Introduction
Venous malformation is the most common type of
vascular malformation (1,2). Typically, venous malformations are
present at birth and develop gradually throughout life. The lesions
grow in proportion to the body over time, and unlike hemangioma, do
not spontaneously regress (3). The
pathological feature of venous malformation is the absence of
endothelial cell mitotic activity, which is the most fundamental
difference from hemangioma (4).
Venous malformation may be characterized by thin vascular walls, a
reduced number of smooth muscle cells and a larger than normal
ratio of vascular wall radius to vascular wall thickness. Also,
veins are typically small and/or medium-sized (5). Additionally, the vein wall only has one
or no layer of smooth muscle cells and is therefore abnormally
expanded. Due to the lack of valves in venous malformations, blood
readily flows backward and stops when it accumulates in the cavity.
As a result, a thrombus can form after coming into contact with
endothelial cells. This state is defined as local intravascular
coagulation, which is the leading cause of pain in venous
malformation. The incidence of venous malformations is low, without
predisposition for sex. Unlike hemangioma, venous malformation is
not associated with endothelial cell proliferation. Furthermore,
the malformations can vary according to the location of malformed
lesions. If the surface is violaceous with a protuberant
subcutaneous mass, which can be reduced by pressing Currently,
interventional sclerotherapy is recommended as the first choice
treatment by the International Union of Phlebology (6,7).
However, due to rapid reflux rate following the injection and the
short half-life of the sclerosing agent, the efficacy of this
method is typically unsatisfactory (8). Furthermore, postoperative recurrence in
Puig's classified advanced venous malformation is common (9). In the current study, a total of 121
patients with venous malformations who underwent interventional
sclerotherapy between April 2009 and October 2014 were
retrospectively analyzed A total of 21 patients with Puig's
classified advanced venous malformations were successfully treated
with absolute ethanol combined with n-butyl cyanoacrylate.
Materials and methods
Clinical data
A total of 121 patients with venous malformations,
who underwent interventional sclerotherapy under general anesthesia
between April 2009 and October 2014 at the Department of
Interventional Radiology and Vascular Anomalies, Guangzhou Women
and Children's Medical Center, Guangzhou, China were retrospective
enrolled in the present study. This retrospective study was
approved by the Ethics Committee of Guangzhou Women and Children's
Medical Center, Guangzhou, China.
All patients (52 males and 69 females; age range, 5
months to 16 years) met the International Society for the Study of
Vascular Anomalies diagnostic criteria for venous malformations
(10,11). Most lesions were present since birth
and had grown proportionally with the patients. The superficial
foci appeared blue, the masses were compressible and the texture
was soft. The skin temperatures in the lesion areas were not higher
compared with the surrounding area (a key point for hemangioma
identification) and the lesions tested positive in the posture
experiment. During this experiment, changing the position or
pressing the proximal part of lesion increased the local venous
pressure and mass swelling. In addition, when venous draining was
unimpeded, the venous malformation could be partly or largely
retracted. All patients underwent magnetic resonance imaging (MRI)
examinations (Philips Achieva 3.0 T dual gradient MRI imager). The
lesions exhibited equal or low signals on T1WI and high
signals on T2WI. Ultrasound examinations revealed that
the lesions were expressed as uneven internal echoes with irregular
sharp and pipe-like echoes. Venous blood flow was probed using the
Doppler test.
A total of 121 patients with venous malformations
were initially selected on the basis of admission time. The venous
malformations were classified according to Puig's classification
(12) using the angiography of
venous malformations, for which 21 cases met the inclusion
criteria. The inclusion criteria were as follows: Complete
follow-up records; no previous intervention sclerotherapy;
diagnosis was confirmed by direct puncture angiography under
digital subtraction angiography (DSA) guidance and belonged to type
III or type IV, according to Puig's classification; and the
patient/guardian provided informed consent for treatment. Exclusion
criteria consisted of the following: Incomplete data; the effective
lesion had been previously treated with sclerotherapy; and other
vascular diseases were also present, including venolymphatic
malformation and/or arteriovenous malformations.
Among the 21/121 selected patients (9 males and 12
females; age range, 6 months to 14 years), the lesion in 9 patients
occurred at birth and gradually increased with age, and the lesion
in 12 cases occurred 3–24 months after birth. With regard to the
lesion distribution, the lesions were located in the maxillofacial
region in 8 patients (38%), in the limbs in 6 patients (29%), in
the trunk in 4 patients (19%) and in the gluteal region in 3 cases
(14%). A total of 7 patients had superficial venous malformations
and the foci were subcutaneously located in 5 patients but in the
mucous membrane in 2 patients. A total of 14 cases had
intramuscular venous malformations; the foci were located in the
deep muscle tissue and the mass was only palpable when painful.
Foci involving the skin or mucous surface were bluish-violet in
color and elevated from the skin surface or mucous membrane; the
foci located in deep muscle tissue were expressed as a mass in MRI
imaging, the texture of which was soft and tested positive in the
posture experiment. A total of 9 patients exhibited a single
localized lesion, whereas 12 patients exhibited multiple diffuse
lesions (≥2). The lesion sizes ranged from 1.5×2.2×1.0 to
14.0×11.0×7.0 cm. There were 14 patients who had chief complaints
of irregular pain at the lesion site, which could be relieved
without further treatment.
Angiography and classification of
venous malformations
All 121 patients underwent local puncture
angiography of the lesion prior to treatment. With regard to the
angiography method applied, the most protuberant part of the venous
malformation or the most painful site was labeled according to the
child's complaint and punctured directly using a 6.5 disposable
venous transfusion needle. When the lesion was successfully
punctured, venous blood was smoothly pumped back. If there was no
venous blood or the pumping was not smooth, the puncture was
performed again. Subsequently, iohexol contrast agent (30% iodine
content) was slowly injected under fluoroscopy until the lesion was
fully opacified. A small quantity of contrast agent was injected to
observe any draining vein and its diameter. Furthermore, it was
observed whether vascular malformations could be fully filled. In
addition, the size, whether there was definite drainage, the vein
development and the direction of vein drainage was recorded. Based
on lesion morphology and the characteristics of draining veins, the
venous malformations were classified as type I–IV according to
Puig's classification as follows: Type I, isolated malformation
without peripheral drainage; Type II, malformation that drains into
normal veins; Type III, malformation that drains into dilated
veins; and Type IV, malformation that represents dysplastic venous
ectasia. The draining rate was slow in the type I and II
malformations, and the sclerosing agent worked more effectively. By
contrast, the draining rate was fast in type III and IV
malformations and the injected sclerosing agent did not have
adequate contact with the blood vessel wall, and thus the effect
was poor, leading to ready recurrence of the lesions. Of the
selected patients, 13 were diagnosed with type III and 8 with type
IV venous malformations.
Drug allocation
All operations were performed under the guidance of
Innova 3100 (American GE Corp., Fairfield, CT, USA). The drugs used
during the operation included absolute ethanol, n-butyl
cyanoacrylate (NBCA glue, 0.5 ml; B. Braun Melsungen AG, Melsungen,
Germany), lipiodol injection (10 ml/tube; Guerbet, Roissy, France)
and iohexol injection (120 mg/ml; GE Pharmaceutical Co., Ltd.,
Shanghai, China). The ratio of absolute ethanol to lipiodol in the
sclerosing agent was 5:1 (v/v). The maximum dosage of absolute
ethanol was 1 ml/kg, with a total dosage of ≤50 ml. When the dosage
of absolute ethanol exceeded 0.5 ml/kg, the pulmonary artery
pressure was monitored. The ratio of NBCA glue to lipiodol was 1:3
to 1:5 (v/v).
Treatment method
All interventional sclerotherapies were performed on
patients under general anesthesia as the patients were children.
The surgical sites were labeled according to the description given
by the parents. Following successful anesthesia, the most
protuberant part of the venous malformation was directly punctured
using a 6.5 disposable venous transfusion needle containing the
contrast agent. When the lesion was successfully punctured, venous
blood was smoothly pumped back. If there was no venous blood or the
pumping was not smooth, the puncture was performed again. Following
this, iohexol contrast agent (30% iodine content) was injected
under fluoroscopy and the filling of venous malformation was
continuously observed. Prior to treatment with sclerosing agent,
the patients were intramuscularly injected with 0.3 mg/kg
dexamethasone and the proximal-end draining veins of the venous
malformation were pressed using a tourniquet to expand lesions. The
NBCA glue was slowly injected into the lesion under fluoroscopy via
a venous transfusion needle, which was used for injecting the
contrast agent. The ratio of glue and lipiodol mixture used was
1:4. During the injection process, NBCA glue was observed under
fluoroscopy to monitor whether it entered into the draining veins
and to assess the filling of the vessel mass. In addition, 5%
glucose water was used prior to and following injection to avoid
solidification of the NBCA glue. The use of salt water was
prohibited as NBCA glue solidifies quickly following ion exposure.
When angiography exhibited a marked reduction in the draining
velocity of lesions, absolute ethanol was injected. When the
draining vein was embolized completely, injection of the sclerosing
agent was stopped. If the lesion could not be completely filled via
one injection point, another puncture site was used to inject the
embolization agent. Subsequently, a second needle was used next to
the original puncture point. All patients were followed-up 2 months
after treatment. If the symptoms persisted, the treatment was
continued. The time interval between treatments was 2 months. The
patients were followed-up for 6–24 months (average, 15 months)
following treatment.
Efficacy criteria and follow-up
All patients were reviewed 2 months after treatment
and efficacy of the treatment was evaluated. If the lesions were
reduced by <80% or if the symptoms persisted, the treatment was
continued and the aforementioned therapeutic method was used. The
efficacy of treatment was evaluated by MRI examination. The final
efficacy was determined by an MRI performed 6 months after the
final treatment. The treatment efficacy was classified into three
levels (11,13): i) Controlled, the majority of lesions
disappeared after injection (lesions were reduced by >50%) and
the pain symptoms were relieved; ii) no change, the lesions were
reduced by <50% and the pain symptoms persisted; and iii)
failed, lesions remained unchanged or continued to increase. The
following calculation was used to determine the effective rate:
Effective rate=the number of controlled cases/the number of total
cases ×100%. Additionally, the systemic and local adverse reactions
in patients were recorded. Since all patients demonstrated local
swelling and pain following the operation, which was relieved
without treatment 3–7 days after operation, and the swelling was
due to the sclerotherapy mechanism, swelling and pain were not
classified as adverse reactions.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used to calculate the efficacy and adverse reaction
rates in the study. The comparison of effective rates and incidence
of adverse reactions between the two groups were tested using the
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Efficacy
Telephone follow-ups (for patients who resided far
from the treatment center) and questionnaire follow-ups were
performed for 6/21 patients (29%); office visits, imaging and
questionnaire follow-ups were performed for 11/21 patients (52%);
and office visits and imaging follow-ups with no questionnaire were
performed for 4/21 patients (19%). Imaging follow-ups consisted of
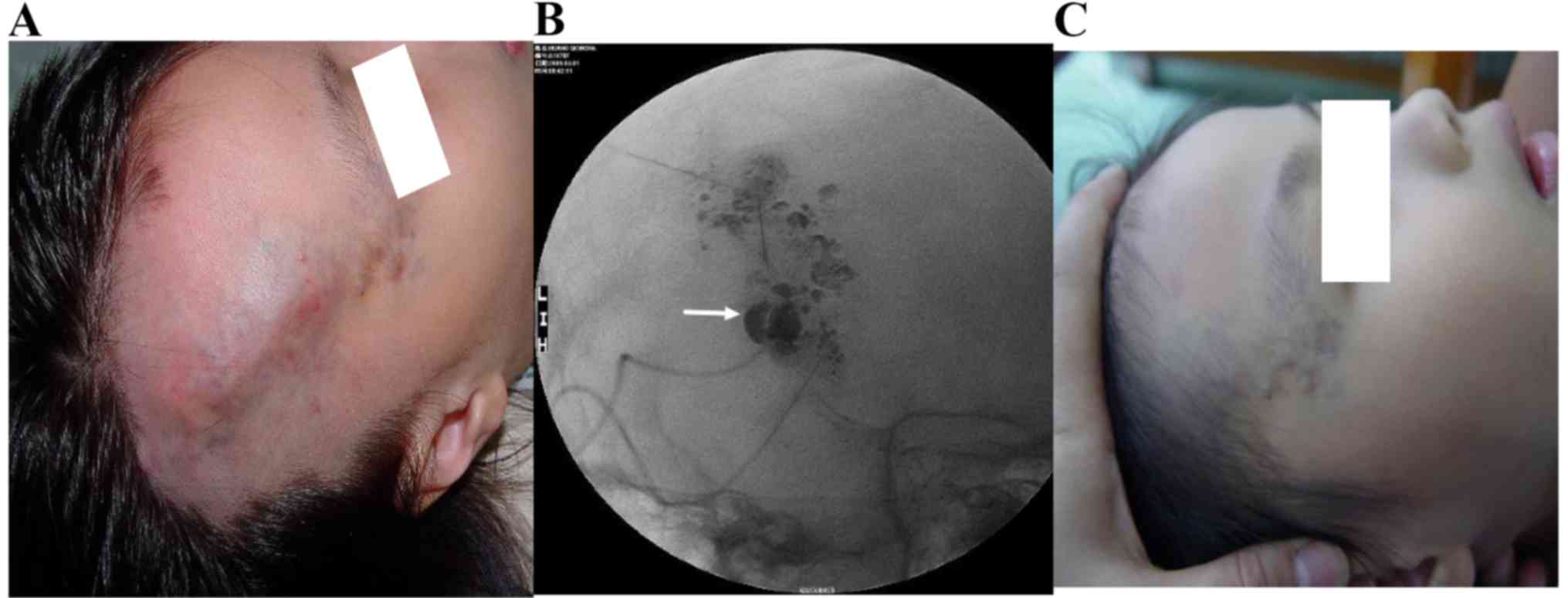
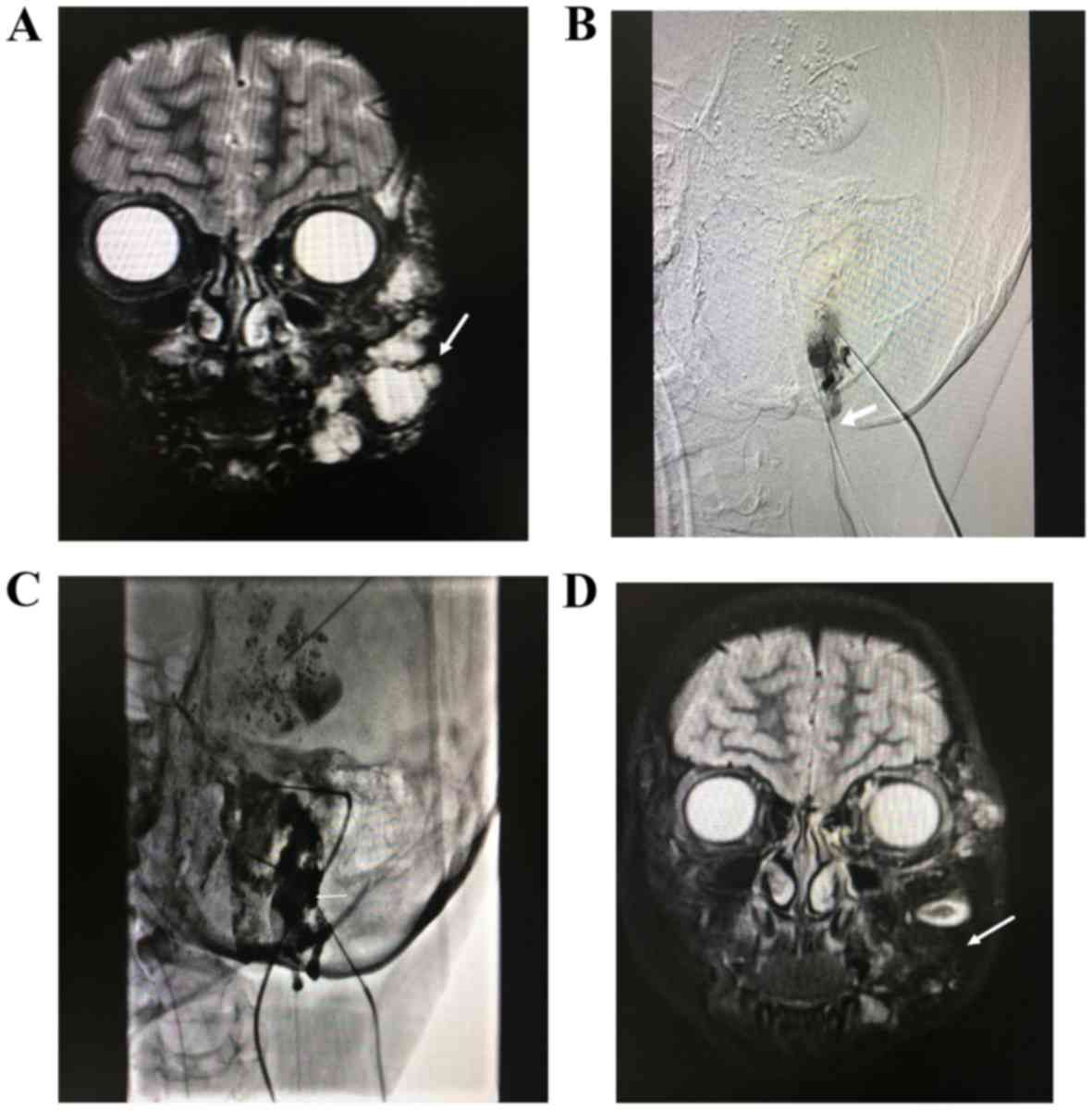
MRI imaging in 16 patients and angiography in 5 patients (Figs. 1 and 2).
Treatment outcome and adverse
events
A total of 21 patients with Puig's classified
advanced venous malformations were treated with absolute ethanol
combined with n-butyl cyanoacrylate, of whom in 15 patients the
syndrome was controlled and the symptoms disappeared; thus the
total effective rate was 71% (15/21). Notably, 1 patient developed
skin ulcerations, which classified as a minor complication, 1
patient developed ectopic embolism caused by n-butyl cyanoacrylate
reflux and 1 patient developed transient pulmonary hypertension.
The incidence rate of adverse reactions was 14.3% (Table I).
 | Table I.Patient demographic and clinical
characteristics. |
Table I.
Patient demographic and clinical
characteristics.
| Patient no. | Age (years) | Sex | Lesion location | Number of procedures
for each patient | Follow-up result |
|---|
| 1 | 3.0 | F | Maxillofacial
region | 2 | Controlled |
| 2 | 1.5 | F | Upper limbs | 2 | Controlled |
| 3 | 6.0 | M | Maxillofacial
region | 3 | Controlled |
| 4 | 5.0 | F | Trunk | 1 | No change |
| 5a | 7.0 | M | Lower limbs | 3 | Controlled |
| 6 | 3.6 | M | Maxillofacial
region | 2 | Controlled |
| 7 | 11.0 | F | Trunk | 4 | Controlled |
| 8 | 6.0 | F | Lower limbs | 2 | Controlled |
| 9b | 8.0 | M | Maxillofacial
region | 3 | No change |
| 10 | 3.0 | F | Lower limbs | 2 | Controlled |
| 11 | 14.0 | M | Trunk | 1 | No change |
| 12 | 7.0 | M | Maxillofacial
region | 3 | Controlled |
| 13 | 5.0 | F | Gluteal region | 2 | Failed |
| 14 | 8.0 | F | Trunk | 1 | Controlled |
| 15 | 0.5 | F | Upper limbs | 1 | Controlled |
| 16c | 5.0 | M | Maxillofacial
region | 2 | Controlled |
| 17 | 4.0 | M | Gluteal region | 2 | Controlled |
| 18 | 3.0 | F | Maxillofacial
region | 3 | Failed |
| 19 | 9.0 | F | Upper limbs | 2 | Controlled |
| 20 | 2.0 | M | Gluteal region | 1 | No change |
| 21 | 10.0 | F | Maxillofacial
region | 2 | Controlled |
Discussion
Puig's classified advanced venous malformation is
difficult to treat clinically. Extensive lesions cannot be
surgically removed and the efficacy of sclerosing embolization is
poor since large draining veins cannot be embolized by liquid
embolic agents (14–16) or foam embolic agents (17,18).
Furthermore, the contact time between the sclerosing agent and vein
endothelial cells is short, and thus proper treatment of vascular
malformation sclerosis cannot be achieved (19). It has also been indicated that
notable sclerosing agent reflux causes serious complications. The
effective rate of absolute ethanol treatment for low draining
venous malformations has been reported as 75–95% (14,15,20);
however, the efficacy of interventional therapy for Puig's
classified advanced venous malformations has been unsatisfactory,
which was reported to be at only 41% (21). To the best of our knowledge, this is
the first study to report the use of absolute ethanol combined with
n-butyl cyanoacrylate sclerotherapy in patients for the treatment
of Puig's classified advanced venous malformation. The present
findings indicated that the effective rate was 70%, which was
higher compared with rates described in a previous study, which was
55% (15).
NBCA is an acrylic adhesive. In the blood, it
rapidly polymerizes with free hydroxide in plasma and causes blood
clots (22). As the acrylic adhesive
is in liquid form, it can be evenly dispersed throughout the
lesions and can promote embolic effects (23). Furthermore, NBCA is spongy following
polymerization and does not easily form lumps (24). Lipiodol can delay the polymerization
of NBCA and also the NBCA can be monitored under DSA (25). Hence, NBCA is considered safe and
accurate for embolizing lesions. Previously, NBCA glue has been
predominantly used in the treatment of encephalic angioma, carotid
body lesions and arteriovenous fistulas (22,26). It
is less aggressive than absolute ethanol, instantly polymerizes in
the blood and is considered non-toxic and non-carcinogenic. In the
current study, NBCA glue was used to embolize venous drainage such
that blood flow was substantially reduced in Puig's classified
advanced venous malformations, and the reported efficacy was ~70%
in combination with absolute ethanol. NBCA and lipiodol are
commonly mixed at a 1:3-5 ratio for intraoperative use. In the
present study, when the proportion of lipiodol was low, NBCA could
easily solidify, therefore, it could not fully reach the distal end
of the draining vein. If the proportion of lipiodol was high, the
concentration of NBCA decreased and NBCA was easily refluxed,
therefore, it failed to fully embolize the draining vein. Notably,
injection of an appropriate volume was crucial. If the quantity of
sclerosing agent injected was too low, the efficacy was limited; if
too high, the sclerosing agent refluxed, which can lead to serious
consequences. According to the present results, the sclerosing
agent injection should be stopped in any of the following cases: If
the injection pressure increased, if the sclerosing agent diffused
into the interstitial space outside the lesions or if the skin
color changed. In the present study, all injection procedures
followed the above principles.
It was critical to accurately determine the endpoint
for the application of sclerotherapy in the treatment of venous
malformations. It was difficult to cure large venous malformations,
and therefore the other objective of the treatment used in the
present study was to control the development of the lesion,
decrease the volume and improve the appearance, rather than
radically attempt to cure the lesion (7,27–29). In
a clinical setting, it was determined that the lesions could not be
removed in certain patients with venous malformations who had
undergone sclerotherapy >10 times. Therefore, the concept of
‘cure’ for venous malformation was not the removal of the lesion
but the disappearance of symptoms. In addition, sclerotherapy may
be unable to prevent the continued progression of remaining lesions
according to previous reports (30).
For Puig's classified advanced venous malformations, the
indications for treatment must be carefully considered. There is no
consensus on the selection of sclerosing agent in the clinical
practice and large randomized clinical trials are insufficient.
Most clinicians select the sclerosing agent according to their own
familiarity with sclerosing agents and the focus size (8,31,32). To
the best of our knowledge, this is the first study to report the
use of NBCA glue combined with absolute ethanol in the treatment of
Puig's classified advanced venous malformations. The findings in
the present study provide a novel option for the sclerotherapy of
venous malformations.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HBL and CQN acquired data. LGL and JZ designed the
present study. XML, ZYL and SYZ interpreted of data. The final
version of the manuscript has been read and approved by all
authors, and each author believes that the manuscript represents
honest work.
Ethics approval and consent to
participate
Ethical approval was granted by the Ethics Committee
of Guangzhou Women and Children's Medical Center, Guangzhou, China.
All procedures performed involving human participants were in
accordance with the ethical standards of the Ethics Committee of
Guangzhou Women and Children's Medical Center and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards.
Patient consent for publication
Informed consent, which included agreement to the
publication of patient data and accompanying images (following
anonymization), was obtained from the parents or guardians of
participants included in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blatt J, McLean TW, Castellino SM and
Burkhart CN: A review of contemporary options for medical
management of hemangiomas, other vascular tumors, and vascular
malformations. Pharmacol Ther. 139:327–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burrows PE and Mason KP: Percutaneous
treatment of low flow vascular malformations. J Vasc Interv Radiol.
15:431–445. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Puig S, Casati B, Staudenherz A and Paya
K: Vascular low-flow malformations in children: Current concepts
for classification, diagnosis and therapy. Eur J Radiol. 53:35–45.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Childs DD and Emory CL: Successful
treatment of intramuscular venous malformation with image-guided
radiofrequency ablation. J Vasc Interv Radiol. 23:1391–1393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scorletti F, Patel MN, Hammill AM, Ricci
KW, Myer CM IV and Dasgupta R: Sclerotherapy for intramuscular
vascular malformations: A single-center experience. J Pediatr Surg.
53:1056–1059. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gurgacz S, Zamora L and Scott NA:
Percutaneous sclerotherapy for vascular malformations: A systematic
review. Ann Vasc Surg. 28:1335–1349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horbach SE, Lokhorst MM, Saeed P, de
Goüyon Matignon de Pontouraude CM, Rothova A and van der Horst CM:
Sclerotherapy for low-flow vascular malformations of the head and
neck: A systematic review of sclerosing agents. J Plast Reconstr
Aesthet Surg. 69:295–304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cornelis FH, Marin F, Labrèze C, Pinsolle
V, Le Bras Y, Midy D and Grenier N: Percutaneous cryoablation of
symptomatic venous malformations as a second-line therapeutic
option: A five-year single institution experience. Eur Radiol.
27:5015–5023. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teusch VI, Piehler AP, Uller W,
Müller-Wille R, Prantl L, Stroszczynski C, Wohlgemuth WA and Jung
EM: Value of different ultrasound elastography techniques in
patients with venous malformations prior to and after
sclerotherapy. Clin Hemorheol Microcirc. 66:347–355. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee BB, Baumgartner I, Berlien P,
Bianchini G, Burrows P, Gloviczki P, Huang Y, Laredo J, Loose DA,
Markovic J, et al: Diagnosis and treatment of venous malformations.
Consensus document of the international union of phlebology (IUP):
Updated 2013. Int Angiol. 34:97–149. 2015.PubMed/NCBI
|
|
11
|
Behravesh S, Yakes W, Gupta N, Naidu S,
Chong BW, Khademhosseini A and Oklu R: Venous malformations:
Clinical diagnosis and treatment. Cardiovasc Diagn Ther. 6:557–569.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puig S, Aref H, Chigot V, Bonin B and
Brunelle F: Classification of venous malformations in children and
implications for sclerotherapy. Pediatr Radiol. 33:99–103. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van der Vleuten CJ, Kater A, Wijnen MH,
Schultze Kool LJ and Rovers MM: Effectiveness of sclerotherapy,
surgery, and laser therapy in patients with venous malformations: A
systematic review. Cardiovasc Intervent Radiol. 37:977–989.
2014.PubMed/NCBI
|
|
14
|
Vogelzang RL, Atassi R, Vouche M, Resnick
S and Salem R: Ethanol embolotherapy of vascular malformations:
Clinical outcomes at a single center. J Vasc Interv Radiol.
25:206–213; quiz 214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steiner F, FitzJohn T and Tan ST: Ethanol
sclerotherapy for venous malformation. ANZ J Surg. 86:790–795.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee BB, Do YS, Byun HS, Choo IW, Kim DI
and Huh SH: Advanced management of venous malformation with ethanol
sclerotherapy: Mid-term results. J Vasc Surg. 37:533–538. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ul Haq F, Mitchell SE, Tekes A and Weiss
CR: Bleomycin foam treatment of venous malformations: A promising
agent for effective treatment with minimal swelling. J Vasc Interv
Radiol. 26:1484–1493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colletti G, Deganello A, Bardazzi A,
Mattassi R, Dalmonte P, Gazzabin L and Stillo F: Complications
after treatment of head and neck venous malformations with sodium
tetradecyl sulfate foam. J Craniofac Surg. 28:e388–e392. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang SK, Drucker NA, Gupta AK, Marshalleck
FE and Dalsing MC: Diagnosis and management of the venous
malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous
Lymphat Disord. 5:587–595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wohlgemuth WA, Müller-Wille R, Teusch V,
Hammer S, Wildgruber M and Uller W: Ethanolgel sclerotherapy of
venous malformations improves health-related quality-of-life in
adults and children-results of a prospective study. Eur Radiol.
27:2482–2488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hung JW, Leung MW, Liu CS, Fung DH, Poon
WL, Yam FS, Leung YC, Chung KL, Tang PM, Chao NS and Liu KK: Venous
malformation and localized intravascular coagulopathy in children.
Eur J Pediatr Surg. 27:181–184. 2017.PubMed/NCBI
|
|
22
|
Kuklik E, Sojka M, Karska K and Szajner M:
Endovascular treatment of renal arteriovenous fistula with N-Butyl
cyanoacrylate (NBCA). Pol J Radiol. 82:304–306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Won Y, Lee SL, Kim Y and Ku YM: Clinical
efficacy of transcatheter embolization of visceral artery
pseudoaneurysms using N-butyl cyanoacrylate (NBCA). Diagn Interv
Imaging. 96:563–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jang HY, Kim KW, Kwon JH, Kwon HJ, Kim B,
Seo N, Lee J, Song GW and Lee SG: N-butyl-2 cyanoacrylate (NBCA)
embolus in the graft portal vein after portosystemic collateral
embolization in liver transplantation recipient: What is the
clinical significance? Acta Radiol. 58:1326–1333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen YM, Liu YT, Chen J and Sun L:
Efficacy and safety of NBCA (n-butyl-2-cyanoacrylate) medical
adhesive for patch fixation in totally extraperitoneal prosthesis
(TEP): A prospective, randomized, controlled trial. Eur Rev Med
Pharmacol Sci. 21:680–686. 2017.PubMed/NCBI
|
|
26
|
Guziński M, Kurcz J, Kukulska M, Neska M
and Garcarek J: Embolization of a true giant splenic artery
aneurysm using nbca glue-case report and literature review. Pol J
Radiol. 80:155–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morgan P, Keller R and Patel K:
Evidence-based management of vascular malformations. Facial Plast
Surg. 32:162–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mulliken JB, Zetter BR and Folkman J: In
vitro characteristics of endothelium from hemangiomas and vascular
malformations. Surgery. 92:348–353. 1982.PubMed/NCBI
|
|
29
|
Odeyinde SO, Kangesu L and Badran M:
Sclerotherapy for vascular malformations: Complications and a
review of techniques to avoid them. J Plast Reconstr Aesthet Surg.
66:215–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fishman SJ and Mulliken JB: Hemangiomas
and vascular malformations of infancy and childhood. Pediatr Clin
North Am. 40:1177–1200. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiramel GK, Keshava SN, Moses V, Mammen
S, David S and Sen S: Percutaneous sclerotherapy of congenital
slow-flow vascular malformations of the orbit. Cardiovasc Intervent
Radiol. 38:270–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harmoush S, Chinnadurai P, El Salek K,
Metwalli Z, Herce H, Bhatt A, Steinkuller P, Vece T, Siddiqui S,
Pimpalwar A, et al: Multimodality image-guided sclerotherapy of
low-flow orbital vascular malformations: Report of Single-center
experience. J Vasc Interv Radiol. 27:987–995.e4. 2016. View Article : Google Scholar : PubMed/NCBI
|