Introduction
Cardiac valve disease is basically an abnormal heart
valve structure caused by rheumatic fever, which leads to a
disorder of cardiac hemodynamics and impairs the quality of life of
affected patients as well as posing a threat to their lives
(1–3). Artificial cardiac valve replacement has
effectively improved the life quality of patients and has an
important role in the treatment of advanced cardiac valve disease
(4). Chikwe et al (5) determined, in a long-term follow-up
study, that the mean follow-up time was 10.8 years, and the 15-year
survival rate following biological and mechanical valve replacement
was as high as 57.5 and 59.9%, respectively. An estimated 100,000
patients worldwide undergo cardiac valve replacement due to
diseases including rheumatic heart valves and degenerative valves
(6). However, patients are required
to adhere to anti-coagulant therapy after replacement.
Warfarin effectively inhibits the synthesis of
vitamin K cyclase, which is the most commonly used as
anti-coagulant drug for patients after cardiac valve replacement
(7,8). A study has indicated that after
treatment with warfarin, the synthesis of coagulation factors was
effectively inhibited by >40% (9). However, excessive or insufficient
anti-coagulant effects may cause severe clinical symptoms. The
prothrombin time (PT) and international normalized ratio (INR) as
preferable clinical indicators of anti-coagulant effects are able
to reflect the degree of blood agglutination in patients, providing
a basis for clinicians to propose treatment plans for patients.
However, the dosage of warfarin and the optimal INR value after
cardiac valve replacement in the elderly patients have remained
elusive, which was therefore investigated in the present study.
Materials and methods
Patients
In the present study, 213 patients who received
anti-coagulation treatment with warfarin after cardiac valve
replacement at Linyi Central Hospital (Linyi, China) between
January 2010 and May 2013 and met the inclusion criteria were
selected as research subjects and retrospectively analyzed. Of
these patients, 70 were males and 143 were females with an age
ranging from 57 to 73 years and a mean age of 65.7±6.4 years. The
cohort included 187 patients with rheumatic heart disease, 13 with
infective endocarditis and 13 with congenital cardiomyopathy.
Pre-operative color Doppler ultrasound was used to classify cardiac
function.
The inclusion criteria were as follows: Course of
disease, ≥6 months; no drug therapy, radiotherapy or chemotherapy
within 3 months; the complete clinical information was available;
and the warfarin anti-coagulation and monitoring strategy were in
line with the conventional requirements (10). The exclusion criteria were as
follows: Autism, or a memory or hearing impairment; patients with a
respiratory system disease, liver or kidney dysfunction, or
diabetes; a blood transfusion was not performed 1 month prior to
admission; an inherited genetic disease; allergy to warfarin.
Warfarin anti-coagulant and monitoring routine were
as follows: Warfarin was applied for anti-coagulant treatment at 48
h after surgery. The initial dosage was 3–5 mg/day. After 24 h, 3–5
ml peripheral blood was collected in an EDTA anti-coagulant tube
and sent to the laboratory for PT and INR detection. The dosage of
warfarin was adjusted according to the PT/INR index (10). Within the first two weeks after the
surgery, PT and INR were examined daily. After the patient's index
was stable, monitoring was reduced to once a week and then to once
a month after discharge. The frequency of monitoring was determined
according to the patient's condition. Patients' complications and
adverse reactions, as well as PT and INR values were recorded by
querying patients' electronic medical records.
Extraction of patient information
The clinical data of the patients were extracted
from the database, including gender, age, type of surgery, body
mass index, education level, smoking and drinking habits, and area
of residence. Baseline laboratory parameters included white blood
cell count (µg/l), platelets (µg/l), cardiac troponin I (µg/l),
creatine kinase (CK, µg/l), CK-muscle/brain (MB; µg/l), lactate
dehydrogenase (µg/l), hydroxybutyrate dehydrogenase (µg/l),
C-reactive protein (mg/l) and aspartate transaminase (µg/l).
Changes in INR and PT, the adjustment of the dosage of warfarin and
end-of-follow-up events (bleeding, embolism and associated
intervention measures), as well as the above indexes were extracted
from the hospital's electronic medical record system and the
outpatient electronic system.
Grouping
Patients were grouped according to
warfarin-associated adverse reactions: The normal group comprised
patients with no symptoms and complications, while those with
hemorrhage, including hematuria, epistaxis and gastrointestinal
hemorrhage, were assigned to the hemorrhage group and those with
embolism-associated symptoms, including cerebral infarction and
lower limb arterial infarction, were assigned to the embolism
group.
Observation indexes
The primary observation indexes of the present study
were the differences in PT, INR and warfarin dosage among the
normal, hemorrhage and embolism groups. This data was normally
distributed, thus the 95% confidence interval was calculated to
evaluate the safety range of PT and INR in elderly patients of the
normal group.
Statistical analysis
SPSS version 20.0 software (IBM Corp., Armonk, NY,
USA) was used for statistical analysis of all of the data collected
in the present study. The measurement data were expressed as the
mean ± standard deviation. One-way analysis of variance was used
for comparison among multiple groups, followed by the
least-significant differences test for comparison between pairs of
groups. The count data were expressed as n (%) and compared between
groups using the chi-squared test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics
Significant differences in the cTnI levels were
identified among the three groups, of which the embolism group was
significantly greater than the hemorrhage and the normal groups; no
significant difference was identified between the hemorrhage group
and the normal group. No significant differences were identified in
other indicators (Table I).
 | Table I.Characteristics of the patients. |
Table I.
Characteristics of the patients.
| Parameter | Normal group | Embolism group | Hemorrhage group | F/χ2 | P |
|---|
| Gender |
|
|
| 3.004 | 0.223 |
| Male | 64 | 2 | 4 |
|
|
|
Female | 118 | 8 | 17 |
|
|
| Age (years) |
|
|
| 3.045 | 0.218 |
|
>60 | 139 | 9 | 19 |
|
|
| ≤60 | 43 | 1 | 2 |
|
|
| Body mass index
(kg/m2) | 26.7±2.5 | 25.4±2.8 | 27.2±1.9 | 1.978 | 0.141 |
| Degree of
education |
|
|
| 2.233 | 0.327 |
|
<Senior high school | 154 | 7 | 16 |
|
|
| ≥Senior
high school | 28 | 3 | 5 |
|
|
| Smoking |
|
|
| 1.606 | 0.448 |
| Yes | 122 | 8 | 18 |
|
|
| No | 60 | 2 | 3 |
|
|
| Intemperance |
|
|
| 3.159 | 0.206 |
| Yes | 43 | 4 | 8 |
|
|
| No | 139 | 6 | 13 |
|
|
| Place of
residence |
|
|
| 2.431 | 0.296 |
|
Countryside | 102 | 7 | 15 |
|
|
| City | 80 | 3 | 6 |
|
|
| Cardiac functional
grading |
|
|
| 4.849 | 0.303 |
| II | 77 | 2 | 5 |
|
|
| III | 60 | 4 | 8 |
|
|
| IV | 45 | 4 | 8 |
|
|
| Valve
replacement |
|
|
| 5.829 | 0.212 |
| Aortic
valve replacement | 53 | 3 | 5 |
|
|
| Mitral
valve replacement | 93 | 3 | 8 |
|
|
| Aortic
valve + mitral valve replacement | 36 | 4 | 8 |
|
|
| WBC
(4.0–10.0×109/l) |
5.58±1.32 |
5.62±1.48 |
5.54±1.68 | 0.013 | 0.987 |
| PLT
(100–300×109/l) | 165.15±45.25 | 157.35±41.73 | 157.37±48.95 | 0.139 | 0.870 |
| cTnI (0–0.3
µg/l) |
2.68±1.17 |
3.49±0.15 |
3.29±0.42 | 5.145 | 0.007 |
| CK (8–60 µg/l) |
53.45±14.63 | 53.76±7.12 |
56.70±20.99 | 0.435 | 0.648 |
| CK-MB (0–24
µg/l) |
6.09±3.39 |
4.84±0.82 |
6.02±0.45 | 0.744 | 0.476 |
| LDH (109–245
µg/l) | 295.41±69.89 | 267.72±11.07 | 301.87±41.57 | 0.965 | 0.383 |
Adverse events
All of the 213 patients were followed up after
cardiac valve replacement. A total of 31 patients (14.6%)
experienced adverse reactions and complications, including 21
patients (9.9%) with hemorrhage, 10 patients (4.7%) with embolism,
while the remaining 182 patients (85.4%) had no hemorrhage or
embolism (Table II).
 | Table II.Adverse events in the different group
and their management. |
Table II.
Adverse events in the different group
and their management.
| Group/adverse
effect | Cases (n=213) | PT (sec) | INR | Measure of
intervention |
|---|
| Normal control
(n=182) |
|
None | 182 (85.45) | 19.54
(14.83–24.94) | 2.05
(1.28–2.77) | None |
| Hemorrhage group
(n=10) |
|
Cerebral infarction | 6 (2.82) | 13.78
(11.86–15.62) | 1.68
(1.07–1.46) | Dosage of warfarin
was increased over time |
| Lower
limb artery infarction | 4 (1.88) | 13.75
(12.13–15.28) | 1.27
(1.03–1.43) | Dosage of warfarin
was increased over time |
| Embolism group
(n=21) |
|
Alimentary tract
hemorrhage | 3 (1.41) | 30.61
(29.42–31.65) | 3.30
(3.12–3.47) | The drug dosage was
reduced according to the patient's condition |
| Nasal
hemorrhage | 8 (3.76) | 27.63
(26.76–28.44) | 3.01
(2.69–3.30) | Drug dose was
reduced according to the patient's condition |
|
Hematuresis | 3 (1.41) | 29.25
(28.37–30.12) | 3.63
(3.54–3.71) | The drug dosage was
reduced according to the patient's condition |
|
Gingival bleeding | 7 (3.29) | 27.23
(25.03–29.43) | 2.90
(2.71–3.12) | Drug dose was
reduced according to the patient's condition |
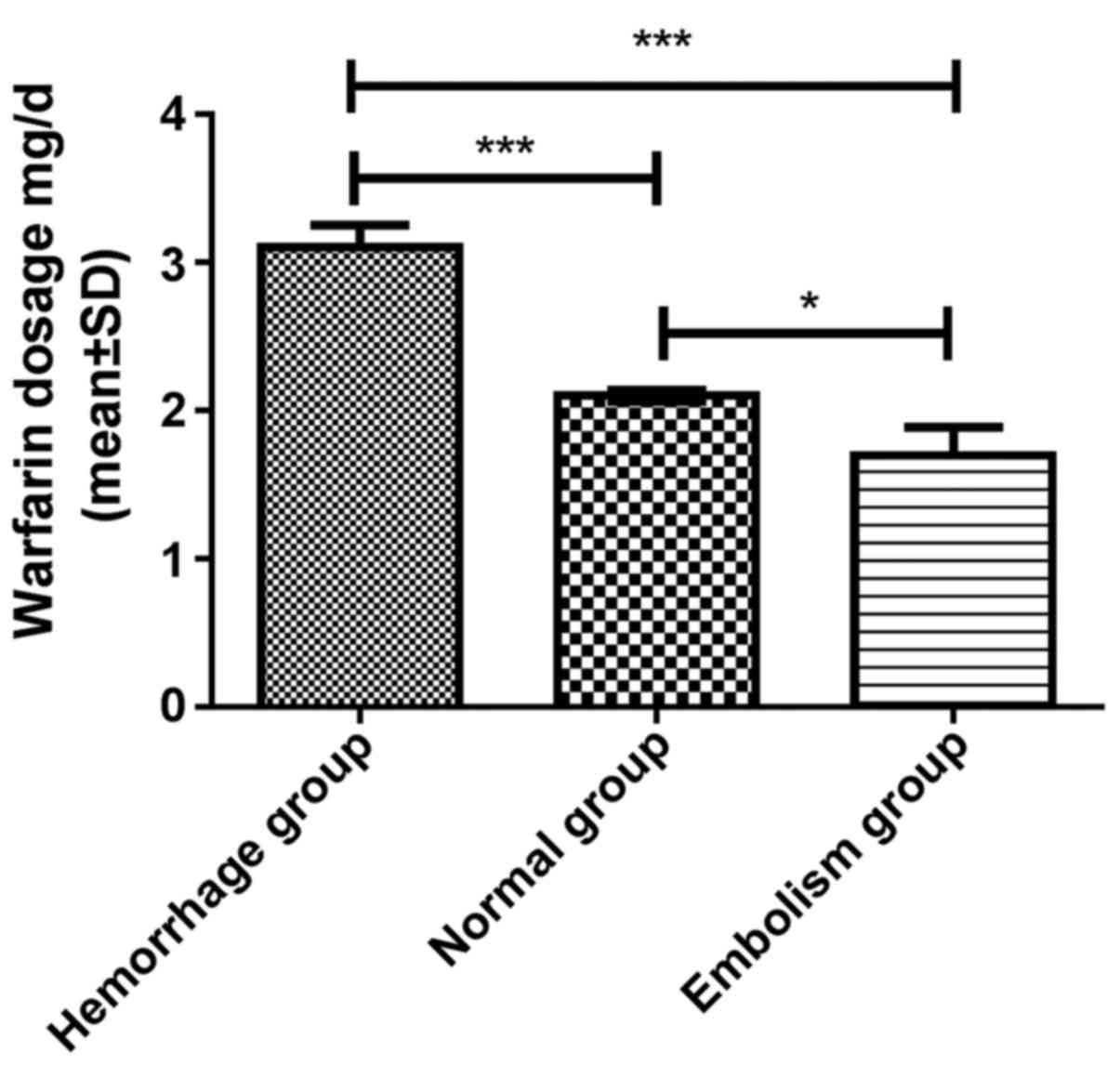
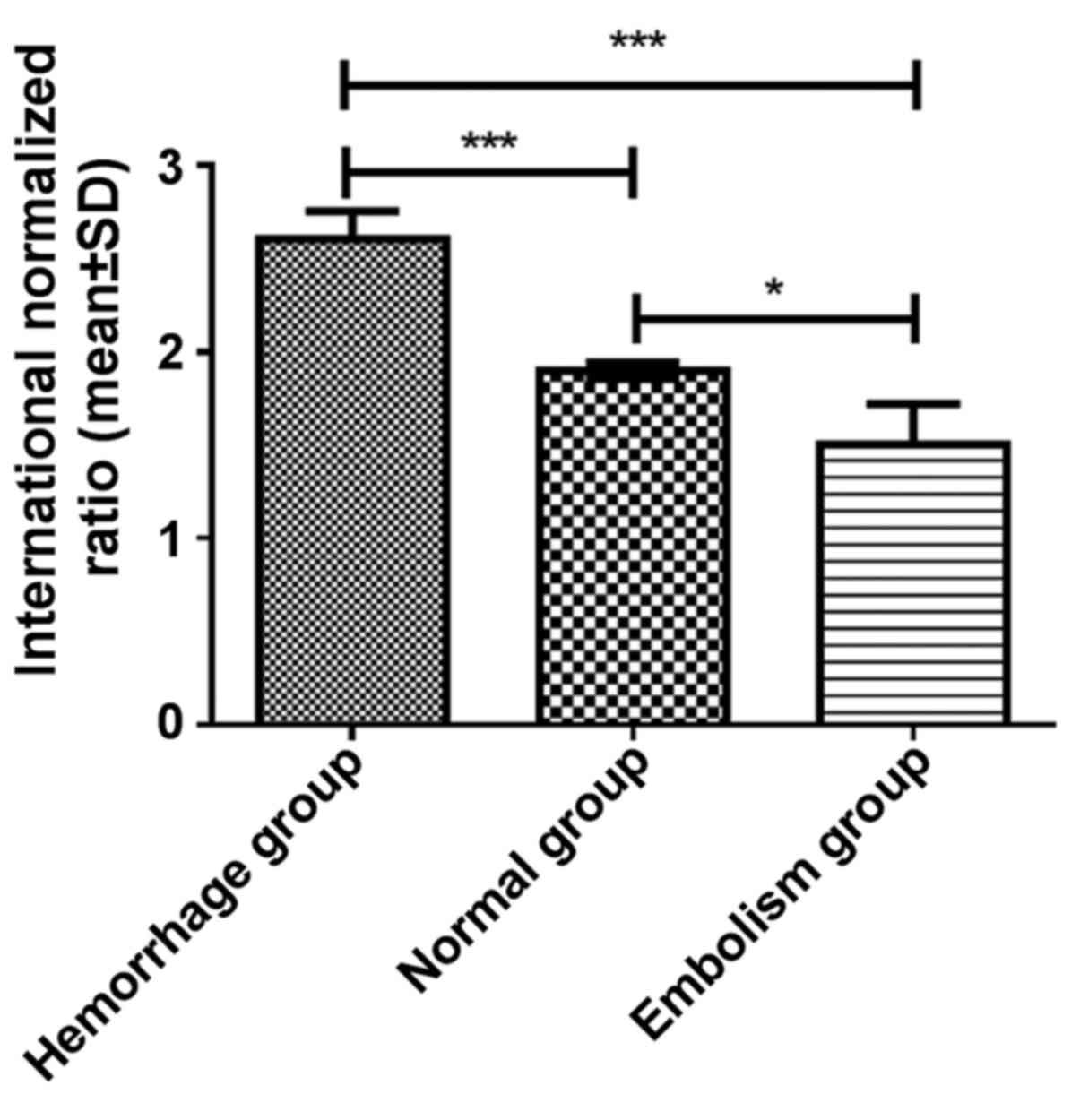
Comparison of warfarin dosage, and the
PT and INR between the patient groups
Significant differences in the warfarin dosage
(F=27.780, P<0.001), PT (F=45.496, P<0.001) and INR
(F=16.644, P<0.001) were identified among the three groups after
6 months. The post-hoc test indicated that in the hemorrhage group,
the warfarin dosage (3.1±0.7 mg/day), PT (24.6±2.7 sec) and INR
(2.6±0.7) after 6 months were significantly different from those in
the normal group (warfarin dosage, 2.1±0.5 mg/day; PT, 19.4±3.1 sec
and INR, 1.9±0.6) and the embolism group (warfarin dosage, 1.7±0.6
mg/day; PT, 13.4±1.8 sec and INR 1.5±0.7; all P<0.001; Figs. 1–3).
After 6 months, the warfarin dosage (P<0.05), PT (P<0.001)
and INR (P<0.05) of the normal group were all significantly
higher compared with those of the embolism group.
Discussion
Cardiac valve replacement is one of the most
effective methods for the treatment of mid- to late-stage cardiac
valvular diseases. Over the past half century, the number of
patients receiving cardiac valve replacement was estimated to be
100,000 per annum worldwide. In China, the cardiac valve
replacements account for 30% of those performed worldwide (11). Rheumatic heart disease is one of the
most common types of heart disease, the major causative factor of
which is the valve damage caused by inflammation, which leads to
problems in the process of transfusion of blood in the patient's
heart, including ventricular hypertrophy and dyspnea, while the
severe form of the disease may pose a threat to the life of
affected patients (12,13). However, the replacement of a cardiac
valve is not completely risk-free, and its biggest drawback is that
patients require long-term or permanent use of blood
anti-coagulants to prevent the occurrence of thromboembolism.
Warfarin as the most widely used clinical
anti-coagulant drug, with the mechanism of action being the
impairment of the blood coagulation system by inhibiting
coagulation factors (including types II, VII, IX and X) synthesized
by vitamin K in the liver. Due to its relatively low cost compared
with that of other drugs, its use may reduce the economic burden of
patients (14,15). However, the level of warfarin
required to achieve a pharmaceutical effect is close to the toxic
dose, and ethnic and regional differences with this regard are
huge, which requires strict control of the dosage of this drug. In
addition, insufficient blood coagulation, thromboembolism and
excessive bleeding are likely to occur. Therefore, patients need to
be monitored closely for a long time to determine if their
condition is stable. At present, patients undergoing cardiac valve
replacement are mainly assessed using their PT and INR values as
indicators. PT is a very important index reflecting liver synthesis
function, reserve function, lesion severity and prognosis (16). INR refers to the ratio of the
patient's prothrombin time to the normal prothrombin time, which is
an important criterion for judging the dosage of oral anticoagulant
drugs (17).
In the present study, the PT and INR were measured
in 213 patients who received cardiac valve replacement and
subsequently took warfarin. Post-operative adverse events and
complications occurring during the follow-up of the patients were
recorded and statistically analyzed. It was identified that the
percentage of patients with adverse events of hemorrhage was as
high as 9.9%, which was slightly higher than the domestic
statistics of 0.8–9.2% (18). This
may be because it is difficult to achieve proper anticoagulant
strength with self-medication because, as demonstrated by Jiang
et al (19), the majority of
patients undergoing heart valve replacements are elderly, possess a
low educational level and lack of professional knowledge. While PT
detection may exhibit differences in different laboratories,
leading to a lack of comparability, INR is able to effectively and
accurately determine the anti-coagulant effect in patients. At
present, there is a certain controversy regarding the normal range
of INR after cardiac valve replacement. It has been reported that
the coagulation function in the Japanese population is lower than
that in western countries, and Japanese patients are more likely to
have hemorrhage symptoms (20). The
PT and INR analyses performed on 213 patients using warfarin found
that the PT (19.4±3.1 sec) and the INR (1.9±0.6) in the normal
group were basically consistent with those reported in domestic
studies (21–23). In recent years, an increasing number
of anti-coagulant drugs have been gradually promoted, which have a
fast onset and long-lasting effect compared with that of warfarin.
However, the time to market for such drugs is relatively short,
their price is relatively high compared with that of warfarin, and
large-scale controlled trials to verify their efficacy and safety
are currently lacking. Therefore, the safety and applicability of
these drugs requires further confirmation (24–26).
Of note, the present study had certain shortcomings,
including the small number of subjects. This may be the reason for
the larger INR standard deviations. Secondly, the ideal range of
INR was not predicted. Therefore, the number of samples will be
increased and an ideal INR range may be determined in future
studies, so as to verify the results of the current study.
In conclusion, INR-guided warfarin anti-coagulation
treatment is recommended to ensure the safety of elderly patients
after cardiac valve replacement, but a reasonable target range of
the INR still requires further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JHF contributed to the conception of the current
study, performed the experimental studies and data analysis,
acquired the data, and edited and reviewed the manuscript. QHM
contributed to study design, statistical analysis, literature
research, clinical studies and manuscript preparation. The final
version of the manuscript has been read and approved by the two
authors and each author believes that the manuscript represents
honest work.
Ethical approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Linyi Central Hospital (Linyi, China), and the
spouses/guardians of all patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coffey S, Harper AR, Cairns BJ, Roberts IS
and Prendergast BD: Clinical information has low sensitivity for
postmortem diagnosis of heart valve disease. Heart. 103:1031–1035.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chambers JB, Prendergast B, Iung B,
Rosenhek R, Zamorano JL, Piérard LA, Modine T, Falk V, Kappetein
AP, Pibarot P, et al: Standards defining a ‘Heart Valve Centre’:
ESC Working Group on Valvular Heart Disease and European
Association for Cardiothoracic Surgery Viewpoint. Eur Heart J.
38:2177–2183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schaefer A, Sill B, Schoenebeck J,
Schneeberger Y, Reichenspurner H and Gulbins H: Failing stentless
Bioprostheses in patients with carcinoid heart valve disease. J
Cardiothorac Surg. 10:412015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duffy N, Xue YQ and Hughes L: Prosthetic
heart valve devices and methods of valve repair. US Patent
US9795482B2. Filed April 27, 2010; issued October 24, 2017.
|
|
5
|
Chikwe J, Chiang YP, Egorova NN, Itagaki S
and Adams DH: Survival and outcomes following bioprosthetic vs
mechanical mitral valve replacement in patients aged 50 to 69
years. JAMA. 313:1435–1442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spenser B, Benichou N, Bash A and Zakai A:
Prosthetic heart valve and method. US Patent US 20170224481 A1.
Filed April 24, 2017; issued August 10, 2017.
|
|
7
|
Eikelboom JW, Connolly SJ, Brueckmann M,
Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K,
Friedman J, Guiver K, et al: Dabigatran versus warfarin in patients
with mechanical heart valves. N Engl J Med. 369:1206–1214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwann TA, Habib RH, Suri RM, Brennan JM,
He X, Thourani VH, Engoren M, Ailawadi G, Englum BR, Bonnell MR and
Gammie JS: Variation in warfarin use at hospital discharge after
isolated bioprosthetic mitral valve replacement: An analysis of the
society of thoracic surgeons adult cardiac surgery database. Chest.
150:597–605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gudmundsdottir BR, Jonsson PI and
Onundarson PT: INR and vitamin K Dependent coagulation factor
fluctuation during warfarin initiation and stable therapy in
patients dosed with the fiix-prothrombin time or the quick
prothrombin time. The Fiix Trial. Blood. 124:42782014.
|
|
10
|
Qian X: Influence of pharmacist
intervention on the knowledge of anticoagulation therapy with
warfarin by the patient. Pharmacotherapy. 35:2015.
|
|
11
|
Nakano T, Nakamura T, Nakamura Y, Irie K,
Sato K, Matsuo K, Imakyure O, Ogata K, Mishima K and Kamimura H:
Effects of Teicoplanin on the PT-INR controlled by warfarin in
infection patients. Yakugaku Zasshi. 137:909–916. 2017.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang X, Li Z, Zhao X, Wang C, Liu L, Wang
C, Pan Y, Li H, Wang D, Hart RG, et al: Use of warfarin at
discharge among acute ischemic stroke patients with nonvalvular
atrial fibrillation in China. Stroke. 47:464–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong L, Shi YK, Xu JP, Zhang EY, Liu JC,
Li YX, Ni YM, Yang Q, Han T, Fu B, et al: The multicenter study on
the registration and follow-up of low anticoagulation therapy for
the heart valve operation in China. Zhonghua Yi Xue Za Zhi.
96:1489–1494. 2016.(In Chinese). PubMed/NCBI
|
|
14
|
Parks T, Mirabel MM, Kado J, Auckland K,
Nowak J, Rautanen A, Mentzer AJ, Marijon E, Jouven X, Perman ML, et
al: Association between a common immunoglobulin heavy chain allele
and rheumatic heart disease risk in Oceania. Nat Commun.
8:149462017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zuhlke L, Engel ME, Karthikeyan G,
Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A,
Daniels R, et al: Characteristics, complications, and gaps in
evidence-based interventions in rheumatic heart disease: The Global
Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J.
36:1115–1122a. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matchar DB, Love SR, Jacobson AK, Edson R,
Uyeda L, Phibbs CS and Dolor RJ: The impact of frequency of patient
self-testing of prothrombin time on time in target range within VA
Cooperative Study #481: The Home INR Study (THINRS), a randomized,
controlled trial. J Thromb Thrombolysis. 40:17–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mcdowell TY, Lawrence J, Florian J,
Southworth MR, Grant S and Stockbridge N: Relationship between
International normalized ratio and outcomes in modern trials with
warfarin controls. Pharmacotherapy. 38:899–906. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van de Werf F, Brueckmann M, Connolly SJ,
Friedman J, Granger CB, Härtter S, Harper R, Kappetein AP, Lehr T,
Mack MJ, et al: A comparison of dabigatran etexilate with warfarin
in patients with mechanical heart valves: THE Randomized, phase II
study to evaluate the safety and pharmacokinetics of oral
dabigatran etexilate in patients after heart valve replacement
(RE-ALIGN). Am Heart J. 163:931–937.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang X, Huang X and Bao J: Application
and discussion of health education in Warfarin therapy of atrial
fibrillation patients. China Med Herald (Zhong Guo Yi Yao Dao Bao).
5:0992011.(In Chinese).
|
|
20
|
Liu Y, Yu XY, Zhong SL, Yang M, Tan HH,
Fei HW and Chen JY: Clinical application of anticoagulation
treatment with warfarin after prosthetic heart valve replacement: A
single center-based survey. Nan Fang Yi Ke Da Xue Xue Bao.
30:2242–2245. 2010.(In Chinese). PubMed/NCBI
|
|
21
|
Go AS, Singer DE, Toh S, Cheetham TC,
Reichman ME, Graham DJ, Southworth MR, Zhang R, Izem R, Goulding
MR, et al: Outcomes of Dabigatran and warfarin for atrial
fibrillation in contemporary practice: A retrospective cohort
study. Ann Intern Med. 167:845–854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan Y, Dong L, Zhang D, Xiang D and Li Y:
Correlation between demographic factors and warfarin stable dosage
in population of Western China. Biomed Res. 28:8249–8253. 2017.
|
|
23
|
Yu Z, Ding YL, Lu F, Miao LY, Shen ZY and
Ye WX: Warfarin dosage adjustment strategy in Chinese population.
Int J Clin Exp Med. 8:9904–9910. 2015.PubMed/NCBI
|
|
24
|
You JH, Chan FW, Wong RS and Cheng G: Is
INR between 2.0 and 3. 0 the optimal level for Chinese patients on
warfarin therapy for moderate-intensity anticoagulation? Br J Clin
Pharmacol. 59:582–587. 2005.PubMed/NCBI
|
|
25
|
Volpp KG, Loewenstein G, Troxel AB, Doshi
J, Price M, Laskin M and Kimmel SE: A test of financial incentives
to improve warfarin adherence. BMC Health Serv Res. 8:2722008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Larsen TB, Rasmussen LH, Skjøth F, Due KM,
Callréus T, Rosenzweig M and Lip GY: Efficacy and safety of
dabigatran etexilate and warfarin in ‘real-world’ patients with
atrial fibrillation: A prospective nationwide cohort study. J Am
Coll Cardiol. 61:2264–2273. 2013. View Article : Google Scholar : PubMed/NCBI
|