Introduction
Hepatocellular carcinoma (HCC) is a complicated type
of malignant tumor with a high global incidence rate compared with
other malignant tumor types (1). The
percentage of cases of nonalcoholic steatohepatitis HCC has
increased in the past; the overall survival has not. Solely 31% of
patients with HCC identified via screening/surveillance received
any curative treatment (2,3). Despite the reasonable progression in
the understanding of the disease mechanism and its therapeutic
possibilities in the past three decades, poor therapeutic outcomes
have been reported in response to conventional treatments,
including liver transplantation and surgical resection, and the
recurrence rate remains considerably high (4,5). In
addition, the presence of multifocal tumors in the liver is
considered to be a notable risk factor for HCC incidence and due to
tumor cell invasion and intrahepatic metastasis, alternative
treatments have been described that may improve clinical outcomes,
including intratumorally injected gene therapy (6).
Gene-mediated cytotoxic immunotherapy (GMCI) is a
clinical intervention that forms a tumor-specific vaccine effect
via intratumoral delivery of adenovirus (ADV)-mediated herpes
simplex virus thymidine kinase (ADV-TK) followed by a systemic
anti-herpetic prodrug, such as valacyclovir or ganciclovir (GCV),
in combination with standard tumor resection surgery or radiation
(7). Necrotic and apoptotic cell
death, and acute inflammation form surgery activate and entice
antigens, which induce immune cells and T-cell expansion (7). Stimulation of T cell proliferation and
the production of inflammatory cytokines may be induced by herpes
simplex virus type 1 thymidine kinase (HSV-TK) protein, which has
been described to resemble a super-antigen molecule (7). This immunostimulatory environment forms
a systemic anti-tumor immune response that leads to the release of
autologous tumor-associated antigens (TAAs) (7). Notably, GMCI treatment towards local
tumors led to protection against metastases in mouse syngeneic
models (8–10). In addition, tumor growth inhibition
has been presented in splenocytes from tumors treated with ADV-TK
combined with GCV but not controls treated with ADV-TK plus saline
(11). These findings suggest that
TAA release and tumor cell death are required to induce a
tumor-specific response. Therefore, GMCI may induce immune
protection against recurrence from minimal residual disease after
tumor debulking.
A previous study on hepatic metastasis in lung
cancer has demonstrated that treatment with HSV-TK followed by
ADV-TK led to significant tumor regression and prolongation of
survival (12). In an interleukin
(IL)-2 adenoviral vector expressing murine model, IL-2 treatment
alone was ineffective, whereas combination therapy with HSV-TK
resulted in further tumor regression and prolonged animal survival
(12). In addition, a previous study
indicated a similar trend was also exhibited in liver metastases in
breast cancer, whereby significant tumor regression was presented
in response to treatment with ADV-TK combined with GCV, which was
assessed by computerized morphometric analysis towards the residual
tumor (13). In addition to this
result, a significant prolongation of survival was also indicated
in ADV-TK and GCV-treated animals (13). Notably, recombinant ADV-TK and GCV
exposure significantly suppressed the growth of SMMC-7721 liver
cells in vitro in a previous study (14). In the examination of nonvascular
invasion, recurrence-free survival and overall survival were also
remarkably higher in the patients receiving liver transplantation
combined with ADV-TK treatment compared with patients receiving
liver transplantation only (15).
Combined ADV-TK and GCV therapy was proposed as a promising
treatment for HCC due to the notable anti-tumor effects observed,
which included the promotion of apoptosis and inhibition of
angiogenesis in a HCC model in vivo (16). However, to best of our knowledge, no
study has reported the underlying mechanism of combined ADV-TK and
GCV treatment in clinically collected tissue-based samples. The
present study aimed to illustrate the therapeutic effect of ADV-TK
and GCV combined with partial hepatectomy via examining the cell
death and apoptosis-associated protein expression levels in
patient's tissue.
Materials and methods
Specimens
A total of 34 hepatocellular carcinoma tissues
(1×1×0.5 cm3) were obtained from surgical specimens of
hospitalized patients between January 2004 and January 2012 at the
Department of General Surgery, Beijing Youan Hospital, Capital
Medical University (Beijing, China). A partial liver resection was
performed in all patients as previously described (17). A total of 14 patients received ADV-TK
(Shenzhen Tiandaxing Gene Engineering Co., Ltd., Shenzhen, China)
therapy prior to partial hepatectomy. An intratumoral dose of
5.0×1011 ADV-TK particles was administered and caused an
objective response with no significant toxicity. The dose was based
on a phase I dose escalation trial (15). To ensure uniform dosing, a total of
5.0×1011 ADV-TK particles in 60 ml of 0.9% saline were
injected into peritoneum tissues around liver at a doses of
1.25×1011 viral particles each, including the lesser
curvature of stomach, abdominal aorta side, head of the pancreas
surface of the right kidney and the area under the right diaphragm.
The first dose of GCV (5 mg/kg; Roche Diagnostics, Basel,
Switzerland) was administered intravenously and following 24 h
further doses were administered twice daily for 10 days until
partial hepatectomy. Patients in control group received liver
resection only and were not treated with ADV-TK/GCV. During partial
hepatectomy, tumor tissue specimens were collected; necrotic and
tumor junction areas were not included in the analysis. Detailed
patient information is presented in Table I. Written, informed consent was
obtained from all patients and the present study was approved by
the Institutional Review Board of Beijing Youan Hospital, Capital
Medical University. All tissues were sliced into 5-µm sections and
were fixed with 10% neutral formaldehyde solution for 12–18 h at
room temperature prior to conventional dehydration and paraffin
embedding.
 | Table I.General clinical data of all
patients. |
Table I.
General clinical data of all
patients.
|
Characteristics | Experimental (14
cases) | Control (20
cases) | P-value |
|---|
| Age (years) | 51.14±12.64 | 60.40±9.80 | 0.109a |
| Sex |
|
|
|
|
Male | 10/14 | 2/20 |
|
|
Female | 4/14 | 18/20 | 0.354a |
| AFP before surgery
(ng/ml) | 2227±5474 | 101±161 | 0.232a |
| Neoplasm stage |
|
|
|
| Phase
I | 10 cases | 10 cases |
|
| Phase
II | 2 cases | 6 cases |
|
| Phase
III | 2 cases | 4 cases | 0.439b |
| Postoperative
pathological grades |
|
|
|
| High
differentiation | 2 case | 6 cases |
|
|
Moderate differentiation | 4 cases | 8 cases |
|
| Poor
differentiation | 8 cases | 6 cases | 0.274b |
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
With xylene and gradient concentrated ethanol (100,
95, 90, 80 and 70%), each section was washed twice and subsequently
rinsed with phosphate-buffered saline (PBS). Sections were fixed
with Proteinase K solution for 20 min at 37°C and rinsed twice with
PBS. Once sections were dry, 50 µl of TUNEL reaction mixture from
in-situ cell death kit (11684809910; Roche Diagnostics) were
diluted 1:2 with PBS, added to each slice and incubated at 37°C for
1 h. All slides were washed with PBS three times, treated with 1
µg/ml 4′,6-diamidino-2-phenylindole (DAPI) working solution and
incubated at 37°C for 4 min. Following, the coverslip was treated
with mounting media. Images were acquired using a conventional
fluorescent microscope under an excitation wavelength of 500 nm and
a detection wavelength of 550 nm. Six fields of view on each slide
were observed. Magnifications of ×20 or ×40 were used to confirmed
whether the labeling was successful or not and to observe the
labeled nuclei (intact or fragmented). TUNEL-positive puncta were
quantified using an ×10 objective. The following equation was used
to calculate the apoptosis index (AI): (number of TUNEL-positive
cells/total number of cells) ×100%.
Immunohistochemical staining
For the immunohistochemical staining of targeted
proteins, HCC tissue sections were deparaffinized and dehydrated.
Once sections were washed with distilled water, all sections were
soaked in methanol with 3.0% hydrogen peroxide for 15 min at room
temperature in order to block the peroxidase in tissues and further
washed with distilled water. Antigens were retrieved with citric
acid buffer by being heating samples at 100°C for 20 min prior to
cooling for 20 min at room temperature. Once sections were washed
with PBS five times, the slides were incubated with primary rabbit
polyclonal antibody against nuclear factor (NK)-κB (1:1,000;
ab86299; Abcam, Cambridge, UK) and caspase-3 (1:500; ab2302;
Abcam); and primary mouse monoclonal antibodies against B-cell
lymphoma (Bcl-2; 1:1,100; ZA-0611; OriGene Technologies, Inc.,
Beijing, China) and B-cl2-assoicated protein X (Bax; 1:200;
ZA-0611; OriGene Technologies, Inc.) overnight at 4°C. The slides
were subsequently washed five times with PBS and then incubated
with horseradish peroxidase (HRP)-conjugated goat secondary
antibody (1:500; TA130005; OriGene Technologies, Inc.) for 10 min
at room temperature. Slides were washed with PBS and treated with
diaminobenzidine substrate from a kit (OriGene Technologies, Inc.)
for 5 min at room temperature to visualize the reaction between
antigen and antibody. Sections were counterstained at room
temperature with Mayer's hematoxylin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 30 sec, dehydrated, cleared and mounted.
Class- and species-matched irrelevant antibodies and incubations
were used as controls. Sections were observed under an Olympus BX53
fluorescence microscope (magnification, ×10; Olympus Corporation,
Tokyo, Japan).
Western blot analysis
For assessing cytochrome c release,
subcellular fractions were separated using the technique indicated
by Ott et al (18). Liver
tissues were homogenized in radioimmunoprecipitation assay buffer
(Sigma Aldrich; Merck KGaA). Cell lysates were mixed with Laemmli
sample buffer and boiled for 3 min. Protein concentrations were
determined by bicinchoninic acid assay (Thermo Fisher Scientific,
Inc.). Equal amounts of proteins (20 µg) were separated on 10%
SDS-PAGE gels and transferred onto nitrocellulose membranes.
Membranes were blocked with 5% skimmed milk with TBS for 1 h at
room temperature and incubated with anti-Bax antibody (1:500;
ab32503; Abcam), anti-Bcl-2 antibody (1:200; ab196495; Abcam) and
anti-cytochrome c antibody (1:200; ab13575; Abcam) overnight
at 4°C. Mitochondrial and cytosolic fractions (20 µg each) were
resolved in 1-mm thick 12% Novex Tris-glycine polyacrylamide gel
and immunoblotted as described above. Subsequently, the membranes
were incubated with a HRP-conjugated secondary antibody (1:500;
ZB-2306; OriGene Technologies, Inc.) at room temperature for 1 h.
Protein bands were visualized with an ECL system (Clinx Scientific
Instruments, Shanghai, China). The relative band intensity was
determined using a gel image analysis system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Densitometry was performed using
Quantity-One image analysis Software (Version 4.6.9; Bio-Rad
Laboratories, Inc.). Protein expression levels in each sample were
quantified and the ratio of protein to GAPDH (1:200; ab8245; Abcam)
was defined as the protein expression.
Statistical analysis
All statistical analyses were performed using SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as
mean ± standard deviation for normal distribution, whereas the
median was representative of skewed distribution. When data were
satisfactory for qualification of normal distribution, the
independent sample Student's t-test was performed. When data did
not meet this qualification, the Kruskal-Wallis test was then used.
χ2 test was used for enumeration data. One-way analysis
of variance was used for multiple group comparison followed by a
Bonferroni post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
ADV-TK combined with GCV significantly
induced apoptosis
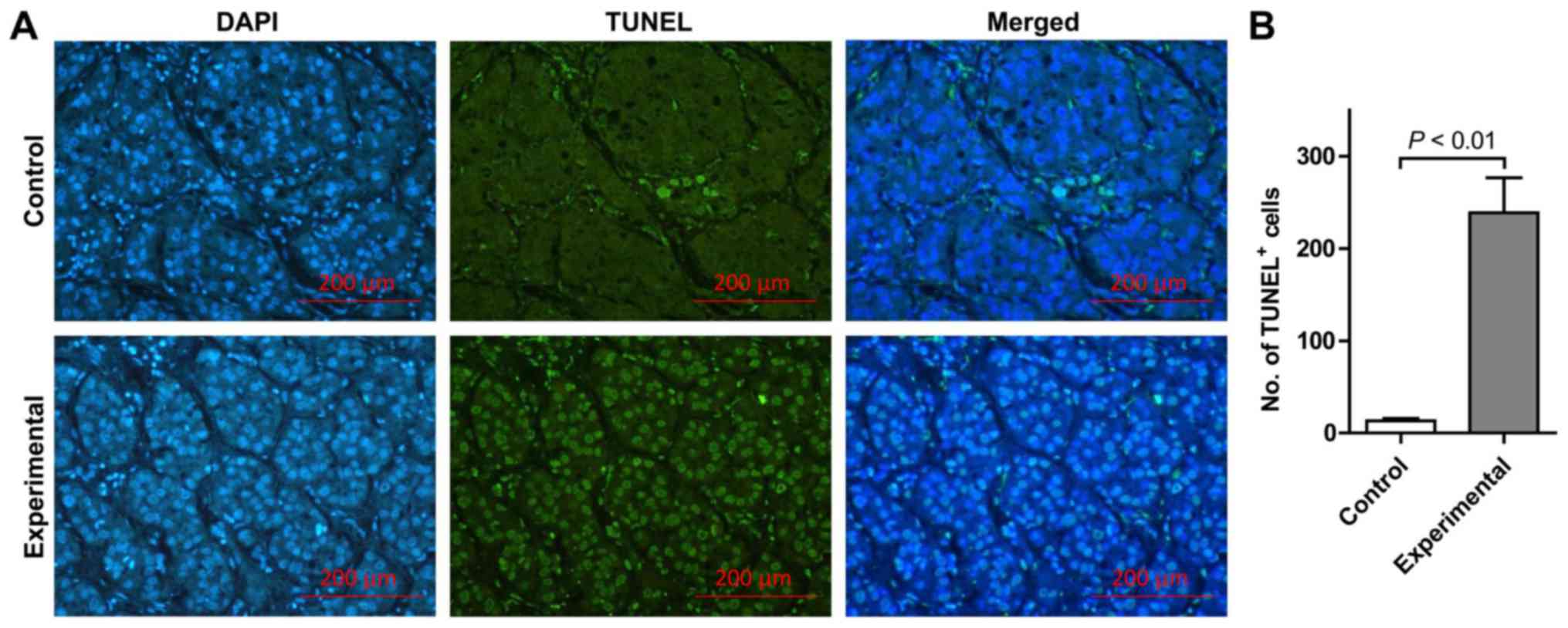
TUNEL assay examination revealed that few
TUNEL-positive apoptotic cells and limited DAPI staining of the
same tissue section was indicated in the control group. Conversely,
the number of TUNEL-positive cells was markedly increased in the
experimental group (Fig. 1A). In
addition, DAPI staining in the experimental group revealed multiple
condensed and fragmented nuclei, suggesting the cells were going
through apoptosis in the identical tissue sections subjected to
combined ADV-TK and GCV treatment. In the experimental group, the
AI in HCC tissues was significantly elevated compared with the
control group (P<0.01; Fig. 1B).
These findings suggest that treatment with ADV-TK combined with GCV
may lead to significantly increased apoptosis.
ADV-TK combined with GCV induces
apoptosis through alternating the protein expression of caspase-3
and NF-κB, the Bax/Bcl-2 expression ratio and cytochrome c
release
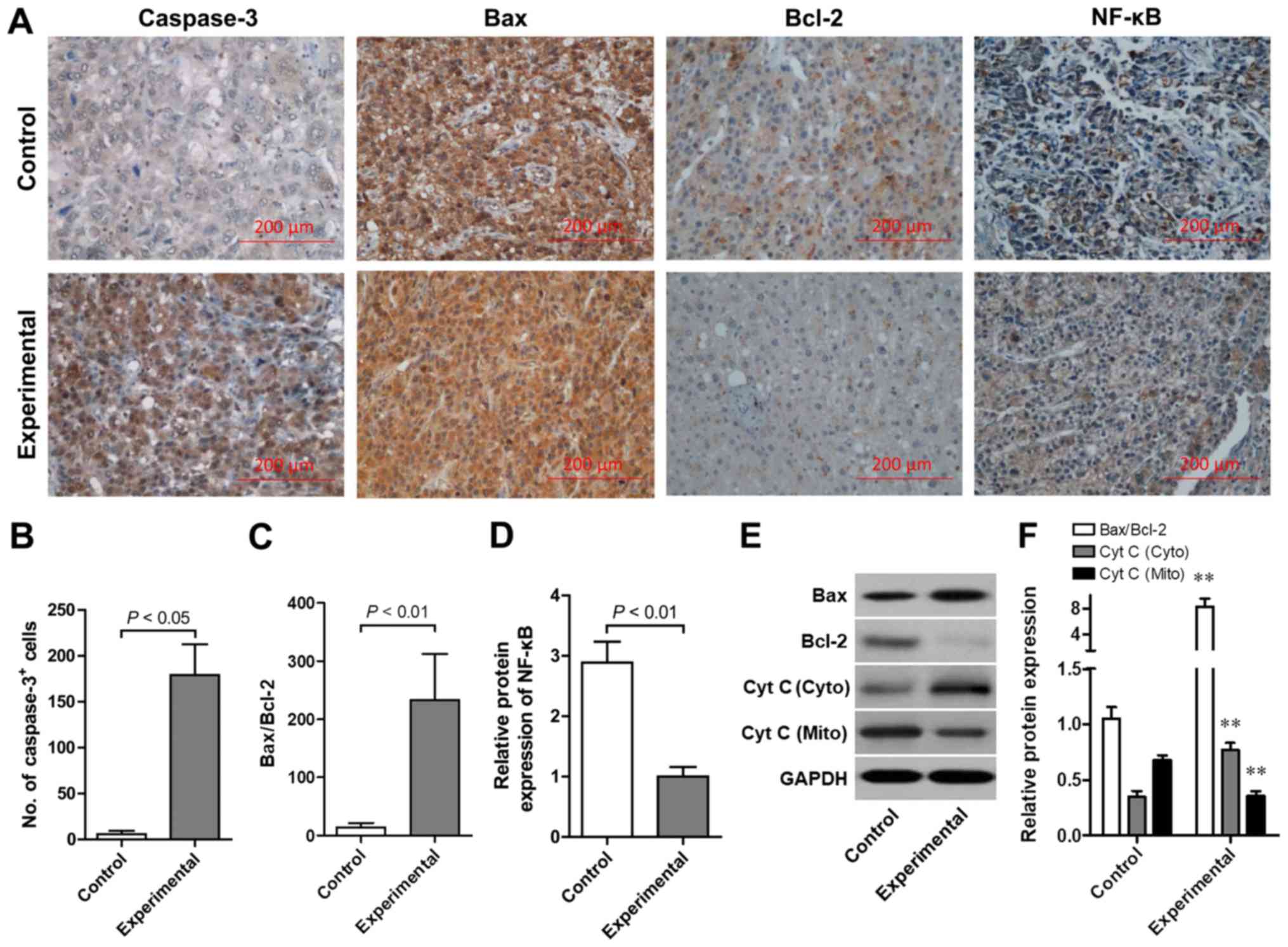
As indicated in Fig.
2A, the protein expression levels of Bax, Bcl-2, caspase-3 and
NF-κB was detected by immunohistochemistry. Brown particles
revealed positive protein expression, which was primarily located
in the cell membrane, cell nucleus and cytoplasm. The positive
staining was diffuse and focal distribution was exhibited. Notably,
the number of caspase-3-positive cells was significantly elevated
in the experimental group compared with the normal control
(P<0.05; Fig. 2B). By contrast,
the Bax/Bcl-2-positive cell ratio was significantly increased in
the experimental group compared with the normal control group
(P<0.01; Fig. 2C). Furthermore, a
significantly increased number of NF-κB-positive cells was observed
in the control group compared with the experimental group
(P<0.01; Fig. 2D). In order to
confirm the effect of combined ADV-TK and GCV therapy on the
apoptotic signaling pathway, western blot analysis was performed to
further assess the Bax/Bcl-2 ratio and cytochrome c release.
Representative western blot analysis images were demonstrated
(Fig. 2E). Results indicated that
the Bax/Bcl-2 protein ratio was significantly increased with
combined ADV-TK and GCV treatment compared with the control
(P<0.01). Furthermore, the release of cytochrome c from
the mitochondria was significantly increased with combined ADV-TK
and GCV treatment compared with the control (P<0.01; Fig. 2F).
Discussion
The present study was performed to assess the
therapeutic effects of ADV-TK and GCV treatment combined with
partial hepatectomy by comparing the biological activities in
tissues from patients with HCC. The present results demonstrated
that increased apoptotic cell death was observed in patients with
HCC who underwent ADV-TK and GCV treatment combined with partial
hepatectomy compared with those who were only subjected to partial
hepatectomy. Furthermore, the expression levels of
apoptosis-associated proteins were assessed in the present study.
Notably, the protein expression levels of caspase-3, the ratio of
Bax/Bcl-2 protein and the release of cytochrome C were
significantly increased, whereas the protein expression levels of
NF-κB were decreased in HCC tissues obtained from patients treated
with combined ADV-TK and GCV therapy compared with the control.
Suicide gene therapy using HSV-TK gene transduction
in combination with GCV is a therapeutic strategy that has been
used in a wide variety of cancer treatments, and its application
has been assessed in over 17 clinical trials in the Unites States
(19). Phosphorylation of GCV
results in its conversion into a non-diffusible nucleoside
analogue, which terminates DNA synthesis and consequently results
in cell death (20). Another benefit
of this approach is the ‘bystander effect’, in which the cytotoxic
GCV-triphosphate molecule is taken up by untransduced cells through
gap junctions, which ultimately enhances tumor cell death (21).
The role of HSV-TK and GCV in the promotion of cell
death has been demonstrated in the treatment of diverse types of
cancer in vitro and in vivo (22–24). One
of the mechanisms involved in combined ADV-TK and GCV-induced
cell-death is apoptosis (25,26). A
study on colorectal cancer with combined ADV-TK and GCV treatment
suggested that its cytocidal effect may be dependent on the tumor
cell type, as evidenced by early apoptosis in the G1 phase of cell
cycle and late apoptotic or necrotic cell death at the sub-G1 phase
with DNA fragmentation (27). Cell
death in ADV-TK and GCV transduced oral carcinoma has been revealed
to be mediated through the apoptotic signaling pathway (28). By contrast, it was proposed that
nonapoptotic biological activity may be a central manifestation in
cell death induced by combined ADV-TK and GCV therapy (29). The present results demonstrated
combined ADV-TK and GCV treatment significantly increased the
number of apoptotic cells according to TUNEL analysis, which
suggested that combined ADV-TK and GCV treatment induced apoptotic
cell death in HCC tissues.
Caspase-3 is one of the key effector caspases in the
cell. Inactive procaspase is cleaved into an active molecule with a
lower molecule weight (~17KD), which activates other proteins to
trigger the apoptotic process (30).
In ADV-TK and GCV transduced NT8e cells, cleaved caspase could not
be detected (31). Similarly, it has
also been reported that no cleaved or activated caspase-3 band was
observed following combined HSV-TK and GCV treatment (32). These findings suggest that the
biological activity serving a role in the cytocidal effect of
HSV-TK and GCV treatment on NT8e cells other than apoptosis
requires further investigation. The results in the present study
suggested that combined ADV-TK and GCV treatment increased the
caspase-3 protein expression levels and was subsequently
responsible for the activation of the early stage apoptosis
signaling pathway.
The mitochondrial apoptosis-induced channel is
responsible for cytochrome c release in the early stage of
apoptosis (33). It has been
reported that the combined ADV-TK and GCV system effectively
inhibited the proliferation of non-small cell lung cancer cells
in vitro and in vivo via the potent induction of
apoptosis, in which the release of the apoptosis initiator
cytochrome c was increased (34). The therapeutic mechanism of suicide
gene therapy was further investigated by Beltinger et al
(26). Their study suggested that
treatment with ADV-TK and GCV led to mitochondrial perturbations,
including loss of the mitochondrial membrane potential and release
of cytochrome c from the mitochondria into the cytosol, as
well as caspase activation and nuclear fragmentation. As major
members of the Bcl-2 family, Bcl-2 and Bax serve a crucial role in
the inhibition of the intrinsic apoptotic signaling pathway and
tumor progression triggered by mitochondrial dysfunction (35). In a previous study, the expression
level of Bcl protein was significantly lower, whereas Bax protein
expression level was significantly higher following 70-HSV-TK and
GCV administration combined with
Mn0.5Zn0.5Fe2O4
nanoparticles for HCC treatment in vitro and in vivo
(36). A study suggested that the
balance between Bcl and Bax proteins is important in assessing the
sensitivity of tumor cells to GCV (37). Following the induction of glioma cell
death, it was suggested that cytosine deaminase/5-fluorocytosine-
and ADV-TK/GCV-induced apoptosis does not require cell death
receptors or p53, but gathering at a mitochondrial pathway caused
by different mechanisms of Bcl-2 modulation (38). A previous study suggested that
downregulation of Bcl-2 and increased caspase-3 expression was a
possible apoptosis mechanism in BIU87 cells induced by a HSV-TK/GCV
system combined with allitride (39). Along with the present analysis of
caspase-3 activity and apoptosis suggest that combined ADV-TK and
GCV treatment induces apoptosis in host tumor cells by increasing
the Bax/Bcl-2 ratio and inducing the release of cytochrome c
from mitochondria to the cytosol.
NF-κB-inducing kinase serves an important role in
promoting the processing of p100/NF-κB2, which is defined as the
non-canonical NF-κB signaling pathway (40). Furthermore, NF-κB was overexpressed
in various types of cancer and the inhibition of NF-κB was implied
to reduce cell growth and enhance cell apoptosis (41,42). A
previous study indicated that apoptosis is the central mechanism in
cell death and tumor progression, whereby latent membrane protein 1
regulated the NF-κB-positive cells upon their treatment with the
pVLTR-TK and GCV system (43).
However, to the best of our knowledge, no study has ever reported
the NF-κB-associated mechanism in combined ADV-TK and GCV-induced
apoptosis. The present results demonstrated that combined treatment
with ADV-TK and GCV could significantly reduce the expression of
NF-κB protein, suggesting that NF-κB may be associated with ADV-TK
and GCV-induced apoptosis in HCC. However, further investigations
are required to fully elucidate this potential mechanism.
In the present study, a simple approach was applied
to understand the cell death modulation in tissues from patients
with HCC who had received combined ADV-TK and GCV therapy. The
results concluded that combined ADV-TK and GCV treatment promoted
cell death in HCC tissues. Furthermore, the present findings
suggested that cell death was primarily mediated by the apoptotic
signaling pathway.
Acknowledgements
Not applicable.
Funding
The study was supported by National Science and
Technology Major Project (grant no. 2017ZX10203205-006-003),
Changes of T Cell Immune Mechanism Before and After Hepatectomy for
Hepatocellular Carcinoma (grant no. 20150303), Beijing Key
Laboratory (grant no. BZ0373), the National Key Research and
Development Program of China: The cluster construction of human
genetic resource Bio-bank in North China (grant no.
2016YFC1201703).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NL conceived and designed the study. HTZ and LQ
performed experiments, and were major contributors in writing the
manuscript. CLL and JYJ made significant contributions to
acquisition and analysis of data. LBS and XFZ performed the
statistical analysis. HTZ, LQ and LBS were responsible for
biological samples collection. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Youan Hospital, Capital Medical University.
Written informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wallace MC, Preen D, Jeffrey GP and Adams
LA: The evolving epidemiology of hepatocellular carcinoma: A global
perspective. Expert Rev Gastroenterol Hepatol. 9:765–779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim NG, Nguyen PP, Dang H, Kumari R,
Garcia G, Esquivel CO and Nguyen MH: Temporal trends in disease
presentation and survival of patients with hepatocellular
carcinoma: A real-world experience from 1998 to 2015. Cancer.
124:2588–2598. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libbrecht L, Desmet V and Roskams T:
Preneoplastic lesions in human hepatocarcinogenesis. Liver Int.
25:16–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Faloppi L, Scartozzi M, Maccaroni E, Di
Pietro Paolo M, Berardi R, Del Prete M and Cascinu S: Evolving
strategies for the treatment of hepatocellular carcinoma: From
clinical-guided to molecularly-tailored therapeutic options. Cancer
Treat Rev. 37:169–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lou L, Ye W, Chen Y, Wu S, Jin L, He J,
Tao X, Zhu J, Chen X, Deng A and Wang J: Ardipusilloside inhibits
survival, invasion and metastasis of human hepatocellular carcinoma
cells. Phytomedicine. 19:603–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aguilar LK, Guzik BW and Aguilar-Cordova
E: Cytotoxic immunotherapy strategies for cancer: Mechanisms and
clinical development. J Cell Biochem. 112:1969–1977. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hall SJ, Mutchnik SE, Chen SH, Woo SL and
Thompson TC: Adenovirus-mediated herpes simplex virus thymidine
kinase gene and ganciclovir therapy leads to systemic activity
against spontaneous and induced metastasis in an orthotopic mouse
model of prostate cancer. Int J Cancer. 70:183–187. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perez-Cruet MJ, Trask TW, Chen SH, Goodman
JC, Woo SL, Grossman RG and Shine HD: Adenovirus-mediated gene
therapy of experimental gliomas. J Neurosci Res. 39:506–511. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vile RG, Nelson JA, Castleden S, Chong H
and Hart IR: Systemic gene therapy of murine melanoma using tissue
specific expression of the HSVtk gene involves an immune component.
Cancer Res. 54:6228–6234. 1994.PubMed/NCBI
|
|
11
|
Agard C, Ligeza C, Dupas B, Izembart A, El
Kouri C, Moullier P and Ferry N: Immune-dependent distant bystander
effect after adenovirus-mediated suicide gene transfer in a rat
model of liver colorectal metastasis. Cancer Gene Ther. 8:128–136.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwong YL, Chen SH, Kosai K, Finegold M and
Woo SL: Combination therapy with suicide and cytokine genes for
hepatic metastases of lung cancer. Chest. 112:1332–1337. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwong YL, Chen SH, Kosai K, Finegold MJ
and Woo SL: Adenoviral-mediated suicide gene therapy for hepatic
metastases of breast cancer. Cancer Gene Ther. 3:339–344.
1996.PubMed/NCBI
|
|
14
|
Chen GZ, Hu H, Xu MY and Deng XL:
Construction of recombinant adenovirus containing TK gene and its
effect against human liver cancer cells. Nan Fang Yi Ke Da Xue Xue
Bao. 30:1887–1889. 2010.(In Chinese). PubMed/NCBI
|
|
15
|
Li N, Zhou J, Weng D, Zhang C, Li L, Wang
B, Song Y, He Q, Lin D, Chen D, et al: Adjuvant adenovirus-mediated
delivery of herpes simplex virus thymidine kinase administration
improves outcome of liver transplantation in patients with advanced
hepatocellular carcinoma. Clin Cancer Res. 13:5847–5854. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu R, Chen D, Lin D, Lu F, Yin J and Li
N: Adenovirus vector-mediated herpes simplex virus-thymidine kinase
gene/ganciclovir system exhibits anti-tumor effects in an
orthotopic hepatocellular carcinoma model. Pharmazie. 69:547–552.
2014.PubMed/NCBI
|
|
17
|
Chan ACY, Chok K, Dai JWC and Lo CM:
Impact of split completeness on future liver remnant hypertrophy in
associating liver partition and portal vein ligation for staged
hepatectomy (ALPPS) in hepatocellular carcinoma: Complete-ALPPS
versus partial-ALPPS. Surgery. 161:357–364. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ott M, Robertson JD, Gogvadze V,
Zhivotovsky B and Orrenius S: Cytochrome c release from
mitochondria proceeds by a two-step process. Proc Natl Acad Sci
USA. 99:1259–1263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenberg SA, Blaese RM, Brenner MK,
Deisseroth AB, Ledley FD, Lotze MT, Wilson JM, Nabel GJ, Cornetta
K, Economou JS, et al: Human gene marker/therapy clinical
protocols. Human Gene Ther. 8:2301–2338. 1997. View Article : Google Scholar
|
|
20
|
Moolten FL: Tumor chemosensitivity
conferred by inserted herpes thymidine kinase genes: Paradigm for a
prospective cancer control strategy. Cancer Res. 46:5276–5281.
1986.PubMed/NCBI
|
|
21
|
Kothari V, Joshi G, Nama S, Somasundaram K
and Mulherkar R: HDAC inhibitor valproic acid enhances tumor cell
kill in adenovirus-HSVtk mediated suicide gene therapy in HNSCC
xenograft mouse model. Int J Cancer. 126:733–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ketola A, Määttä AM, Pasanen T, Tulimäki K
and Wahlfors J: Osteosarcoma and chondrosarcoma as targets for
virus vectors and herpes simplex virus thymidine kinase/ganciclovir
gene therapy. Int J Mol Med. 13:705–710. 2004.PubMed/NCBI
|
|
23
|
Määttä AM, Tenhunen A, Pasanen T,
Meriläinen O, Pellinen R, Mäkinen K, Alhava E and Wahlfors J:
Non-small cell lung cancer as a target disease for herpes simplex
type 1 thymidine kinase-ganciclovir gene therapy. Int J Oncol.
24:943–949. 2004.PubMed/NCBI
|
|
24
|
Kwong KY, Zou Y, Day CP and Hung MC: The
suppression of colon cancer cell growth in nude mice by targeting
beta-catenin/TCF pathway. Oncogene. 21:8340–8346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chu QD, Sun L, Li J, Byrnes K, Chervenak
D, DeBenedetti A, Mathis JM and Li BD: Rat adenocarcinoma cell line
infected with an adenovirus carrying a novel herpes-simplex
virus-thymidine kinase suicide gene construct dies by apoptosis
upon treatment with ganciclovir. J Surg Res. 143:189–194. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beltinger C, Fulda S, Kammertoens T,
Uckert W and Debatin KM: Mitochondrial amplification of death
signals determines thymidine kinase/ganciclovir-triggered
activation of apoptosis. Cancer Res. 60:3212–3217. 2000.PubMed/NCBI
|
|
27
|
Todryk S, Melcher A, Bottley G, Gough M
and Vile R: Cell death associated with genetic prodrug activation
therapy of colorectal cancer. Cancer Lett. 174:25–33. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishikawa M, Hayashi Y, Yamamoto N, Fukui
T, Fukuhara H, Mitsudo K, Tohnai I, Ueda M, Mizuno M and Yoshida J:
Cell death of human oral squamous cell carcinoma cell line induced
by herpes simplex virus thymidine kinase gene and ganciclovir.
Nagoya J Med Sci. 66:129–137. 2003.PubMed/NCBI
|
|
29
|
Katabi M, Yuan S, Chan H, Galipeau J and
Batist G: The nonapoptotic pathway mediating thymidine
kinase/ganciclovir toxicity is reduced by signal from adenovirus
type 5 early region 4. Mol Ther. 5:170–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sergeeva TF, Shirmanova MV, Zlobovskaya
OA, Gavrina AI, Dudenkova VV, Lukina MM, Lukyanov KA and Zagaynova
EV: Relationship between intracellular pH, metabolic co-factors and
caspase-3 activation in cancer cells during apoptosis. Biochim
Biophys Acta. 1864:604–611. 2017. View Article : Google Scholar
|
|
31
|
Srivastava D, Joshi G, Somasundaram K and
Mulherkar R: Mode of cell death associated with adenovirus-mediated
suicide gene therapy in HNSCC tumor model. Anticancer Res.
31:3851–3857. 2011.PubMed/NCBI
|
|
32
|
Kuratate I: Cell death induction of
thymidine kinase gene transfer followed by ganciclovir treatment in
oral squamous cell carcinoma cell lines. Yonago Acta Med. 48:63–74.
2005.
|
|
33
|
Martinez-Caballero S, Dejean LM, Jonas EA
and Kinnally KW: The role of the mitochondrial apoptosis induced
channel MAC in cytochrome c release. J Bioenerg Biomembr.
37:155–164. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiu CC, Kang YL, Yang TH, Huang CH and
Fang K: Ectopic expression of herpes simplex virus-thymidine kinase
gene in human non-small cell lung cancer cells conferred
caspase-activated apoptosis sensitized by ganciclovir. Int J
Cancer. 102:328–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thangarajan S, Ramachandran S and
Krishnamurthy P: Chrysin exerts neuroprotective effects against
3-Nitropropionic acid induced behavioral despair-Mitochondrial
dysfunction and striatal apoptosis via upregulating Bcl-2 gene and
downregulating Bax-Bad genes in male wistar rats. Biomed
Pharmacother. 84:514–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang Q, Lu M, Chen D and Liu P:
Combination of PEI-Mn0.5Zn0.5Fe2O4 nanoparticles and pHsp
70-HSV-TK/GCV with magnet-induced heating for treatment of
hepatoma. Int J Nanomedicine. 10:7129–7143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Craperi D, Vicat JM, Nissou MF, Mathieu J,
Baudier J, Benabid AL and Verna JM: Increased bax expression is
associated with cell death induced by ganciclovir in a herpes
thymidine kinase gene-expressing glioma cell line. Hum Gene Ther.
10:679–688. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fischer U, Steffens S, Frank S, Rainov NG,
Schulze-Osthoff K and Kramm CM: Mechanisms of thymidine
kinase/ganciclovir and cytosine deaminase/ 5-fluorocytosine suicide
gene therapy-induced cell death in glioma cells. Oncogene.
24:1231–1243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qiu SP, Mao XP, Cao KY, Chen XJ, Yuan GQ,
Xu L and Huang XR: The effect of cell killing and apoptosis by
human herpes simplex virus-thymidine kinase/ganciclovir system
combined with allitride in BIU87 cells. Zhonghua Wai Ke Za Zhi.
43:382–386. 2005.(In Chinese). PubMed/NCBI
|
|
40
|
Boutaffala L, Bertrand MJ, Remouchamps C,
Seleznik G, Reisinger F, Janas M, Bénézech C, Fernandes MT,
Marchetti S, Mair F, et al: NIK promotes tissue destruction
independently of the alternative NF-κB pathway through
TNFR1/RIP1-induced apoptosis. Cell Death Differ. 22:2020–2033.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seubwai W, Wongkham C, Puapairoj A,
Khuntikeo N, Pugkhem A, Hahnvajanawong C, Chaiyagool J, Umezawa K,
Okada S and Wongkham S: Aberrant expression of NF-κB in liver fluke
associated cholangiocarcinoma: Implications for targeted therapy.
PLoS One. 9:e1060562014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shinoda K, Kuboki S, Shimizu H, Ohtsuka M,
Kato A, Yoshitomi H, Furukawa K and Miyazaki M: Pin1 facilitates
NF-κB activation and promotes tumour progression in human
hepatocellular carcinoma. Br J Cancer. 113:1323–1331. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lifang Y, Min T, Midan A and Ya C:
HSV-tk/GCV gene therapy mediated by EBV-LMP1 for EBV-associated
cancer. J Exp Clin Cancer Res. 27:422008. View Article : Google Scholar : PubMed/NCBI
|