Introduction
Lichen planus (LP), a chronic inflammatory
dermatosis, usually affects middle aged people and both sexes with
a minor female predominance (1–4). It
frequently involves the skin, nails and scalp hair or mucous
membranes (oral, esophageal, laryngeal, vulvovaginal and
conjunctival mucosa) (2,4,5). It
often affects the flexor surface of the extremities. It has been
shown that patients with oral LP also present cutaneous lesions in
16% of the cases, while 19% also present genital disease (6). The mean age is between 50–60 years for
oral LP and between 40–45 years for cutaneous LP (7–9). The
lesions are frequently bilateral and symmetric. The clinical
presentation of lichen planus depends on the affected area. It is a
disease with various clinical features. Based on the morphology and
location of the lesions, it has different clinical subtypes, which
include: papular (common), annular, actinic, atrophic,
hypertrophic, vesiculobullous, follicular, linear, pigmentosus and
pigmentosus-inversus (2). The
classic skin lesions consists of polygonal papules that are flat
and vary from erythematous to violaceus (2). The top of the lesion can have a thin or
adherent scale (5). The
pathognomonic findings are the lines called Wickham striae which
are fine white lines that cross the plaque (5).
The precise cause of lichen planus is still unclear
but studies suggest the interaction of genetic factors (in
cutaneous lichen planus the presence of antigen HLA-DR1) with
autoimmune mechanisms (association with autoimmune disorders such
as alopecia areata and ulcerative colitis) or viral infections (the
development rate of lichen planus is 2.5–4.5 higher in HCV
seropositive patients) (10–13). Other factors include environmental
factors, stress, anxiety, internal malignancies and dyslipidemia
(14,15). In lichen planus, CD8+ T
lymphocytes have a major role as they are the most important
components of the infiltrate that damages keratinocytes (2,16,17).
Until now, in order to confirm the diagnosis of LP
and start a correct treatment, biopsy and histopathological
examination are required (2). The
usual histopathological changes observed in lichen planus include
orthokeratosis and compact hyperkeratosis, thickening of the
granular layer, acanthosis, keratinocyte damage at the level of the
basal layer, decreased population of epidermal melanocytes,
inflammatory cells in the papillary dermis (18,19).
Current trends for the diagnosis and especially the
monitoring treatment response of certain skin and mucosal
inflammatory diseases, such as psoriasis and acne, use modern
non-invasive or minimally invasive techniques without the necessity
of biopsy (20–23).
The aim of this study is to review the role of
modern in vivo imaging techniques such as dermoscopy,
reflectance confocal microscopy (RCM), optical coherence tomography
(OCT), diffuse reflection spectrophotometry and ultrasound in the
investigation of LP lesions and their correlation with classical
histopathological features.
Dermoscopy in LP
Dermoscopy is a non-invasive method described for
the first time by Braun in the 1990s and it was initially used for
the diagnosis of pigmented skin tumors (24). Subsequently, the field of use of this
investigation has expanded, and it is useful in a wide range of
disorders such as benign or malignant, pigmented or non-pigmented
skin tumors, as well as inflammatory or infectious skin diseases:
psoriasis, lichen planus, sarcoidosis and scabies (25–27).
As stated before, the diagnosis of lichen planus is
based on skin biopsy. Without minimizing the importance of the
histopathological examination, dermoscopy has three important roles
in LP. Firstly, it increases the accuracy of LP diagnosis,
especially in the context in which this condition can embrace
various clinical forms, often difficult to diagnose by clinical
examination alone. Thus, differential diagnosis with syphilis,
Darier's disease, acanthosis nigricans, lupus erythematous and
psoriasis can be performed using a non-invasive technique (25). Secondly, using dermoscopy avoids skin
biopsies commonly used in diagnosis of LP, but difficult to accept
by the patient. Thirdly, dermoscopy can also be useful in the
therapeutic monitoring of this condition by repeated investigations
at different stages of treatment.
The dermoscopic semiology in LP includes mainly
three elements: Wickham striae (WS), vascular patterns and pigment
patterns, elements that vary depending on the clinical form of LP,
the age of the disease and localization.
WS were first described by Wickham (28) ‘as reticular streaks, dots or other
varied configurations superimposed on a LP papule’ being considered
to date a pathognomonic sign for the diagnosis of LP (24). WS pathogenesis has caused numerous
debates. Thus, Summerly and his collaborators considered that the
presence of WS is due to a functional anomaly of keratinocytes,
while Ryan and colleagues associated their presence with other
causes and the diminution of the vascular network in the
superficial dermis (24). In 1909,
Darier correlated the formation of WS with hypergranulosis
(24,25). In the classical forms of LP, WS are
commonly found, but there are other forms of LP in which they are
absent (invisible WS) probably due to acute forms of the disease in
which hypergranulosis has not yet been formed. The data from the
literature show, on the one hand, a correlation between the
presence or absence of WS and the histopathological changes
specific to various clinical forms of LP, and on the other hand
their presence correlates with the degree of disease activity or
evolution under treatment (26,27,29). WS
are present in classical forms of LP, disappear after the
treatment, and reappear after disease recurrences, being considered
a marker of disease activity (26).
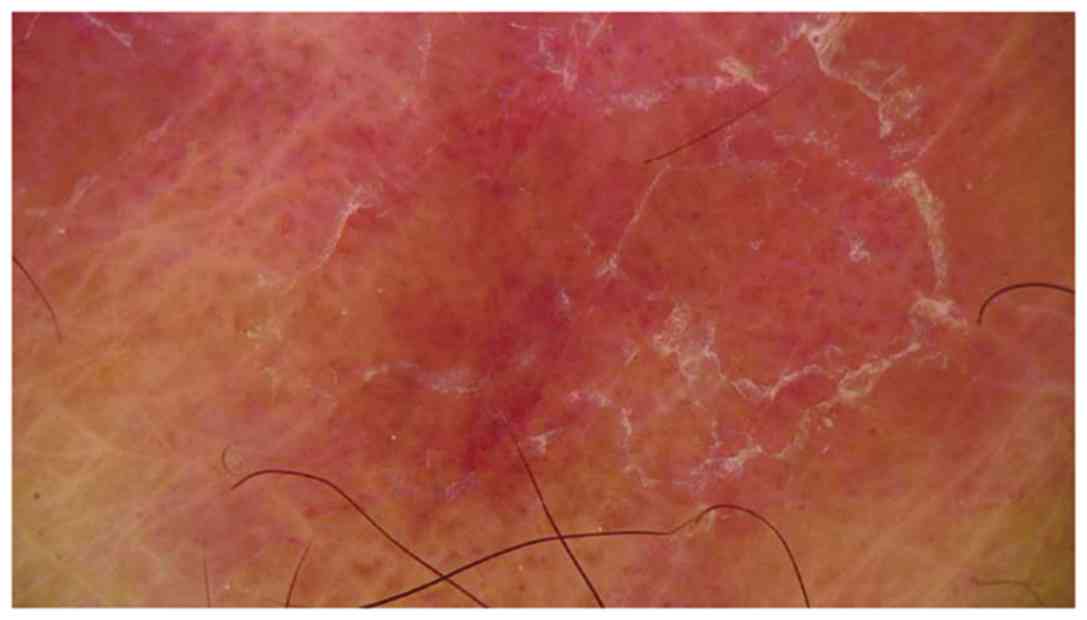
From a dermoscopic point of view, we meet the classic reticular
pattern of WS as white crossing lines (25). However, recent studies suggest
introduction of new dermoscopic patterns for LP, such as the starry
sky or leaf venation patterns (24).
The starry sky pattern signifies clustered, follicular white dots
of WS, possibly being a sequela of hypergranulosis (29,30). The
leaf venation pattern consists of side strips that branch out from
the central WS venation and join together to mimic the snow
crystals. Besides these dermoscopic aspects of WS, other patterns,
such as linear, globular, perpendicular, veil-like or
structureless, have also been described in the literature (29). Moreover, at the periphery of WS there
were white, thin lines and blue-white veils probably as a
consequence of the presence of melanophages in the deep dermis
(26,31). Also, sometimes yellowish or white
non-structured patterns can be identified as associated with WS,
probably due to dermal spongiosis and degeneration of the basal
layer (32–34). In lichen planopilaris of the scalp,
WS patterns are significant in recent lesions, while the veil-like
structureless WS pattern is the main feature in this clinical form
of LP (35).
The vascular patterns are a second feature of LP,
the blood vessels being well visible through dermoscopy (36,37).
They appear in the form of red dots which represent the most
important dermoscopic feature in the LP. In early lesions of
actinic LP, peripheral homogeneous vascular patterns have been
encountered, this feature being significantly reduced or even
absent after treatment (38). In
conclusion, the most common dermoscopic aspects of WS have three
characteristics: white, cross-linked and, as stated below, with red
dots.
Pigment patterns are different depending on the form
of LP. However, they show several important features (32,39)such
as: Are always present in certain clinical forms of LP, such as
pigmented LP; different dermoscopic appearance can be observed in
the same lesion; their dermoscopic appearance does not change after
treatment.
Melanocyte proliferation is a dermoscopic marker
(34,39)expressed through pigmented dots,
globules and diffuse hyperpigmentation, which are among the most
common dermoscopic aspects in LP. Peppering pigment pattern shows
the presence of melanophages in the superficial dermis. Moreover,
crowding of melanophages around the blood vessels in the dermal
papillae induces the dermoscopic pattern of pigment dots to papilla
(24). Other pigment patterns
encountered in LP lesions are perifollicular/annular/granular
pattern, linear, reticular or cobblestone pattern, homogeneous
cloud like pattern (Fig. 1).
Reflectance confocal microscopy of LP
Reflectance confocal microscopy (RCM) is a novel
non-invasive imaging technique that is prevalently used in skin
tumor diagnosis (40–44), also proving useful in clinical
decision management (45–47). RCM provides ‘optical skin biopsies’,
revealing microscopic-level changes across multiple skin layers
with an en-face point of view (48).
Various confocal criteria for melanocytic and non-melanocytic
lesions have demonstrated both high sensitivity and specificity
values, suggesting that in the future, the dissemination of RCM
technique could, at least in selected cases, avoid unnecessary skin
biopsies (49). Reflectance confocal
microscopy represents, at the moment, the best possible bridge
between dermoscopy and histology, giving clinicians the possibility
of carrying out non-invasive, real-time, virtual skin biopsies.
While in dermato-oncology RCM can deliver information regarding the
nature of skin lesions and their malignant potential, in
inflammatory skin diseases this technology has been mostly employed
for bedside, real-time, microscopic evaluation of psoriasis
(20,50–52),
lichen planus (18,48), contact dermatitis (53), revealing specific confocal features
to support clinical diagnosis and assist with patient management
(54–57). Unfortunately, available data on
confocal descriptors of inflammatory skin diseases focuses mainly
on RCM-histology correlation and treatment follow-up, and very
rarely on differential diagnosis (51,58).
In LP, RCM examination reveals a well represented
granular layer with large (25–35 µm) polygonal cells containing a
luminous grainy cytoplasm, corresponding to histological
hypergranulosis (59–61). In normal skin, the difference between
the granular and spinous layers is not readily distinguishable
during RCM examination, due to the fact that granular cells are
normally arranged in a thin layer. In LP however, due to
hypergranulosis, the passage from granular to spinous layer is more
easily observable. The spinous layer commonly shows localized
bright areas with loss of the normal honeycomb pattern, and dark
areas with thickened intercellular spaces corresponding to moderate
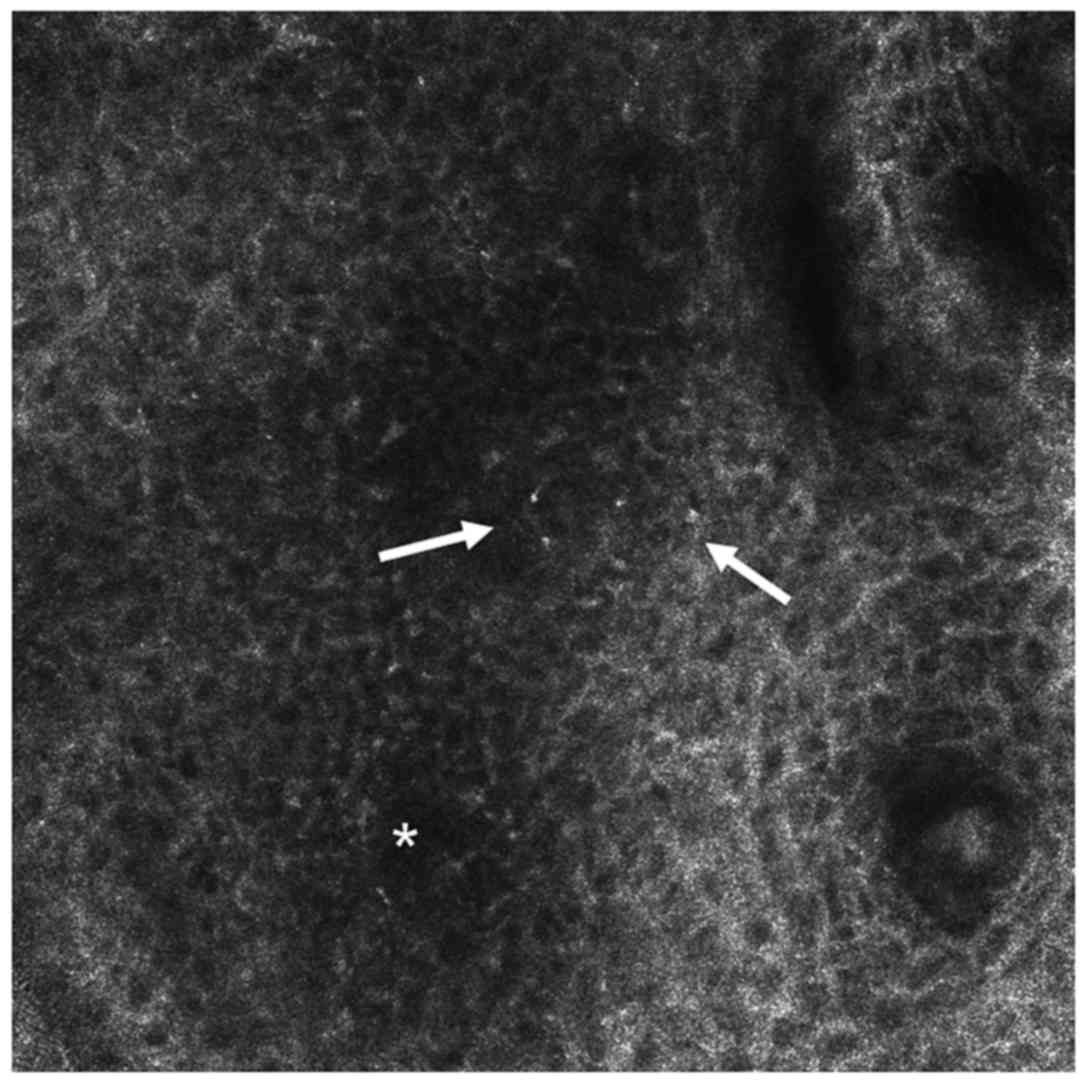
spongiosis (Fig. 2). Numerous
inflammatory infiltrates in the form of circular and polygonal
bright elements can be observed throughout the epidermis (54). One characteristic confocal feature of
LP is considered to be the presence of necrotic keratinocytes
visualized as uniformly bright, polygonal cells, larger than
surrounding keratinocytes (18) both
in the spinous and basal layers, noticeable as Civatte bodies in
histology (62). Some of the
features noted above are part of probably the most common global
pattern identified upon RCM examination of LP lesions, the
interface dermatitis (18,62,63).
The histological term ‘interface dermatitis’ refers
to skin diseases in which an inflammatory process prevalently
involves the dermo-epidermal junction (DEJ) showing the presence of
focal or diffuse inflammatory infiltrates, injury or necrosis of
basal keratinocytes, associated with vacuolar or lichenoid changes.
Lichen planus, along with discoid lupus erythematosus (DLE),
represent the prototypes for this group of cutaneous inflammatory
diseases as their pathological processes both involve the DEJ. RCM
has been employed in interface dermatitis and major and minor
descriptors of this group of inflammatory diseases have already
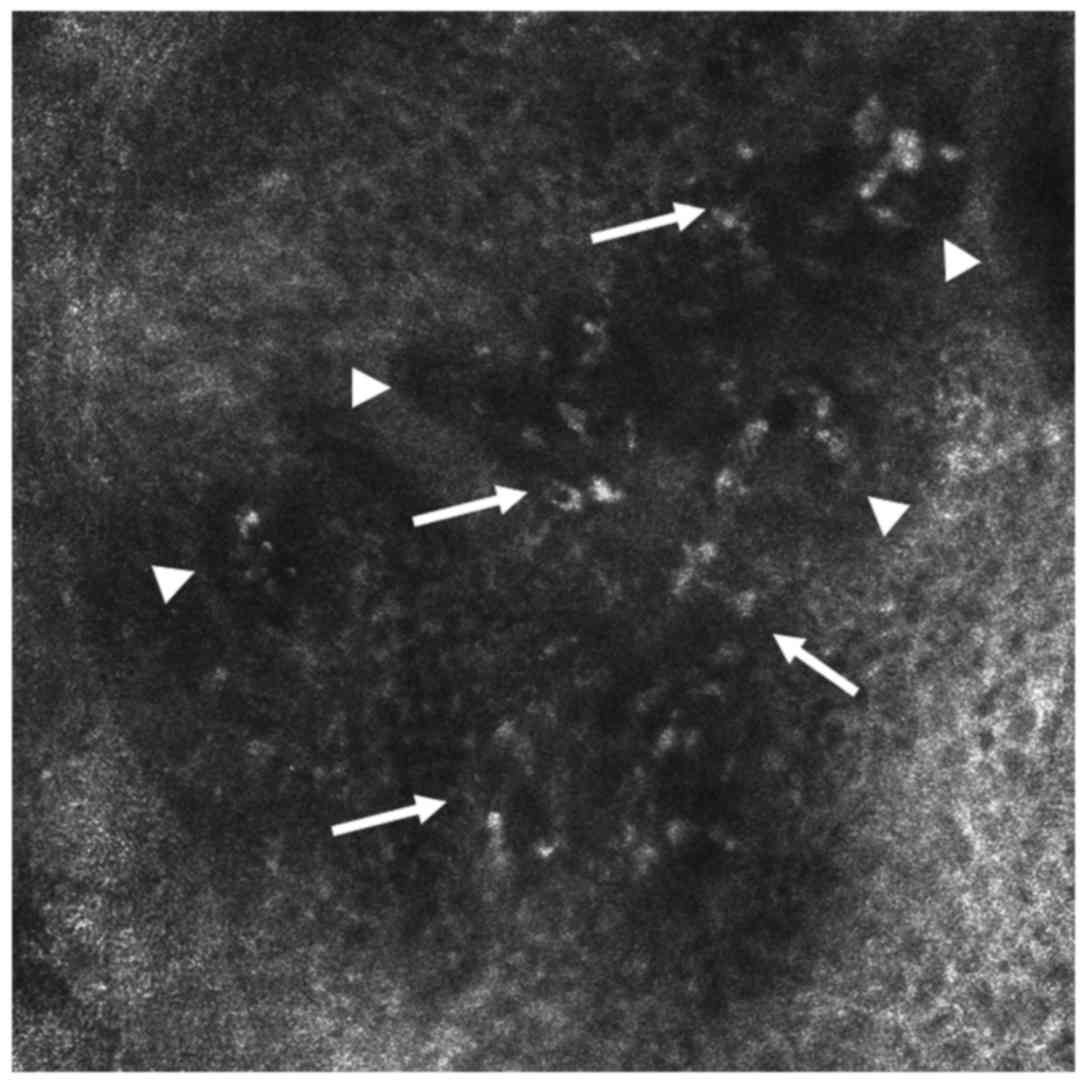
been characterized (18,64). In accordance with optical microscopy
findings, RCM has shown in LP lesions the presence of round, bright
elements arranged in sheets, representing inflammatory infiltrates
that obscure the DEJ (59). The
dermal papillae (DP) are visible as dark, round areas. In interface
dermatitis such as LP, RCM reveals a non-edged papillae pattern
(Fig. 3).
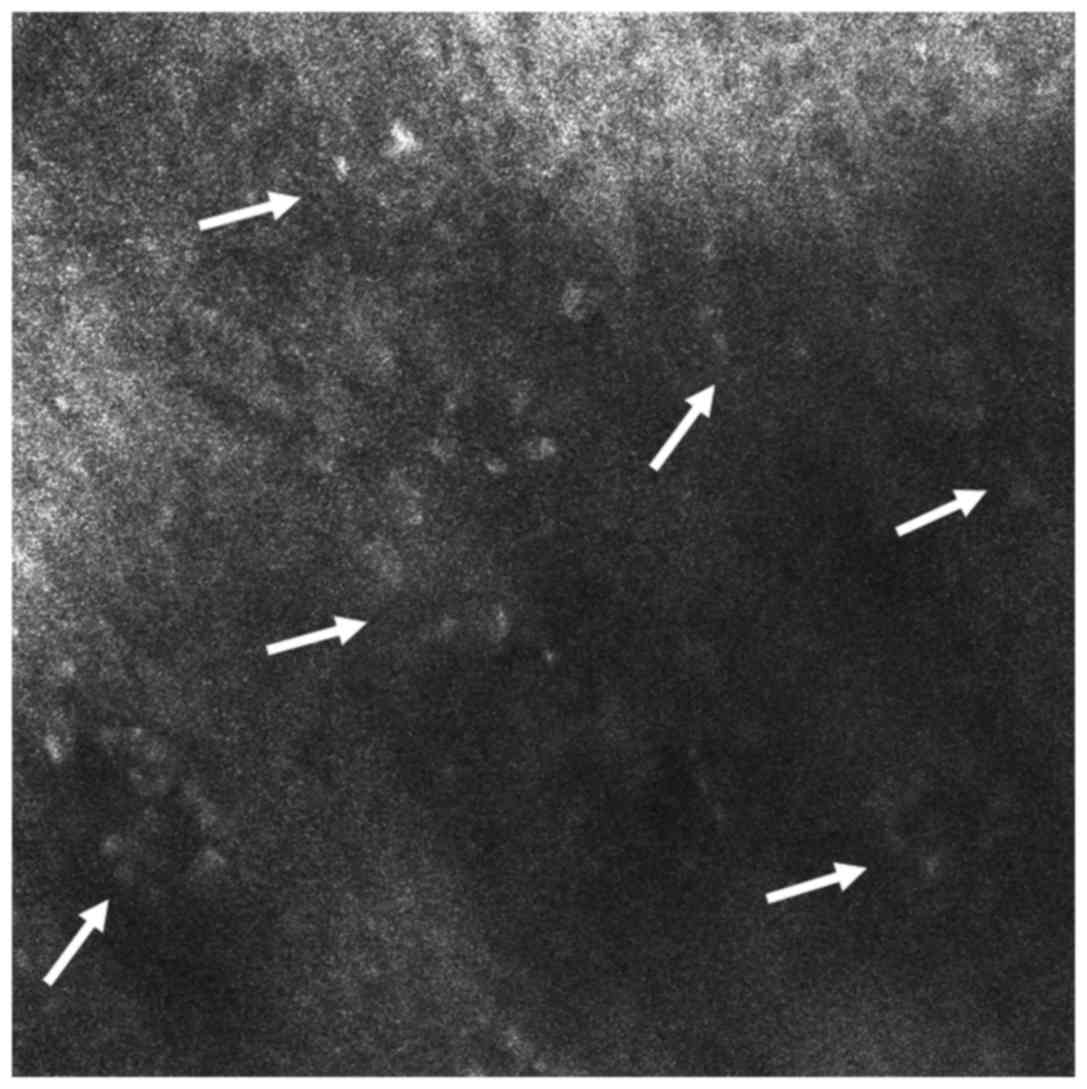
Moving on from the shallow epidermal layers to the
level of the upper dermis, a noteworthy finding is the presence of
plump refractile structures, with shapes that vary from oval to
stellar, representing melanophages. Round to polygonal, somewhat
refractile cells, clearly smaller than melanophages, correlated to
inflammatory cells, are also visible in the dermis (Fig. 4). Dilated dermal blood vessels appear
as dark, canalicular structures, placed horizontally on confocal
sections (65), in contrast to the
vertical appearance of vessels depicted in other cutaneous
inflammatory diseases such as psoriasis.
Summing up, nine common features of LP observable by
RCM have been described: spongiosis (in various degrees),
hypergranulosis, necrotic keratinocytes, single inflammatory cells,
focal or diffuse inflammatory infiltrates, dilated blood vessels,
interface dermatitis, dermal inflammatory infiltrates, melanophages
in the upper dermis (18,66,67).
Considering the aforementioned findings, it is
without doubt that RCM can be of great aid in supporting the
clinical diagnosis of LP. However, it is unable to distinguish
between different leukocyte cell types thus limiting the
interpretation of the inflammatory cell infiltrate, and has a
limited depth of penetration of down to about 250 µm below the skin
surface (48) without the
possibility of having a clear ‘picture’ of reticular dermis.
Therefore, it is less helpful in the differential diagnosis of
interface dermatitis (e.g., DLE vs. LP) (19) or in particular cases of lichen planus
in which the diagnosis is hard to establish even after
histopathological examination (68).
In spite of these limitations, the advantage of a
non-invasive, real-time microscopic skin examination resides in the
possibility of immediate clinical-microscopic correlations which
clinicians can use in several clinical applications: major
differential diagnosis, therapeutic follow-up examinations, and
identification of the best possible site for a skin biopsy
(19). Larger studies demonstrating
the diagnostic and differential diagnosis efficiency of RCM in the
field of interface dermatitis are still needed.
Optical coherence tomography of LP
Optical coherence tomography (OCT) is an emergent
imaging technique, developed over the last decade, based on the
interaction of the infrared radiation (900–1500 nm) and the living
tissues. It allows the non-invasive, in vivo visualization
at high resolution of the micro-structural morphology of skin
components (69). OCT is based on
the principle of Michelson interferometry. A light source emits a
light beam that is split into a reference beam and a probe beam,
which is sent to the investigated tissue. The OCT device records
the signals generated by the interference of the probe light
back-scattered from the tissue with the reference beam. Measurement
of the interference pattern allows determining the position of
different absorbent or reflective tissues components, such as cell
membranes or melanin pigment. The optical properties of the tissue
and the central wavelength of the light beam determine the depth of
penetration. Light absorption and scattering in the tissue sample
should be minimized to allow maximal imaging depth, and this is
best achieved for wavelengths in the window 700–1300 nm, with
longer wavelengths performing better for deeper visualization
(70).
The method was introduced in the medical practice in
the field of ophthalmology in the 1990s, and expanded quickly to
other specialties, from cardio-vascular surgery to
gastroenterology, urology, neurology or gynecology (70). Its use in dermatology started to be
explored in the 1990s, and currently several OCT systems have
become commercially available for research and clinical practice
for skin diseases. These various systems follow mainly two
technological concepts, the time domain and frequency or spectral
domain OCT (71). Wavelengths are
used in the range between 930 and 1300 nm to achieve a
visualization of superficial layers of the skin, as deep as 2 mm,
corresponding usually to the papillary dermis, with a lateral
resolution of 3–25 µm (72). The
axial resolution varies between 3 and 12 µm, and the field of view
between 1.6 to 10 mm diameter. The broadband light sources are
preferred to improve axial resolution and depth visualization, and
with longer wavelengths achieving deeper penetration but lower
lateral resolution (71). Most
systems provide in vivo, real-time, bi-dimensional
cross-sectional images of the skin layers, comparable with
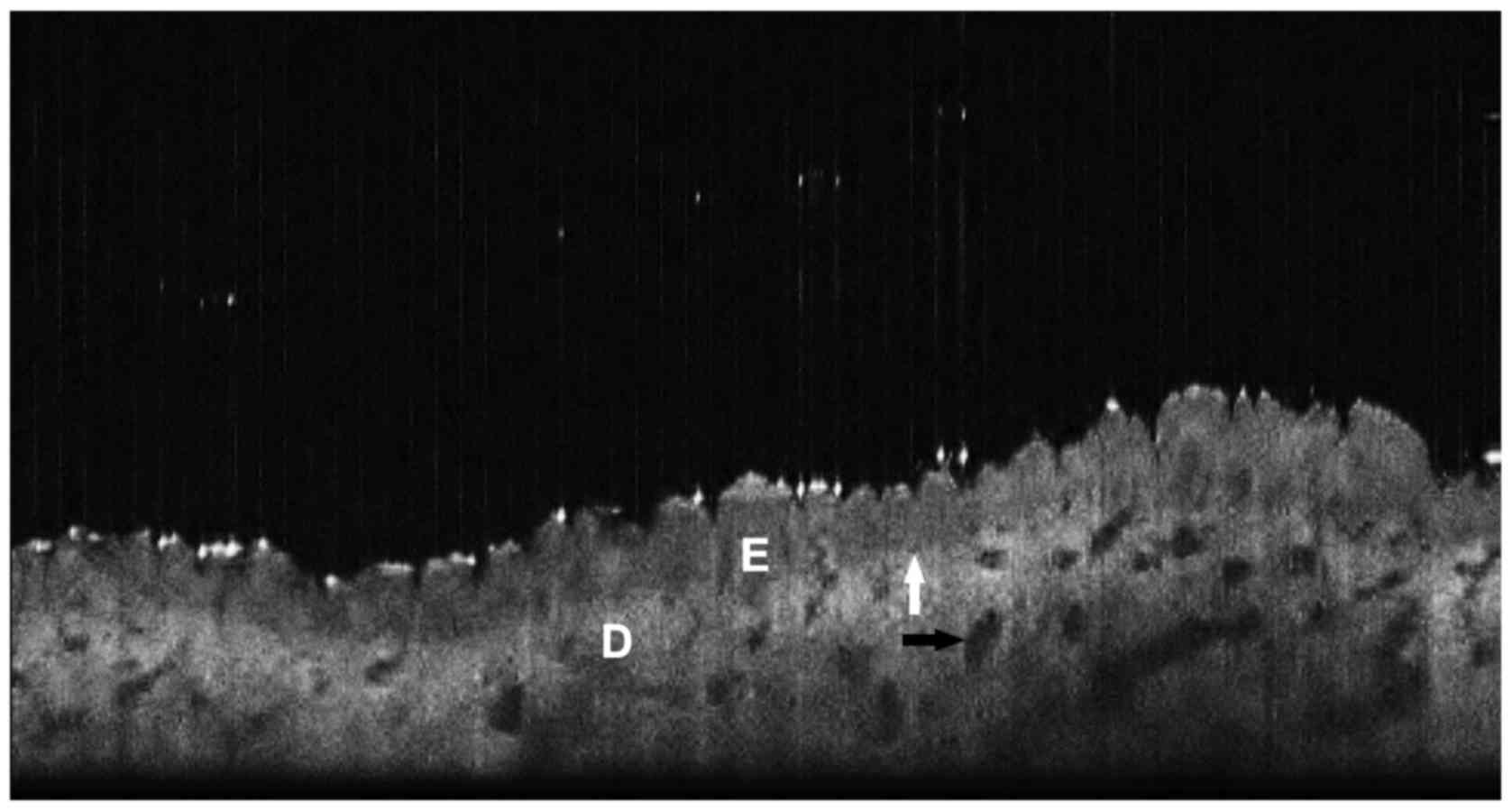
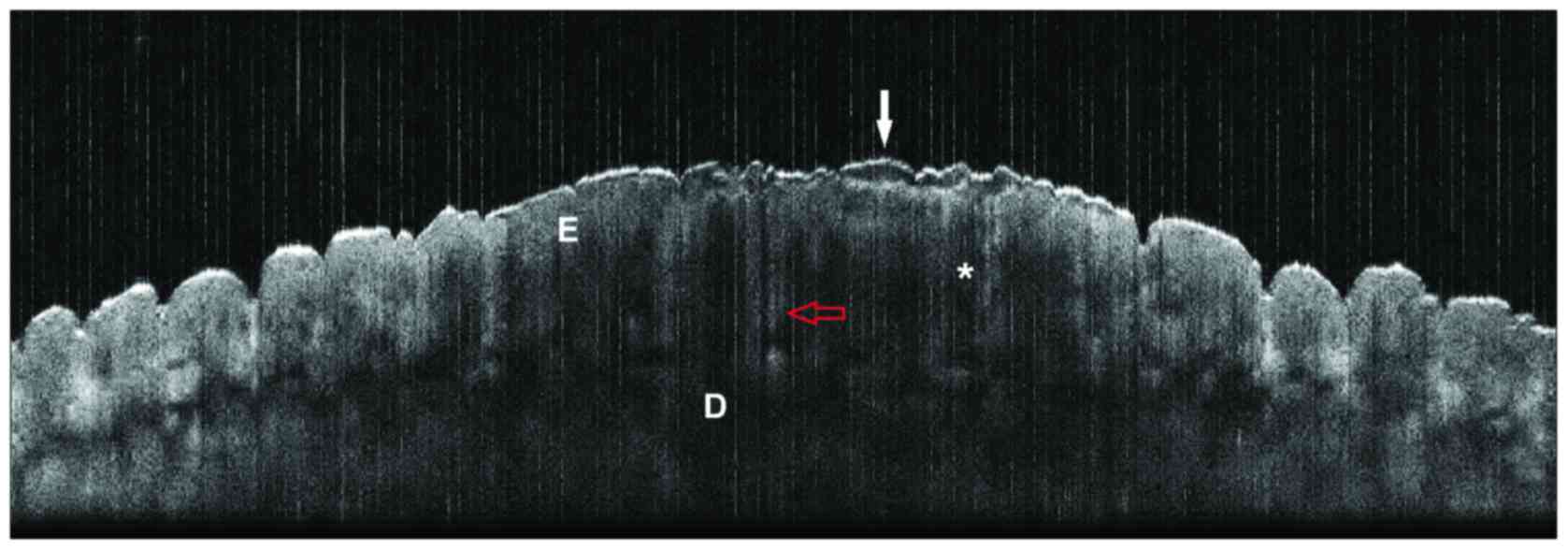
low-magnification histopathological sections (Fig. 5). Some, like high-definition OCT
(HD-OCT) system (Skintell; Agfa Healthcare, Mortsel, Belgium)
delivers horizontal, en-face images, allowing a three dimensional
visualization of skin structures.
OCT has shown promising results in the in
vivo assessment of normal or diseased skin structure and
properties, like the epidermal thickness, the architectural
characteristics of skin appendages such as hair, glands and nails,
the degree of skin fibrosis and the aspect of superficial dermal
vessels (71,72). Importantly, OCT allows monitoring of
the dynamic changes of these characteristics in response to
conservative treatments, without the need of invasive maneuvers
(73,74). The main body of research has been
dedicated so far to the role of OCT in the pre-biopsy evaluation of
skin tumors (72,74,75),
especially non-melanocytic neoplasms. Studies support the role of
OCT in differentiating non-melanoma skin cancers from benign
lesions and normal skin with sensitivities of 79–94% and
specificities of 85–96% (72,76).
Newer high-resolution HD-OCT systems are reported to allow for
in vivo differentiating of histological subtypes of basal
cell carcinoma, and grading of actinic keratoses (77,78). OCT
shows promising results in the pre-surgical estimation of lateral
margins of tumors helping orientate the extent of surgery for
complete resection (72,79). Comparatively much less information is
available on the value of OCT for the non-invasive assessment of
inflammatory skin diseases. OCT can show the thickening of the
epidermis and stratum corneum in psoriasis, the epidermic
spongiosis in atopic or contact dermatitis and the dynamics of
these alterations under topical treatment (73,74,80,81). The
changes at the dermo-epidermal junction (DEJ) such as the shape of
the interline or the effacement of DEJ can be visualized. However,
deeper inflammatory changes are hard to evaluate, especially in
thicker skin, due to low penetration of this technique that is
usually confined to the papillary dermis.
In this context very limited information exists on
the benefits of OCT technology for the in vivo diagnosis of
LP. The main study was performed by Boone et al who reported
a series of 9 patients with histopathologically confirmed LP,
investigated by HD-OCT (Skintell; Agfa Healthcare) (82). The time domain HD-OCT system used in
their study provides 2D images of a 1.8×1.5 mm2 field
and a penetration depth of up to 0.57 mm at axial and lateral
resolution of about 3 µm each. This system natively collects
stacked en-face images of the tissues, which can be reconstructed
by proprietary algorithm into conventional b-mode, cross-sectional
images. The authors reported to be able to visualize by HD-OCT
important LP characteristics like irregular acanthosis with
increased epidermal layer thickness, with saw-tooth appearance of
the dermo-epidermal interline, focal hypergranulosis, and the
typical interface inflammatory infiltrate, manifest as effacement
of the DEJ interline (82). More
subtle signs such as basal vacuolar degeneration visualized as
total obliteration of the ring-like structures around the dermal
papillae, or the presence of inflammatory and necrotic cells in the
epidermis as bright spots were reported thanks to the availability
of high-resolution en-face images. Importantly, these aspects
allowed the authors to differentiate in vivo LP from more
frequent inflammatory diseases with different infiltrate patterns
like psoriasis or eczema (82). We
observed comparable aspects on cross section using a conventional
OCT system, at 930 nm wavelength (Thorlabs OCP930SR Spectral Radar;
Thorlabs Inc., Newton, NJ, USA) (Fig.
6) (unpublished results). Schmitz et al, reported on one
case where OCT was used to follow in vivo the dynamic
changes in the lesions of palmo-plantar LP treated with UVA
phototherapy (83).
Other authors reported on the use of OCT for the
in vivo differentiation of oral mucosal lesions LP from
malignant or premalignant oral cavity changes, or to monitor the
evolution under treatment of recalcitrant oral LP (84). OCT can be used to identify
architectural changes in the keratin cell layer, epithelial layer,
basement membrane, lamina propria, and rete pegs of oral mucosa
(85). Initial studies found that
OCT could differentiate between oral squamous cell carcinoma versus
all other oral pathologies, including LP with a sensitivity of
0.931 and specificity of 0.97 (86,87).
Later reports contested, however, the OCT ability to differentiate
between different oral mucosal abnormalities (87).
These initial reports suggest that OCT could be an
useful auxiliary tool in the in vivo differential diagnosis
of LP, especially in clinical equivocal settings such as mucosal
lesions, and in monitoring the response to treatment (69,70).
Confirmation of its use requires indication in larger series of
patients. So far, the limits of the technology relate to the low
depth penetration, which can make the visualization of DEJ
difficult in areas with thicker skin, and the insufficient
resolution of conventional OCT systems in order to allow
identification of cellular changes. The higher resolution of the
HD-OCT systems is counter-weighted by the lower penetration depth,
of about 570 µm, and the small field of view. These features
enhance the impact of the skin compression, patient's and
examiner's movements during image acquisition, inducing higher
variability of the results. Further drawbacks of the technique
include the difficulties to visualize elevated lesions, the
inter-observer variability and the longer learning curve, which
demands solid knowledge of histopathology for the clinician who is
evaluating the images (26,71,72).
Nonetheless, even if this technique's performance is not yet
sufficient to replace histopathological examination for the nuanced
diagnosis of LP and its variants, it has still the important
advantages of a non-invasive investigation method, quick and well
accepted by patients that can provide in real time helpful
information to orientate and monitor the clinical approach. New
developments, like image enhancement algorithms or systems that
combine OCT with other imaging techniques such as Raman
spectroscopy, fluorescence, Doppler and ultrasound, may further
improve the diagnostic performance of OCT for the clinical research
and practice of inflammatory skin diseases, while enhancing the
cost-effectiveness and convenience of use (88).
Other techniques
Ultrasound is well known for over 30 years, being
considered a non-invasive method useful in differential diagnosis
of skin tumors, inflammatory or sclerotic skin diseases, or in
evaluation of skin patch test and tuberculin test (88). Introduced in dermatological practice
in the early 1970s, ultrasound used 1.5–5 MHz transducers with less
satisfactory resolution because it allowed only visualization of
deep structures: Large glands, veins and arteries, muscles and
fatty tissue (89). Alexander and
Miller in 1979 using a 15 MHz transducer were able to measure the
thickness of the skin, but the first 15–20 MHz transducers, which
appeared in the 1980s, allowed the visualization of dermis,
subcutaneous tissue, arterioles and venules (89–92).
Nowadays it is necessary to use a transducer with a
high frequency (25–100 MHz) for the examination of superficial skin
layers (epidermis, dermis) and mucous membranes with a thickness of
<2 mm (90,93,94).
Examining the papules in LP, a hypoechogenic fusiform band, the
maximum of the hypoechogenic zone corresponding to the maximum area
of epidermal acanthosis and dermal inflammatory infiltrate, is
observed (90).
Another non-invasive optic technique used to
diagnose and monitor LP evolution is diffuse reflection
spectrophotometry (DRS). Interaction between light and human tissue
structures allows observation of the process of absorption and
dispersion of light on biological tissues, with an essential role
in obtaining spectral curves to give veracity to the diagnosis
(95). Depending on the absorption
power of the different skin structures, spectrophotometric images
are born. Regarding LP, DRS allows the detection of inflammatory
cells such as localized T-lymphocytes in the epidermis (95,96). DRS
acts in the spectral range 400–450 nm, highlighting the presence of
inflammatory cells in the LP, leading to the formation of a
spectral reflection curve that helps the diagnosis and monitoring
of this pathology (97).
Conclusion
LP is a chronic inflammatory skin condition
affecting skin and or mucosal surfaces. There are several clinical
types of LP that share similar histopathological features. Our
review shows the possibility of using modern imaging techniques for
the in vivo diagnosis and also for evaluation of the
treatment response. In vivo techniques such dermoscopy,
reflectance confocal microscopy, optical coherence tomography,
diffuse reflection spectrophotometry and ultrasound, allow
identification of specific aspects in LP lesions and to correlate
them with histological findings. Moreover, combining these
techniques may improve the accuracy of the diagnosis.
Acknowledgements
Not applicable.
Funding
This work was partially supported by a grant of the
Romanian Ministry of Research and Innovation, CCCDI-UEFISCDI
(project nο. 61PCCDI⁄2018 PN-III-P1-1.2-PCCDI-2017-0341), within
PNCDI-III.
Availability of data and materials
Not applicable.
Authors' contributions
SLI, AMF, ML, MAI, SZ, BD, GI, DN, CT, CMP and CC
contributed to the acquisition and design, analysis and
systematization of data, manuscript drafting and critical revision
of manuscript for important intellectual content. All authors read
and approved the final version of manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Le Cleach L and Chosidow O: Clinical
practice. Lichen planus. N Engl J Med. 366:723–732. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gorouhi F, Davari P and Fazel N: Cutaneous
and mucosal lichen planus: A comprehensive review of clinical
subtypes, risk factors, diagnosis, and prognosis.
ScientificWorldJournal. 2014:7428262014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fazel N: Cutaneous lichen planus: A
systematic review of treatments. J Dermatolog Treat. 26:280–283.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arora SK, Chhabra S, Saikia UN, Dogra S
and Minz RW: Lichen planus: A clinical and immuno-histological
analysis. Indian J Dermatol. 59:257–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedman P, Sabban EC, Marcucci C, Peralta
R and Cabo H: Dermoscopic findings in different clinical variants
of lichen planus. Is dermoscopy useful? Dermatol Pract Concept.
5:51–55. 2015.PubMed/NCBI
|
|
6
|
Eisen D: The clinical features, malignant
potential, and systemic associations of oral lichen planus: A study
of 723 patients. J Am Acad Dermatol. 46:207–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carbone M, Arduino PG, Carrozzo M,
Gandolfo S, Argiolas MR, Bertolusso G, Conrotto D, Pentenero M and
Broccoletti R: Course of oral lichen planus: A retrospective study
of 808 northern Italian patients. Oral Dis. 15:235–243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bermejo-Fenoll A, Sánchez-Siles M,
López-Jornet P, Camacho-Alonso F and Salazar-Sánchez N: A
retrospective clinicopathological study of 550 patients with oral
lichen planus in south-eastern Spain. J Oral Pathol Med.
39:491–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Irvine C, Irvine F and Champion RH:
Long-term follow-up of lichen planus. Acta Derm Venereol.
71:242–244. 1991.PubMed/NCBI
|
|
10
|
Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ,
Zhou XJ, Khan A, Seymour GJ and Bigby M: The pathogenesis of oral
lichen planus. Crit Rev Oral Biol Med. 13:350–365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Epidemiological evidence of the
association between lichen planus and two immune-related diseases.
Alopecia areata and ulcerative colitis. Gruppo Italiano Studi
Epidemiologici in Dermatologia. Arch Dermatol. 127:688–691. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lodi G, Pellicano R and Carrozzo M:
Hepatitis C virus infection and lichen planus: A systematic review
with meta-analysis. Oral Dis. 16:601–612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shengyuan L, Songpo Y, Wen W, Wenjing T,
Haitao Z, Binyou W and Hepatitis C: Hepatitis C virus and lichen
planus: A reciprocal association determined by a meta-analysis.
Arch Dermatol. 145:1040–1047. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Girardi C, Luz C, Cherubini K, de
Figueiredo MA, Nunes ML and Salum FG: Salivary cortisol and
dehydroepiandrosterone (DHEA) levels, psychological factors in
patients with oral lichen planus. Arch Oral Biol. 56:864–868. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Constantin C, Corina G, Ana C, Adriana D
and Daniel B: The role of stress in skin diseases. Intern Med.
8:73–84. 2011.
|
|
16
|
Iijima W, Ohtani H, Nakayama T, Sugawara
Y, Sato E, Nagura H, Yoshie O and Sasano T: Infiltrating
CD8+ T cells in oral lichen planus predominantly express
CCR5 and CXCR3 and carry respective chemokine ligands RANTES/CCL5
and IP-10/CXCL10 in their cytolytic granules: A potential
self-recruiting mechanism. Am J Pathol. 163:261–268. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma A, Białynicki-Birula R, Schwartz RA
and Janniger CK: Lichen planus: An update and review. Cutis.
90:17–23. 2012.PubMed/NCBI
|
|
18
|
Moscarella E, González S, Agozzino M,
Sánchez-Mateos JL, Panetta C, Contaldo M and Ardigò M: Pilot study
on reflectance confocal microscopy imaging of lichen planus: A
real-time, non-invasive aid for clinical diagnosis. J Eur Acad
Dermatol Venereol. 26:1258–1265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agozzino M, Gonzalez S and Ardigò M:
Reflectance confocal microscopy for inflammatory skin diseases.
Actas Dermosifiliogr. 107:631–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in
vivo reflectance confocal microscopy. Exp Ther Med.
15:1241–1246. 2018.PubMed/NCBI
|
|
21
|
Manfredini M, Greco M, Farnetani F, Ciardo
S, De Carvalho N, Mandel VD, Starace M and Pellacani G: Acne:
Morphologic and vascular study of lesions and surrounding skin by
means of optical coherence tomography. J Eur Acad Dermatol
Venereol. 31:1541–1546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ianosi S, Neagoe D, Calbureanu M and
Ianosi G: Investigator-blind, placebo-controlled, randomized
comparative study on combined vacuum and intense pulsed light
versus intense pulsed light devices in both comedonal and
papulopustular acne. J Cosmet Laser Ther. 15:248–254. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015. View Article : Google Scholar
|
|
24
|
Tan C, Min ZS, Xue Y and Zhu WY: Spectrum
of dermoscopic patterns in lichen planus: A case series from China.
J Cutan Med Surg. 18:28–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vázquez-López F, Gómez-Díez S, Sánchez J
and Pérez-Oliva N: Dermoscopy of active lichen planus. Arch
Dermatol. 143:10922007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zalaudek I and Argenziano G: Dermoscopy
subpatterns of inflammatory skin disorders. Arch Dermatol.
142:8082006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lallas A, Kyrgidis A, Tzellos TG, Apalla
Z, Karakyriou E, Karatolias A, Lefaki I, Sotiriou E, Ioannides D,
Argenziano G, et al: Accuracy of dermoscopic criteria for the
diagnosis of psoriasis, dermatitis, lichen planus and pityriasis
rosea. Br J Dermatol. 166:1198–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wickham L: Sur un signe pathognomonique du
lichen de Wilson (lichen plan) Stries et ponctuations grisatres.
Ann Dermatol Syph. 6:517–520. 1895.
|
|
29
|
Vázquez-López F, Manjón-Haces JA,
Maldonado-Seral C, Raya-Aguado C, Pérez-Oliva N and Marghoob AA:
Dermoscopic features of plaque psoriasis and lichen planus: New
observations. Dermatology. 207:151–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Güngör Ş, Topal IO and Göncü EK:
Dermoscopic patterns in active and regressive lichen planus and
lichen planus variants: A morphological study. Dermatol Pract
Concept. 5:45–53. 2015.
|
|
31
|
Vazquez-Lopez F, Palacios-Garcia L,
Gomez-Diez S and Argenziano G: Dermoscopy for discriminating
between lichenoid sarcoidosis and lichen planus. Arch Dermatol.
147:1130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Güngör Ş, Topal IO, Erdogan Ş and Özcan D:
Classical lichen planus and lichen planus pigmentosus inversus
overlap with dermoscopic features. Our Dermatol Online. 5:42–44.
2014. View Article : Google Scholar
|
|
33
|
Vázquez-López F, Vidal AM and Zalaudek I:
Dermoscopic subpatterns of ashy dermatosis related to lichen
planus. Arch Dermatol. 146:1102010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vázquez-López F, Maldonado-Seral C,
López-Escobar M and Pérez-Oliva N: Dermoscopy of pigmented lichen
planus lesions. Clin Exp Dermatol. 28:554–555. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Summerly R and Jones EW: The
microarchitecture of Wickham's striae. Trans St Johns Hosp Dermatol
Soc. 50:157–161. 1964.PubMed/NCBI
|
|
36
|
Ryan TJ: Lichen planus, Whickham's striae
and blood vessels. Br J Dermatol. 85:497–498. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liebman TN, Rabinovitz HS, Dusza SW and
Marghoob AA: White shiny structures: dermoscopic features revealed
under polarized light. J Eur Acad Dermatol Venereol. 26:1493–1497.
2012.PubMed/NCBI
|
|
38
|
Vázquez-López F, Alvarez-Cuesta C,
Hidalgo-García Y and Pérez-Oliva N: The handheld dermatoscope
improves the recognition of Wickham striae and capillaries in
Lichen planus lesions. Arch Dermatol. 137:1376. 2001.
|
|
39
|
Soyer HP, Argenziano G, Chimenti S and
Ruocco V: Dermoscopy of pigmented skin lesions. Eur J Dermatol.
11:270–276. 2001.PubMed/NCBI
|
|
40
|
Diaconeasa A, Boda D, Neagu M, Constantin
C, Căruntu C, Vlădău L and Guţu D: The role of confocal microscopy
in the dermato-oncology practice. J Med Life. 4:63–74.
2011.PubMed/NCBI
|
|
41
|
Ghita MA, Caruntu C, Rosca AE, Kaleshi H,
Caruntu A, Moraru L, Docea AO, Zurac S, Boda D, Neagu M, et al:
Reflectance confocal microscopy and dermoscopy for in vivo,
non-invasive skin imaging of superficial basal cell carcinoma.
Oncol Lett. 11:3019–3024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Căruntu C, Boda D, Guţu DE and Căruntu A:
In vivo reflectance confocal microscopy of basal cell
carcinoma with cystic degeneration. Rom J Morphol Embryol.
55:1437–1441. 2014.PubMed/NCBI
|
|
43
|
Lupu M, Caruntu C, Solomon I, Popa A,
Lisievici C, Draghici C, Papagheorghe L, Voiculescu VM and
Giurcaneanu C: The use of in vivo reflectance confocal
microscopy and dermoscopy in the preoperative determination of
basal cell carcinoma histopathological subtypes. DermatoVenerol.
62:7–13. 2017.
|
|
44
|
Lupu M, Caruntu A, Caruntu C, Boda D,
Moraru L, Voiculescu V and Bastian A: Non-invasive imaging of
actinic cheilitis and squamous cell carcinoma of the lip. Mol Clin
Oncol. 8:640–646. 2018.PubMed/NCBI
|
|
45
|
Langley RGB, Rajadhyaksha M, Dwyer PJ,
Sober AJ, Flotte TJ and Anderson RR: Confocal scanning laser
microscopy of benign and malignant melanocytic skin lesions in
vivo. J Am Acad Dermatol. 45:365–376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
González S: Confocal reflectance
microscopy in dermatology: Promise and reality of non-invasive
diagnosis and monitoring. Actas Dermosifiliogr. 100 Suppl 2:59–69.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guida S, Longo C, Casari A, Ciardo S,
Manfredini M, Reggiani C, Pellacani G and Farnetani F: Update on
the use of confocal microscopy in melanoma and non-melanoma skin
cancer. G Ital Dermatol Venereol. 150:547–563. 2015.PubMed/NCBI
|
|
48
|
Alessi SS, Nico MMS, Fernandes JD and
Lourenço SV: Reflectance confocal microscopy as a new tool in the
in vivo evaluation of desquamative gingivitis: Patterns in
mucous membrane pemphigoid, pemphigus vulgaris and oral lichen
planus. Br J Dermatol. 168:257–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Farnetani F, Scope A, Braun RP, Gonzalez
S, Guitera P, Malvehy J, Manfredini M, Marghoob AA, Moscarella E,
Oliviero M, et al: Skin cancer diagnosis with reflectance confocal
microscopy: Reproducibility of feature recognition and accuracy of
diagnosis. JAMA Dermatol. 151:1075–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for
psoriatic lesions. Rom J Morphol Embryol. 55:1191–1196.
2014.PubMed/NCBI
|
|
51
|
Agozzino M, Berardesca E, Donadio C,
Franceschini C, de Felice CM, Cavallotti C, Sperduti I and Ardigò
M: Reflectance confocal microscopy features of seborrheic
dermatitis for plaque psoriasis differentiation. Dermatology.
229:215–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ardigo M, Cota C, Berardesca E and
González S: Concordance between in vivo reflectance confocal
microscopy and histology in the evaluation of plaque psoriasis. J
Eur Acad Dermatol Venereol. 23:660–667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
González S, González E, White WM,
Rajadhyaksha M and Anderson RR: Allergic contact dermatitis:
Correlation of in vivo confocal imaging to routine
histology. J Am Acad Dermatol. 40:708–713. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Białek-Galas K, Wielowieyska-Szybińska D,
Dyduch G and Wojas-Pelc A: The use of reflectance confocal
microscopy in selected inflammatory skin diseases. Pol J Pathol.
66:103–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Başaran YK, Gürel MS, Erdemir AT, Turan E,
Yurt N and Bağci IS: Evaluation of the response to treatment of
psoriasis vulgaris with reflectance confocal microscopy. Skin Res
Technol. 21:18–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Căruntu C and Boda D: Evaluation through
in vivo reflectance confocal microscopy of the cutaneous
neurogenic inflammatory reaction induced by capsaicin in human
subjects. J Biomed Opt. 17:0850032012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ghiţă MA, Căruntu C, Rosca AE, Căruntu A,
Moraru L, Constantin C, Neagu M and Boda D: Real-time investigation
of skin blood flow changes induced by topical capsaicin. Acta
Dermatovenerol Croat. 25:223–227. 2017.PubMed/NCBI
|
|
58
|
Hoogedoorn L, Peppelman M, van de Kerkhof
PC, van Erp PE and Gerritsen MJ: The value of in vivo
reflectance confocal microscopy in the diagnosis and monitoring of
inflammatory and infectious skin diseases: A systematic review. Br
J Dermatol. 172:1222–1248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rajadhyaksha M, González S, Zavislan JM,
Anderson RR and Webb RH: In vivo confocal scanning laser
microscopy of human skin II: Advances in instrumentation and
comparison with histology. J Invest Dermatol. 113:293–303. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hofmann-Wellenhof R, Pellacani G, Malvehy
J and Soyer HP: Interface dermatitis. Reflectance Confocal
Microscopy for Skin Diseases. Springer; Berlin: pp. 392–400.
2012
|
|
61
|
Bağcı IS, Gürel MS, Aksu AEK, Erdemir AT,
Yüksel Eİ and Başaran YK: Reflectance confocal microscopic
evaluation of nonmelanocytic lip lesions. Lasers Med Sci.
32:1497–1506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Longo C, Zalaudek I, Argenziano G and
Pellacani G: New directions in dermatopathology: In vivo
confocal microscopy in clinical practice. Dermatol Clin. 30799–814.
(viii)2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Contaldo M, Agozzino M, Moscarella E,
Esposito S, Serpico R and Ardigò M: In vivo characterization
of healthy oral mucosa by reflectance confocal microscopy: A
translational research for optical biopsy. Ultrastruct Pathol.
37:151–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ardigò M, Maliszewski I, Cota C, Scope A,
Sacerdoti G, Gonzalez S and Berardesca E: Preliminary evaluation of
in vivo reflectance confocal microscopy features of discoid
lupus erythematosus. Br J Dermatol. 156:1196–1203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kelloff GJ, Sullivan DC, Baker H, Clarke
LP, Nordstrom R, Tatum JL, Dorfman GS, Jacobs P, Berg CD, Pomper
MG, et al: Workshop Program Committee: Workshop on imaging science
development for cancer prevention and preemption. Cancer Biomark.
3:1–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Calzavara-Pinton P, Longo C, Venturini M,
Sala R and Pellacani G: Reflectance confocal microscopy for in
vivo skin imaging. Photochem Photobiol. 84:1421–1430. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ardigo M, Donadio C, Franceschini C,
Catricalà C and Agozzino M: Interest of reflectance confocal
microscopy for inflammatory oral mucosal diseases. J Eur Acad
Dermatol Venereol. 29:1850–1853. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Baderca F, Lighezan R, Alexa A, Zăhoi D,
Raica M, Izvernariu D and Ardelean L: Atypical variant of lichen
planus mimicking normal skin histology. Rom J Morphol Embryol.
52:1355–1360. 2011.PubMed/NCBI
|
|
69
|
Serup J, Jemec GBE and Grove GL: Optical
coherence tomography in dermatology. Handbook of Non-Invasive
Methods and the Skin. 2nd. CRC Press; Boca Raton, FL: 2006
|
|
70
|
Gambichler T, Jaedicke V and Terras S:
Optical coherence tomography in dermatology: Technical and clinical
aspects. Arch Dermatol Res. 303:457–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sattler E, Kästle R and Welzel J: Optical
coherence tomography in dermatology. J Biomed Opt. 18:0612242013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gambichler T, Pljakic A and Schmitz L:
Recent advances in clinical application of optical coherence
tomography of human skin. Clin Cosmet Investig Dermatol. 8:345–354.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jung S, Lademann J, Darvin ME, Richter C,
Pedersen CB, Richter H, Schanzer S, Kottner J, Blume-Peytavi U and
Røpke MA: In vivo characterization of structural changes
after topical application of glucocorticoids in healthy human skin.
J Biomed Opt. 22:760182017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Levine A, Wang K and Markowitz O: Optical
coherence tomography in the diagnosis of skin cancer. Dermatol
Clin. 35:465–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Popescu I, Carstea E, Turcu G, Giurcaneanu
C and Forsea A: Multimodal optospectral investigation of
melanocytic skin lesions: A correlation study using optical
coherence tomography and dermoscopy. Rom Rep Phys. 66:672–682.
2014.
|
|
76
|
Mogensen M, Joergensen TM, Nürnberg BM,
Morsy HA, Thomsen JB, Thrane L and Jemec GB: Assessment of optical
coherence tomography imaging in the diagnosis of non-melanoma skin
cancer and benign lesions versus normal skin. Dermatol Surg.
35:965–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Boone MALM, Suppa M, Pellacani G, Marneffe
A, Miyamoto M, Alarcon I, Ruini C, Hofmann-Wellenhof R, Malvehy J,
Jemec GBE, et al: High-definition optical coherence tomography
algorithm for discrimination of basal cell carcinoma from clinical
BCC imitators and differentiation between common subtypes. J Eur
Acad Dermatol Venereol. 29:1771–1780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Boone MALM, Suppa M, Marneffe A, Miyamoto
M, Jemec GBE and Del Marmol V: A new algorithm for the
discrimination of actinic keratosis from normal skin and squamous
cell carcinoma based on in vivo analysis of optical
properties by high-definition optical coherence tomography. J Eur
Acad Dermatol Venereol. 30:1714–1725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang KX, Meekings A, Fluhr JW, McKenzie G,
Lee DA, Fisher J, Markowitz O and Siegel DM: Optical coherence
tomography-based optimization of mohs micrographic surgery of Basal
cell carcinoma: A pilot study. Dermatol Surg. 39:627–633. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Olsen J, Themstrup L and Jemec GB: Optical
coherence tomography in dermatology. G Ital Dermatol Venereol.
150:603–615. 2015.PubMed/NCBI
|
|
81
|
Forsea A-M, Carstea EM, Ghervase L,
Giurcaneanu C and Pavelescu G: Clinical application of optical
coherence tomography for the imaging of non-melanocytic cutaneous
tumors: A pilot multi-modal study. J Med Life. 3:381–389.
2010.PubMed/NCBI
|
|
82
|
Boone M, Norrenberg S, Jemec G and Del
Marmol V: High-definition optical coherence tomography: adapted
algorithmic method for pattern analysis of inflammatory skin
diseases: a pilot study. Arch Dermatol Res. 305:283–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Schmitz L, Gambichler T, Zerbinati N and
Dirschka T: Optical coherence tomography of palmoplantar lichen
planus and ultraviolet A1 laser treatment: A case report. J Dtsch
Dermatol Ges. 12:e9–e10. 2014.
|
|
84
|
Fomina IuV, Gladkova ND, Leont'ev VK,
Urutina MN, Gazhva SI, Snopova LB, Gelikonov VM and Kamenskiĭ VA:
Optical coherence tomography in the evaluation of the oral cavity
mucosa. Part II. Benign and malignant diseases. Stomatologiia
(Mosk). 83:25–32. 2004.(In Russian).
|
|
85
|
Hamdoon Z, Jerjes W, Al-Delayme R,
McKenzie G, Jay A and Hopper C: Structural validation of oral
mucosal tissue using optical coherence tomography. Head Neck Oncol.
4:292012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wilder-Smith P, Lee K, Guo S, Zhang J,
Osann K, Chen Z and Messadi D: In vivo diagnosis of oral
dysplasia and malignancy using optical coherence tomography:
Preliminary studies in 50 patients. Lasers Surg Med. 41:353–357.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bhatia N, Lalla Y, Vu AN and Farah CS:
Advances in optical adjunctive AIDS for visualisation and detection
of oral malignant and potentially malignant lesions. Int J Dent.
2013:1940292013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Adabi S, Turani Z, Fatemizadeh E, Clayton
A and Nasiriavanaki M: Optical coherence tomography technology and
quality improvement methods for optical coherence Tomography images
of skin: A short review. Biomed Eng Comput Biol.
8:11795972177134752017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Alexander H and Miller DL: Determining
skin thickness with pulsed ultra sound. J Invest Dermatol.
72:17–19. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
El Gammal S, El Gammal C, Kaspar K, Pieck
C, Altmeyer P, Vogt M and Ermert H: Sonography of the skin at 100
MHz enables in vivo visualization of stratum corneum and
viable epidermis in palmar skin and psoriatic plaques. J Invest
Dermatol. 113:821–829. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
El Gammal S, Auer T, Hoffmann K, Altmeyer
P, Passmann C and Ermert H: Grundlagen, anwendungsgebiete und
grenzen des hochfrequenten (20–50 MHz) ultraschalls in der
dermatologie. Zbl Haut. 162:817–838. 1993.(In German).
|
|
92
|
El-Gammal S, Auer T, Hoffmann K, Matthes U
and Altmeyer P: Möglichkeiten und Grenzen der hochauflösenden (20
und 50 MHz) Sonographie in der Dermatologie. Aktuelle Derm.
18:197–208. 1992.(In German).
|
|
93
|
Seidenari S and Di Nardo A: B scanning
evaluation of irritant reactions with binary transformation and
image analysis. Acta Derm Venereol Suppl (Stockh). 175:9–13.
1992.PubMed/NCBI
|
|
94
|
Seidenari S: High-frequency sonography
combined with image analysis: A noninvasive objective method for
skin evaluation and description. Clin Dermatol. 13:349–359. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jayanthi JL, Nisha GU, Manju S, Philip EK,
Jeemon P, Baiju KV, Beena VT and Subhash N: Diffuse reflectance
spectroscopy: Diagnostic accuracy of a non-invasive screening
technique for early detection of malignant changes in the oral
cavity. BMJ Open. 1:e0000712011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Messadi DV, Younai FS, Liu HH, Guo G and
Wang CY: The clinical effectiveness of reflectance optical
spectroscopy for the in vivo diagnosis of oral lesions. Int
J Oral Sci. 6:162–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gupta S and Jawanda MK: Oral Lichen
Planus: An update on etiology, pathogenesis, clinical presentation,
diagnosis and management. Indian J Dermatol. 60:222–229. 2015.
View Article : Google Scholar : PubMed/NCBI
|