non-linear regression
fits using the Michaelis-Menten equation. The maximal reaction
velocity (Vmax) and the substrate concentration at 50%
of Vmax (Km) were determined using GraphPad
Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
Statistical analyses
Statistical analyses were performed with SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). The effect
of BCRP on PAL accumulation in 293 and 293/BCRP cells was analyzed
with one-way analysis of variance, using Fisher's least significant
difference test for post hoc comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
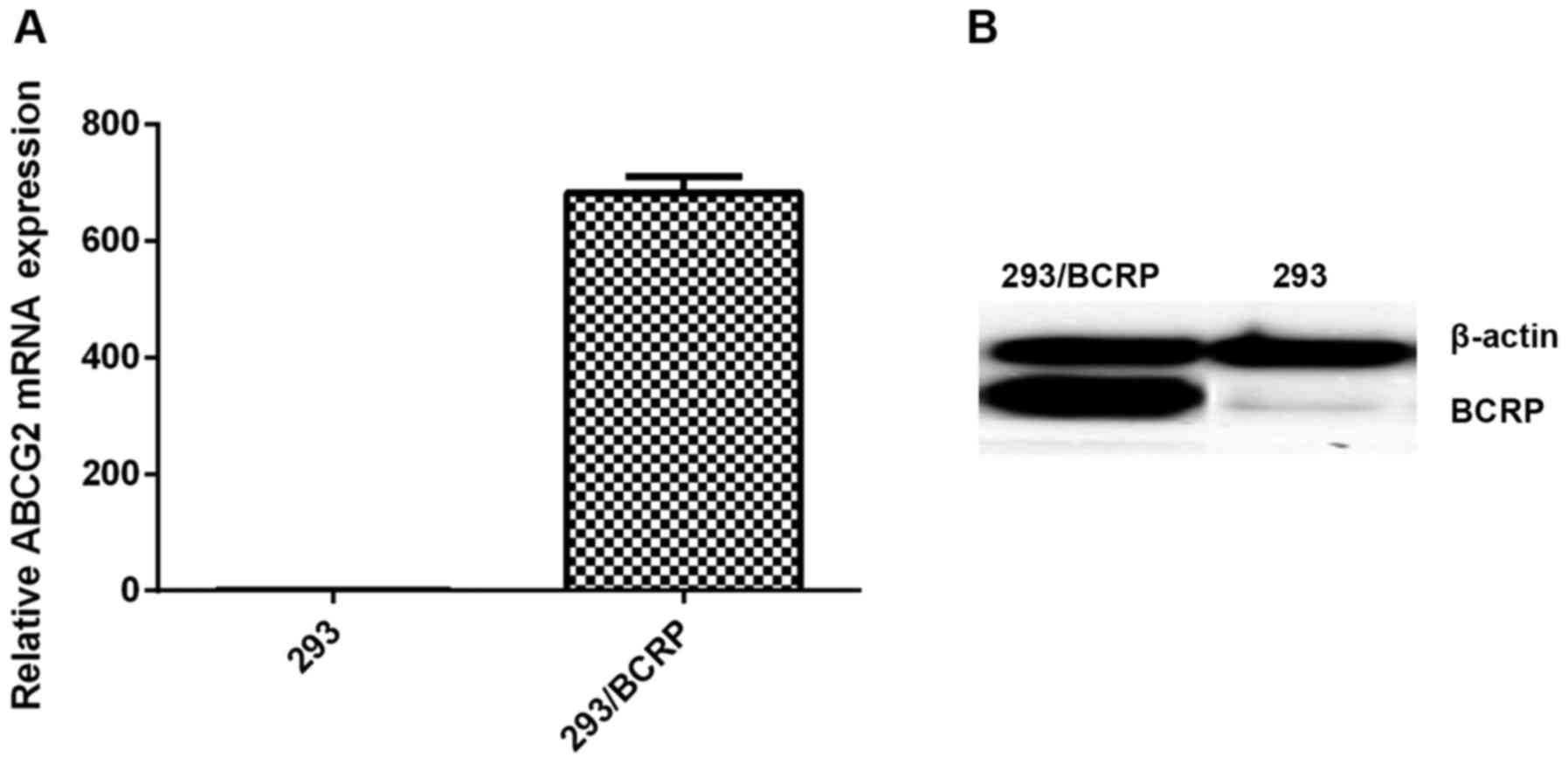
Expression of BCRP in 293/BCRP
cells
The results from the RT-qPCR and western blotting
assays indicated high expression levels of BCRP mRNA and protein in
293 cells transfected with the BCRP gene, whereas no notable BCRP
was detected in the 293 cells (Fig.
1).
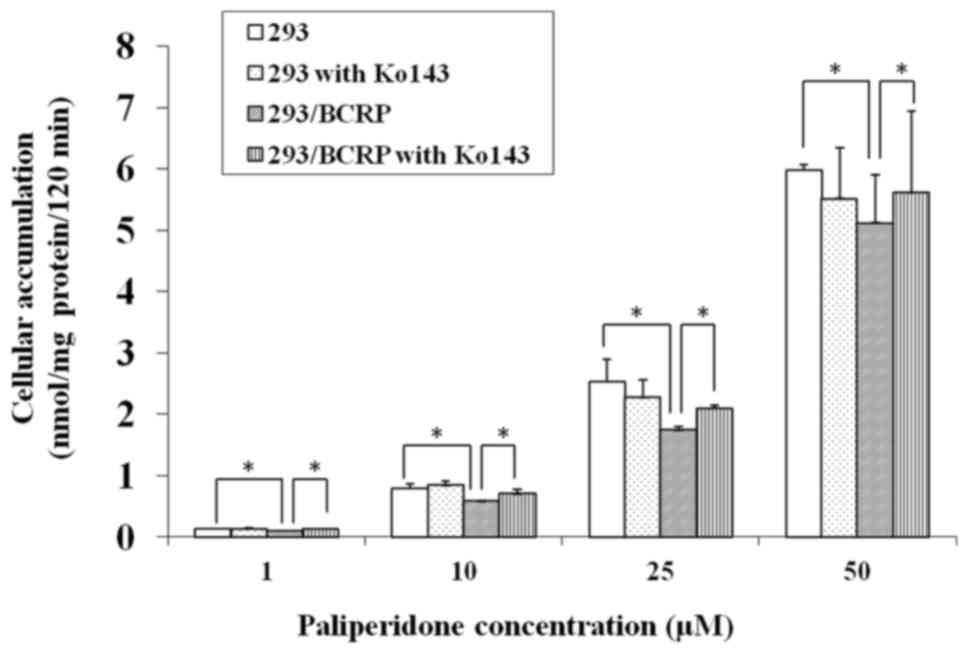
Cellular accumulation
The cellular accumulation of PAL in both 293 and
293/BCRP cells increased markedly with the drug concentration.
However, the rate of increase was markedly lower in 293/BCRP cells
than in 293 cells (Fig. 2),
indicating that the cellular bioavailability of PAL was attenuated
by BCRP. Conversely, combination treatment with 5 µM Ko143
significantly increased the PAL accumulation in 293/BCRP cells but
had no influence on its accumulation in 293 cells. These results
suggest that PAL can be effluxed by BCRP.
Bidirectional transport
Table I lists the
permeability parameters of the transcellular transport of PAL. The
transport of PAL was asymmetric, with the transport rates being
distinctly lower in the AP→BL direction than in the BL→AP
direction. Transfection of the BCRP gene further reduced the
transport rates in the AP→BL direction, while it increased the
rates in the BL→AP direction. The net efflux ratios of PAL in were
>1.7 for concentrations from 0.1 to 25 µM, which indicated that
PAL may be a substrate of BCRP. The net efflux ratio of PAL
decreased as the concentration increased to 50 µM, for which the
net efflux ratio was 1.22, suggesting that the BCRP in PAL
transport had saturated.
 | Table I.Permeability of PAL across LLC-PK1
and LLC-PK1/BCRP cell monolayers. |
Table I.
Permeability of PAL across LLC-PK1
and LLC-PK1/BCRP cell monolayers.
| A, Without
Ko143 |
|---|
|
|---|
|
| LLC-PK1/BCRP | LLC-PK1 |
|
|---|
|
|
|
|
|
|---|
| PAL (µM) | Pab
(×10−6 cm/sec) | Pbax
(10−6 cm/sec) | ER | Pab
(×10−6 cm/sec) | Pba
(×10−6 cm/sec) | ER | Net ER |
|---|
| 0.1 | 3.38±0.27 | 17.61±1.24 | 5.21 | 6.75±0.60 | 9.97±0.71 | 1.47 | 3.53 |
| 1 | 3.68±0.10 | 17.72±0.72 | 4.80 | 7.07±0.58 | 13.02±0.85 | 1.84 | 2.61 |
| 10 | 8.24±0.60 | 33.38±0.54 | 4.05 | 11.34±0.44 | 21.68±0.75 | 1.91 | 2.12 |
| 25 | 8.69±0.77 | 35.29±3.51 | 4.06 | 10.14±0.72 | 24.13±1.32 | 2.38 | 1.71 |
| 50 | 11.66±1.51 | 29.81±1.40 | 2.56 | 10.80±0.54 | 22.55±0.97 | 2.09 | 1.22 |
|
| B, With Ko143 (5
µM) |
|
|
|
LLC-PK1/BCRP | LLC-PK1 |
|
|
|
|
|
|
| PAL
(µM) |
Papp(BL→AP)
(×10−6 cm/sec) |
Papp(BL→AP)
(×10−6 cm/sec) | ER |
Papp(BL→AP)
(×10−6 cm/sec) |
Papp(BL→AP)
(×10−6 cm/sec) | ER | Net ER |
|
| 0.1 | 5.74±0.37 | 9.30±0.92 | 1.62 | 7.15±0.72 | 12.21±0.70 | 1.71 | 0.95 |
| 1 | 7.32±0.57 | 17.19±0.68 | 2.35 | 7.12±0.73 | 14.07±0.52 | 1.98 | 1.19 |
| 10 | 11.84±0.80 | 21.21±0.68 | 1.79 | 11.09±0.89 | 23.11±0.54 | 2.08 | 0.85 |
| 25 | 13.89±0.87 | 25.98±0.81 | 1.87 | 11.46±1.13 | 25.31±1.26 | 2.21 | 0.85 |
| 50 | 15.14±0.39 | 23.99±1.33 | 1.58 | 13.16±0.64 | 20.75±1.35 | 1.57 | 1.01 |
The addition of the BCRP inhibitor Ko143 (5 µM)
increased transport rates in the AP→BL direction but decreased
those in the BL→AP direction were observed in LLC-PK1/BCRP cells
and the net ratios of PAL were markedly decreased. These results
further support that PAL is a substrate of BCRP.
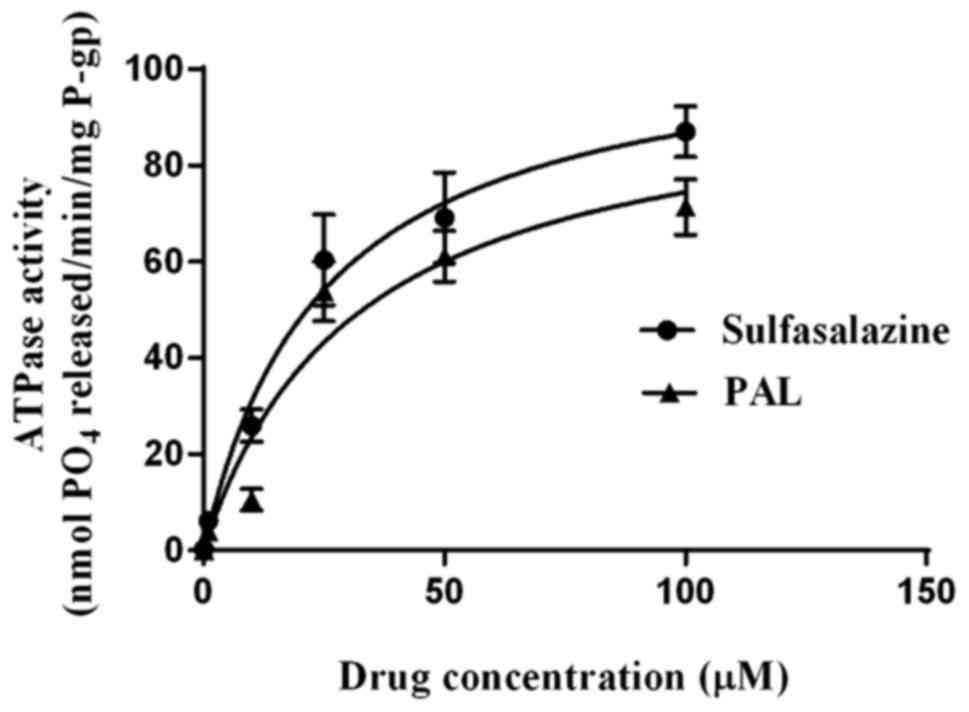
In vitro BCRP affinity for PAL
It is known that ABC drug transporters utilize the
energy of ATP hydrolysis to transport drugs outside cells (11,21). As
presented in Fig. 3, both
sulfasalazine and PAL stimulated vanadate-sensitive BCRP ATPase
activity in a concentration-dependent manner, with the degree of
stimulation being less for PAL than for sulfasalazine at all
concentrations. The concentrations of sulfasalazine and PAL
required for half-maximal stimulation (i.e., EC50
values) of the vanadate-sensitive ATPase activity were 25.09±4.96
and 31.68±9.60 µM, respectively, and the Vmax values
were 108.5 and 98.01 nM/min; the Vmax/Km
values of the two compounds were therefore 4.32×10−3 and
3.09×10−3 min−1.
Discussion
PAL is a relatively novel atypical antipsychotic
drug that has been demonstrated to be effective in reducing the
symptoms of schizophrenia and in improving personal and social
functioning in both short- and long-term studies (6). However, like other atypical
antipsychotic drugs, there are significant individual differences
in the treatment efficacy and adverse effects of PAL (8). A number of studies have demonstrated
that ABC transporters are associated with the efflux of an
antipsychotic, which may influence the drug absorption and
transport in vivo, and hence may be associated with the
individual differences in clinical treatment responses (22,23). The
present study is, to the best of our knowledge, the first to use
three independent methods to investigate the potency of PAL
transport by BCRP.
The cellular accumulation experiments performed in
the present study were based on BCRP being able to pump its
substrates out of cells so as to decrease their intracellular
concentrations (11). The results
demonstrated that the concentration of PAL was significantly lower
in 293/BCRP cells than in 293 cells, and that this difference could
be ameliorated by the addition of 5 µM Ko143. These observations
indicate that BCRP can export PAL and reduce its intracellular
concentration. It was also demonstrated that the difference in the
intracellular accumulation between wild-type (293) and 293/BCRP
cells decreased for 50 µM PAL concentrations compared with 25 µM
PAL, which may be due to saturation of the transporter.
LLC-PK1/BCRP transgenic cells were used to explore
the association of BCRP with the transport of PAL, as this cell
line exhibits high BCRP protein expression and strongly maintains
the characteristics of the parent LLC-PK1 cell (11). The results demonstrated that for
concentrations ranging from 0.1–50 µM, the bidirectional transport
of PAL was highly polarized in LLC-PK1/BCRP cells, and could be
eliminated by the BCRP inhibitor Ko143, with this not being
observed in the LLC-PK1 cells. These results were highly consistent
with those from the PAL accumulation experiments, with both sets of
results indicating that PAL can be effluxed by BCRP.
The LLC-PK1 cells were obtained from pig kidney
cells to form a polarity monolayer spontaneously in the cultivation
condition, which differentiates the characteristics of microvilli
and tight junctions so as to simulate a membrane barrier in
vivo (18,24). When transfected with BCRP, the
protein was located on the apical side of the cells, which is
similar to the findings for BCRP under physiological conditions
(25). This indicates that the
results from in vitro studies may predict the efflux effect
of BCRP on PAL in the BBB.
ATPase assays were used to investigate the direct
interaction between PAL and BCRP, and they confirmed that PAL may
have a moderate affinity for BCRP (with
Vm/Km=3.09×103 min−1).
Three classes of drug-stimulated ATPase activity in ABC
transporters have been proposed previously (26,27):
Class I compounds, which stimulate ATPase activity at low
concentrations but inhibit the activity at high concentrations
predominate; class II compounds, which exhibit
concentration-dependent ATPase activity with no inhibition at high
concentrations; and class III compounds, which inhibit both basal
and class I or II ATPase activity. Wang et al (15) previously reported that PAL could
inhibit the function of BCRP with a concentration where the
response is reduced by half of 51 µM; combined with the present
results, this indicates that PAL acts as class I compounds, being
substrates at lower concentrations and competitive inhibitors at
higher concentrations.
It has been reported that residue 482 of BCRP may be
the site of drug interaction in human cell lines, and that mutation
of the ABCG2 gene in exon 5 leads to the substitution of Arg482 for
a threonine (R482T) or a glycine (R482G) (28). The affinity of BCRP to several
substrates has also been demonstrated to be altered in the
wild-type and mutant cell lines (29,30).
Cells having a threonine or glycine at position 482 were able to
efflux rhodamine 123, whereas cells having an arginine at that
position were not (29). The
findings of cross-resistance studies suggest that cells carrying an
R482T mutation exhibit higher anthracycline resistance, whereas an
R482G mutation seems to confer lower resistances to SN-38 and
topotecan along with a higher affinity to etoposide (30).
The Arg482 BCRP membrane was used in the ATPase
affinity assay, and the ABCG2 gene was transfected to both 293 and
LLC-PK1. Therefore, the present results only demonstrated the
substrate affinity of wild-type BCRP to PAL, and so the affinity of
PAL to the mutant ABCG2 gene may require further studies
that may elucidate the possible genetic reason for the individual
variations in the responses to PAL.
Pharmacokinetics studies have demonstrated that a
PAL dosage of 6 mg/day produces a plasma concentration of ~0.1 µM
(13,31). A previous in vitro study
demonstrated that BCRP may significantly efflux PAL at that
concentration, and then may block it through the BBB. It has also
been demonstrated that PAL is the substrate of P-gp, and that the
3435C>T SNP impacts the pharmacokinetics of PAL (14). These two proteins may therefore act
together in effluxing PAL out of the brain, and this may be the
reason for the significantly higher concentration of risperidone,
which is a substrate of P-gp but not of BCRP, compared with PAL,
considering the similarity of their structures (31).
Multiple psychotropic drugs are reportedly combined
in approximately 50% of patients, and especially in
treatment-resistance cases (32).
Furthermore, a number of antipsychotics have been demonstrated to
interact with BCRP (16,17), and so when used together with other
drugs they may inhibit the function of BCRP, thereby increasing the
plasma and brain concentrations of PAL and eventually leading to
better efficacy or serious adverse reactions.
In conclusion, the novel atypical antipsychotic drug
PAL is a substrate of BCRP that may have moderate affinity for BCRP
at clinical dosages, and its penetration through the BBB may be
influenced by BCRP. These mechanisms may provide a clue for
explaining the significant individual differences observed
clinically in the treatment efficacy and adverse effects of
PAL.
Acknowledgements
The present authors would like to thank Professor
Zeng Su for providing the LLC-PK1 and LLC-PK1/BCRP cells used in
the present experiments.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ performed cellular accumulation experiments and
prepared the manuscript. HL was involved in the study design and
data analysis. PX and LS performed cell culturing and transfection
experiments. QW and QL performed polymerase chain reaction and
western blot analysis. HY and YL performed the bidirectional
transports experiment and in vitro BCRP affinity assays. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflicts of
interest.
References
|
1
|
Tandon R, Belmaker RH, Gattaz WF,
Lopez-Ibor JJ Jr, Okasha A, Singh B, Stein DJ and Olie JP:
Fleischhacker WW and Moeller HJ; Section of Pharmacopsychiatry,
World Psychiatric Association: World Psychiatric Association
Pharmacopsychiatry Section statement on comparative effectiveness
of antipsychotics in the treatment of schizophrenia. Schizophr Res.
100:20–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou
CF and Chaiyakunapruk N: Global economic burden of schizophrenia: A
systematic review. Neuropsychiatr Dis Treat. 12:357–373.
2016.PubMed/NCBI
|
|
3
|
Samara MT, Dold M, Gianatsi M,
Nikolakopoulou A, Helfer B, Salanti G and Leucht S: Efficacy,
acceptability, and tolerability of antipsychotics in
treatment-resistant schizophrenia: A network meta-analysis. JAMA
Psychiatry. 73:199–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Owen MJ, Sawa A and Mortensen PB:
Schizophrenia. Lancet. 388:86–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki T, Remington G, Mulsant BH, Rajji
TK, Uchida H, Graff-Guerrero A and Mamo DC: Treatment resistant
schizophrenia and response to antipsychotics: A review. Schizophr
Res. 133:54–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chwieduk CM and Keating GM: Paliperidone
extended release: A review of its use in the management of
schizophrenia. Drugs. 70:1295–1317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang SM, Han C, Lee SJ, Patkar AA, Pae CU
and Fleischhacker WW: Paliperidone: A review of clinical trial data
and clinical implications. Clin Drug Investig. 32:497–512. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nussbaum AM and Stroup TS: Paliperidone
for treatment of schizophrenia. Schizophr Bull. 34:419–422. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spina E and Cavallaro R: The pharmacology
and safety of paliperidone extended-release in the treatment of
schizophrenia. Expert Opin Drug Saf. 6:651–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Breedvel P, Benjnen JH and Schellens JH:
Use of P-glycoprotein and BCRP inhibitors to improve oral
bioavailability and CNS penetration of anticancer drugs. Trends
Pharmacol Sci. 27:17–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ueno M, Nakagawa T, Wu B, Onodera M, Huang
CL, Kusaka T, Araki N and Sakamoto H: Transporters in the brain
endothelial barrier. Curr Med Chem. 17:1125–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Begley DJ: ABC transporters and the
blood-brain barrier. Curr Pharm Des. 10:1295–1312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vermeir M, Naessens I, Remmerie B, Mannens
G, Hendrickx J, Sterkens P, Talluri K, Boom S, Eerdekens M, van
Osselaer N and Cleton A: Absorption, metabolism, and excretion of
paliperidone, a new monoaminergic antagonist, in humans. Drug Metab
Dispos. 36:769–779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki Y, Tsuneyama N, Fukui N, Sugai T,
Watanabe J, Ono S, Saito M and Someya T: Impact of the ABCB1 gene
polymorphism on plasma 9-hydroxyrisperidone and active moiety
levels in Japanese patients with schizophrenia. J Clin
Psychopharmacol. 33:411–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang JS, Ruan Y, Taylor RM, Donovan JL,
Markowitz JS and DeVane CL: The brain entry of risperidone and
9-hydroxyrisperidone is greatly limited by P-glycoprotein. Int J
Neuropsychopharmacol. 7:415–419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jani M, Ambrus C, Magnan R, Jakab KT,
Beéry E, Zolnerciks JK and Krajcsi P: Structure and function of
BCRP, a broad specificity transporter of xenobiotics and
endobiotics. Arch Toxicol. 88:1205–1248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang JS, Zhu HJ, Markowitz JS, Donovan JL,
Yuan HJ and Devane CL: Antipsychotic drugs inhibit the function of
breast cancer resistance protein. Basic Clin Pharmacol Toxicol.
103:336–341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian Y, Qu BX, Yao Y and Zeng S:
Establishment of BCRP expressed pig kidney cell line LLC-PK1/BCRP
and its biological profile. Yao Xue Xue Bao. 47:1599–1604. 2012.(In
Chinese). PubMed/NCBI
|
|
19
|
Yan H, Zhang DY, Li X, Yuan XQ, Yang YL,
Zhu KW, Zeng H, Li XL, Cao S, Zhou HH, et al: Long non-coding RNA
GAS5 polymorphism predicts a poor prognosis of acute myeloid
leukemia in Chinese patients via affecting hematopoietic
reconstitution. Leuk Lymphoma. 58:1948–1957. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng B, Mills JB, Davidson RE, Mireles RJ,
Janiszewski JS, Troutman MD and de Morais SM: In vitro
P-glycoprotein assays to predict the in vivo interactions of
P-glycoprotein with drugs in the central nervous system. Drug Metab
Dispos. 36:268–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boulton DW, DeVane CL, Liston HL and
Markowitz JS: In vitro P-glycoprotein affinity for atypical and
conventional antipsychotics. Life Sci. 71:163–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim KA, Joo HJ, Lee HM and Park JY:
Influence of ABCB1 and CYP3A5 genetic polymorphisms on the
pharmacokinetics of quetiapine in healthy volunteers. Pharmacogenet
Genomics. 24:35–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoosain FG, Choonara YE, Tomar LK, Kumar
P, Tyagi C, du Toit LC and Pillay V: Bypassing P-glycoprotein drug
efflux mechanisms: Possible applications in pharmacoresistant
schizophrenia therapy. Biomed Res Int. 2015:4849632015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian Y, Qian S, Jiang Y, Shen Q, Zheng J,
Zhou H and Zeng S: The interaction between human breast cancer
resistance protein (BCRP) and five bisbenzylisoquinoline alkaloids.
Int J Pharm. 453:371–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takada T, Suzuki H and Sugiyama Y:
Characterization of polarized expression of point- or
deletion-mutated human BCRP/ABCG2 in LLC-PK1 cells. Pharm Res.
22:458–464. 2005.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M,
Pastan I and Gottesman MM: Biochemical, cellular, and
pharmacological aspects of the multidrug transporter. Annu Rev
Pharmacol Toxicol. 39:361–398, 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ambudkar SV, Dey S, Hrycyna CA,
Ramachandra M, Pastan I and Gottesman MM: Biochemical, cellular,
and pharmacological aspects of the multidrug transporter. Annu Rev
Pharmacol Toxicol. 39:361–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hrycyna CA, Ramachandra M, Ambudkar SV, Ko
YH, Pedersen PL, Pastan I and Gottesman MM: Mechanism of action of
human P-glycoprotein ATPase activity. Photochemical cleavage during
a catalytic transition state using orthovanadate reveals cross-talk
between the two ATP sites. J Biol Chem. 273:16631–16634. 1998.
|
|
28
|
Ozvegy-Laczka C, Köblös G, Sarkadi B and
Váradi A: Single amino acid (482) variants of the ABCG2 multidrug
transporter: Major differences in transport capacity and substrate
recognition. Biochim Biophys Acta. 1668:53–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shukla S, Robey RW, Bates SE and Ambudkar
SV: The calcium channel blockers, 1,4-dihydropyridines, are
substrates of the multidrug resistance-linked ABC drug transporter,
ABCG2. Biochemistry. 45:8940–8951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eddabra L, Wenner T, El Btaouri H, Baranek
T, Madoulet C, Cornillet-Lefebvre P and Morjani H: Arginine 482 to
glycine mutation in ABCG2/BCRP increases etoposide transport and
resistance to the drug in HEK-293 cells. Oncol Rep. 27:232–237.
2012.PubMed/NCBI
|
|
31
|
de Leon J, Wynn G and Sandson NB: The
pharmacokinetics of paliperidone versus risperidone.
Psychosomatics. 51:80–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lindenmayer JP and Kaur A: Antipsychotic
management of schizoaffective disorder: A review. Drugs.
76:589–604. 2016. View Article : Google Scholar : PubMed/NCBI
|