Introduction
The window of endometrial receptivity is essential
in the implantation of blastocysts and triggers multiple reactions
arising from the endometrium as well as blastocysts (1). The reaction is complex and the
receptive endometrium or uterus are closely associated with the
stimulated/competent/implanting blastocysts (2). The receptive endometrium and stimulated
blastocysts simultaneous function to achieve blastocyst
implantation, leading to pregnancy (3).
Mouse blastocyst implantation occurs in a series of
pre-receptive [day 4 (1000 h)], early receptive [day 4 (1600 h)],
receptive [day 5 (0500 h)] and refractory [day 5 (1000 h)] periods
(4). Regardless of the species,
interactions between the uterus and developing conceptus occur
during the endometrial receptive period. The uterine condition must
be conducive to assisting in implantation and development of the
embryo to establish pregnancy (5).
Cytokines, steroid hormones, peptides, growth factors and enzymes
are essential for implantation (6).
The transforming growth factor (TGF)-β family has
been highly conserved throughout evolution and consists of secreted
elements that modulate developmental reactions including
proliferation and differentiation (7,8). Bone
morphogenetic protein (BMP) belongs to the TGF-β family and binds
to receptor complexes made up of two BMP type 1 receptors [activin
receptor-like kinase (ALK)6, ALK3 or ALK2] and two type 2 receptors
[activin A receptor type (ACVR2) B, ACVR2A or BMP receptor (BMPR)
2] (9). Following BMP binding,
mothers against decapentaplegic homolog (SMAD)5 and/or SMAD1 are
phosphorylated, which was demonstrated to be associated with SMAD4
(10). These proteins are then
translocated to the nucleus to regulate the expression of specific
genes depending on the context. The BMP pathway participates in
modulating the implantation of blastocysts, endometrial epithelial
cells decidualization, and placenta development (11). BMP2 and its corresponding receptor
ALK2 are indispensable to the fertility of females. Conditional
deletion of BMP2 and ALK2 inhibits endometrial epithelial cell
decidualization (7). BMPR2 deletion
in the female reproductive tract leads to development-retarded
embryos, aberrant generation of uterine vessels, and limited
placental development. However, the influence of BMP7 on the female
reproductive tract as well as the potential additional effects of
BMP7 and BMP5 has not been examined.
Endoglin is a type 1 transmembrane glycoprotein,
which serves as an assisted TGF-β receptor and causes
phosphorylation of downstream factors including Smad transcription
factor family members (12). Type 1
and 2 TGF-β receptors act on type 3 co-receptors such as endoglin,
although the understanding of endoglin activity inside or outside
receptor complexes is insufficient (13,14).
Endoglin expression mainly occurs in the proliferating endothelium,
stroma and placental syncytiotrophoblasts (15).
Although previous studies have demonstrated that
endoglin is present in the uterine stroma and endothelium, its
effect on uterine receptivity in terms of embryo implantation
remains unclear. Previous studies also demonstrated that BMP7 is
expressed in the endometrium during the early stages of pregnancy.
However, the role of BMP7 in the blastocyst implantation has not
been assessed. In the current study, the authors demonstrated that
endoglin was stimulated by Rac1 signaling in reaction to BMP7 and
endoglin signaling occurred in endometrial epithelial cells during
the acquisition of endometrial epithelial cell receptivity to
establish embryo implantation.
Materials and methods
Antibodies and reagents
Progesterone, 17-β-estradiol, collagenase type II,
non-essential amino acids, propidium iodide, minimum essential
medium Eagle, sodium bicarbonate, phosphatase inhibitor, protease
inhibitor cocktail, anti-BMP7 (1:1,000; cat. no. SAB1403611),
anti-RAC1 (1:1,000; cat. no. SAB4300461), anti-endoglin (1:1,000;
cat. no. SRP6015), and anti-actin (1:4,000; cat. no. A5441)
antibodies were all obtained from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany).
Pregnancy animal model
A total of 4 wild-type female Swiss albino mice
(age, 6–8 weeks; weight, 22 g) were purchased from Beijing Vital
River Laboratory Animal Technology Co., Ltd. (Beijing, China). Mice
were kept in a chamber in a room with controlled temperature
(20–23°C) under a 12-h light/dark cycle with relative humidity
(40–60%). Mice were given water and food ad libitum. The present
study was approved by the Ethics Committee of Qilu Hospital,
Shandong University (Jinan, China). Mice were sacrificed on
different days of embryo implantation, i. e., day 4 (1000 h; prior
to implantation), day 4 (1600 h; final stage prior to
implantation), day 5 (0500 h; around implantation) and day 5 (1000
h; following implantation), and uterine specimens were obtained.
Embryos were examined under a light microscope (NIKON ECLIPSE-801;
Nikon Corporation, Tokyo, Japan) to confirm the different periods
of receptivity and different stages of endometrial receptivity for
embryo implantation by the presence of morula, blastocyst, hatched
blastocyst and implanting blastocyst stages, as previously
described (16). Strips of uterine
tissue (~2×5 mm) were obtained from the mesometrium between
placental discs as previously described and used in subsequent
experimentation (17,18).
Mouse endometrial epithelial cells
isolation, cell culture, and transient knockdown assay
Fat and connective tissues were removed by washing
with phosphate-buffered saline, and the uterus was cut
longitudinally into small pieces. The uteri pieces were incubated
in PBS containing collagenase type 2 and 0.1% dispase-2 (both
Sigma-Aldrich; Merck KGaA) for 10 min at 37°C. The supernatant was
added to a 15-ml tube containing 2 ml fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA), with 4 tubes for each sample.
Subsequently, the supernatant was centrifuged for at 400 × g for 5
min at 4°C. Epithelial cells were obtained using the EasySep™ Mouse
Epithelial Cell Enrichment kits (Stemcell Technologies, Inc.,
Vancouver, BC, Canada), according to the manufacturer's protocol.
Briefly, mice endometrial epithelial cells were harvested and grown
in 1% Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
FBS and 100 U/ml penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C; this time point was defined as 0 h.
Following 12-h incubation, transient BMP7 knockdown was performed
using small interfering (si)RNA targeting BMP7 (Thermo Fisher
Scientific, Inc.). Briefly, cells were serum starved for 4 h at
37°C prior to 12-h transfection with 200 pmol BMP7 siRNA
(5′-CAGCCGAATTCCGGATCT-3′) in Opti-MEM® (Gibco; Thermo
Fisher Scientific, Inc.) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The medium was
changed to DMEM supplemented with 10% FBS and 100 U/ml
penicillin/streptomycin and cells were maintained at 37°C for 12
h.
Immunofluorescence
Mouse endometrial epithelial cells were seeded at a
density of 2×106 cells/well into six-well plates, with each well
containing sterile coverslips. Following 24 h growth, 4%
paraformaldehyde in PBS was used to fix cells for 20 min at 37°C.
Cells were washed with PBS and subsequently permeabilized with PBS
containing 1% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA)
and 0.2% Triton X-100 for 20 min at room temperature. Cells were
blocked for 2 h at room temperature with 1% BSA in PBS followed by
washing with PBS. Cells were incubated with anti-cytokeratin
pan-fluorescein isothiocyanate (1:1,000; F3418; Sigma-Aldrich;
Merck KGaA) overnight at 4°C. Cells were washed with PBS three
times and nuclei were stained with DAPI (1 µg/ml) for 1 min at room
temperature. Subsequently 70% glycerol was utilized to place
coverslips on the slides and cells were imaged using a laser
scanning confocal microscope (magnification, ×100).
BMP7 and endoglin siRNA transfection
of human endometrial cells
Ishiwaka cells, a human endometrial adenocarcinoma
cell line was purchased from Sigma-Aldrich; Merck KGaA. Ishiwaka
cells were cultured in minimum essential medium Eagle (MEM;
Sigma-Aldrich; Merck KGaA) supplemented with 10% FBS and maintained
at 37°C in a 5% CO2-humidified incubator. Human choriocarcinoma
cells JAr was purchased from the American Type Culture Collection
(Manassas, VA, USA). JAr cells were cultured in DMEM/F12
supplemented with 10% FBS, 1% antibiotics and antimycotics (100X;
Thermo Fisher Scientific, Inc.) and maintained at 37°C in a 5%
CO2-humidified incubator.
JAr cells were seeded into non-adherent 96-well
plates at density of 1×104 cells/well, which facilitated the
cultivation of homogenous cellular aggregates with high performance
efficiencies in parallel. The size can be controlled by the initial
cell density per well. Ishikawa cells were seeded into 12-well
plates at density of 1×104 cells/well and incubated at 37°C for 12
h in a 5% CO2-humidified incubator. A total of 1 µl BMP7
(5′-CAGCCGAATTCCGGATCT-3′) or endoglin
(5′-UGACCUGUCUGGUUGCACATT-3′) siRNA (50 ng/µl) was diluted in 50 µl
Opti-MEM® in microcentrifuge tubes with no RNase; and 1
µl of Lipofectamine® 2000 was diluted in 50 µl
Opti-MEM® in microcentrifuge tubes were incubated for 20
min at room temperature. The Opti-MEM®-siRNA and
Lipofectamine® 2000-Opti-MEM® suspension were
mixed and incubated for an additional 30 min at room temperature,
and then added to the wells containing serum-free media (900 µl).
The cells were then incubated at 37°C in a 5% CO2-humidified
incubator for 12 h. Cells were grown at 37°C in complete media for
an additional 12 h.
In vitro embryo implantation
JAr cells were incubated in DMEM/F12 supplemented
with 10% FBS, 1% antibiotics and antimycotics (100X; Thermo Fisher
Scientific, Inc.) for 12 h on a shaker at 200 rpm at 37°C in a 5%
CO2-humidified incubator. The first filtration was carried out
using a 100-µm membrane, whilst the second filtration was carried
out using a 70-µm membrane to obtain 70–100 µm spheroids. Samples
were incubated with Cell Tracker Red CMTPX (cat. no. C34552; Thermo
Fisher Scientific, Inc.) for 10 min at room temperature. Spheroids
were resuspended in phenol red-free media (cat. no. 2104025; Thermo
Fisher Scientific, Inc.). A 10-min co-culture of spheroids was
carried out in the presence of endoglin siRNA, BMP7 siRNA or
control siRNA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
room temperature prior to washing with DMEM. Spheroid attachment
was assessed under a light microscope.
Intraluminal Morpholino
oligonucleotide (MO) delivery for endometrial BMP7 silencing
Antisense MO sequences targeting mouse BMP7 were
designed and provided by Gene Tools, LLC (Philomath, OR, USA) and
used to knockdown BMP7 in mice. Gravid mice received laparotomy
under local anesthesia and narcosis on the fourth day (1000 h) of
the receptivity period to inject BMP7 MO
(5′-CTGTTTTACTTACGAAACTGTCATT-3′; Gene Tools, LLC) into one of the
uterine horns. The remaining uterine horn was treated with control
MO (5′-CCTCTTACCTCAGTTACAATTTAT-3′; Gene Tools, LLC, USA). Evans
blue dye (100%) was injected into mice prior to being sacrifice on
the fifth day (1000 h) to examine the location of embryo
implantation. Images of the uterus were captured to assess embryo
implantation using a light microscope (magnification, ×40).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from uteri tissue using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA (1 µg) was reverse transcribed into cDNA using the
SuperScript III kit (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. PCR samples for each specimen
contained cDNA, PCR Super Mix (Thermo Fisher Scientific, Inc.) and
forward and reverse primers (20 pmol). qPCR was subsequently
performed in triplicate using the SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd., Dalian, China) and SsoFast™ Probes
Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and a
standard thermocycling procedure was performed on a Bio-Rad CFX96™
Real-time PCR System (Bio-Rad Laboratories, Inc.). The following
primer pairs listed were used for qPCR: Bmp7 forward,
5′-GGATTTTTAGGTTTGTTGGTTG-3′ and reverse,
5′-CAACTCACAATAAACACACATACAT-3′; endoglin, forward,
5′-GCCgGCTTGTCTCCTTCATG-3′ and reverse,
5′-GCAACAAGCTCTTTCTTTAGTACCA-3′; and β-actin forward,
5′-AAATCTGGCACCACACCTTC-3′ and reverse,
5′-GGGGTGTTGAAGGTCTCAAA-3′.
The following thermocycling conditions were used for
the qPCR: Initial denaturation at 95°C for 1 min; 38 cycles of 95°C
for 1 min, 57°C for 1 min, 72°C for 1 min; and a terminal extension
at 72°C for 10 min. The 2−ΔΔCq method (19) was used to analyze the relative
changes in gene expression and normalized to the internal reference
gene β-actin.
Protein extraction
Uterine tissue samples were washed with washing
buffer [100 mM KCl (pH 7.4), 3.5 mM MgCl2, protease and phosphatase
inhibitor cocktail, 3 mM NaCl and 10 mM PIPES] was used for uterine
tissue washing. Following homogenization, tissues were centrifuged
at 200 × g for 15 min at 4°C. The supernatant was removed and
centrifuged for 10 min at 1,500 × g at 4°C. The acquired
post-nuclear supernatant was further centrifuged at 12,000 × g for
10 min at 4°C. The harvested post-mitochondrial supernatant served
as raw protein extract linked to cytosol and mitochondrial
membranes and were stored at −80°C. Total protein was extracted
from Ishiwaka cell lysates and separated primary epithelium using
radioimmunoprecipitation assay buffer (25 mM Tris, 150 mM NaCl,
0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100). Total protein
was quantified using the Pierce BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.).
Immunoprecipitation
Protein A-Agarose (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for preliminary elimination of protein
extracts obtained at various receptivity periods. Protein extracts
(100 µg) mixed with protein A-Agarose were incubated with anti-BMP7
antibody (1:100) overnight at 4°C. Protein A-Agarose was washed
with PBS and a Protein A-Agarose slurry was prepared using
cytosolic buffers. Protein complexes were captured on Protein
A-Agarose at 4°C for 2 h. Subsequently, 10% SDS-PAGE was applied to
resolve proteins for western blotting.
Western blotting
Laemmli sample buffer containing 2.5%
β-mercaptoethanol (Sigma-Aldrich; Merck KGaA) was used for protein
denaturation. Denatured protein (20 µg protein/lane) were separated
via SDS-PAGE on a 10% gel. The separated proteins were transferred
to polyvinylidene difluoride membranes and blocked at room
temperature for 1 h with 5% skimmed milk. Membranes were incubated
with primary antibodies (1:1,000) overnight at 4°C. Following
primary incubation, membranes were incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (anti-mouse,
AP192P; 1:4,000 and anti-rabbit, AP182P 1:4,000; both
Sigma-Aldrich; Merck KGaA) at 25°C for 1 h. The membranes were
washed with Tris buffered-saline containing 0.1% Tween®
20 and protein bands were visualized using the Immobilon Western
Chemiluminescent HSP Substrate (cat. no. WBKLS0500; EMD Millipore,
Billerica, MA, USA). Protein expression was quantified using Total
Lab Quant 1D gel analysis software (version 5.01; Nonlinear
Dynamics, Ltd., Newcastle, UK).
Rac1 activation assay
Rac1 activation was analyzed using Rac1 Activation
Assay kit (cat. no. ab211161; Abcam, Cambridge, MA, USA), according
to the manufacturer's protocol. Briefly, protein extracts (60 µg)
were added to multi-well plates pre-coated with Rac-GTP-binding
protein for 30 min at 4°C. Following incubation, 50 µl anti-RAC1
antibody was added to samples and incubated for 45 min at 4°C. A
total of 50 µl HRP-conjugated secondary antibodies (Abcam) were
added to samples and incubated for 45 min at 4°C. A total of 50 µl
HRP detection agent (Abcam) was added to samples for 20 min at 4°C
for color development. The reaction was stopped by adding 50 µl HRP
halting solution (cat. no. ab211161; Abcam) and a microplate
spectrophotometer was used to measure the optical density at a
wavelength of 490 nm.
Statistical analysis
All experiments were performed 3–5 times using
different mice as replicates. Data presented as the mean ± standard
error of the mean. All statistical analyses were performed using
GraphPad Prism software (version 6.0; GraphPad Software, Inc., La
Jolla, CA, USA). Student's t-test was used to identify the
significance of difference between two groups, and one-way analysis
of variance followed by the Newman-Keuls test was used for multiple
groups. The results of western blot band intensity are presented as
a ratio (protein of interest/β-actin) to correct for loading error
for each sample. P<0.05 was considered to indicate a
statistically significant difference.
Results
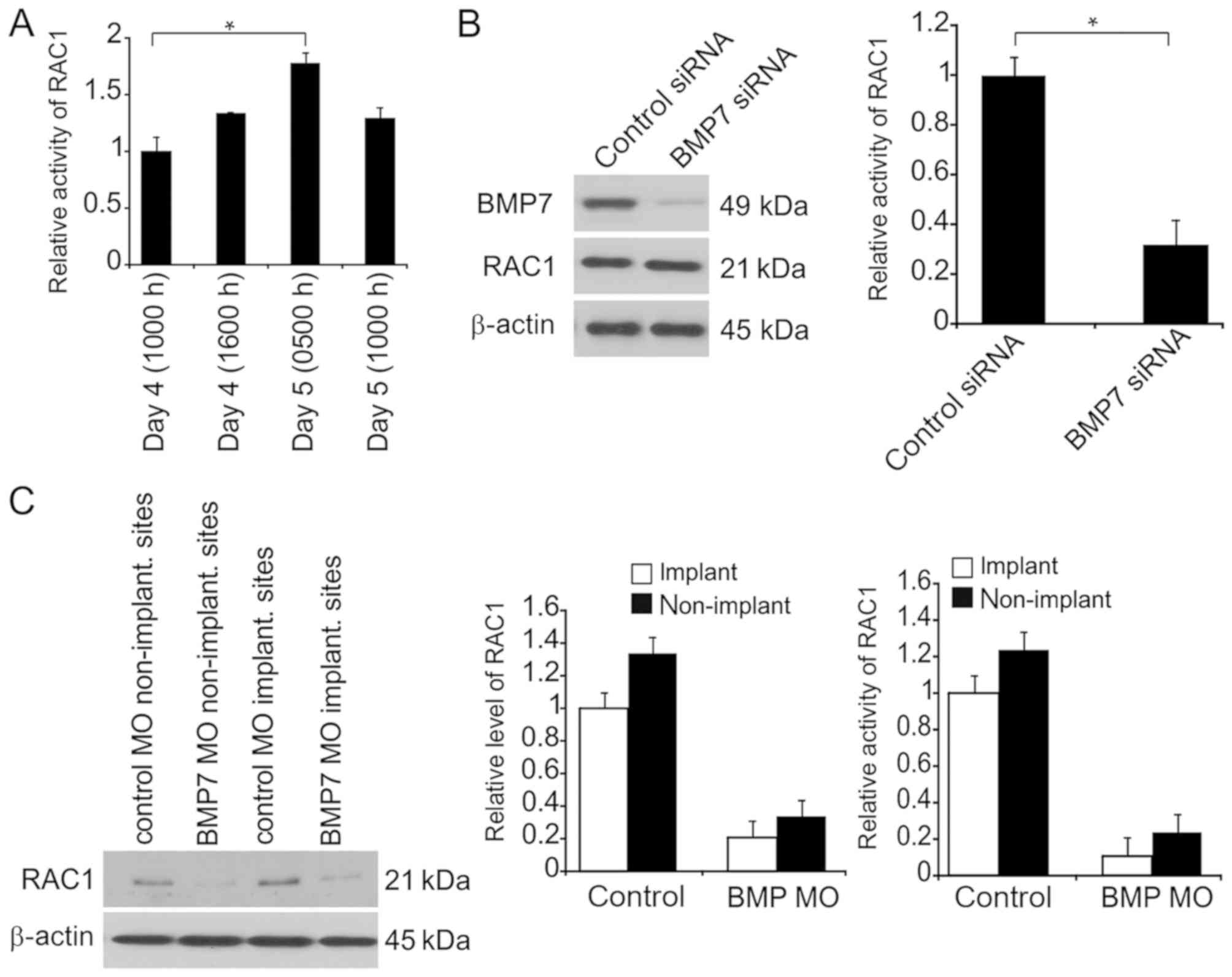
BMP7 is upregulated at receptive
status in endometrial epithelial cells during endometrial
receptivity period in mice and mediates downstream pathway via
endoglin
To evaluate the influence of BMP7 on embryo
implantation, BMP7 was detected in endometrial epithelial cells
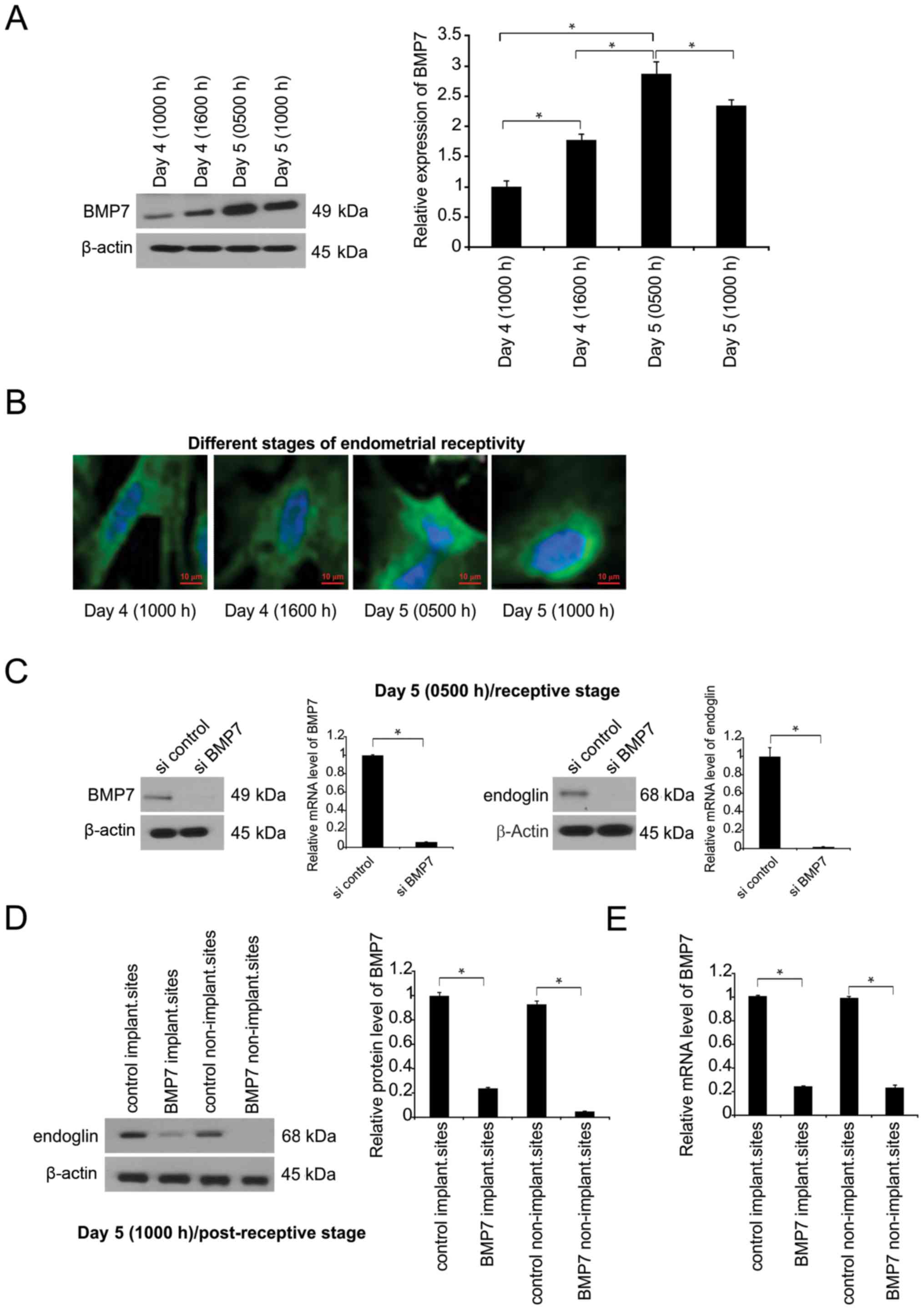
during early gravidity (Fig. 1A).
Prior to implantation, BMP7 expression was enhanced during the
receptive period compared with its basal concentration in
endometrial epithelial cells (Fig.
1A). At advanced stages following the receptivity period, BMP7
expression was generally decreased (Fig.
1A). BMP7 was expressed throughout the cells, but was most
prevalent in vesicular compartments (Fig. 1B). To evaluate the interaction
between endoglin and BMP7, BMP7 expression was silenced in
endometrial epithelial cells, which resulted in a significant
decrease in endoglin expression (Fig.
1C).
In vivo, BMP7 was knocked down via MO at 4 days,
1000 h. At 5 days (1000 h), BMP transcription was inhibited with or
without implantation compared with in MO control, demonstrating
BMP7 knockdown at day 5 at 1000 h (Fig.
1D). Endoglin expression was suppressed following BMP7
exhaustion in the uteri at day 5, 1000 h stage (Fig. 1D). The expression of the tested
factors (BMP-7, endoglin) was also determined in non-pregnant
endometrium. The findings demonstrated that BMP7 is expressed in
the endometrium during the early stages of pregnancy.
Endoglin is associated with
endometrial epithelial cell preparation for receptivity
construction
Endoglin has an essential role in embryo invasion,
and its expression can be found in uteri luminal and glandular
epithelium. It was revealed that BMP7 silencing led to suppression
of endoglin expression in the endometria (Fig. 1D and E).
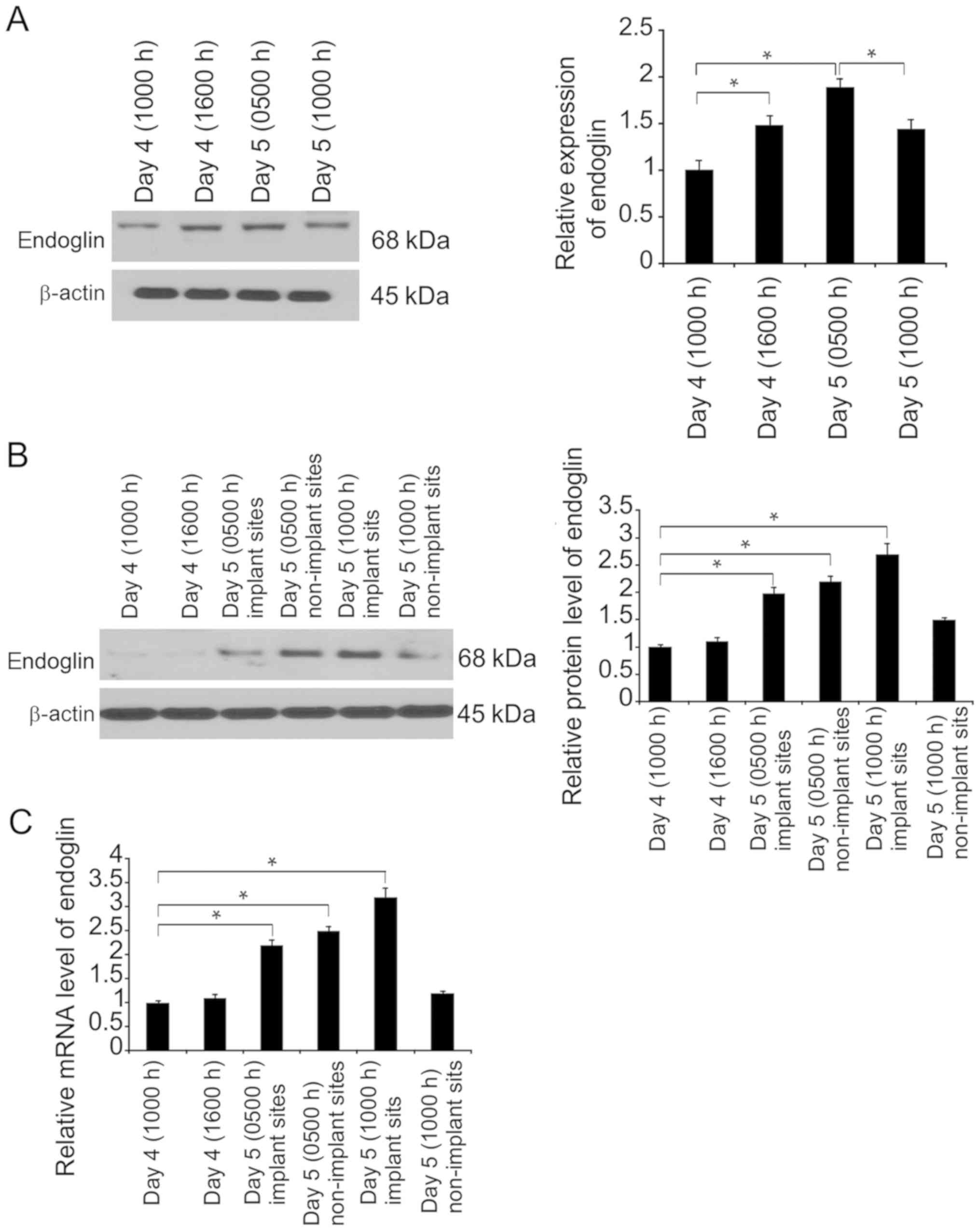
Subsequently, endoglin expression was evaluated by
western blotting at different time points of endometrial
receptivity in the uteri and separated endometrial epithelial cells
acquired from receptive endometria. Primary endometrial epithelial
cells subjected to separation and 24-h culture displayed slight
elevations in endoglin expression during the period of receptivity
or around implantation (Fig. 2A).
Endoglin expression was examined in mouse uteri at different time
points of endometrial receptivity. The results demonstrated that
endoglin expression was limited prior to receptivity, but enhanced
during and following receptivity (Fig.
2B and C).
Endoglin knockdown impaired
implantation in mice
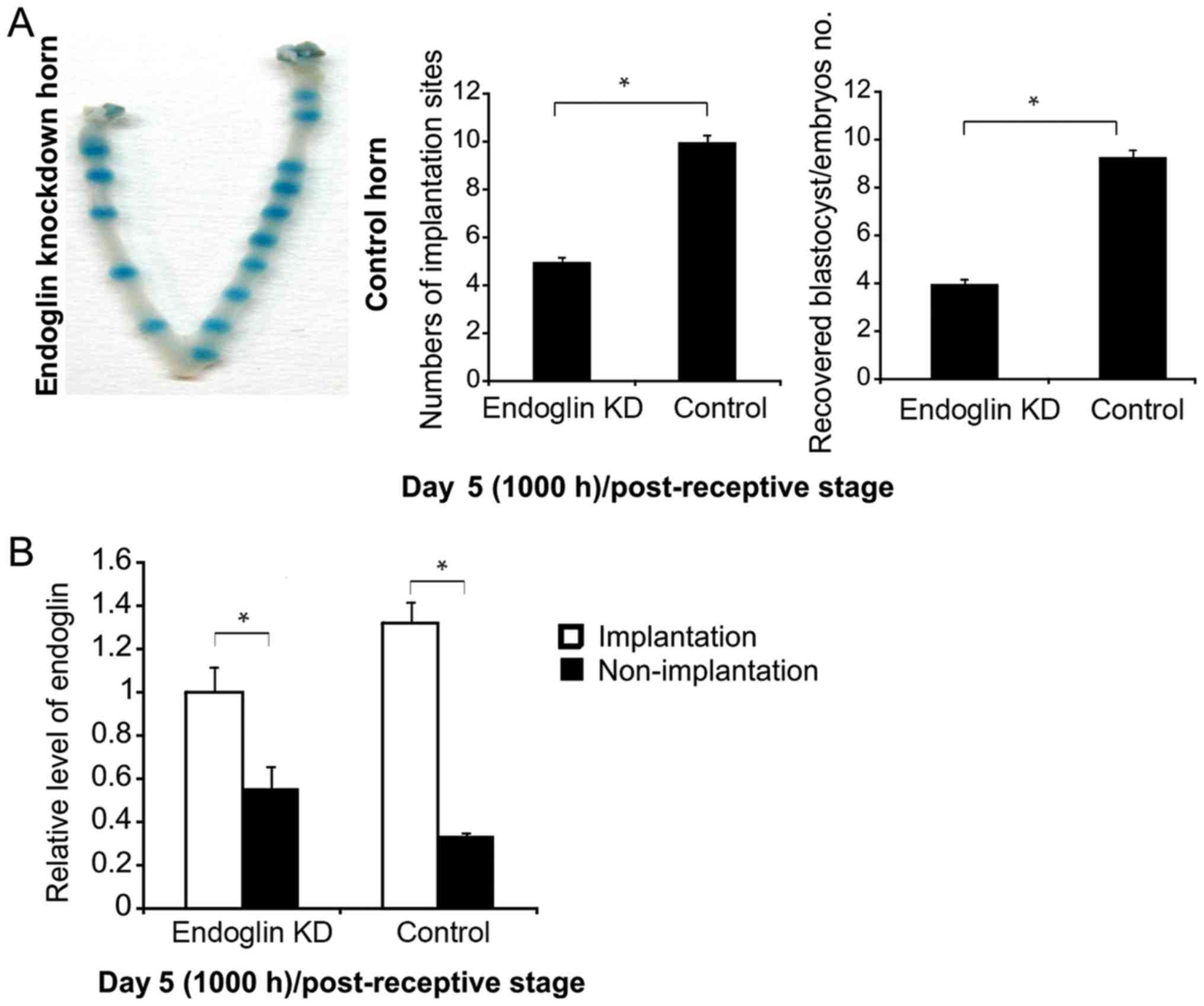
To evaluate endoglin activity in endometrial
receptivity, endoglin was knocked down prior to implantation (day
4, 1000 h) and cells were evaluated at day 5 (1000 h) following
receptivity. The results revealed that implantation sites were
decreased (Fig. 3A) and endoglin
expression was unchanged at endoglin-suppressed implantation sites
in the uterus (Fig. 3B); however,
non-implantation sites were decreased in the two groups (Fig. 3B).
Rac1-GTP is regulated via endoglin in
endometrial epithelial cells during endometrial receptivity
As a G-protein belonging to the RHOGTPase family,
Rac1 promotes endometrial receptivity and its suppression decreases
embryo implantation (20). Previous
studies demonstrated that Rac1 stimulation occurs via BMP7 in the
renal epithelium (21).
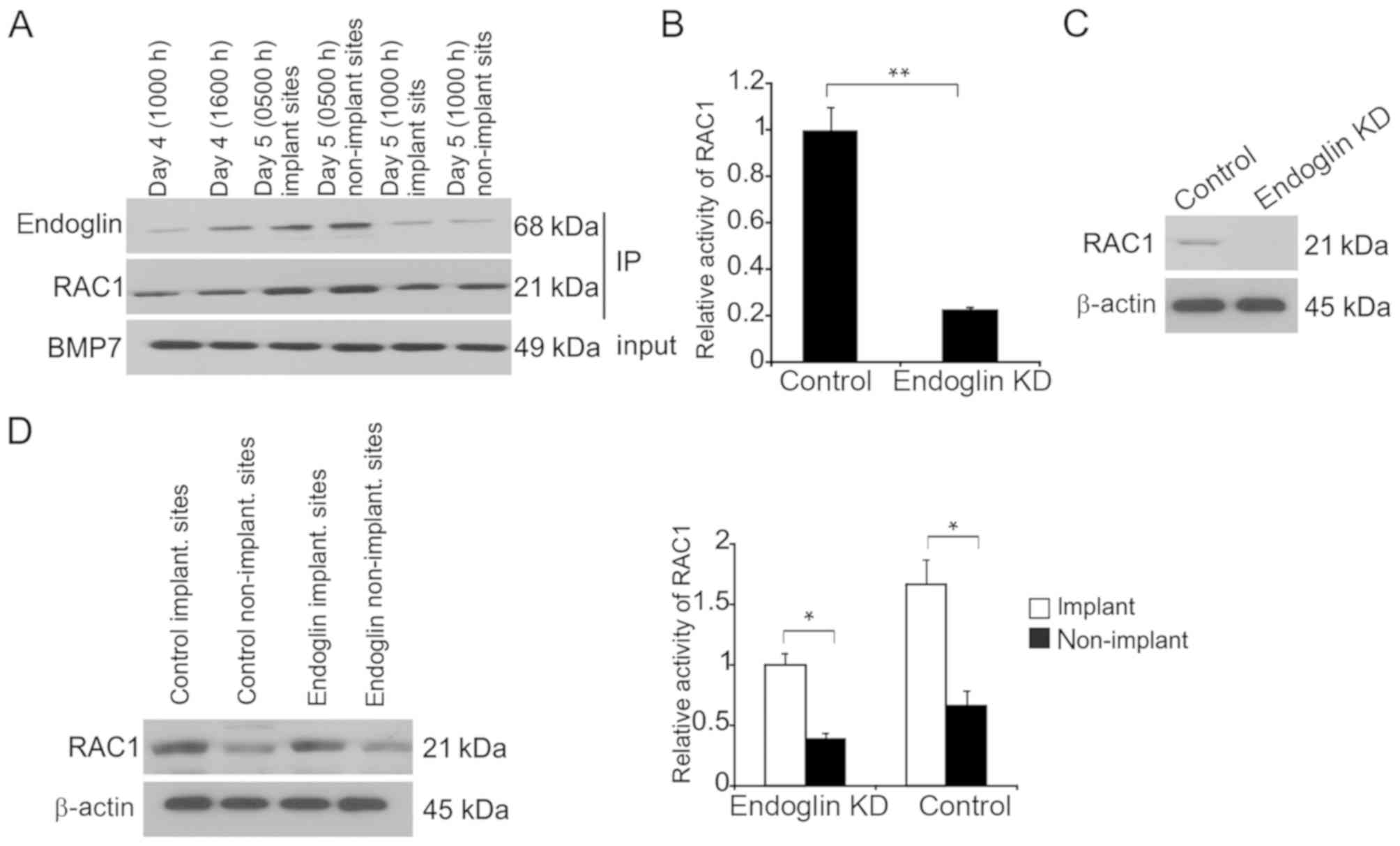
Prior to examining the interaction between endoglin
and BMP7, immunoprecipitation experiments were carried to determine
the physical interactions between these proteins. Upon
immunoprecipitation of BMP7, endoglin- as well as Rac1-positive
bands were detected (Fig. 4A). There
were no bands for independent secondary antibodies (anti-mouse and
anti-rabbit immunoglobulin G) on the immunoblots of endoglin, Rac1,
or BMP7, suggesting an interaction between BMP7 and Rac1 as well as
endoglin in the endometria to spread the BMP7 signals.
Rac1 activity was impaired when endoglin was
exhausted in separated endometrial epithelial cells following 24-h
culture from day 5 (0500 h; Fig.
4B). Furthermore, Rac1 expression was markedly suppressed when
endoglin was exhausted (Fig. 4C).
In vivo evaluation of endoglin regulation was carried out.
Rac1 activity was inhibited when endoglin was exhausted at both
implantation and non-implantation sites at day 5 (1000 h) compared
with control implantation and non-implantation sites, respectively
(Fig. 4D).
Rac1-GTP is regulated via endoglin
stimulator, BMP7, in endometrial epithelial cells
Next, Rac1 activity was determined using the Rac1
Activation Assay kit in endometrial epithelial cells at various
periods of endometrial receptivity. Rac1 activity was enhanced at
day 5 (0500 h) in endometrial epithelial cells (Fig. 5A). Rac1 stimulation was previously
demonstrated to be regulated via BMP7 in mesangial
cell-myofibroblast differentiation and stimulated via deactivating
or separating RHOGDI, which suppresses Rac1 stimulation (21). Thus, BMP7 response to the activity of
Rac1-GTP in endometrial epithelial cells and the uterus tissue was
examined. Rac1-GTP levels were three-fold lower following BMP7
silencing in separated endometrial cells on day 5 (0500 h)
(Fig. 5B). Rac1 expression was
suppressed in BMP7 MO with or without implantation (Fig. 5C). The results also indicated that
Rac1-GTP was suppressed in BMP7 MO with or without implantation
(Fig. 5C).
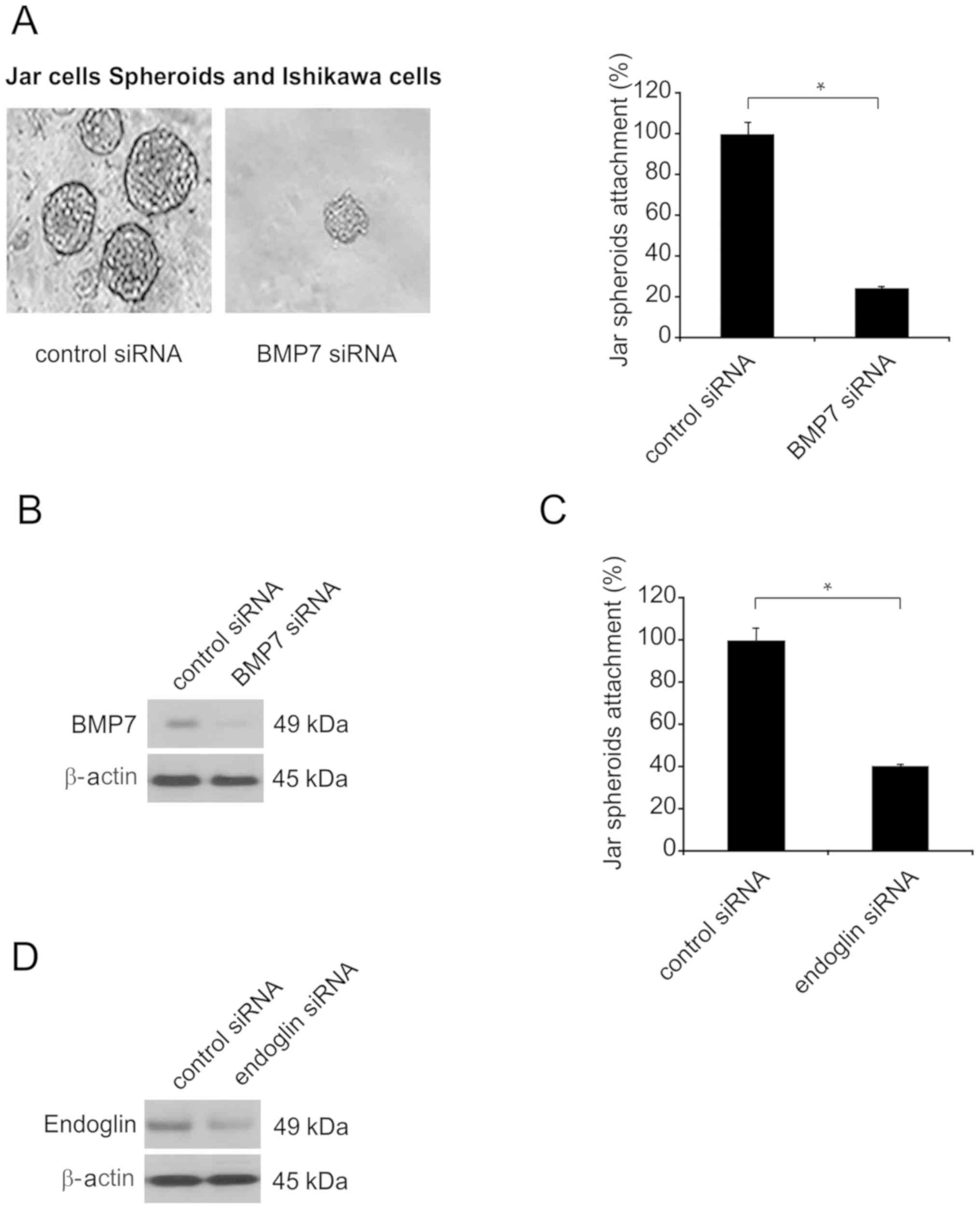
Coculture of JAr spheroids following
BMP7 knockdown/endoglin exhaustion in Ishikawa cell single layer
revealed inhibited attachment
To assess blastocyst attachment modulated by
endoglin or BMP7 on endometrial epithelial cells, spheroids served
as embryonic bodies and were cocultured on a single layer of
endometria. JAr spheroids 80–100 µm in size were added to a single
layer of Ishikawa cells in which BMP7 had been exhausted (siRNA; 60
nmol) or that had received transfection of scrambled siRNA.
Coculture was conducted for 6 h, and spheroid attachment was
subsequently quantified (n=3; Fig.
6A). Attachment of spheroids cocultured with a single
endometrial epithelial cell layer was inhibited when BMP7 was
silenced (Fig. 6A). In total, ~20%
of spheroids were adhered (Fig. 6A).
BMP7 knockdown was confirmed by western blotting in endometrial
epithelial cells (Fig. 6B).
Additional experiments were conducted to examine
co-culture of the Ishikawa layer with exhausted endoglin.
Suppression of endoglin function reduced spheroid attachment by
~60% (Fig. 6C). Endoglin knockdown
was confirmed by western blotting in endometrial epithelial cells
(Fig. 6D).
Discussion
The present authors observed that BMP7 participates
in endometrial receptivity during embryo implantation. The
noticeable BMP7 expression in endometrial epithelial cells suggests
its participation in the promotion of blastocyst attachment
following the epithelium is processed for blastocyst attachment.
BMP7 expression was promoted at day 5 (0500) during endometrial
receptivity in endometrial epithelial cells, which modulates the
receptivity of the epithelium as the receptive biomarkers were
inhibited when BMP7 was silenced in endometrial epithelial cells.
This suggests that BMP7 affects the endometrial receptivity status
for the interaction and attachment of the blastocyst. This is
supported by the fact that endometrial receptivity biomarkers are
inhibited in recurrent miscarriage (22). The present study is the first to
demonstrate that BMP7 is associated with receptivity of the
endometrium. In addition, the results indicated that BMP7 regulates
receptivity of endometrial epithelial cells for implantation of
blastocysts via the endoglin pathway.
It was previously reported that mouse endometria
expressing BMP2 and ALK2 failed to decidualize, leading to
infertility (23). However,
elimination of BMP7 only partly influenced natural gravidity
(24) and did not affect artificial
triggers of decidualization, indicating that BMP2 and extra ligands
serve as primary targets of the TGF-β group to promote
decidualization (25). Additional
studies are required to determine the influence of BMP7 on
decidualization.
Downstream endoglin was demonstrated to be involved
in endometrial receptivity for the adhesion of blastocysts
(26). Endoglin stimulation in a
reaction to BMP7 was identified in endometrial and endometrial
epithelial cells in the endometrial receptivity stage. BMP7
interacts with endoglin, and upregulation of BMP7 during
implantation may trigger endoglin migration to locations at the
apex of uterine luminal epithelium to assist in promoting
conditions for cross-talk between the endometrium and embryo
(27). Thus, BMP7 may function in
focal adhesions by increasing endometrial receptivity.
In addition to the interaction between BMP7 and
endoglin, Rac1 stimulation occurs through BMP7. It has been
demonstrated that Rac1 is necessary for endometrial receptivity
(28). Rac1 stimulation was impaired
when BMP7 was eliminated from endometrial epithelial cells in
receptivity obtainment, demonstrating that Rac1 stimulation
occurred via BMP7.
Reactions modulated by endoglin can assist in
forming the loose structure of the luminal epithelium, making it
less adhesive in the basal lamina and allowing for invasion of
blastocysts (29). Endoglin
concentration was highest at implantation sites during the
endometrial receptivity period, resembling endoglin function in the
endometrium. The separated and cultured endometrial epithelial
cells reflected the influence observed in vivo, indicating
that endoglin functions in endometrial receptivity or development,
which was unexpected. Spreading of endoglin occurred mainly at the
apex prior to receptivity and during the advanced period. The
localization of endoglin returned to the basal areas of endometrial
epithelial cells, which correlated with a previous study (30). Rac1 activity via endoglin modulation
was detected in the entire uterus and endometrial epithelial cells
in endometrial receptivity. Endoglin was stimulated by Rac1
signaling in reaction to BMP7.
In a recent study, defective junction remodeling as
well as premature decline of the apical to basal polarity of the
epithelium was observed when Rac1 was exhausted, disrupting
endometrial receptivity and implantation (31). Rac1 was observed to function in
endometrial epithelial cells regardless of endometrial receptivity
status, but was greatest during implantation. The association
between Rac1 and endometrial receptivity for implantation of
blastocysts was examined. The results indicated that Rac1 activity
was enhanced in implantation regions, revealing the function of
Rac1 in the endometrium.
To achieve endometrial receptivity, BMP7 expression
is based on 17-β-estradiol, as BMP7 transcription and translation
is promoted by 17-β-estradiol availability. Reactions in the
glandular and luminal mouse epithelium are strongest towards
17-β-estradiol. However, the BMP7 reaction in humans is distinct
because adding estradiol and/or progesterone does not enhance BMP7
expression in Ishikawa cells; these cells are derived from
carcinoma and cannot withstand normal conditions (32). Consequently, associating the
reactions of Ishikawa cells with BMP expression in a mouse model is
difficult. P4 enhanced the expression and activity of Rac1 in
endometria of humans, indicating that Rac1 activity is regulated by
available P4 to modulate endometrial receptivity, and thus Rac1 is
promising for promoting receptivity (33).
In conclusion, the BMP7-endoglin-Rac1 axis
functioned in endometrial epithelial cells to achieve receptivity
to assist in blastocyst adhesion to construct gravidity.
Additionally, the highest expression of BMP7 was observed in the
presence of 17-β-estradiol in endometrial receptivity specific to
the epithelium during embryo implantation of mouse gravidity
models. The present results indicated that BMP7 can be used as a
biomarker to predict the endometrial epithelial cell receptivity
for the attachment of blastocyst.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY, XL, HS and BD designed the experiments; CY, LF
and SS performed the experiments; CY and BD analyzed the data; BD
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Qilu Hospital, Shandong University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoshinaga K: Research on blastocyst
implantation essential factors (BIEFs). Am J Reprod Immunol.
63:413–424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haouzi D and Hamamah S: The acquisition of
the human endometrial receptivity phenotype: Lessons from proteomic
studies. Reproductomics Elsevier. 303–314. 2018.
|
|
3
|
Propst AM, Hansard L, Silverberg K,
Hegtvedt M, Burger NZ and Vaughn TC: Endometrial receptivity and
pregnancy rates are higher after 7 days of progesterone in
medicated FET cycles. Fertil Steril. 108:e357–e358. 2017.
View Article : Google Scholar
|
|
4
|
Kumar V, Soni UK, Maurya VK, Singh K and
Jha RK: Integrin beta8 (ITGB8) activates VAV-RAC1 signaling via FAK
in the acquisition of endometrial epithelial cell receptivity for
blastocyst implantation. Sci Rep. 7:18852017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Achache H and Revel A: Endometrial
receptivity markers, the journey to successful embryo implantation.
Hum Reprod Update. 12:731–746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aghajanova L, Hamilton AE and Giudice LC:
Uterine receptivity to human embryonic implantation: Histology,
biomarkers, and transcriptomics. Semin Cell Dev Biol. 19:204–211.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hill CR, Sanchez NS, Love JD, Arrieta JA,
Hong CC, Brown CB, Austin AF and Barnett JV: BMP2 signals loss of
epithelial character in epicardial cells but requires the Type III
TGFbeta receptor to promote invasion. Cell Signal. 24:1012–1022.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christensen ST, Morthorst SK, Mogensen JB
and Pedersen LB: Primary cilia and coordination of receptor
tyrosine kinase (RTK) and transforming growth factor β (TGF-β)
signaling. Cold Spring Harb Perspect Biol. 9(pii): a0281672017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang RN, Green J, Wang Z, Deng Y, Qiao M,
Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, et al: Bone
morphogenetic protein (BMP) signaling in development and human
diseases. Genes Dis. 1:87–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
López-Rovira T, Chalaux E and Massagué J:
Rosa JL and Ventura F: Direct binding of Smad1 and Smad4 to two
distinct motifs mediates bone morphogenetic protein-specific
transcriptional activation of Id1 gene. J Biol Chem. 277:3176–3185.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frum T and Ralston A: Cell signaling and
transcription factors regulating cell fate during formation of the
mouse blastocyst. Trends Genet. 31:402–410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Velasco S, Alvarez-Munoz P, Pericacho M,
Dijke PT, Bernabéu C, López-Novoa JM and Rodríguez-Barbero A: L-
and S-endoglin differentially modulate TGFbeta1 signaling mediated
by ALK1 and ALK5 in L6E9 myoblasts. J Cell Sci. 121:913–919. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lebrin F, Goumans MJ, Jonker L, Carvalho
RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM and ten
Dijke P: Endoglin promotes endothelial cell proliferation and
TGF-beta/ALK1 signal transduction. EMBO J. 23:4018–4028. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruiz-Llorente L, Gallardo-Vara E, Rossi E,
Smadja DM, Botella LM and Bernabeu C: Endoglin and alk1 as
therapeutic targets for hereditary hemorrhagic telangiectasia.
Expert Opin Ther Targets. 21:933–947. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pece-Barbara N, Vera S, Kathirkamathamby
K, Liebner S, Di Guglielmo GM, Dejana E, Wrana JL and Letarte M:
Endoglin null endothelial cells proliferate faster and are more
responsive to transforming growth factor beta1 with higher affinity
receptors and an activated Alk1 pathway. J Biol Chem.
280:27800–27808. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maurya VK, Jha RK, Kumar V, Joshi A,
Chadchan S, Mohan JJ and Laloraya M: Transforming growth
factor-beta 1 (TGF-B1) liberation from its latent complex during
embryo implantation and its regulation by estradiol in mouse. Biol
Reprod. 89:842013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
St-Jean G, Boyer A, Zamberlam G, Godin P,
Paquet M and Boerboom D: Targeted ablation of Wnt4 and Wnt5a in
Müllerian duct mesenchyme impedes endometrial gland development and
causes partial Müllerian agenesis. Biol Reprod. Jul 13–2018.(Epub
ahead of print). doi: 10.1093/biolre/ioy160.
|
|
18
|
Richardson KA, Hannon PR, Johnson-Walker
YJ, Myint MS, Flaws JA and Nowak RA: Di (2-ethylhexyl) phthalate
(DEHP) alters proliferation and uterine gland numbers in the uteri
of adult exposed mice. Reprod Toxicol. 77:70–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leone DP, Srinivasan K, Brakebusch C and
McConnell SK: The rho GTPase Rac1 is required for proliferation and
survival of progenitors in the developing forebrain. Dev Neurobiol.
70:659–678. 2010.PubMed/NCBI
|
|
21
|
Grijelmo C, Rodrigue C, Svrcek M, Bruyneel
E, Hendrix A, de Wever O and Gespach C: Proinvasive activity of
BMP-7 through SMAD4/src-independent and ERK/Rac/JNK-dependent
signaling pathways in colon cancer cells. Cell Signal.
19:1722–1732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan SY, Hang F, Purvarshi G, Li MQ, Meng
DH and Huang LL: Decreased endometrial vascularity and receptivity
in unexplained recurrent miscarriage patients during midluteal and
early pregnancy phases. Taiwan J Obstet Gynecol. 54:522–526. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bollum LK, Huse K, Oksvold MP, Bai B,
Hilden VI, Forfang L, Yoon SO, Wälchli S, Smeland EB and Myklebust
JH: BMP-7 induces apoptosis in human germinal center B cells and is
influenced by TGF-β receptor type I ALK5. PLoS One.
12:e01771882017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akiyama I, Yoshino O, Osuga Y and Yano T:
Bone morphogenetic protein-7(BMP-7) might contribute to
folliculogenesis by promoting angiogenesis. Fertil Steril. 98
(Suppl):S2002012. View Article : Google Scholar
|
|
25
|
Ying Y and Zhao GQ: Detection of multiple
bone morphogenetic protein messenger ribonucleic acids and their
signal transducer, Smad1, during mouse decidualization. Biol
Reprod. 63:1781–1786. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chadchan SB, Kumar V, Maurya VK, Soni UK
and Jha RK: Endoglin (CD105) coordinates the process of endometrial
receptivity for embryo implantation. Mol Cell Endocrinol.
425:69–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Monsivais D, Clementi C, Peng J, Fullerton
PT Jr, Prunskaite-Hyyryläinen R, Vainio SJ and Matzuk MM: BMP7
induces uterine receptivity and blastocyst attachment.
Endocrinology. 158:979–992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu Z, Wang Q, Cui T, Wang J, Ran H, Bao H,
Lu J, Wang B, Lydon JP, DeMayo F, et al: Uterine RAC1 via Pak1-ERM
signaling directs normal luminal epithelial integrity conducive to
on-time embryo implantation in mice. Cell Death Differ. 23:169–181.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rossi E, Lopez-Novoa JM and Bernabeu C:
Endoglin involvement in integrin-mediated cell adhesion as a
putative pathogenic mechanism in hereditary hemorrhagic
telangiectasia type 1 (HHT1). Front Genet. 5:4572014.PubMed/NCBI
|
|
30
|
Chadchan SB, Kumar V, Maurya VK, Soni UK
and Jha RK: Endoglin (CD105) coordinates the process of endometrial
receptivity for embryo implantation. Mol Cell Endocrinol.
425:69–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma Y, Zou H, Zhu XX, Pang J, Xu Q, Jin QY,
Ding YH, Zhou B and Huang DS: Transforming growth factor β: A
potential biomarker and therapeutic target of ventricular
remodeling. Oncotarget. 8:53780–53790. 2017.PubMed/NCBI
|
|
32
|
Bradshaw FJ and Bradshaw D: Progesterone
and reproduction in marsupials: A review. Gen Comp Endocrinol.
170:18–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang W, Ju YY, Zhou QX, Tang JX, Li M,
Zhang L, Kang S, Chen ZG, Wang YJ, Ji H, et al: The Small GTPase
rac1 contributes to extinction of aversive memories of drug
withdrawal by facilitating GABAA receptor endocytosis in the vmPFC.
J Neurosci. 37:7096–7110. 2017. View Article : Google Scholar : PubMed/NCBI
|