Introduction
The incidence of nonmelanoma skin cancer (NMSC),
which includes squamous cell carcinoma (SCC) and basal cell
carcinoma (BCC), has exhibited a marked increase in the previous
decade and at present is the most common malignancy in Caucasian
populations (1). NMSC is associated
with a low rate of mortality but a high rate of disfigurement in
cases where skin lesions are located on the head and neck. In
addition, SCC occurs less frequently compared with BCC but is
generally more aggressive. Sunlight (2), viral infection (3), diet (4),
immunosuppression in organ transplant recipients (5) and induction of spontaneous genetic
mutations (6) have been regarded as
causes for NMSC. Tumors are markedly associated with chronic
ultraviolet (UV) radiation exposure and occur primarily on
sun-exposed areas of the body (7).
Early detection and surgical removal may prevent the majority of
complications. However, skin cancer has a high rate of recurrence
and occasionally tumors progress to advanced stages that are
difficult to treat with present therapeutic modalities;
additionally, advanced-stage tumors become associated with high
morbidity and decreased survival rates (8). At present, treatment options have
remained limited for locally advanced or metastatic NMSC.
Therefore, an in-depth understanding of the molecular basis of skin
tumorigenesis is necessary in order to develop novel and specific
diagnostic biomarkers and efficient therapies.
Src homology 3 and multiple ankyrin repeat domains
protein (SHANK)-associated RH domain-interacting protein (SHARPIN)
is a 387-amino acid protein that was originally identified as a
SHANK-binding protein, which is enriched in the postsynaptic
density of excitatory neurotransmitters (9). In addition, SHARPIN has been detected
in cancer in the brain, spleen, lungs and other organs. Seymour
et al (10) have identified
SHARPIN as a gene mutated in chronic proliferative
dermatitis (cpdm) mice (Sharpincpdm/cpdm) which
spontaneously causes chronic inflammation, primarily in the skin,
but also in other tissues including the gut, lung, liver and
esophagus. SHARPIN has been previously identified as a common
component of the linear ubiquitin chain assembly complex (LUBAC)
which also contains E3 ubiquitin protein ligase ring finger protein
31 and RanBP-type and C3HC4-type zinc finger-containing protein 1
(HOIL-1L) (11). The C-terminal
portion of SHARPIN consists of a ubiquitin-like (UBL) domain
followed by an Npl4-zinc finger (NZF) domain and is important for
the formation of a complex with the LUBAC component, haem-oxidized
iron-regulatory protein 2 ubiquitin ligase-1 interacting protein
and ubiquitin (9). LUBAC is an
important component of the nuclear factor
kappa-light-chain-enhancer of activated B cells (NF-κB) signaling
pathway, which is a critical regulator of inflammation, immune
response and lymphoid tissue development (12). NF-κB signaling is generally
classified into canonical and non-canonical pathways. The canonical
pathway, primarily triggered by tumor necrosis factor (TNF),
lipopolysaccharides, and T and B cell receptors, occurs in the
majority of cells as the principal NF-κB pathway. Upon stimulation,
the downstream kinase inhibitor of κB (IκB) kinase (IKK) complex,
composed of two catalytic subunits (IKKα and IKKβ) and one
regulatory subunit [NF-κB essential modulator (NEMO)], is
activated, allowing the phosphorylation of the IκBα inhibitory
protein. A linear form of polyubiquitin chains was previously
identified in the NF-κB signaling pathway following TNF stimulation
(13). The generation of linear
ubiquitin polymers is catalyzed by LUBAC. Previous evidence
indicates that LUBAC is recruited to TNF receptor complexes upon
TNF induction, and then conjugates linear ubiquitin chains to the
regulatory subunit NEMO of the IKK complex (14). This activates the kinase activity of
IKK and ubiquitin-dependent degradation of phosphorylated IκBα,
therefore enabling the nuclear translocation of NF-κB dimers and
downstream gene expression (15).
SHARPIN contains a PH (pleckstrin homology) domain at the
N-terminus, which serves as a dimerization domain and may serve a
role in other physiological functions of SHARPIN, including its
tumor-associated role and its ability to inhibit β1-integrin
activation (16). Furthermore,
SHARPIN has been identified as a commonly overexpressed
proto-oncogene and functionally serves tumor-associated roles
during cancer progression according to previous studies (17–23).
However, data regarding the function of SHARPIN in the pathogenesis
and development of NMSC is lacking. These background data prompted
the present study to investigate the expression and mutations of
SHARPIN in skin tumors and identify a promising prognostic
biomarker and therapeutic target for NMSC. Immunohistochemistry was
utilized in the current study to assess SHARPIN expression in NMSCs
and polymerase chain reaction (PCR) was used to detect mutations of
SHARPIN in NMSCs. It was revealed that the expression of
SHARPIN was absent in cancer nests and was significantly low in
precancerous NMSC lesions. The total mutation frequency of SHARPIN
was 21.8% in BCC and 17.0% in SCC.
Materials and methods
Literature retrieval
To acquire all literature regarding SHARPIN and
NMSCs, PubMed (https://www.ncbi.nlm.nih.gov/pubmed) was searched
using the following search string to identify relevant papers:
(NMSC) OR non-melanoma skin cancer AND SHARPIN. No restrictions on
publication date or language were imposed during the search
strategy. No articles were identified.
Specimen selection
Anonymized control DNA samples from blood specimens
of 100 normal individuals and skin tissues from 12 healthy
volunteers who received cosmetic surgeries were obtained according
to a protocol approved by the Southern Medical University Shenzhen
Hospital Subject Review Board. All 100 normal individuals and 12
healthy volunteers did not have skin diseases. Formalin-fixed
paraffin-embedded (FFPE) samples were retrieved from the Department
of Dermatology of Shenzhen Hospital in Southern Medical University
(Shenzhen, China). All samples from January 2012 to June 2017 were
biopsied. All samples were fixed for 24 h in 10% formalin solution
at room temperature. The thickness of the sections was 4 µm. A
total of 85 BCC, 77 SCC and 21 keratoacanthoma (KA) FFPE samples
were collected. The diagnoses of the samples were confirmed by
pathologists from the Department of Dermatology of Shenzhen
Hospital in Southern Medical University. Informed consent was
obtained from all patients.
DNA extraction and mutation
sequencing
DNA was extracted from the blood using the
phenol-chloroform method (24). The
FFPE genomic DNA was extracted using a QIAamp DNA FFPE Tissue kit
(Qiagen GmbH, Hilden, Germany). To detect hotspot mutations, 8
exons and exon-intron adjacent sequences of the SHARPIN gene were
amplified using PCR. In the DNA from the tumor samples, each
amplification reaction was performed under standard conditions in a
20 µl PCR mixture containing 70–150 ng template DNA, 10 pmol
primers, and 10 µl 2X Taq Master Mix (Dye Plus) (Vazyme,
Piscataway, NJ, USA). The GC percentage of Exon 1 was relatively
high; therefore, the 2X Taq Master Mix (Dye Plus) was replaced by
2X Phanta Max Master Mix (Vazyme) in the amplification of Exon 1.
The 8 primer pairs that were used are listed in Table I. Exon 3 was amplified by PCR. The
thermocycler conditions for the standard and nested PCR protocols
are listed in Table II. PCR
products were purified using QIAquick reagent (Qiagen GmbH) and
directly sequenced based on the Big Dye Terminator sequencing
chemistry (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA USA) in an ABI3130 automated sequencer (Applied
Biosystems; Thermo Fisher Scientific, Inc.). All mutations were
confirmed through repeated bidirectional sequencing on the ABI
sequencer. Gene sequences were blasted using DNASTAR Lasergene 7.1
(DNASTAR Inc., Madison, WI, USA).
 | Table I.Primers used in the screening of Src
homology 3 and multiple ankyrin repeat domains protein-associated
RH domain-interacting protein gene mutations. |
Table I.
Primers used in the screening of Src
homology 3 and multiple ankyrin repeat domains protein-associated
RH domain-interacting protein gene mutations.
| Exon | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| 1 |
CAGGTTCGCGGCCCGTGTTT |
AAGAGGACTGACCGCGCGCC |
| 2 |
ATTTCTTTGCTCCTCGTGCG |
CTTCCCAGACATCCAGCAGT |
| 3 |
CAGCACAGCACACCCATATC |
GGGACTATCTGCTATCCCCG |
| 4 |
AGCAGATAGTCCCCAGTGGT |
GTGGGTTCAGGGATGGATGG |
| 5 |
CATCAGGTGAGGCCTGGG |
CCGAGCTCTGAGAACACCTG |
| 6 |
ATCACCTGCCCTGATGCTC |
GTGGAGCTCAGGACTGTGG |
| 7 |
CACAGTCCTGAGCTCCACC |
GTTGCTTCCCTGCTCTTTCC |
| 8 |
CAGGGAAGCAACAACTGTCT |
ATTCCTGTGGATTCTGCCCT |
 | Table II.PCR amplification thermocycler
conditions of Src homology 3 and multiple ankyrin repeat domains
protein-associated RH domain-interacting protein gene. |
Table II.
PCR amplification thermocycler
conditions of Src homology 3 and multiple ankyrin repeat domains
protein-associated RH domain-interacting protein gene.
|
| Touchdown PCR | Ordinary PCR |
|---|
|
|
|
|
|---|
| Steps | Temperature,
°C | Duration | Steps | Temperature,
°C | Duration |
|---|
|
1 | 94 | 5 min | 1 | 94 | 5 min |
|
2 | 94 | 30 sec | 2 | 94 | 30 sec |
|
3 | 63, EXa −0.5 | 30 sec | 3 | 60/56/57b | 30 sec |
|
4 | 72 | 20 sec | 4 | 72 | 20 sec |
|
5 | Back to step 2 | 16 times | 5 | Back to step 2 | 35 times |
|
6 | 94 | 30 sec | 6 | 72 | 7 min |
|
7 | 54 | 30 sec | 7 | 4 | Until use |
|
8 | 72 | 20 sec | – | – | – |
|
9 | Back to step 5 | 20 times | – | – | – |
| 10 | 72 | 7 min | – | – | – |
| 11 | 4 | Until use | – | – | – |
Immunohistochemistry
FFPE sections were deparaffinized in xylene at room
temperature and rehydrated in 100, 95, 90, 80 and 70% alcohol
solutions prepared with absolute ethyl alcohol and distilled water.
For antigen retrieval, sections were heated in citrate buffer (pH
6.0) for 15 min at 100°C in a microwave oven and naturally cooled
to room temperature. Subsequently, the samples were blocked with a
mixture of methanol and 0.75% hydrogen peroxide for 20 min at room
temperature. Following washing with PBS, samples were incubated
with SHARPIN antibody (cat. no., sc-98127; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; dilution, 1:100) at 4°C
overnight. Subsequent to incubation, slides were washed three times
with PBS. The slides were then processed using a 2-step
Plus® Poly-horseradish peroxidase Anti-Mouse/Rabbit IgG
Detection System (cat. no., PV-9000; ZSGB-BIO; OriGene
Technologies, Inc., Beijing, China) and were developed with a DAB
Detection kit (Enhanced Polymer; cat. no., PV-9000-D; ZSGB-BIO;
OriGene Technologies, Inc.) for 3 min at room temperature. SHARPIN
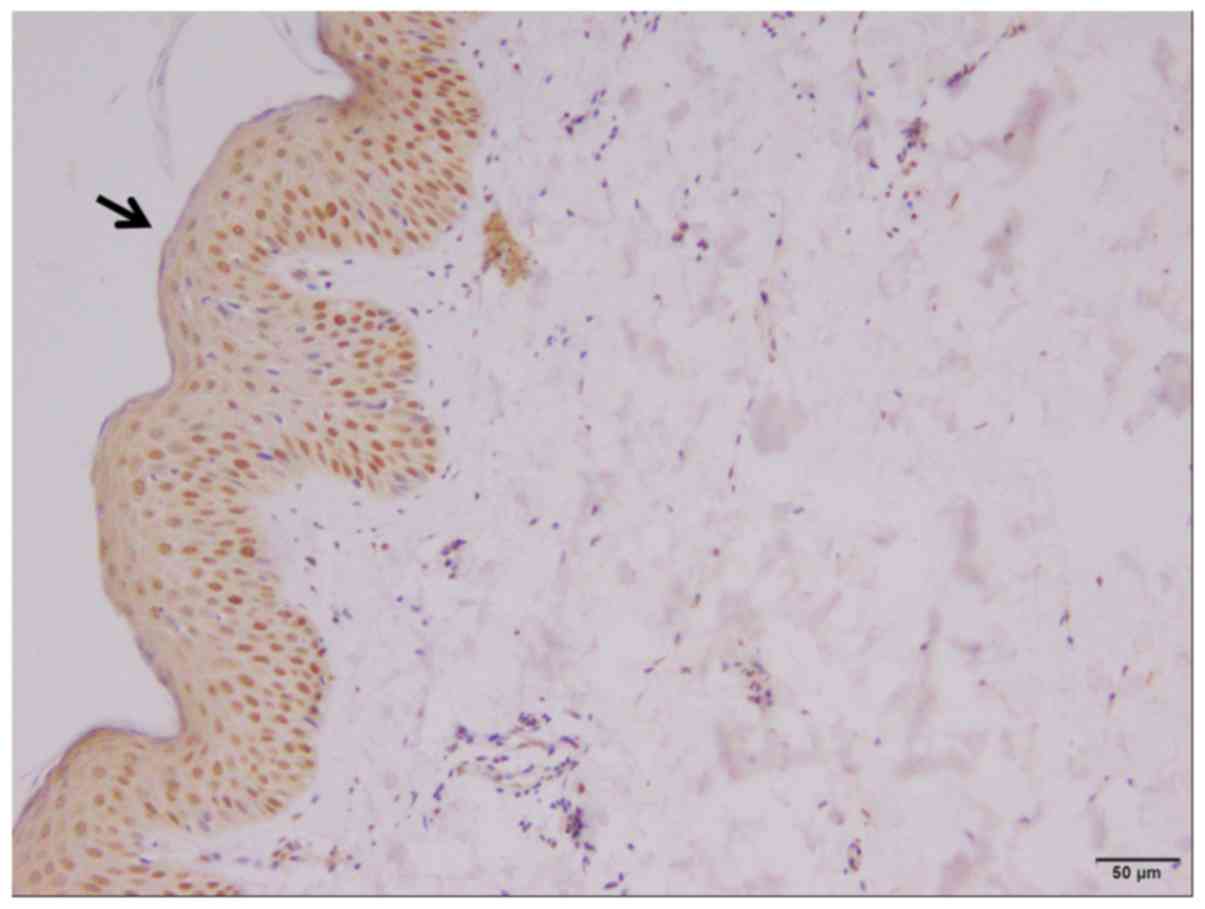
immunohistochemical staining was expected to be localized to the
cytoplasm.
Histologic scoring and analysis
Samples were evaluated using a standard light
microscopic technique (magnification, ×200) as performed by two
pathologists (Shenzhen Hospital in Southern Medical University).
Staining for the SHARPIN protein was evaluated in the tumors and in
the normal skin tissues, which were invariably SHARPIN-positive and
served as positive controls. Each tumor sample was scored by the
cross-product (H score) of the percentage of tumor cell staining at
each of the 3 staining intensities. Degrees of staining were
divided into four levels: None, 0; weak, 1; moderate, 2; and
strong, 3. For example, a particular tumor may have 30% cell
staining at intensity =1 and 70% of cell staining at intensity =3,
for a combined H score of 240 [(30×1) + (70×3) =240] out of a
maximum of 300. This system was performed as described previously
by Bollag et al (25).
Concordance was observed between the scores given by the two
pathologists (81% of the scores were in agreement within a 40-point
range). Cases with discrepancies of <50 points were recorded and
reconciled on a two-headed microscope. Final H scores for each case
were averaged by each pathologist. The expression scale of SHARPIN
was graded by H score as follows: Low, H score 1–100; moderate, H
score 101–200; and high, H score 201–300.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). Data were presented as the mean ±
standard deviation. Differences in SHARPIN expression levels
between normal skin and SCC, BCC and KA samples were analyzed using
one-way analysis of variance and Tamhane's T2 post hoc test. The
Broder grading system of SCC is commonly utilized to assess
prognosis. It divides SCC into four categories based on
histological grade. Grade I is composed of well-differentiated
tumors, in which 75–100% of squamous cells are differentiated.
Grade II is composed of moderately differentiated tumors in which
50–75% of squamous cells are differentiated. Grade III is composed
of poorly differentiated tumors in which only 25–50% of cells are
differentiated. Grade IV is an anaplastic tumor in which 0–25% of
cells are differentiated (26). Main
histologic variants of BCC include nodular type, adenoidal type,
superficial type and sclerosing type (27). Associations between SHARPIN
expression levels and aforementioned clinicopathological parameters
were analyzed using the χ2 test for categorical variables.
P<0.05 was considered to indicate a statistically significant
difference.
Results
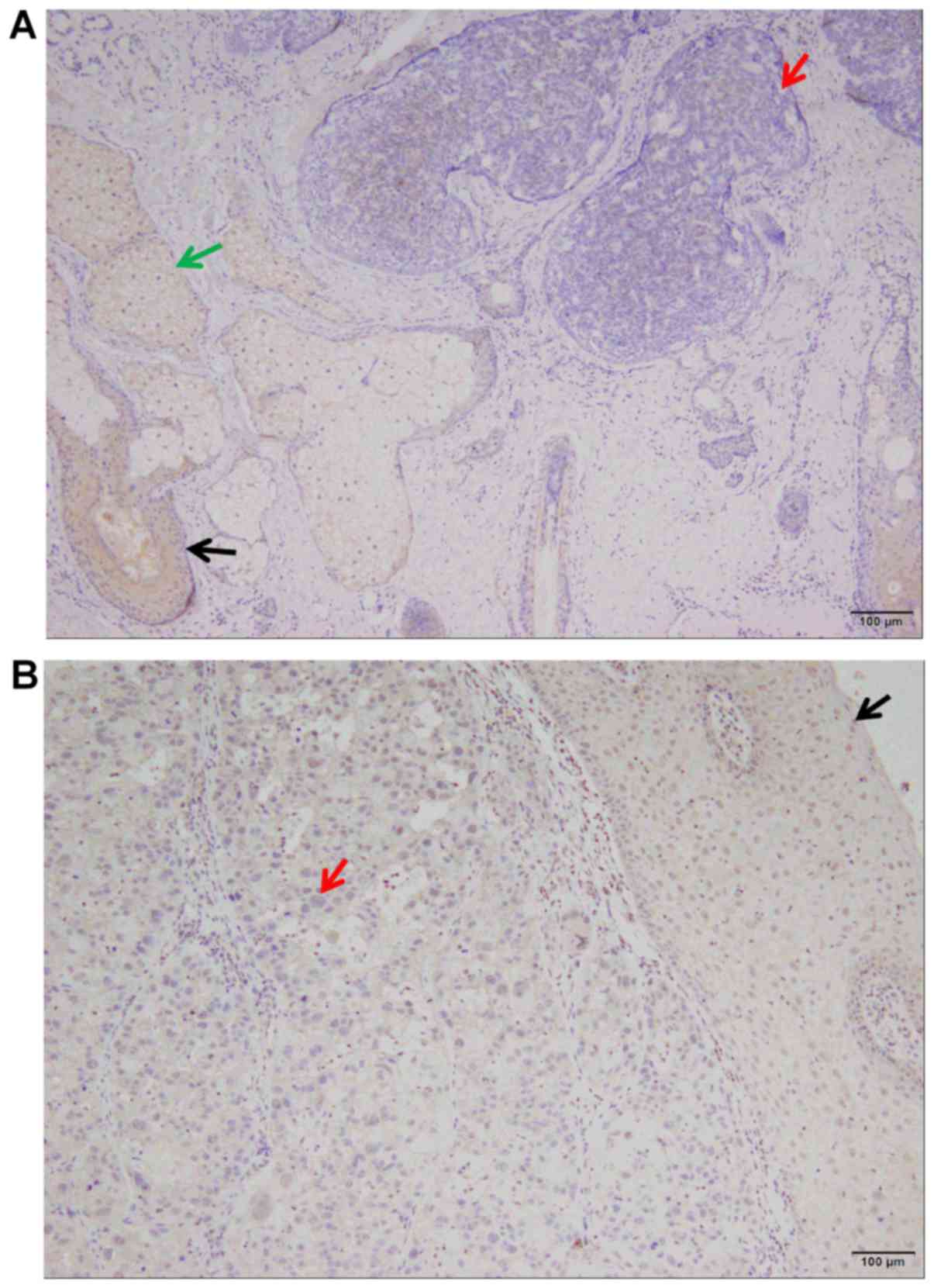
SHARPIN is aberrantly decreased in
human NMSC
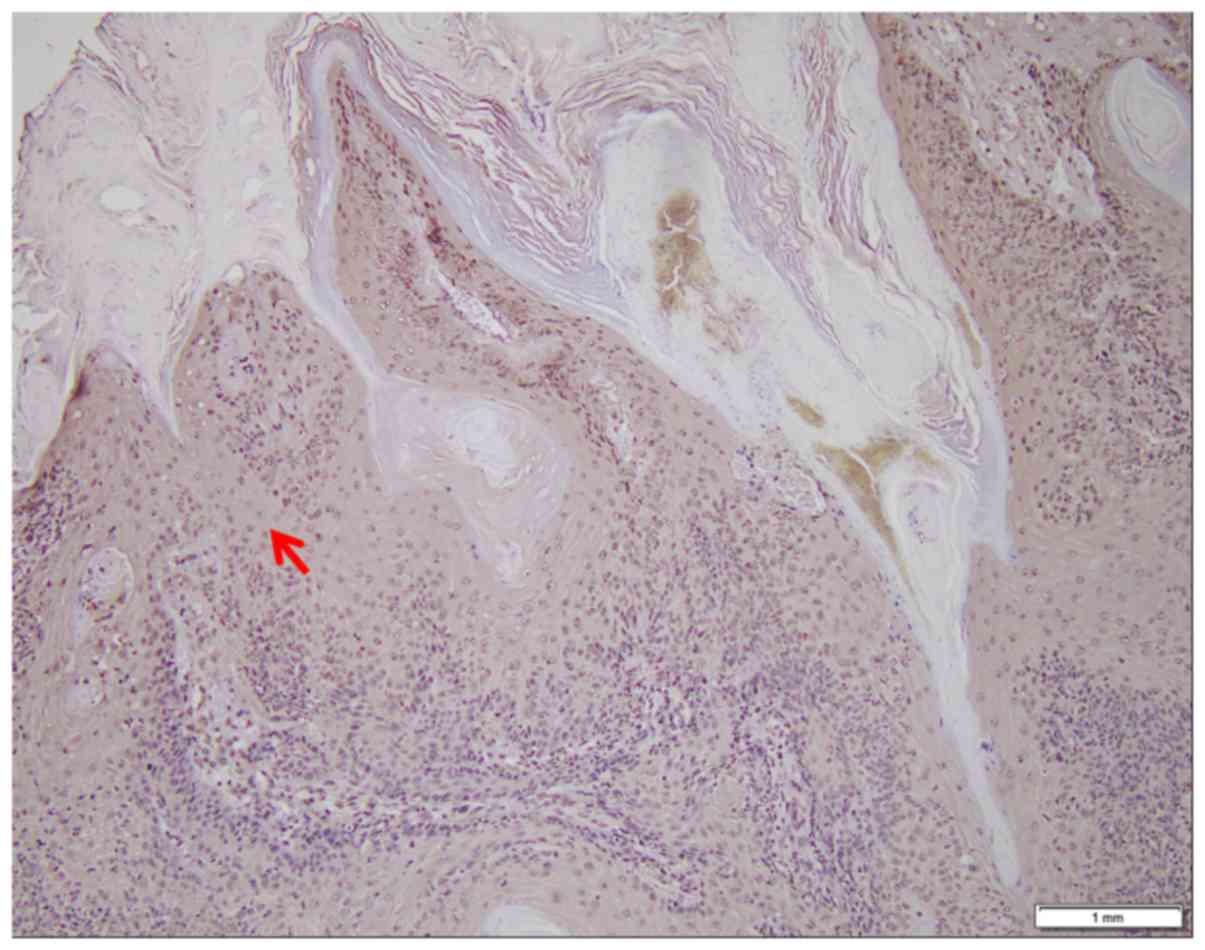
The SHARPIN protein was absent in the tumor nests
and significantly decreased in precancerous lesions of SCC and BCC
(Fig. 1) when compared to normal
epithelium (Fig. 2). In addition,
SHARPIN was moderately to highly expressed in KA samples (Fig. 3).
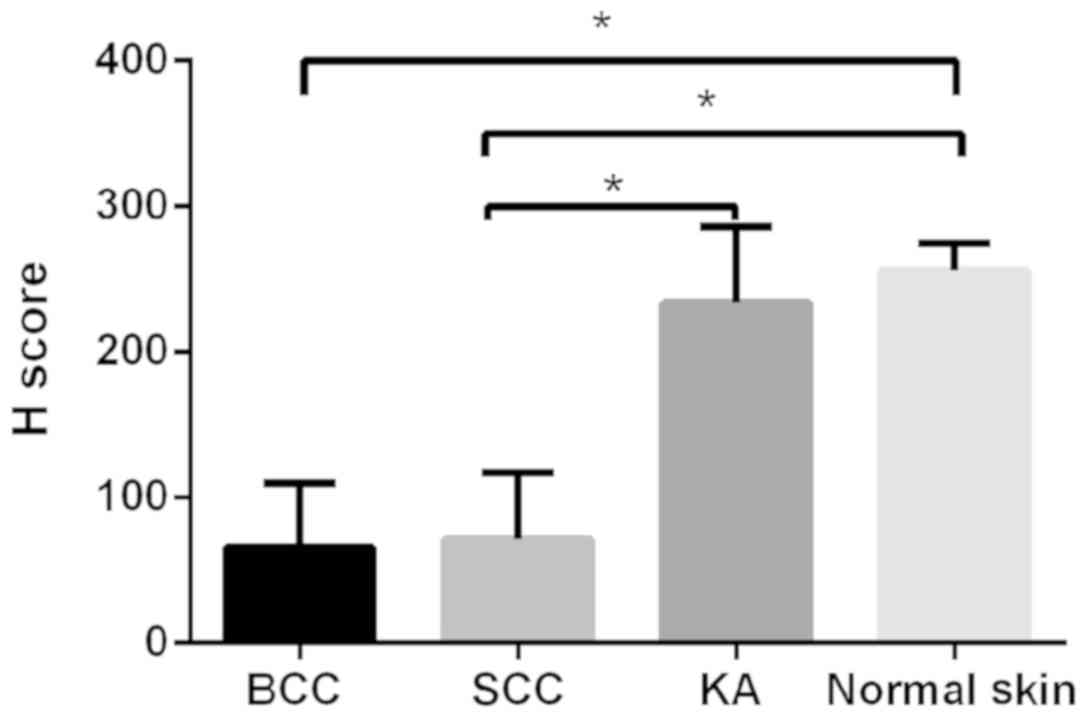
In BCC, SHARPIN expression was low in 63 cases
(74.5%) and moderate in 22 cases (25.5%). In SCC, SHARPIN
expression was low in 52 cases (68.1%) and moderate in 25 cases
(31.9%). Furthermore, the difference in SHARPIN expression levels
between BCC and normal skin, SCC and normal skin, and SCC and KA
were all significant (P<0.05) (Fig.
4). However, no significant association was observed between
SHARPIN expression and tumor grading of SCC. Demographics of all
the patients and their H scores are summarized in Tables III–VI.
 | Table III.Demographics and H scores of patients
with basal cell carcinoma. |
Table III.
Demographics and H scores of patients
with basal cell carcinoma.
| ID | Sex | Age, y | Location | Type | H score |
|---|
| B01 | M | 74 | Nose | adenoidal type | 10 |
| B02 | F | 52 | Right ear | adenoidal type | 10 |
| B03 | M | 72 | Trunk | superficial
type | 10 |
| B04 | F | 70 | Right hand | superficial
type | 10 |
| B05 | M | 68 | Right shoulder | superficial
type | 10 |
| B06 | M | 62 | Upper lip | nodular type | 10 |
| B07 | M | 63 | Nose | adenoidal type | 10 |
| B08 | M | 44 | Lower lip | sclerosing
type | 10 |
| B09 | F | 82 | Lower lip | sclerosing
type | 10 |
| B10 | F | 45 | Head | adenoidal type | 10 |
| B11 | F | 70 | Nose | adenoidal type | 15 |
| B12 | F | 63 | Right hip | adenoidal type | 20 |
| B13 | F | 49 | Right thigh | sclerosing
type | 20 |
| B14 | F | 73 | Left forearm | superficial
type | 20 |
| B15 | M | 69 | Upper lip | nodular type | 20 |
| B16 | M | 69 | Nose | adenoidal type | 20 |
| B17 | M | 65 | Left hand | sclerosing
type | 20 |
| B18 | M | 67 | Nose | adenoidal type | 20 |
| B19 | F | 47 | Nose | adenoidal type | 20 |
| B20 | M | 65 | Neck | superficial
type | 20 |
| B21 | F | 73 | Head | nodular type | 25 |
| B22 | M | 22 | Nose | nodular type | 30 |
| B23 | M | 58 | Lower lip | adenoidal type | 30 |
| B24 | F | 58 | Nose | superficial
type | 30 |
| B25 | F | 57 | Left cheek | adenoidal type | 35 |
| B26 | M | 59 | Upper lip | adenoidal type | 35 |
| B27 | F | 68 | Right tempus | superficial
type | 40 |
| B28 | F | 80 | Upper lip | pigmented type | 40 |
| B29 | F | 95 | Back | nodular type | 40 |
| B30 | M | 78 | Back | nodular type | 40 |
| B31 | F | 65 | Left tempus | nodular type | 40 |
| B32 | F | 76 | Back | pigmented type | 40 |
| B33 | M | 64 | Left leg | superficial
type | 40 |
| B34 | M | 43 | Head | sclerosing
type | 45 |
| B35 | F | 41 | Left forehead | pigmented type | 50 |
| B36 | F | 68 | Chest | pigmented type | 50 |
| B37 | F | 83 | Right hand | nodular type | 50 |
| B38 | M | 89 | Nose | superficial
type | 50 |
| B39 | F | 75 | Left hand | pigmented type | 50 |
| B40 | F | 75 | Lower lip | superficial
type | 55 |
| B41 | M | 63 | Nose | sclerosing
type | 55 |
| B42 | M | 45 | Upper lip | nodular type | 60 |
| B43 | F | 64 | Left thigh | adenoidal type | 60 |
| B44 | M | 50 | Back | nodular type | 60 |
| B45 | M | 59 | Lower lip | nodular type | 60 |
| B46 | M | 46 | Upper lip | adenoidal type | 70 |
| B47 | M | 81 | Head | nodular type | 70 |
| B48 | F | 60 | Lower lip | nodular type | 70 |
| B49 | M | 58 | Left thigh | adenoidal type | 70 |
| B50 | F | 43 | Anus | nodular type | 70 |
| B51 | F | 78 | Left thigh | nodular type | 75 |
| B52 | F | 61 | Nose | nodular type | 80 |
| B53 | M | 56 | Nose | nodular type | 80 |
| B54 | M | 53 | Left thigh | nodular type | 80 |
| B55 | F | 45 | Right cheek | pigmented type | 80 |
| B56 | F | 29 | Left check | superficial
type | 80 |
| B57 | F | 44 | Left thigh | adenoidal type | 80 |
| B58 | F | 58 | Left thigh | nodular type | 80 |
| B59 | F | 73 | Back | nodular type | 85 |
| B60 | M | 58 | Nose | superficial
type | 90 |
| B61 | F | 73 | Head | nodular type | 90 |
| B62 | M | 73 | Left cheek | sclerosing
type | 90 |
| B63 | F | 57 | Nose | nodular type | 90 |
| B64 | F | 34 | Lower lip | superficial
type | 100 |
| B65 | F | 41 | Nose | nodular type | 100 |
| B66 | F | 34 | Lower lip | superficial
type | 100 |
| B67 | M | 73 | Lower lip | nodular type | 100 |
| B68 | M | 82 | Nose | nodular type | 100 |
| B69 | M | 63 | Back | nodular type | 100 |
| B70 | F | 38 | Upper lip | superficial
type | 105 |
| B71 | F | 53 | Right tempus | nodular type | 105 |
| B72 | F | 64 | Back | nodular type | 110 |
| B73 | F | 53 | Lower lip | nodular type | 120 |
| B74 | F | 63 | Back | nodular type | 120 |
| B75 | M | 35 | Upper lip | superficial
type | 120 |
| B76 | F | 44 | Nose | superficial
type | 130 |
| B77 | F | 60 | Right tempus | nodular type | 135 |
| B78 | F | 70 | Nose | superficial
type | 140 |
| B79 | M | 37 | Nose | nodular type | 140 |
| B80 | F | 36 | Nose | nodular type | 140 |
| B81 | F | 64 | Right tempus | adenoidal type | 150 |
| B82 | M | 56 | Lower lip | sclerosing
type | 150 |
| B83 | M | 73 | Back | superficial
type | 160 |
| B84 | F | 47 | Right hand | nodular type | 160 |
| B85 | M | 58 | Lower lip | nodular type | 190 |
 | Table VI.Demographics and H scores of negative
control patients. |
Table VI.
Demographics and H scores of negative
control patients.
| ID | Sex | Age, y | H score |
|---|
| N01 | M | 30 | 220 |
| N02 | F | 22 | 260 |
| N03 | F | 57 | 260 |
| N04 | F | 48 | 275 |
| N05 | M | 32 | 280 |
| N06 | F | 50 | 240 |
| N07 | M | 40 | 280 |
| N08 | M | 18 | 245 |
| N09 | M | 39 | 245 |
| N10 | F | 28 | 250 |
| N11 | F | 69 | 270 |
| N12 | M | 55 | 250 |
SHARPIN mutation analysis
A total of 8 exons and exon-intron adjacent
sequences of SHARPIN were analyzed using DNA extracts from FFPE
blocks of BCC, SCC and KA samples and healthy skin specimens, and
DNA extracts from peripheral blood samples of 100 normal controls.
Complete descriptions of the mutations detected in BCC and SCC are
presented in Table VII. Total
mutation rates were 21.8% in BCC and 17.0% in SCC samples. The
C>T substitutions were 5.5% in BCC and 6.4% in SCC.
Additionally, no mutations of SHARPIN were detected in DNA extracts
from one benign skin tumor, 12 healthy skin tissues and blood
samples from 100 normal individuals.
 | Table VII.Distribution of Src homology 3 and
multiple ankyrin repeat domains protein-associated RH
domain-interacting protein gene mutations in patients with BCC and
SCC. |
Table VII.
Distribution of Src homology 3 and
multiple ankyrin repeat domains protein-associated RH
domain-interacting protein gene mutations in patients with BCC and
SCC.
| Tumor type | Exon | Mutation | Modified
protein | Frequency | Domain |
|---|
| BCC | E1 | c.10 C>T | p.Pro4Leu | 1/55 | – |
|
| – | c.68 C>T | p.Ala23Val | 1/55 | – |
|
| – | c.146 A>G | p.Asp49Gly | 1/55 | – |
|
| E2 | c.329 T>C | p.Gln110Arg | 1/55 | PH |
|
| E5 | c.733 C>A | p.His245Thr | 1/55 | UBL |
|
| E7 | c.937 C>T | p.Pro313Ser | 1/55 | – |
|
| – | c.944 A>G | p.His315Arg | 1/55 | – |
|
| – | c.992 T>C | p.Leu332Ser | 3/55 | – |
|
| E8 | c.1109 T>C | p.Met370Thr | 1/55 | NZF |
|
| – | c.1137 G>A | p.Trp379Gln | 1/55 | – |
| SCC | E1 | c.53 C>A | p.Ala18Asp | 1/47 | – |
|
| E2 | c.214 T>C | p.Trp72Arg | 1/47 | PH |
|
| E3 | c.421 C>T | p.Pro141Ser | 1/47 | – |
|
| – | c.466 C>T | p.Pro156Ser | 1/47 | – |
|
| – | c.469 C>T | p.Pro157Ser | 1/47 | – |
|
| – | c.478 G>A | p.Ala160Thr | 1/47 | – |
|
| E5 | c.709 T>C | p.Ser237Pro | 1/47 | – |
|
| E8 | c.1007 G>T | p.Gly336Val | 1/47 | – |
Discussion
The present study evaluated the expression of
SHARPIN protein and analyzed the sequences of SHARPIN in
NMSC. To the best of our knowledge, this is the first study to
comprehensively investigate the expression and mutations of SHARPIN
in a large series of patients with NMSC.
The essential contribution of SHARPIN to the
activation of NF-κB supports the possibility that SHARPIN promotes
tumorigenesis, as NF-κB signaling possesses well-demonstrated
tumorigenic properties (12). This
is supported by the SHARPIN-mediated suppression of apoptosis in
the keratinocytes and hepatocytes of cpdm mice (18). Additionally, SHARPIN promotes the
migration of Chinese hamster ovary cells in vitro and
lymphocytes in vivo, and increases the lung metastasis of
osteosarcoma in vivo in immunocompromised mice (19). In addition, the upregulation of
SHARPIN has been observed in different types of internal
solid cancer, including ovarian cancer, renal cell carcinoma, and
cervical and prostate cancer (20,21).
Furthermore, SHARPIN induces PTEN polyubiquitination independently
of the K48 linkage. This process requires the UBL domain, which
mediates SHARPIN's association with PTEN and its ability to bind
ubiquitin via the NZF motif (28).
Rantala et al (16)
demonstrated that SHARPIN inactivates integrins in a number of
different cell types and affects integrin-dependent cellular
functions. Bii et al (22)
identified SHARPIN as a metastasis gene in breast cancer using a
replication-incompetent gammaretroviral vector, suggesting the
potential of SHARPIN as a biomarker for stratifying patients with
breast cancer. Additionally, Haris et al (23) identified that SHARPIN was
significantly upregulated in U87 glioblastoma cells upon treatment
with Aloe-emodin. Collectively, substantial evidence has
demonstrated the role of SHARPIN in promoting tumorigenesis.
Despite these data, a PubMed search did not identify any studies
examining the expression of SHARPIN in NMSC. Therefore, the present
study explored the expression of SHARPIN in three types of
skin tumors, including the malignant forms BCC and SCC.
Firstly, the expression of SHARPIN was detected via
immunohistochemistry. Contrary to the results of examination of
internal solid tumors (17), SHARPIN
expression was downregulated or absent in the majority of NMSC
samples compared with normal skin tissues and KA. KA is commonly
diagnosed clinically as it rapidly appears and develops as a raised
lesion; however, as a non-pigmented lesion with a central keratin
plug, SCC may also exhibit the same appearance. Furthermore, cases
of KA with SCC arising from the base have been identified (29). Differential diagnosis between KA and
SCC is challenging due to their similarities and the lack of
reliable diagnostic criteria to distinguish them. Therefore,
whether KA is a separate benign entity, or a variant of SCC, is
controversial. At present, no biomarkers exist to distinguish SCC
from KA, and KA lesions are commonly treated in the same way as
SCC. However, SCC has a poorer prognosis than KA and is treated
more aggressively; therefore, distinguishing between these two
malignancies would be advantageous in order to implement the
appropriate treatment, thereby decreasing unnecessary surgeries,
the burden on the healthcare system and, importantly, the anxiety
of the patients (30). Based on the
results of the present study, that SHARPIN is absent or exhibits
low expression in SCC but a high expression in KA, we hypothesize
that SHARPIN may allow early differentiation and in situ
treatment of SCC and KA to avoid metastasis and tissue destruction
of SCC and the overtreatment of KA.
At present, the mechanism of how the downregulation
of SHARPIN promotes skin tumorigenesis remains to be elucidated.
Ikeda et al (11) identified
that the absence of SHARPIN in cpdm mice caused
dysregulation of NF-κB and increased apoptosis and necrosis of
mouse embryonic fibroblasts. The data from the present study
suggested that the downregulation of SHARPIN in NMSC may impair the
function of LUBAC, and subsequently, the activation of NF-κB. In
the majority of tumors, the aberrant activation of NF-κB signaling
stimulates tumor cell proliferation, invasion and metastasis
(31). Counterintuitively,
Hogerlinden et al (32)
demonstrated that selective inhibition of Rel/NF-κB signaling in
the skin leads to disturbed epidermal homeostasis and hair follicle
development, increased frequency of apoptotic keratinocytes and
spontaneous development of SCC. Notably, a number of data have
challenged the view that apoptotic signaling solely serves to
inhibit cancer, arguing instead that apoptosis is responsible for
various effects that may be tumor-promoting (33–36).
Apoptotic cell death is a cell-autonomous event, but its effects
are not; dying cells affect their surrounding environments in
various ways, which may include stimulating the proliferation of
neighboring cells, affecting intra-tumoral cell competition and
exerting paracrine effects on tumor microenvironments. Various data
support the hypothesis that apoptosis may promote tumorigenesis
through the recruitment and activation of phagocytic macrophages at
the tumor sites (37). Taken
together, we hypothesized that decreased SHARPIN expression may
promote NMSC through the impaired activation of NF-κB and increased
apoptosis and necrosis of epidermal cells, which may disrupt the
homeostasis of the epidermis and lead to tumorigenesis.
Traditional Sanger sequencing has been the gold
standard for identifying mutations for a number of years due to its
low false-positive rate and high specificity (21). Therefore, in the present study, DNA
was extracted from NMSC FFPE blocks and mutations in the exons of
SHARPIN were detected. The results indicated that high
proportions of BCC and SCC contained mutations of the
SHARPIN gene. Mutations in SHARPIN exons were
identified in 21.8% of BCC and 17.0% of SCC in the present study.
The proportions of C>T substitutions were 5.5% in BCC and 6.4%
in SCC samples, which were identified as characteristic of
mutations associated with exposure to UV exposure (38). In addition, the mutations were not
only located in the UBL domain of SHARPIN but also in the PH and
NZF domains, thereby potentially affecting other functions of
SHARPIN besides the formation of LUBAC. Furthermore, SHARPIN has
been indicated to inactivate integrins in a number of cell types
and affect integrin-dependent cellular functions independent of
LUBAC (16). Approximately one-half
of the cellular SHARPIN is not associated with the LUBAC complex
(28). Therefore, the present study
concluded that SHARPIN is a multifunctional molecule and may
promote the pathogenesis of NMSC through different mechanisms.
Overall, the present study contributes to a growing
body of evidence supporting the importance of SHARPIN in
NMSC. The results suggest an association between NMSC and low to
absent SHARPIN expression and SHARPIN mutations.
It was identified that SHARPIN protein expression
was absent in cancer nests and significantly decreased in
precancerous lesions of SCC and BCC, but was high in normal skin or
in KA. The total mutation rates of SHARPIN were 21.8% in BCC
and 17.0% in SCC. These data indicated that SHARPIN may serve a
tumor-suppressing role and act as a promising diagnostic biomarker
in NMSC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no., 81371724) to Dr
Yanhua Liang.
Availability of data and material
The datasets used and/or analyzed during the present
study are available from the corresponding authors on reasonable
request.
Authors' contributions
YL designed and supervised the study. YZ performed
the histological examination of the samples and prepared the draft.
YY performed the DNA extraction, polymerase chain reaction and
sequencing. JW conducted data analysis and interpreted the
data.
Ethics approval and consent to
participate
Blood specimens of 100 normal individuals and skin
tissues from 12 healthy volunteers who received cosmetic surgeries,
and formalin-fixed paraffin-embedded (FFPE) samples from the
Department of Dermatology of Shenzhen Hospital in Southern Medical
University (Shenzhen, China), were obtained according to a protocol
approved by the Southern Medical University Shenzhen Hospital
Subject Review Board (2016–016). All samples were fixed for 24 h in
10% formalin solution at room temperature. The thickness of the
sections was 4 µm. Informed consent was obtained from all
participants.
Patient consent for publication
Consent was gained from the participants for the
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rodust PM, Stockfleth E, Ulrich C,
Leverkus M and Eberle J: UV-induced squamous cell carcinoma-a role
for antiapoptotic signalling pathways. Br J Dermatol. 161 (Suppl
3):S107–S115. 2009. View Article : Google Scholar
|
|
2
|
Molho-Pessach V and Lotem M: Ultraviolet
radiation and cutaneous carcinogenesis. Curr Probl Dermatol.
35:14–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Villiers EM, Ruhland A and Sekarić P:
Human papillomaviruses in non-melanoma skin cancer. Semin Cancer
Biol. 9:413–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McNaughton SA, Marks GC and Green AC: Role
of dietary factors in the development of basal cell cancer and
squamous cell cancer of the skin. Cancer Epidemiol Biomarkers Prev.
14:1596–1607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramsay HM, Fryer AA, Reece S, Smith AG and
Harden PN: Clinical risk factors associated with nonmelanoma skin
cancer in renal transplant recipients. Am J Kidney Dis. 36:167–176.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giglia-Mari G and Sarasin A: TP53
mutations in human skin cancers. Hum Mutat. 21:217–228. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Madan V, Lear JT and Szeimies RM:
Non-melanoma skin cancer. Lancet. 375:673–685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisemann N, Jansen L, Castro FA, Chen T,
Eberle A, Nennecke A, Zeissig SR, Brenner H and Katalinic A; GEKID
Cancer Survival Working Group, : Survival with nonmelanoma skin
cancer in Germany. Br J Dermatol. 174:778–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stieglitz B, Haire LF, Dikic I and
Rittinger K: Structural analysis of SHARPIN, a subunit of a large
multi-protein E3 ubiquitin ligase, reveals a novel dimerization
function for the pleckstrin homology superfold. J Biol Chem.
287:20823–20829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seymour RE, Hasham MG, Cox GA, Shultz LD,
Hogenesch H, Roopenian DC and Sundberg JP: Spontaneous mutations in
the mouse Sharpin gene result in multiorgan inflammation, immune
system dysregulation and dermatitis. Genes Immun. 8:416–421. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikeda F, Deribe YL, Skånland SS, Stieglitz
B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V,
Terzic J, et al: SHARPIN forms a linear ubiquitin ligase complex
regulating NF-κB activity and apoptosis. Nature. 471:637–641. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tokunaga F, Nakagawa T, Nakahara M, Saeki
Y, Taniguchi M, Sakata S, Tanaka K, Nakano H and Iwai K: SHARPIN is
a component of the NF-κB-activating linear ubiquitin chain assembly
complex. Nature. 471:633–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haas AL: Linear polyubiquitylation: The
missing link in NF-kappaB signalling. Nat Cell Biol. 11:116–118.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tokunaga F, Sakata S, Saeki Y, Satomi Y,
Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, et
al: Involvement of linear polyubiquitylation of NEMO in NF-kappaB
activation. Nat Cell Biol. 11:123–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Potter CS, Sundberg JP and
Hogenesch H: SHARPIN is a key regulator of immune and inflammatory
responses. J Cell Mol Med. 16:2271–2279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rantala JK, Pouwels J, Pellinen T, Veltel
S, Laasola P, Mattila E, Potter CS, Duffy T, Sundberg JP,
Kallioniemi O, et al: SHARPIN is an endogenous inhibitor of
β1-integrin activation. Nat Cell Biol. 13:1315–1324. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung J, Kim JM, Park B, Cheon Y, Lee B,
Choo SH, Koh SS and Lee S: Newly identified tumor-associated role
of human Sharpin. Mol Cell Biochem. 340:161–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sieber S, Lange N, Kollmorgen G, Erhardt
A, Quaas A, Gontarewicz A, Sass G, Tiegs G and Kreienkamp HJ:
Sharpin contributes to TNFα dependent NFκB activation and
anti-apoptotic signalling in hepatocytes. PLoS One. 7:e299932012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Melo J and Tang D: Elevation of SIPL1
(SHARPIN) increases breast cancer risk. PLoS One. 10:e01275462015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Huang H, Zhou H, Du T, Zeng L,
Cao Y, Chen J, Lai Y, Li J, Wang G, et al: Activation of nuclear
factor κB pathway and downstream targets survivin and livin by
SHARPIN contributes to the progression and metastasis of prostate
cancer. Cancer. 120:3208–3218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Q, Li M, Ma L, Li W, Wu X, Richards J,
Fu G, Xu W, Bythwood T, Li X, et al: A method to evaluate
genome-wide methylation in archival formalin-fixed,
paraffin-embedded ovarian epithelial cells. PLoS One.
9:e1044812014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bii VM, Rae DT and Trobridge GD: A novel
gammaretroviral shuttle vector insertional mutagenesis screen
identifies SHARPIN as a breast cancer metastasis gene and
prognostic biomarker. Oncotarget. 6:39507–39520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haris K, Ismail S, Idris Z, Abdullah JM
and Yusoff AA: Expression profile of genes modulated by Aloe emodin
in human U87 glioblastoma cells. Asian Pac J Cancer Prev.
15:4499–4505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghaheri M, Kahrizi D, Yari K, Babaie A,
Suthar RS and Kazemi E: A comparative evaluation of four DNA
extraction protocols from whole blood sample. Cell Mol Biol.
62:120–124. 2016.PubMed/NCBI
|
|
25
|
Bollag G, Hirth P, Tsai J, Zhang J,
Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, et al:
Clinical efficacy of a RAF inhibitor needs broad target blockade in
BRAF-mutant melanoma. Nature. 467:596–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kallini JR, Hamed N and Khachemoune A:
Squamous cell carcinoma of the skin: Epidemiology, classification,
management, and novel trends. Int J Dermatol. 54:130–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paolino G, Donati M, Didona D, Mercuri SR
and Cantisani C: Histology of non-melanoma skin cancers: An update.
Biomedicines. 5(pii): E712017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Melo J, Lin X, He L, Wei F, Major P and
Tang D: SIPL1-facilitated PTEN ubiquitination contributes to its
association with PTEN. Cell Signal. 26:2749–2756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weedon DD, Malo J, Brooks D and Williamson
R: Squamous cell carcinoma arising in keratoacanthoma: A neglected
phenomenon in the elderly. Am J Dermatopathol. 32:423–426. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vasiljević N, Andersson K, Bjelkenkrantz
K, Kjellström C, Månsson H, Nilsson E, Landberg G, Dillner J and
Forslund O: The Bcl-xL inhibitor of apoptosis is preferentially
expressed in cutaneous squamous cell carcinoma compared with that
in keratoacanthoma. Int J Cancer. 124:2361–2366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Hogerlinden M, Rozell BL,
Ahrlund-Richter L and Toftgård R: Squamous cell carcinomas and
increased apoptosis in skin with inhibited Rel/nuclear
factor-kappaB signaling. Cancer Res. 59:3299–3303. 1999.PubMed/NCBI
|
|
33
|
Michalak EM, Vandenberg CJ, Delbridge AR,
Wu L, Scott CL, Adams JM and Strasser A: Apoptosis-promoted
tumorigenesis: Gamma-irradiation-induced thymic lymphomagenesis
requires Puma-driven leukocyte death. Genes Dev. 24:1608–1613.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li F, Huang Q, Chen J, Peng Y, Roop DR,
Bedford JS and Li CY: Apoptotic cells activate the ‘phoenix rising’
pathway to promote wound healing and tissue regeneration. Sci
Signal. 3:ra132010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Q, Li F, Liu X, Li W, Shi W, Liu FF,
O'Sullivan B, He Z, Peng Y, Tan AC, et al: Caspase 3-mediated
stimulation of tumor cell repopulation during cancer radiotherapy.
Nat Med. 17:860–866. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiu W, Wang X, Leibowitz B, Yang W, Zhang
L and Yu J: PUMA-mediated apoptosis drives chemical
hepatocarcinogenesis in mice. Hepatology. 54:1249–1258. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ichim G and Tait SW: A fate worse than
death: Apoptosis as an oncogenic process. Nat Rev Cancer.
16:539–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Scott GA, Laughlin TS and Rothberg PG:
Mutations of the TERT promoter are common in basal cell carcinoma
and squamous cell carcinoma. Mod Pathol. 27:516–523. 2014.
View Article : Google Scholar : PubMed/NCBI
|