Introduction
Spinal cord injury (SCI) occurs as a result of a
multifactorial process, involving primary injury subsequently
followed by a secondary injury (1).
Primary injury, caused by an initial physical impact, is
characterized by acute bleeding and ischemia (2). Secondary injury may be induced by
several mechanisms, including inflammation, oxidative stress, ion
balance dysregulation and excitotoxicity (3–5). It has
been reported that allicin may protect rats against SCI via
regulation of oxidative stress and inflammatory response pathways
(6), while inhibition of reactive
oxygen species (ROS) production may improve outcomes following SCI
by mediating acute reductions in oxidative stress and inflammation
(7). Apoptosis has been suggested as
a potential therapeutic target for secondary SCI (8), as one of the most damaging processes of
secondary SCI is neuronal apoptosis (9). Motor neuron apoptosis is the main cause
of dysfunction following SCI, as it is one of the primary obstacles
for locomotor functional recovery (10).
The generation of ROS, such as superoxide anions,
H2O2 and hydroxyl radicals, is a normative
response to disease or injury, including SCI (11). The increased formation of ROS may
exceed the capacity of the antioxidant defense systems and
subsequently lead to oxidative stress (12,13).
Oxidative stress serves an important role in apoptosis induction
under physiologic and pathologic conditions (14,15). A
previous study demonstrated that myricetin may protect cells
against H2O2-induced cell damage via its
anti-apoptotic effects (16).
Nischarin (NISCH) is a cytosolic protein that is
found anchored to the inner layer of the plasma membrane, and has
been demonstrated to interact with cytosolic and intermembrane
proteins (17). NISCH is expressed
in various organs, and a previous study demonstrated that NISCH
serves an inhibitory role in cell migration, invasion and the
carcinogenesis of breast cancer cells (18). NISCH is highly expressed in the brain
of adult rats, and has been confirmed to be expressed in PC12 and
Neuro-2A cell lines (19). It has
been reported that overexpression of NISCH may induce human breast
cancer apoptosis and inhibit cell migration and invasion (20), while inhibition of NISCH expression
promotes neurite outgrowth (18).
Recently, it has been shown that NISCH downregulation by
small-interfering RNA (siRNA) accelerates rat motor function
recovery following SCI (21),
indicating that NISCH may be involved in the pathological
mechanisms of SCI. However, the exact molecular mechanism remains
unclear.
The authors of the present study hypothesized that
NISCH downregulation attenuates oxidative stress-induced apoptosis
in PC12 cells. Therefore, the apoptotic rate of PC12 cells and the
expression of Bcl-2/Bcl-2-associated X (Bax) signaling pathway
members was examined in the present study.
Materials and methods
Cell culture
The PC12 rat pheochromocytoma tumor cell line was
purchased from PeproTech Inc. (Rocky Hill, NJ, USA) and maintained
in RPMI 1640 medium supplemented with 10% heat-inactivated fetal
bovine serum (both Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 100 U/ml penicillin and 100 µg/ml streptomycin (Thermo
Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
containing 5% CO2.
Flow cytometric analysis of cell
apoptosis
Cells were treated with 100 µM
H2O2 for 0, 24, 48, 72 and 96 h. Following
H2O2 treatment, cells were harvested by
trypsinization and washed twice with cold phosphate-buffered saline
(PBS). Cell apoptosis was measured following drug treatment using
the Annexin V-FITC/PI Apoptosis Detection kit (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) according to the manufacturer's
instructions. Cells were resuspended and mixed with 500 µl binding
buffer containing 5 µl Annexin V-fluorescein isothiocyanate (FITC)
and 5 µl propidium iodide (PI). Following incubation for 15 min at
room temperature in the dark, cell apoptosis was detected using
fluorescence-activated cell sorting (FACS) flow cytometers from BD
Biosciences (Franklin Lakes, NJ, USA) or Beckman Coulter, Inc.
(Brea, CA, USA) using BD Cell Quest research software (version
5.2.1; BD Biosciences).
NISCH siRNA preparation and cell
transfection
The NISCH siRNA and negative control sequences were
as follows: NISCH siRNA sense, 5′-GCAAGCACUGACCACUCUATT-3′ and
anti-sense, 5′-UAGAGUGGUCAGUGCUUGCTT-3′; Negative control sense,
5′-UUCUCCGAACGUGUCACGUTT-3′, and anti-sense,
5′-ACGUGACACGUUCGGAGAATT-3′. NISCH siRNA was diluted to 20 µM with
a universal buffer (Beijing Huaxia Ocean Technology Co., Ltd.,
Beijing, China). Cells were divided into control, vehicle and
NISCH-siRNA groups. PC12 cells in the logarithmic growth phase were
seeded at a density of 2.5×105 cells/well in a 12-well
plate (1 ml/well) and incubated at 37°C and 5% CO2
overnight. At 2 h prior to transfection, the culture medium was
replaced with serum-free RPMI-1640 culture medium. To prepare cells
for transfection, 5 µl siRNA was first diluted in 200 µl Opti-MEM
(Gibco; Thermo Fisher Scientific, Inc.) without serum.
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
was gently mixed with the diluted siRNA solution (total volume 400
µl) and the samples were incubated at room temperature for 20 min.
A total of 200 µl of this mixture was added into each well, and the
cell culture plate was gently shaken to mix the culture solution.
The transfection groups were as follows: Control group, no
transfection; vehicle group, transfected with negative control
sequences; NISCH-siRNA group, transfected with siRNA sequences
targeting the rat NISCH gene.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using a TRIzol reagent kit
(Aidlab Biotechnologies Co., Ltd., Beijing, China) and reverse
transcribed into cDNA using the HiScript® Reverse
Transcriptase (RNase H) kit (Vazyme, Piscataway, NJ, USA),
according to the manufacturer's protocol. Real-time PCR was
performed using the 7900HT Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.), according to
manufacturer's protocol. The mRNA expression levels of NISCH were
normalized to the endogenous expression of β-actin. Primers were
purchased from Tsingke (Beijing, China). The following primer pairs
were used for qPCR analyses: NISCH, forward,
5′-TATGTTGTGGCACAGAAGATGG-3′ and reverse,
5′-TTCAGGCAATGGATAGTGGAT-3′; β-actin forward,
5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse,
5′-TAAAGACCTCTATGCCAACACAGT-3′. The PCR reaction was performed in a
total volume of 20 µl, consisting of 4 µl cDNA (5 ng/µl), 0.8 µl
primer mix (10 µM), 10 µl SYBR-Green PCR Master Mix (Vazyme Biotech
Co., Ltd.), 0.4 µl ROX Reference Dye 2 (50X; ShenZhen Hui Nuo
Biotechnology Co., Ltd., Shenzhen, China) and 4.8 µl
H2O. Thermal cycling conditions were as follows: 95°C
for 15 min, followed by 40 cycles at 95°C for 15 sec, and 60°C for
1 min. β-actin was used as an internal control and the expression
levels of target genes were calculated using the 2−ΔΔCq
method (22).
Western blot analysis
Total protein was extracted from
H2O2-treated cells using lysis buffer
(Beyotime Institute of Biotechnology, Shanghai, China) and the
samples were incubated on ice for 50 min. Protein concentration was
measured using a bicinchoninic acid assay kit (Beyotime Institute
of Biotechnology). An equal quantity of protein for each sample was
separated via SDS-PAGE on a 12% gel and then transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked with 10% skim milk in PBS and 0.1%
Tween-20 (pH 7.2) for 2 h at room temperature and incubated at 4°C
overnight with an appropriate quantity of the following primary
antibodies: Mouse monoclonal anti-β-actin (dilution, 1:200; cat.
no. BM0627; Wuhan Boster Biological Technology, Ltd., Wuhan China),
mouse polyclonal anti-NISCH (dilution, 1:500; cat. no. 558262; BD
Biosciences), rabbit polyclonal anti-Bcl-2 (dilution, 1:500; cat.
no. 12789-1-AP; Wuhan Sanying Biotechnology, Wuhan, China), rabbit
polyclonal anti-Bax (dilution, 1:5,000; cat. no. ab32503; Abcam,
Cambridge, UK) and rabbit polyclonal anti-caspase-3 (dilution,
1:800; cat. no. 19677-1-AP; Wuhan Sanying Biotechnology).
Immunoblots were incubated with the horseradish peroxidase-labeled
secondary antibodies goat anti-rabbit Ig (dilution, 1:50,000; cat.
no. BA1054) or goat anti-mouse Ig (dilution, 1:50,000, cat. no.
BA1051; both Wuhan Boster Biological Technology, Ltd.) for 2 h at
21°C, and bands were detected using the Novex™ ECL Chemiluminescent
Substrate Reagent kit (Thermo Fisher Scientific, Inc.).
Densitometry analysis was performed using ImageJ software (version
1.48u; National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence analysis
Cells mounted on slides were placed in a culture
plate and washed with PBS three times. The cells were then fixed
with 4% paraformaldehyde for 15 min at 4°C and incubated with 0.5%
Triton X-100 at room temperature for 20 min. Subsequently, cells
were blocked with 5–10% goat serum (Wuhan Boster Biological
Technology, Ltd.) at room temperature for 30 min. Cells were
subsequently incubated overnight at 4°C with primary antibodies
against NISCH (dilution, 1:50; cat. no. 558262; BD Biosciences),
Bcl-2 (dilution, 1:50; cat. no. 12789-1-AP; Wuhan Sanying
Biotechnology), Bax (dilution, 1:250; cat. no. ab32503; Abcam) and
caspase-3 (dilution, 1:50, cat. no. 19677-1-AP; Wuhan Sanying
Biotechnology). Immunofluorescence was generated by incubating
samples with the Cy3-conjugated secondary antibodies goat
anti-rabbit IgG (dilution, 1:100; cat. no. BA1032) or goat
anti-mouse IgG (1:100; cat. no. BA1031; both Wuhan Boster
Biological Technology, Ltd.) for 1 h at 37°C in the dark. DAPI (5
µg/ml) was added to counterstain the nuclei, and the slides were
incubated for 5 min at room temperature in the dark before being
washed with PBS to remove excess DAPI. Images were obtained using a
fluorescence microscope (Olympus BX53; Olympus Corporation, Tokyo,
Japan).
Statistical analysis
Statistical analysis was performed using GraphPad
6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). The data
are expressed as the mean ± standard error of the mean and are
representative of three independent experiments. Comparisons among
multiple groups were performed by one-way analysis of variance
followed by a Bonferroni post hoc multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
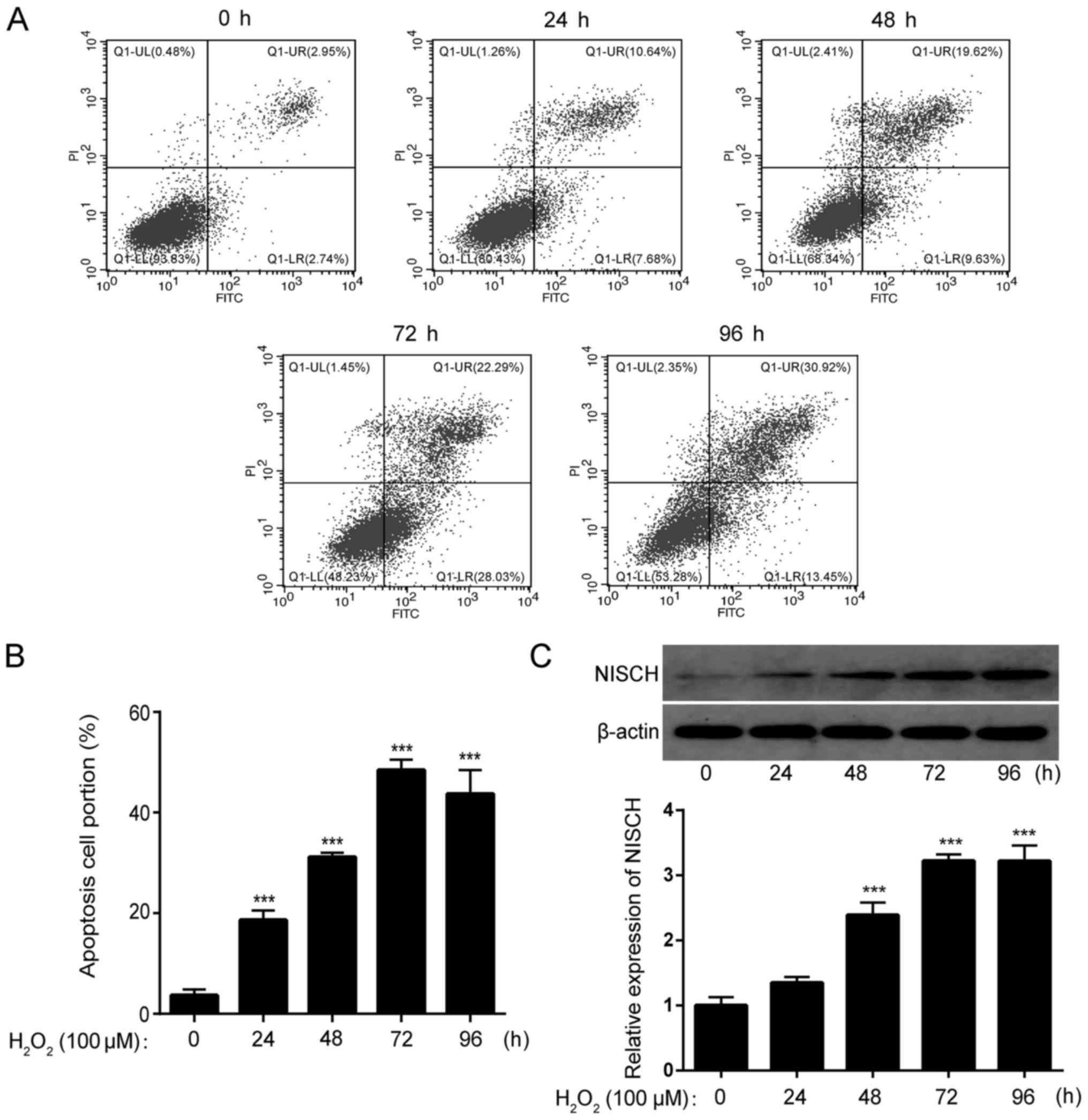
H2O2-induces
cell apoptosis and increases NISCH expression in PC12 cells
Cells were treated with 100 µM
H2O2 for 0, 24, 48, 72 and 96 h,
respectively. By staining cells with Annexin V-FITC and PI, the
apoptotic rate was analyzed by FACS. The results demonstrated that
the proportion of apoptotic cells was significantly increased by
H2O2 treatment of PC12 cells at all time
points (Fig. 1A and B). Furthermore,
the expression of NISCH in PC12 cells was significantly increased
by H2O2 treatment in a time-dependent manner
(Fig. 1C).
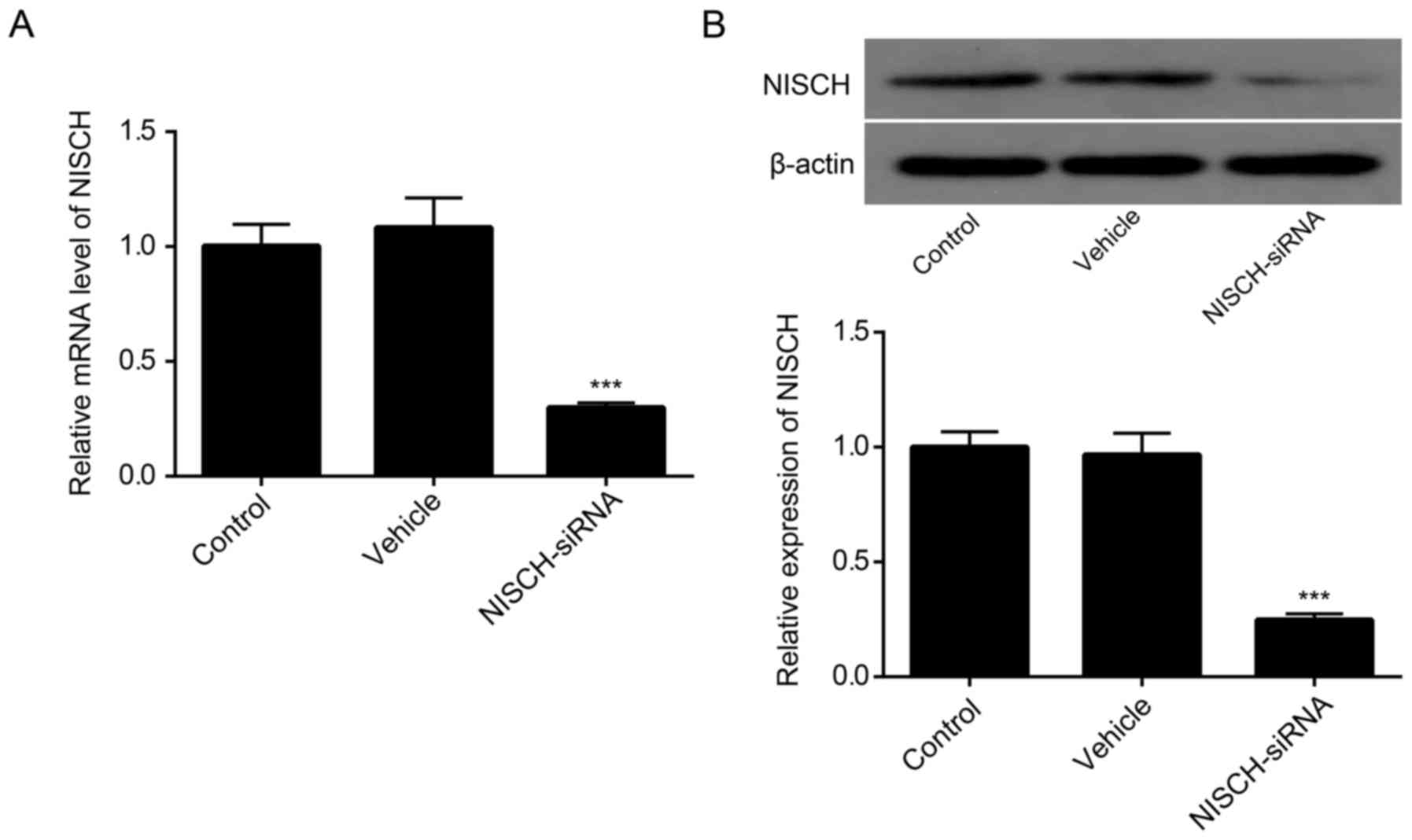
NISCH-siRNA reduces NISCH expression
in PC12 cells
As demonstrated in Fig.
2A, the level of NISCH mRNA was significantly decreased in PC12
cells at 48 h following transfection with NISCH-siRNA. Consistent
with these results, western blot analysis indicated that the
expression of NISCH was reduced in PC12 cells at 48 h following
NISCH-siRNA transfection (Fig.
2B).
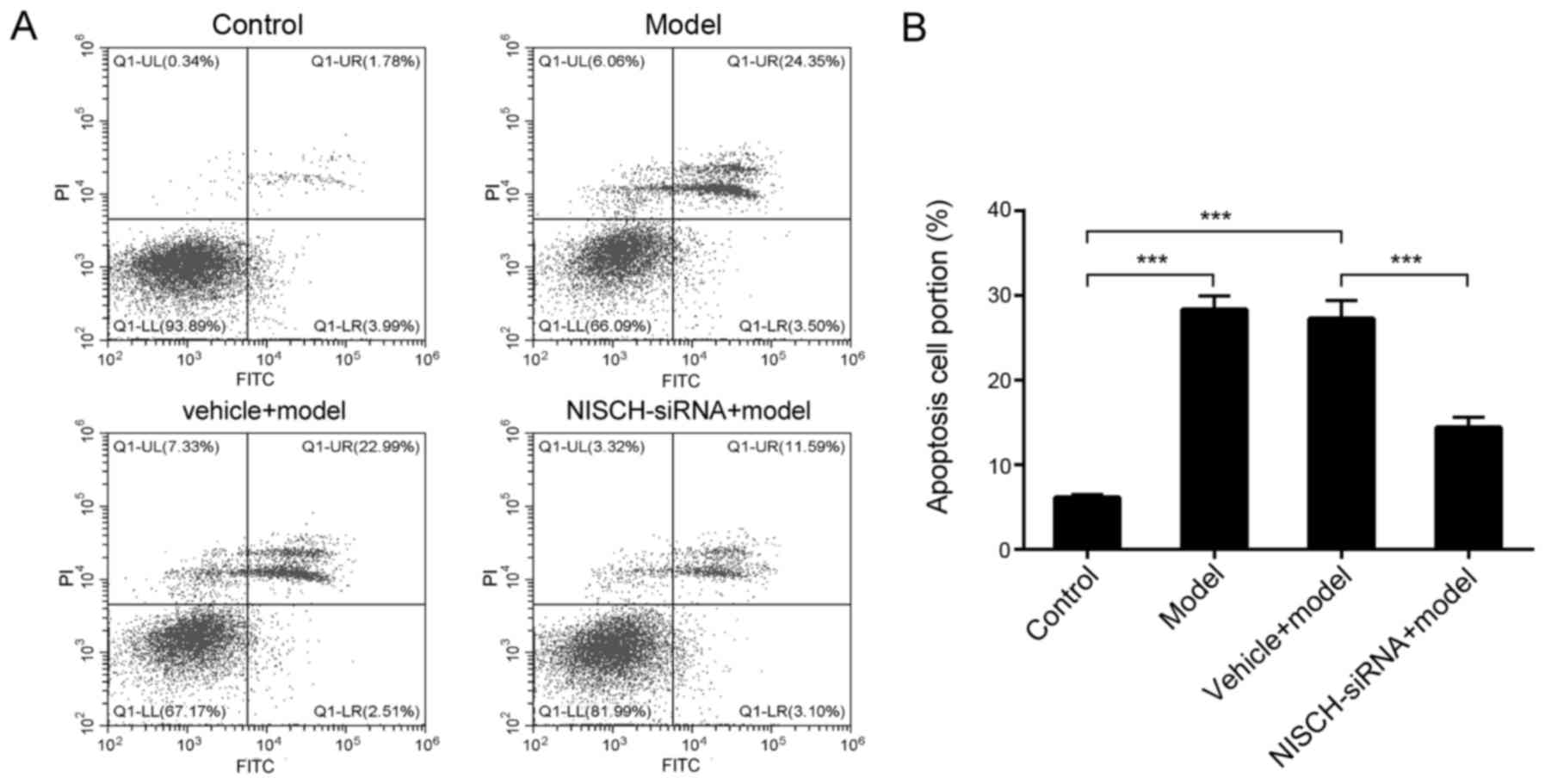
NISCH downregulation attenuates
H2O2-induced apoptosis in PC12 cells
As demonstrated in Fig.
3A and B, H2O2 significantly increased
the level of apoptosis in PC12 cells, while NISCH downregulation
significantly reduced apoptosis levels at 48 h following
H2O2 treatment. These results suggested that
NISCH may function as a pro-apoptotic protein during
H2O2-induced apoptosis, and downregulation of
NISCH may serve a protective role in
H2O2-treated PC12 cells.
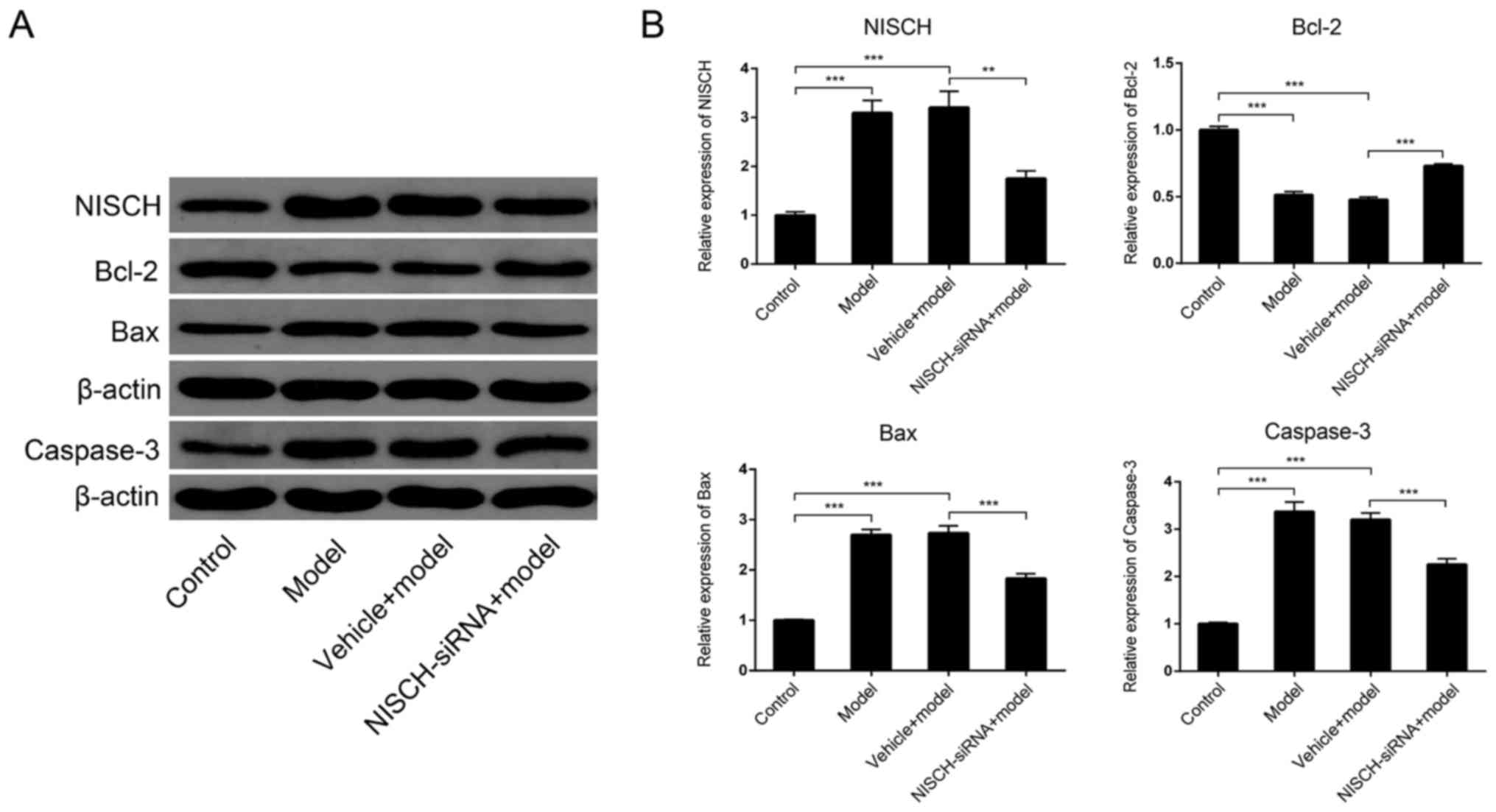
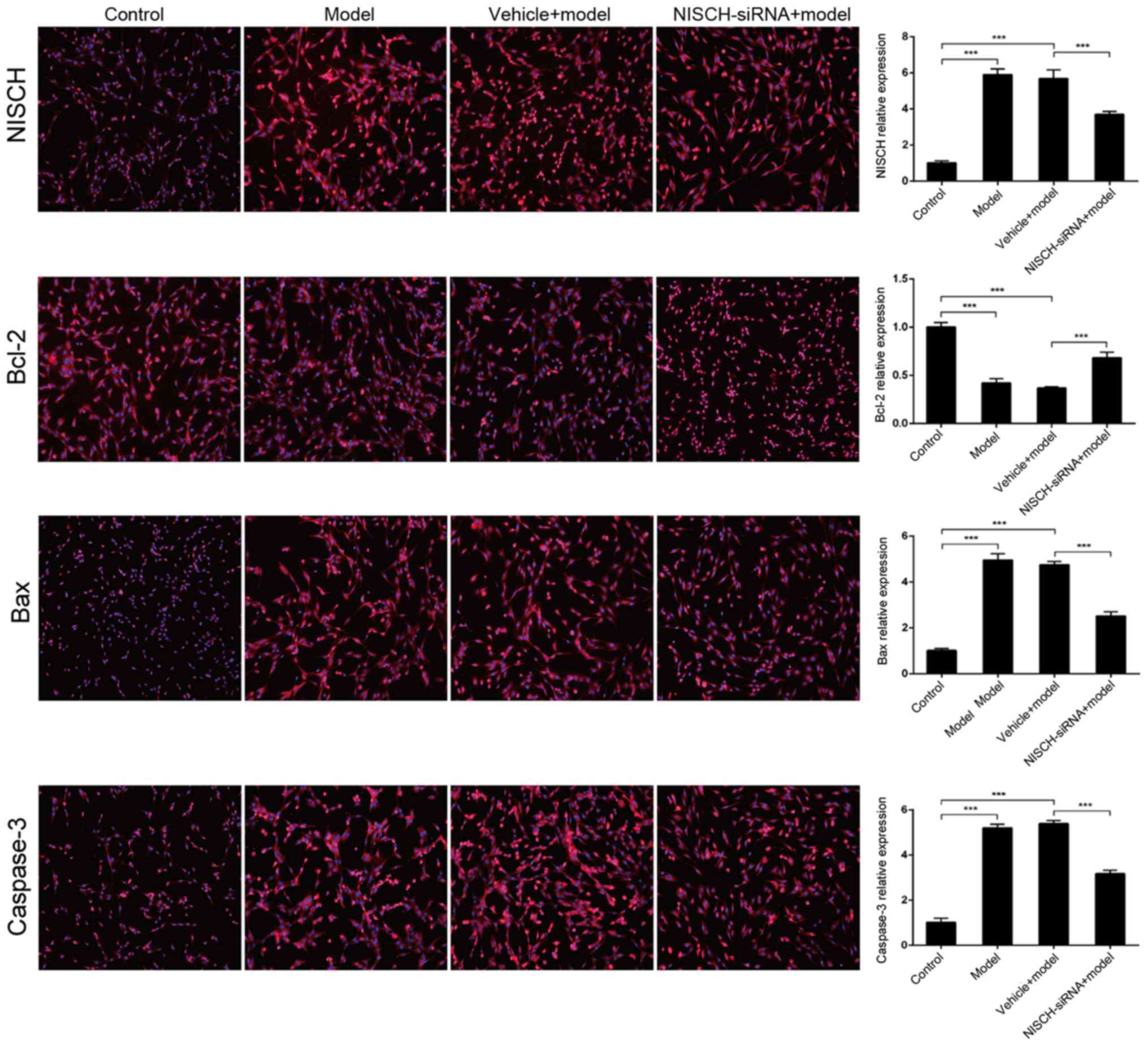
NISCH downregulation alters Bcl-2/Bax
signaling induced by H2O2 in PC12 cells
The Bcl-2/Bax signaling pathway serves an important
role in apoptosis (23). The
pro-survival Bcl-2 and pro-apoptotic Bax are the two most
extensively studied members of the Bcl-2 family. These proteins
function as the primary regulators of the mitochondrial apoptosis
signaling pathway (24). Caspase
enzymes are crucial effectors of the cell death signaling pathway
and are activated by almost all apoptosis-inducing stimuli within
neurons and non-neuronal cells (25). Caspase-3 appears to serve a pivotal
role in this pathway and has been demonstrated to be necessary for
the regulation of development-associated cell death in the brain
(25). Therefore, the expression of
Bcl-2 and Bax in PC12 cells at 48 h following
H2O2 treatment was examined in the present
study. As demonstrated in Fig. 4,
H2O2 significantly increased NISCH
expression, while NISCH downregulation significantly reduced
H2O2-induced NISCH expression. In addition,
Bcl-2 expression was significantly reduced, while Bax and caspase-3
expression levels were significantly increased by
H2O2 treatment (Fig. 4). These
H2O2-induced expression alterations were
partially inhibited by NISCH downregulation. The results indicate
that NISCH downregulation may protect against the apoptosis of PC12
cells by inhibiting the transduction of Bcl-2/Bax signaling
pathways. Immunofluorescence analysis demonstrated the same
alternations in NISCH, Bax, Bcl-2 and caspase-3 expression in
response to H2O2 treatment with or without
transfection with NSCH-siRNA (Fig.
5).
Discussion
Oxidative stress and the generation of free
radicals, as primary or secondary events, have been implicated in a
number of diseases (26). Recent
studies have demonstrated that oxidative stress may serve a role in
the pathogenesis of SCI (27,28). It
has been demonstrated that markers of oxidative stress, such as
malondialdehyde, and advanced oxidation protein products are
significantly increased in rats with SCI, while antioxidants such
as glutathione peroxidase and catalase are significantly decreased
(27). In addition, a previous study
demonstrated that a significant reduction of the expression of
lipid peroxidation factors malondialdehyde may contribute to the
reported neuroprotection of the spinal cord from oxidative damage,
likely induced by the increased SOD (28). NISCH is reportedly involved in
regulating the pathological mechanisms of SCI (21). In the present study, the role of
NISCH in oxidative stress-induced apoptosis was investigated using
a model of H2O2-induced oxidative damage in
PC12 cells. The results indicated that treatment of cells with 100
µM H2O2 significantly induced apoptosis and
increased NISCH expression at different time points. By contrast,
NISCH downregulation significantly attenuated apoptosis levels and
inhibited the expression of Bcl-2/Bax signaling induced by
H2O2.
Apoptosis is important for the development of
neuronal and non-neuronal cells in both the peripheral and central
nervous system (29). Aberrant
apoptosis contributes to the pathogenesis of a variety of disease
states, such as SCI, while inhibition of apoptosis in the
pathological state may improve the pathological injury. For
instance, it has been demonstrated that treadmill exercise can
promote the recovery of motor function by suppressing apoptosis in
the injured spinal cord (30), and
overexpression of neuroglobin can improve functional recovery by
suppressing neuronal apoptosis following SCI (31). In the present study, the expression
of NISCH and levels of apoptosis were significantly increased in
PC12 cells following treatment with H2O2 in a
time-dependent manner, indicating that NISCH may be involved in
H2O2-induced apoptosis in PC12 cells.
Bcl-2 and Bax are two discrete members of a gene
family involved in the regulation of apoptosis. Bcl-2 inhibits cell
death in response to various stimuli, while overexpression of Bax
exerts a pro-apoptotic effect, and antagonizes the anti-apoptotic
activity of Bcl-2 (32,33). Furthermore, the Bcl-2/Bax signaling
pathway reportedly participates in the pathogenesis of SCI
(34–36). Caspase-3 is a crucial mediator of
apoptosis and a frequently activated cell death protease that
catalyzes the specific cleavage of numerous key cellular proteins
(37). A previous study demonstrated
that caspase-3 is activated following SCI (38), and prevention of caspase-3 activation
reduces apoptosis levels (39). In
the current study, NISCH downregulation was demonstrated to inhibit
the H2O2-induced reduction in Bcl-2 and
increase in Bax expression in PC12 cells, indicating that NISCH
downregulation inhibited transduction of the Bcl-2/Bax signaling
pathway. In addition, NISCH downregulation inhibited the activation
of caspase-3 by H2O2, which further
demonstrated that NISCH downregulation may attenuate
H2O2-induced apoptosis in PC12 cells via
inhibition of the Bcl-2/Bax apoptotic signaling pathway.
NISCH has been previously identified as a novel
protein that selectively binds to the proximal transmembrane region
of the integrin α5 subunit cytoplasmic tail (40). Overexpression of the α5 subunit may
protect cells against apoptotic stimuli by modulating the
expression of the anti-apoptotic protein Bcl-2 via activating the
phosphoinositide-3-kinase/Akt signaling pathway (41). NISCH selectively binds to the
integrin α5 subunit and negatively regulates the expression of
integrin a5 subunit (40). In
addition, a previous study demonstrated that overexpression of
NISCH may induce apoptosis in human breast cancer cells (20). Therefore, NISCH may exert
pro-apoptotic activities by regulating the activity of the
Bcl-2/Bax signaling pathway via interaction with the integrin α5
subunit. However, additional apoptosis pathways may be involved in
the pathological mechanism induced by oxidative stress, which
requires further investigation in future studies.
In conclusion, the current study provides evidence
for the role of NISCH in oxidative stress-induced apoptosis, and
suggests a potential mechanism by which NISCH downregulation
attenuates cell apoptosis induced by oxidative stress. The authors
of the present study hypothesize that NISCH downregulation may
inhibit cell apoptosis by inhibiting transduction of the Bcl-2/Bax
signaling pathway. These results may provide a therapeutic
candidate for protecting against oxidative stress-induced
apoptosis.
Acknowledgements
Not applicable.
Funding
The current study was supported by grants from the
Guiding Plan of the Natural Science Fund of Liaoning Province
(grant no. 201602281) and the Science Studies General Projects in
the Department of Education of Liaoning Province (grant no.
L2015314).
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZG, YY, and YG performed the experiments. ZG, HW,
and CS collected the data and prepared the manuscript. ZG and MH
designed the study and analyzed the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang YH, Wang Z, Zheng J and Wang R:
Protective effects of gallic acid against spinal cord
injury-induced oxidative stress. Mol Med Rep. 12:3017–3024. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao B, Zhang Y, Sun H, Ma B and Qian J:
Ryanodine receptor 2 plays a critical role in spinal cord injury
via induction of oxidative stress. Cell Physiol Biochem.
38:1129–1137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: Uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fleming JC, Norenberg MD, Ramsay DA,
Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD
and Weaver LC: The cellular inflammatory response in human spinal
cords after injury. Brain. 129:3249–3269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang N, Yin Y, Xu SJ, Wu YP and Chen WS:
Inflammation & apoptosis in spinal cord injury. Indian J Med
Res. 135:287–296. 2012.PubMed/NCBI
|
|
6
|
Lv R, Mao N, Wu J, Lu C, Ding M, Gu X, Wu
Y and Shi Z: Neuroprotective effect of allicin in a rat model of
acute spinal cord injury. Life Sci. 143:114–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khayrullina G, Bermudez S and Byrnes KR:
Inhibition of NOX2 reduces locomotor impairment, inflammation, and
oxidative stress after spinal cord injury. J Neuroinflammation.
12:1722015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao H, Chen S, Gao K, Zhou Z, Wang C,
Shen Z, Guo Y, Li Z, Wan Z, Liu C and Mei X: Resveratrol protects
against spinal cord injury by activating autophagy and inhibiting
apoptosis mediated by the SIRT1/AMPK signaling pathway.
Neuroscience. 348:241–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lou J, Lenke LG, Ludwig FJ and O'Brien MF:
Apoptosis as a mechanism of neuronal cell death following acute
experimental spinal cord injury. Spinal Cord. 36:683–690. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu J, Ashwell KW and Waite P: Advances in
secondary spinal cord injury: Role of apoptosis. Spine (Phila Pa
1976). 25:1859–1866. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Chen Q, Mao Y, Xu S, Xia C, Shi X,
Zhang JH, Yuan H and Sun X: Hydrogen-rich saline protects against
spinal cord injury in rats. Neurochem Res. 35:1111–1118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bastani NE, Kostovski E, Sakhi AK, Karlsen
A, Carlsen MH, Hjeltnes N, Blomhoff R and Iversen P: Reduced
antioxidant defense and increased oxidative stress in spinal cord
injured patients. Arch Phys Med Rehabil. 93:2223–2228.e2. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Campuzano O, Castillo-Ruiz MM, Acarin L,
Gonzalez B and Castellano B: Decreased myeloperoxidase expressing
cells in the aged rat brain after excitotoxic damage. Exp Gerontol.
46:723–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bergendi L, Benes L, Duracková Z and
Ferencik M: Chemistry, physiology and pathology of free radicals.
Life Sci. 65:1865–1874. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang KA, Wang ZH, Zhang R, Piao MJ, Kim
KC, Kang SS, Kim YW, Lee J, Park D and Hyun JW: Myricetin protects
cells against oxidative stress-induced apoptosis via regulation of
PI3K/Akt and MAPK signaling pathways. Int J Mol Sci. 11:4348–4360.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maziveyi M and Alahari SK: Breast cancer
tumor suppressors: A special emphasis on novel protein nischarin.
Cancer Res. 75:4252–4259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding Y, Li Y, Lu L, Zhang R, Zeng L, Wang
L and Zhang X: Inhibition of nischarin expression promotes neurite
outgrowth through regulation of PAK activity. PLoS One.
10:e01449482015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding Y, Zhang R, Zhang K, Lv X, Chen Y, Li
A, Wang L, Zhang X and Xia Q: Nischarin is differentially expressed
in rat brain and regulates neuronal migration. PLoS One.
8:e545632013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang C, Wei W, Han D, Meng J, Zhu F, Xiao
Y, Wu G, Shi X and Zhang L: Expression of Nischarin negatively
correlates with estrogen receptor and alters apoptosis, migration
and invasion in human breast cancer. Biochem Biophys Res Commun.
484:536–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding YM, Li YY, Wang C, Huang H, Zheng CC,
Huang SH, Xuan Y, Sun XY and Zhang X: Nischarin-siRNA delivered by
polyethylenimine-alginate nanoparticles accelerates motor function
recovery after spinal cord injury. Neural Regen Res. 12:1687–1694.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu S, Liu H and Luo L: Analysis of
relative gene expression using different real-time quantitative
PCR. Acta Agronomica Sinica. 2007.
|
|
23
|
Wang Q and Zhang L, Yuan X, Ou Y, Zhu X,
Cheng Z, Zhang P, Wu X, Meng Y and Zhang L: The relationship
between the Bcl-2/Bax proteins and the mitochondria-mediated
apoptosis pathway in the differentiation of adipose-derived stromal
cells into neurons. PLoS One. 11:e01633272016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Wang R, Wang Y, Peng R, Wu Y and
Yuan Y: Ginkgo biloba extract mitigates liver fibrosis and
apoptosis by regulating p38 MAPK, NF-κB/IκBα, and Bcl-2/Bax
signaling. Drug Des Devel Ther. 9:6303–6317. 2015.PubMed/NCBI
|
|
25
|
D'Mello SR, Kuan CY, Flavell RA and Rakic
P: Caspase-3 is required for apoptosis-associated DNA fragmentation
but not for cell death in neurons deprived of potassium. Journal of
neuroscience research. 59:24–31. 2015. View Article : Google Scholar
|
|
26
|
Fibach E and Rachmilewitz E: The role of
oxidative stress in hemolytic anemia. Curr Mol Med. 8:609–619.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Varija D, Kumar KP, Reddy KP and Reddy VK:
Prolonged constriction of sciatic nerve affecting oxidative
stressors & antioxidant enzymes in rat. Indian J Med Res.
129:587–592. 2009.PubMed/NCBI
|
|
28
|
Wang X, Wu X, Liu Q, Kong G, Zhou J, Jiang
J, Wu X, Huang Z, Su W and Zhu Q: Ketogenic metabolism inhibits
histone deacetylase (HDAC) and reduces oxidative stress after
spinal cord injury in rat. Neuroscience. 366:36–43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Narayanan V: Apoptosis in development and
disease of the nervous system: 1. Naturally occurring cell death in
the developing nervous system. Pediatr Neurol. 16:9–13. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung SY, Kim DY, Yune TY, Shin DH, Baek SB
and Kim CJ: Treadmill exercise reduces spinal cord injury-induced
apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med.
7:587–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lan WB, Lin JH, Chen XW, Wu CY, Zhong GX,
Zhang LQ, Lin WP, Liu WN, Li X and Lin JL: Overexpressing
neuroglobin improves functional recovery by inhibiting neuronal
apoptosis after spinal cord injury. Brain Res. 1562:100–108. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chougule M, Patel AR, Sachdeva P, Jackson
T and Singh M: Anticancer activity of Noscapine, an opioid alkaloid
in combination with Cisplatin in human non-small cell lung cancer.
Lung Cancer. 71:271–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahamed M, Akhtar MJ, Siddiqui MA, Ahmad J,
Musarrat J, Al-Khedhairy AA, AlSalhi MS and Alrokayan SA: Oxidative
stress mediated apoptosis induced by nickel ferrite nanoparticles
in cultured A549 cells. Toxicology. 283:101–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li R, Shang J, Zhou W, Jiang L, Xie D and
Tu G: Overexpression of HIPK2 attenuates spinal cord injury in rats
by modulating apoptosis, oxidative stress, and inflammation. Biomed
Pharmacother. 103:127–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan L, Wang K and Cheng B: Effects of
buyang huanwu decoction on apoptosis of nervous cells and
expressions of Bcl-2 and bax in the spinal cord of
ischemia-reperfusion injury in rabbits. J Tradit Chin Med.
26:153–156. 2006.PubMed/NCBI
|
|
36
|
Guo SZ, Jiang T and Ren XJ: Influence of
neurotrophin-3 on Bcl-2 and Bax expressions in spinal cord injury
of rats. J Med Coll PLA. 22:97–100. 2007. View Article : Google Scholar
|
|
37
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nesic O, Xu GY, McAdoo D, High KW,
Hulsebosch C and Perez-Pol R: IL-1 receptor antagonist prevents
apoptosis and caspase-3 activation after spinal cord injury. J
Neurotrauma. 18:947–956. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim YM, Talanian RV and Billiar TR: Nitric
oxide inhibits apoptosis by preventing increases in caspase-3-like
activity via two distinct mechanisms. J Biol Chem. 272:31138–31148.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alahari SK and Nasrallah H: A membrane
proximal region of the integrin alpha5 subunit is important for its
interaction with nischarin. Biochem J. 377:449–457. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matter ML and Ruoslahti E: A signaling
pathway from the alpha5beta1 and alpha(v)beta3 integrins that
elevates bcl-2 transcription. J Biol Chem. 276:27757–27763. 2001.
View Article : Google Scholar : PubMed/NCBI
|