Introduction
IgA nephropathy (IgAN) is the most common primary
glomerular disease in China. Its incidence rate is increasing year
by year. The disease condition of many patients is chronically
progressive. Approximately 15–40% of patients will progress to
end-stage renal disease after 10 years. Kidney replacement therapy
is needed to maintain their lives, which brings great burden to
patients and society (1). Although
process of IgAN development is relatively benign, it progressively
developes and has a poor prognosis, which lead to the fact that it
becomes the main cause of chronic renal failure (2). Therefore, it is one of the key steps to
improve the level of treatment as finding indicators related to
disease activity, progression and prognosis. It also has important
guiding significance for clinical work.
According to previous studies, many factors are
closely related to the prognosis of IgAN, such as severity of
illness, 24 h urinary protein, GFR levels, and creatinine clearance
(Ccr). These factors also have potential clinical predictive value.
With the deepening and widening of research, more and more factors
have been confirmed to be associated with IgAN. The influence of
pathological type and family history on the outcome of disease
treatment and prognosis are reflected in the research of Kusano
et al (3). It was found by
Yabuki et al (4) that IgAN
patients with IgM deposition had higher urinary protein compared
with those without IgM deposition. Some factors may affect the
prognosis of patients with IgAN to some extent, such as more
immunofluorescence deposition, more severe pathological lesions and
IgM deposition. These factors affecting IgAN are numerous and
complex. The research on the analysis of the disease prognosis and
its impact of the overall factors is not common in China.
Therefore, the authors wanted to compare the general data and
clinical data of IgAN patients with good prognosis and poor
prognosis, analyze the value of Oxford classification, IgM
deposition, and multiple indicators that may affect the prognosis
of patients in predicting the prognosis risk of IgAN patients. The
purpose is to provide the basis for the wide use of technology in
pathological tests on IgM in clinical examination for IgAN
patients.
Patients and methods
Enrolment
A total of 124 patients diagnosed with IgAN in West
China Hospital of Sichuan University (Chengdu, China)from May 2012
to May 2014 were selected as study subjects. They included 55 males
and 69 females. The age of patients was between 27 and 41 years.
The average age was 33.96 years. This study was approved by the
Ethics Committee of West China Hospital of Sichuan University
(Chengdu, China). Signed informed consents were obtained from the
patients or the guardians.
Inclusion/exclusion criteria
Inclusion criteria were: i) The patient was
diagnosed with IgAN in the nephrology department according to the
guideline criteria, and the number of glomeruli in the biopsy
tissue was >10; and ii) the patient had no history of kidney
transplant surgery. Exclusion criteria were: i) Secondary IgAN,
such as cirrhosis-associated nephritis, hepatitis B associated
nephritis and anaphylactoid purpura nephropathy; ii) patients with
acute heart failure, cardiogenic shock and other important organ
diseases; and iii) clinical data was not complete.
Pathology detection method of
kidney
All patients included in the study underwent
ultrasound-guided percutaneous renal biopsy. Then the kidney
tissues were obtained before treatment after diagnosis. Renal
biopsy was embedded in paraffin. Serial sections in the thickness
of 2–3 µm were stained through conventional hematoxylin and eosin
(H&E), periodic acid iodine, periodic acid-silver metheramine
staining and Masson staining. Immunohistochemistry was used to
detect the expression intensity and deposition site of IgG and IgM
by direct immunofluorescence. The sections were washed twice with
PBS for 3 min each time, dripped with rabbit anti-human IgG and
rabbit anti-human IgM monoclonal antibodies (1:100; cat nos.
ab193172 and ab212201; Abcam, Cambridge, MA, USA), incubated in
water bath at 37°C for 60 min, washed twice with PBS for 3 min each
time. Then incubate with Alexa Fluor® 488 goat
anti-rabbit secondary polyclonal antibody (1:200; Cat nos.ab150077;
Abcam, Cambridge, MA, USA). Then the samples were observed under
fluorescence microscope after sealed with glycerol. Pathological
data included data of endothelial cell proliferation, segmental
sclerosis, nevus necrosis, mesangial proliferation, proportion of
sclerosing, percentage of crescents, and extent of
tubulointerstitial injury. Kidney pathology was performed by Lee
and Oxford classification. Katafuchi semi-quantitative scoring
method was used to score the glomerular, renal tubulointerstitial,
and renal angiopathy. All the analysis of sections were performed
independently on the premise of unknown clinical outcome of
patients. When there were inconsistencies or doubts among
pathologists and clinical investigators, these were submitted to a
higher-level pathologist for review. The Lee classification
included 5 grades. Grade I: Glomerular function was almost normal
with occasional mesangial mild hyperplasia, grade II: Glomerular
mesangial widening accompanied by cell hyperplasia (more than 50%)
without small crescents in general, grade III: Mesangial diffuse
widening with cell proliferation, interstitial edema and cell
infiltration, grade IV: Patients with diffuse mesangial hyperplasia
and sclerosis, and glomerular appearance (more than 50%), with
tubular atrophy and interstitial inflammation, and grade V:
Symptoms was similar to IV but more severe. Oxford classification:
Ml for mesangial cell proliferation >0.5; E1 for endothelial
cell proliferation; s1 for segmental sclerosis; T1 for renal
tubular atrophy; and T2 for interstitial fibrosis >25%.
Treatment
According to the Kidney Disease: Improving Global
Outcomes (KDIGO) IgA nephropathy treatment guidelines, treatments
are as follows: i) ARB or ACEI: ARB or ACEI is the preferred
medicine for patients with proteinuria or hypertension. ii)
Glucocorticoid indications: a) After 3–6 months of treatment with
ARB or ACEI, urinary protein in 24 h was still above 1 g and
glomerular filtration rate was above 50 ml/min; b) patients with
clinical manifestations of nephrotic syndrome and pathological
manifestations of minimal change nephropathy; c) crescentic IgA
nephropathy; and d) patients with rapid decline in renal function.
iii) The application of immunosuppressive agents: crescentic IgA
nephropathy with rapid decline in renal function may consider
glucocorticoid combined with cyclophosphamide treatment, no other
use of immunosuppressive agents is recommended. iv) Others: a) Fish
oil: Although the efficacy of fish oil therapy in patients with IgA
nephropathy is inaccurate, it is of low risk and has cardiovascular
benefits. Therefore, fish oil treatment is still considered safe;
b) antiplatelet drugs: Not recommended for use; and c)
tonsillectomy: The curative effect is inaccurate and further large
sample evaluation is needed.
Data collection
This study prospectively analyzed patients and the
data included baseline data such as 24 h urinary protein, GFR
levels, endogenous creatinine clearance (Ccr), erythropoietin
(EPO), and urinary N-acetyl-β-D-glucosidase (NAG). Baseline data
included age on onset, sex, family history (IgAN in close relatives
of the third generation of the family), and pathological type. The
method of GFR measurement is complex. Intravenous injection of 24%
inulin or isotopic markers secreted by the renal tubules is
required. GFR is often estimated by various equations in clinical
practice. The information of Scr, age, and sex are needed for
estimation. Among them, one of the best is the Ruijin equation used
to estimate GFR: 234.96 × Scr −0.926 × age - 0.280 × a (a, female
=0.828, male =1). Ccr is also estimated by using the
Cockcroft-Gault formula: Ccr = (140-age) × body weight (kg) / [72 ×
Scr (mg/dl)] or Ccr = [(140-age) × body weight (kg)] / [0.818 × Scr
(µmol/l)], females are calculated as × 0.85.
Follow-up
All the 124 patients involved in the study were
followed up for 3 years. The first follow-up was 1 month after the
end of treatment, followed by a telephone interview every 3 months.
The end point event was: The patient had a poor prognosis from the
time of enrollment during the follow-up period. The poor prognosis
included: i) GFR decreased by more than 50% compared to baseline
values. ii) Patients were admitted to end-stage renal disease
(ESRD) or required continuity of the kidney replacement therapy or
death from kidney disease. The final follow-up records were used as
follow-up results to record the number of patients who had a poor
prognosis. Censorship was defined as patients of the study who were
lost to follow-up, access denied, withdrawal midway, and dying from
other reasons unrelated to the study.
Follow-up grouping and data
arrangement
Patients with poor prognosis during the follow-up
period were defined as poor prognostic groups. Patients without
worsening were defined as having a good prognosis. At the end of
follow-up, the clinical data such as baseline data, 24 h urinary
protein, GFR level, Ccr, EPO, urinary NAG, IgG and IgM deposition
in the mesangial area and Oxford classification were analyzed.
Statistical analysis
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) scoring
statistical software was used for analysis. Survival analysis was
performed by Kaplan-Meier method to calculate the poor prognosis
rate and the survival curve was drawn. Univariate analysis was used
to explain the factors which may have an impact on the prognosis of
IgAN patients. At the same time, a multi-factor Cox proportional
hazards model was established to further analyze the factors that
may have a significant impact to explore the impact of each
variable on the risk of IgAN patients.
Results
Basic data analysis
In this study, 124 subjects were included. The
clinical data, detection indicators and pathological features of
all patients are shown in Table
I.
 | Table I.Clinical data, detection indicators
and pathological features of 124 patients. |
Table I.
Clinical data, detection indicators
and pathological features of 124 patients.
| Index | (mean ± SD)/n |
|---|
| Age (year) | 33.63±7.63 |
| Course of disease
(month) |
7.72±3.97 |
| Male (case) | 83 |
| Female (case) | 41 |
| Family history
(case) | 34 |
| Systolic blood
pressure (mmHg) | 139.29±40.12 |
| Diastolic blood
pressure (mmHg) |
87.93±29.02 |
| GFR (ml/min) |
92.53±33.64 |
| Ccr (ml/min) | 106.39±41.92 |
| EPO (IU) |
36.36±11.53 |
| NAG (U/l) |
26.73±8.64 |
| IgG deposition
(case) | 19 |
| IgM deposition
(case) | 27 |
| 24 h urine protein
quantity |
| <1.0 g
(case) | 84 |
| 1.0–3.5
(case) | 27 |
| >3.5
(case) | 13 |
|
| Index | n |
|
| Pathological
type |
| Mesangial
proliferative glomerulonephritis (case) | 41 |
|
Endocapillary proliferative
glomerulonephritis (case) | 12 |
|
Membranoproliferative
nephritis (case) | 8 |
|
Crescentic glomerulonephritis
(case) | 26 |
|
Proliferative sclerotic
nephritis (case) | 17 |
| Lee grades |
| I
(case) | 9 |
| II
(case) | 47 |
| III
(case) | 41 |
| IV
(case) | 14 |
| V
(case) | 13 |
| Oxford
classification |
| M1
(case) | 80 |
| E1
(case) | 57 |
| S1
(case) | 97 |
| T1+T2
(case) | 30 |
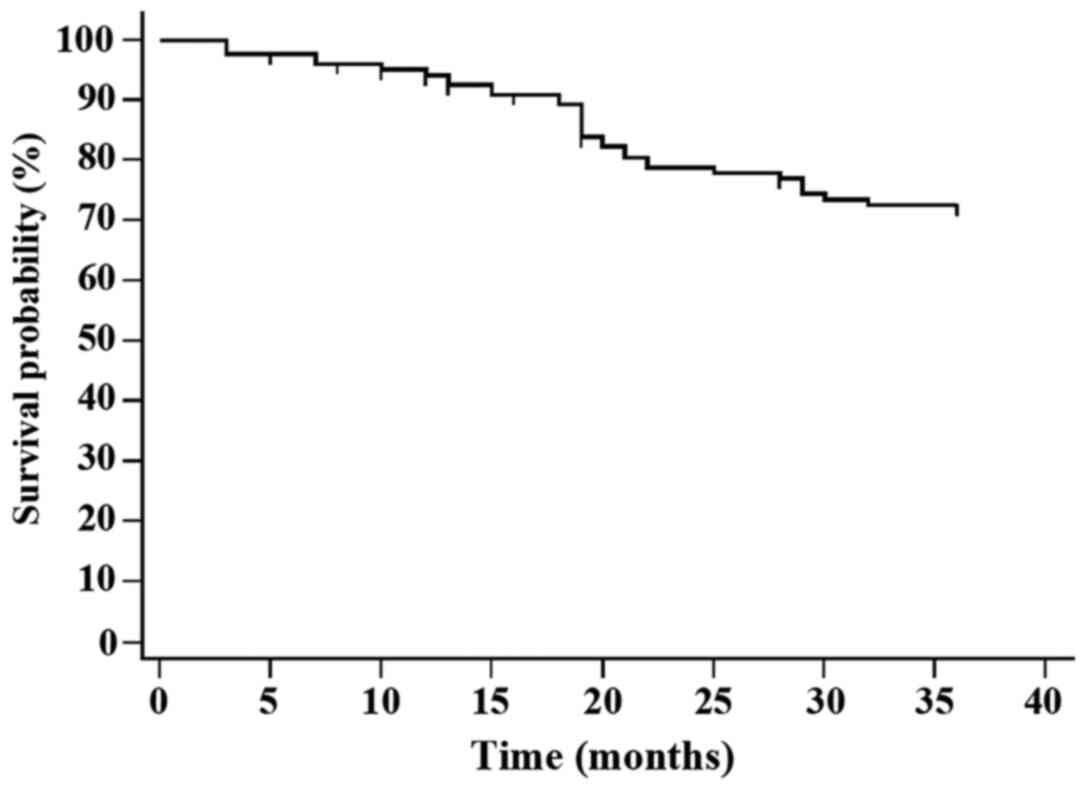
Analysis of survival curve of patients
with poor prognosis
At the end of follow-up, the results showed that
within the total 124 patients, there were 10 patients lost to
follow-up, 82 patients with good prognosis (defined as good
prognosis), 32 patients with poor prognosis (defined as poor
prognosis), and 19 of them had a decrease in GFR more than 50%, 9
people entered end-stage renal disease, and 4 died. Kaplan-Meier
survival curves suggest that the rate of poor prognosis of IgAN
patients is 28.07% (Fig. 1).
Univariate analysis of factors that
may influence the prognosis of patients with IgAN
Factors that may affect the prognosis of the disease
were individually included in the Cox proportional hazards model
for univariate analysis. The results showed that within the
baseline data, family history, pathological type, and Lee
classification (IV and V) affected the prognosis of the disease.
Statistical significance was set at P<0.05. Among other factors,
urinary protein in 24 h, GFR level, Ccr, IgM deposition in the
mesangial area and Oxford classification (T1+T2) could affect the
prognosis of IgAN patients. The difference was statistically
significant at P<0.05 (Table
II).
 | Table II.Single factor Cox regression analysis
on the prognosis of patients. |
Table II.
Single factor Cox regression analysis
on the prognosis of patients.
|
|
|
|
|
|
|
| 95.0% CI |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Characteristics | B | SE | Wald | df | P-value | RR | Lower limit | Upper limit |
|---|
| Age | 0.011 | 0.173 | 4.385 | 1 | 0.594 | 1.011 | 0.720 | 1.419 |
| Sex | −0.409 | 0.325 | 2.113 | 1 | 0.783 | 0.664 | 0.351 | 1.255 |
| Family history | 0.218 | 0.102 | 2.084 | 1 | 0.041 | 1.244 | 1.019 | 1.519 |
| Pathological
type | 0.443 | 0.174 | 2.842 | 1 | 0.033 | 1.642 | 0.456 | 0.903 |
| Lee grades |
| I |
|
|
|
| 0.279 | 1 |
|
|
| II | −0.282 | 0.273 | 3.753 | 1 | 0.174 | 0.754 | 0.442 | 1.288 |
| III | 0.158 | 0.375 | 4.653 | 1 | 0.062 | 0.854 | 0.409 | 1.781 |
| IV | 0.261 | 0.123 | 4.975 | 1 | 0.028 | 1.298 | 1.020 | 1.652 |
| V | 0.233 | 0.107 | 4.128 | 1 | 0.037 | 1.262 | 1.023 | 1.566 |
| 24 h urinary
protein | 0.351 | 0.106 | 5.424 | 1 | 0.031 | 1.421 | 1.154 | 1.749 |
| GFR | 0.583 | 0.118 | 9.389 | 1 | 0.002 | 1.792 | 1.422 | 2.258 |
| Ccr | 0.436 | 0.147 | 9.689 | 1 | 0.001 | 1.546 | 1.159 | 2.062 |
| EPO | −0.167 | 0.232 | 2.374 | 1 | 0.397 | 0.846 | 0.537 | 1.333 |
| NAG | −0.078 | 0.218 | 3.965 | 1 | 0.137 | 0.925 | 0.603 | 1.418 |
| IgG | 0.128 | 0.216 | 2.894 | 1 | 0.157 | 0.745 | 1.736 | 2.145 |
| IgM | 0.513 | 0.187 | 3.71 | 1 | 0.034 | 1.670 | 1.158 | 2.409 |
| Oxford
classification |
| M1 |
|
|
|
| 0.362 | 1 |
|
|
| E1 | −0.178 | 0.183 | 3.846 | 1 | 0.263 | 0.837 | 0.585 | 1.198 |
| S1 | −0.089 | 0.238 | 5.745 | 1 | 0.121 | 0.915 | 0.574 | 1.459 |
|
T1-T2 | 0.291 | 0.125 | 3.175 | 1 | 0.012 | 1.338 | 1.047 | 1.709 |
Multivariate Cox proportional
regression analysis of factors affecting prognosis of IgAN
patients
With further analysis of the above results,
multivariate Cox regression results showed that urinary protein in
24 h, pathological type, Oxford classification (T1+T2), Lee grade
(grade IV) and IgM deposition in the mesangial area are independent
factors influencing patients. The difference was statistically
significant (Table III). The
P-values were 0.041, 0.046, 0.037, 0.043, and 0.028, respectively
(data not shown).
 | Table III.Multiple factor Cox regression
analysis on the prognosis of patients. |
Table III.
Multiple factor Cox regression
analysis on the prognosis of patients.
|
|
|
|
|
|
|
| 95.0% CI |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Factors | B | SE | Wald | df | P-value | RR | Lower limit | Upper limit |
|---|
| Family history | 0.061 | 0.314 | 3.312 | 1 | 0.772 | 1.063 | 0.574 | 1.967 |
| Pathological
type | 1.150 | 0.575 | 3.995 | 1 | 0.046 | 3.159 | 1.023 | 6.758 |
| Lee IV grades |
|
|
|
| 0.043 | 1 |
|
|
| Lee V grades | 0.021 | 0.321 | 3.721 | 1 | 0.169 | 1.021 | 0.544 | 1.915 |
| GFR | 0.066 | 0.384 | 2.267 | 1 | 0.821 | 1.068 | 0.503 | 2.267 |
| Ccr | 0.160 | 0.154 | 2.985 | 1 | 0.395 | 1.173 | 0.867 | 1.586 |
| 24 h urinary
protein | 0.345 | 0.081 | 2.743 | 1 | 0.041 | 1.412 | 1.205 | 1.655 |
| IgM | 0.514 | 0.249 | 3.116 | 1 | 0.028 | 1.672 | 1.026 | 2.724 |
| Oxford
classification |
|
(T1+T2) | 0.264 | 0.113 | 7.338 | 1 | 0.037 | 1.302 | 1.043 | 1.625 |
Discussion
At the time IgAN was reported, IgAN was considered
to be a glomerular disease with a good prognosis. Most of the
patients were able to achieve a good response after receiving
treatment (5). With the deepening of
research, IgAN has gradually developed and individual differences
are relatively large. Although there are some good clinical
treatments, some patients will still enter end-stage renal disease
after treatment. In this study, Kaplan-Meier survival curves were
drawn for the survival of patients with IgAN. The results showed
that in 124 subjects, the survival rate of IgAN kidneys was 94% at
10 months, 89% at 20 months, and 78% at 25 months and 72% at 30
months. It suggested that IgAN patients showed progressive
development and the rate of progression to end-stage renal failure
was fast, which was consistent with the results of long-term
follow-up studies of IgAN patients by scholar Ohata et al
(6).
There are many indicators of prognostic risk and
related risk factors in IgAN patients. Traditional indicators
include age, sex, family history, Scr and GFR. In the study of
D'Amico (7), GFR was considered to
be the most powerful laboratory indicator of IgAN prognosis. The
results of this study also showed that GFR had an impact on disease
prognosis. The calculation of GFR involves multiple indicators such
as age, sex, and Scr, and the assessment of IgAN's progress is more
accurate (8). This report is
slightly different focusing on the factors affecting the prognosis
of IgAN patients and improvements which can prevent patients from
having a poor prognosis. The severity of the patient's condition
has a great deal of clinical concern. From the point of
pathological view, the patient's pathological changes are
different, and each pathological type is of great significance in
guiding the treatment.
For patients with medullary proliferative lesions as
mainly pathological changes have visible hematuria, taking rhubarb
mixture can inhibit the proliferation of mesangial cells. Patients
with changes in glomerulosclerosis and interstitial fibrosis have
large clinical manifestations of proteinuria, they can use either
ARB (ACEI) or glucocorticoids according to specific needs (9). Patients with mesangial hyperplasia and
glomerulosclerosis usually have interstitial lesions of different
severity with different clinical manifestations, and the treatment
methods may be determined based on the condition (10). In the view of the above-mentioned
pathological types, the IgAN grading system has not yet been
applied in the world. The WHO histological classification method,
the Hass classification method, the Lee classification method, and
the Oxford classification score are commonly used. The first two
are more concerned with diffuse lesions and sclerosis, while less
attention is paid to crescents, renal tubules, and renal
interstitial lesions. In the latter two methods, Lee
classifications is more common and its objective is to consider
various pathological changes such as glomerulosclerosis and
interstitial lesions. The Oxford classification score is more
predictive of value and is involved in renal tubules, glomeruli,
and renal interstitium.
In this study, the joint application of the
two-grading system is more comprehensive in describing the severity
of the patient's condition. Previous studies have reported renal
tubular and interstitial injury are the two independent risk
factors that have the greatest adverse renal outcome (11), suggesting that Lee classification
method and Oxford classification score have potential clinical
significance in the prognosis of patients and can be used as an
independent factor influencing the prognosis of patients. However,
it still needs further analysis. Age affects the occurrence and
development of many diseases, but specificity is not ideal
generally. At present, its relationship with the prognosis of IgAN
is still controversial. D'Amico conducted a comparative study of a
certain number of IgAN patients and found that the age may have
larger influence on the univariate analysis, which may affect the
prognosis of patients. However, the conclusion of the single factor
analysis in this study does not have the effect of age. After
comparing the subjects included in this study with those of Sonoda
et al (12) and Al Hussain
et al (1), we found that the
age of the subjects included in this study tends to be younger and
more diffusely distributed. While the latter study suggests that
age above 65 years is a risk factor for IgAN prognosis. It is
speculated that the reason why the disease course and the prognosis
of the disease are not independently related to each other may be
the age distribution. The selection of sample size must also be
considered. The selection of diastolic pressure, systolic blood
pressure, EPO and NAG were all based on relatively recent
literature reports. However, this study did not find statistical
differences between the above factors in univariate analysis. The
reason may be that most patients have taken ACEI or ARB. These
drugs have a good effect on stabilizing blood pressure in patients
(12).
In order to further explore the relationship between
statistically significant factors and patient prognosis in the
above single factor analysis. This study also used methods of
multivariate Cox regression analysis. The analysis results
suggested that the patient's 24 h urine protein, pathological type,
Oxford classification (T1+T2), Lee classification (grade IV) and
IgM deposition in mesangial area are independent factors of
patients. Previous studies have suggested that deposition of IgM in
the mesangial area is only non-specific immunodeposition and is an
immunopathological response of the body (13). There is no pathological value and
pathogenicity in the clinic. Subsequent studies have reported that
IgM deposition in the mesangial area is a sign of worsening of the
disease. Some scholars have also proposed that reducing the IgM
antibody can delay the deterioration of the disease (14). At the same time, some scholars have
also suggested that IgM deposition is related to T cell balance,
production of multiple cytokines and activation of complement. In
IgA nephropathy with IgM deposition, glomerular and
tubulointerstitial lesions are often combined at the same time,
resulting in an impact on prognosis (15), which is also consistent with the
results of this study.
There are still deficiencies in this study. The
number of subjects is small and the time of follow-up is short.
There are not enough factors that may affect the prognosis of IgAN.
In the follow-up study, the authors intend to further increase the
number of samples, extend the follow-up time, include as many
influencing factors as possible, and divide the patients into IgM
deposition group and IgM negative group, analyze and compare the
clinical and pathological features and prognosis of the patients in
the two groups to confirm our conclusion.
In summary, we consider that IgM deposition in the
mesangial area, Oxford classification, 24 h urinary protein,
pathological type and Lee classification can be used as independent
influencing factors for poor prognosis of patients with primary
IgAN. They will provide evidence for the early detection of
high-risk IgAN to establish a reasonable treatment plan, which is
of great significance for improving the prognosis of patients with
IgAN.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH drafted the manuscript. YH and YZ were mainly
devoted to collecting and interpreting the data and H&E
staining. RH and PF were responsible for follow-up. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
West China Hospital of Sichuan University (Chengdu, China). Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al Hussain T, Hussein MH, Al Mana H and
Akhtar M: Pathophysiology of iga nephropathy. Adv Anat Pathol.
24:56–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Daha MR and van Kooten C: Role of
complement in IgA nephropathy. J Nephrol. 29:1–4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kusano T, Takano H, Kang D, Nagahama K,
Aoki M, Morita M, Kaneko T, Tsuruoka S and Shimizu A: Endothelial
cell injury in acute and chronic glomerular lesions in patients
with IgA nephropathy. Hum Pathol. 49:135–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yabuki A, Shimokawa Miyama T, Kohyama M
and Yamato O: Canine IgA nephropathy: A case report. J Vet Med Sci.
78:513–515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
da Silva LS, Almeida BL, de Melo AK, de
Brito DC, Braz AS and Freire EA: IgA nephropathy in systemic lupus
erythematosus patients: Case report and literature review. Rev Bras
Reumatol Engl Ed. 56:270–273. 2016.PubMed/NCBI
|
|
6
|
Ohata C, Ishii N, Koga H and Nakama T: A
clinical and serological study of linear IgA bullous dermatosis
without linear immunoglobulin deposition other than IgA at the
basement membrane zone using direct immunofluorescence. Br J
Dermatol. 177:152–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Amico G: Natural history of idiopathic
IgA nephropathy and factors predictive of disease outcome. Semin
Nephrol. 24:179–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv J, Hou W, Zhou X, Liu G, Zhou F, Zhao
N, Hou P, Zhao M and Zhang H: Interaction between PLA2R1 and
HLA-DQA1 variants associates with anti-PLA2R antibodies and
membranous nephropathy. J Am Soc Nephrol. 24:1323–1329. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patterson R, Schatz M, Fink JN, DeSwarte
RS, Roberts M and Cugell D: Pigeons breeders' disease. I. Serum
immunoglobulin concentrations; IgG, IgM, IgA and IgE antibodies
against pigeon serum. Am J Med. 60:144–151. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han B, Li Y, Han H, Zhao Y, Pan Q and Ren
L: Three IgH isotypes, IgM, IgA and IgY are expressed in Gentoo
penguin and zebra finch. PLoS One. 12:e01733342017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin Q, Chen Y, Lv J, Zhang H, Tang J,
Gunaratnam L, Li X and Yang L: Kidney injury molecule-1 expression
in IgA nephropathy and its correlation with hypoxia and
tubulointerstitial inflammation. Am J Physiol Renal Physiol.
306:885–895. 2014. View Article : Google Scholar
|
|
12
|
Sonoda Y, Gohda T, Suzuki Y, Omote K,
Ishizaka M, Matsuoka J and Tomino Y: Circulating TNF receptors 1
and 2 are associated with the severity of renal interstitial
fibrosis in IgA nephropathy. PLoS One. 10:e01222122015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kosztyu P, Hill M, Jemelkova J, Czernekova
L, Kafkova LR, Hruby M, Matousovic K, Vondrak K, Zadrazil J and
Sterzl I: Glucocorticoids reduce aberrant O-Glycosylation of IgA1
in IgA nephropathy patients. Kidney Blood Press Res. 43:350–359.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lloyd IE, Ahmed F, Revelo MP and Khalighi
MA: De novo immune complex deposition in kidney allografts: A
series of 32 patients. Hum Pathol. 71:109–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CK, Yang AH, Lai HC and Lin BS:
Combined proximal tubulopathy, crystal-storing histiocytosis, and
cast nephropathy in a patient with light chain multiple myeloma.
BMC Nephrol. 18:1702017. View Article : Google Scholar : PubMed/NCBI
|