Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
leading histology-type malignancy in the upper aerodigestive tract
and is characterized by a marked propensity of invasion and
cervical lymph node metastasis associated with poor outcomes.
Despite advancements in multimodality therapy surgery, radiation,
chemotherapy, and recent progress in immunotherapy, HNSCC often
remains fatal, necessitating new and more efficacious therapeutic
strategies to enhance the HNSCC survival (1).
Epithelial-mesenchymal transition (EMT) is a
biological process that allows a polarized epithelial cell, which
normally interacts with basement membrane via its basal surface, to
undergo multiple biochemical changes that enable it to assume a
mesenchymal cell phenotype. These changes include enhanced
migratory capacity, invasiveness, increased resistance to
apoptosis, and a considerably increased production of extracellular
matrix components. Hence, EMT is considered a vital mechanism of
cancer progression, particularly in solid tumors. Different
biomarkers have been used to present EMT. The upregulation of
mesenchymal cell markers (e.g., fibronectin, vimentin, α-smooth
muscle actin, and N-cadherin) and the downregulation of epithelial
markers (e.g., E-cadherin, ZO-1, and α- and γ-catenin) are the
biomarkers often used in monitoring the progression of EMT in
individuals with progressive cancer (2).
Transforming growth factor-β (TGF-β) is a
pleiotropic cytokine involved in various activities, including
differentiation, growth, apoptosis, inflammation, tissue
remodeling, and wound healing (3).
TGF-β can initiate and maintain EMT via different biological
systems and pathophysiological contexts. TGF-β-derived signals can
coordinate the expression and function of Snail, ZEB, and bHLH
factors and promote their interplay in EMT and migration of
malignant tumor (4). Among the TGF-β
subtypes, TGF-β1 was first considered an EMT inducer in normal
mammary epithelial cells (5); since
then, it has been believed that TGF-β1 mediates EMT in cancer cells
and HNSCC (6). Therefore, in cancer,
the mechanisms of EMT have gradually been elucidated over the
years.
CD147, also termed as the extracellular matrix
metalloproteinase inducer (EMMPRIN), is a member of the
immunoglobulin superfamily, and it is highly expressed in cancer
cells. Moreover, it is known to cause different malignancies,
including HNSCC (7,8).
To prevent the progression of malignant tumors,
several studies have attempted to reveal the mechanisms underlying
CD147-induced tumorigenicity in various cancer types (9,10). The
number of studies regarding the contribution of CD147 to HNSCC
progression is also increasing. We have previously reported that
CD147 increased cell invasiveness, proliferation, and drug
resistance via interactions with its ligand and cyclophilin A in
individuals with HNSCC (11). In
addition, CD147 expression correlates with lymph node metastasis in
squamous cell carcinoma of the tongue (12). However, the role of CD147 in
tumorigenicity in HNSCC and the underlying mechanism are not
completely understood. Therefore, further analysis must be
conducted.
Recently, CD147 has been found to promote EMT in
certain individuals with solid tumors (9,13).
However, its role in EMT in individuals with HNSCC remains unclear.
Therefore, the present study aimed to elucidate the role of CD147
in EMT and tumorigenicity in HNSCC.
Materials and methods
Cells and cell culture
We purchased SAS, a human tongue squamous cell
carcinoma cell line, from the RIKEN Cell Bank (Tsukuba, Japan).
FaDu cells, a human hypopharyngeal squamous cell carcinoma cell
line, were kindly gifted by the Department of Cell Biology and
Morphology, Akita University Graduate School of Medicine (Akita,
Japan); both cell lines were used for in vitro studies. All
cells were maintained in the Dulbecco's modified Eagle's medium
(DMEM; Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal
bovine serum in a humidified atmosphere containing 5%
CO2 at 37°C. For stimulation experiments, we
preincubated SAS and FaDu cells with serum-free DMEM and
subsequently incubated with serum-free medium containing 10 or 20
ng/ml of TGF-β1 (Wako, Osaka, Japan).
Immunoblotting
Protein expression was detected by western blot
analysis using actin as an internal control. We lysed cell lines in
detergent containing 1% NP40, 150 mmol/l NaCl, 1 mmol/l EDTA, 0.1
mmol/l phenylmethylsulfonyl fluoride, 1 µg/ml leupeptin, and 1
µg/ml aprotinin and determined the protein levels using the Bio-Rad
Protein Assay Method (Bio-Rad Laboratories Inc., Hercules, CA,
USA). Then, we separated 40 µg of the total protein on 8% SDS-PAGE
gels and transferred it to nitrocellulose membranes using a semidry
transfer machine (Bio-Rad Laboratories, Inc.). Next, we blocked
membranes with 5% skimmed milk/TBS with Tween-20 solution for 1 h
at room temperature, incubated with primary antibodies in 5%
skimmed milk in TBS-T overnight at 4°C. After washing with TBS-T
three times, the membranes were incubated for 1 h with
horseradish-peroxidase-conjugated secondary antibody (Bio-Rad
Laboratories, Inc.) 1:3,000 diluted in 5% skimmed milk in TBS-T.
Then, we rinsed the filters with TBS-T three times and developed
the blot using Luminol Reagent (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) by autoradiography. In this study, we used the
following primary antibodies: rabbit anti-CD147, rabbit
anti-E-cadherin, goat anti-vimentin (1:1,000; Santa Cruz
Biotechnology, Inc.), and mouse anti-β-actin (1:5,000; Merck
Millipore, Tokyo, Japan).
Wound-healing assay
We conducted the wound-healing assay in six-well
tissue culture plates. In addition, we cultured SAS and FaDu cells
as a confluent monolayer. Then, the medium was changed to
serum-free, and after 24 h, a cell-free area was created by gently
scratching the cell monolayer with a sterile 10-µl pipette tip,
resulting in the creation of a 1-mm-wide cell-free area.
Immediately after scratching, the medium was replaced with a fresh
medium or a medium containing 10 ng/ml of TGF-β1. The same wound
areas were observed and photographed under an inverted microscope
(Olympus, Tokyo, Japan), and the distance of the scratch closure
was examined at 0 and 18 h.
Small interfering RNA (siRNA) and
siRNA transfection
CD147 siGENOME siRNA (Dharmacon RNA Technologies,
Lafayette, CO) was transfected into SAS cells for CD147 silencing.
We used the siGENOME nontargeting siRNA as control. Furthermore,
siRNA transfections were performed using Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.). In brief, 1.8×105 of SAS cells
were plated on 6 well plate. After 24 h incubation in complete
media, cells were transfected with 200 pmol of CD147 siRNA or
nontargeting control siRNA. The transfection medium was replaced
with complete media after 4 h of transfection.
Matrigel invasion assay
We evaluated cell invasiveness in vitro using
Matrigel-coated semipermeable-modified Boyden inserts with a pore
size of 8 µm (BD Biosciences, Franklin Lakes, NJ, USA). In
addition, SAS and FaDu cells were plated at a density of 2.5×104
cells/insert in serum-free medium with or without TGF-β (10 ng/ml).
Notably, the lower chamber contained DMEM + 10% FBS and served as a
chemoattractant. Meanwhile, we plated cells in 96-well plates to
serve as loading controls. After 48-h treatment at 37°C in a 5%
CO2 incubator, we removed the cells in the insert by
wiping gently with a cotton swab. Next, cells on the reverse side
of the insert were fixed and stained using Diff-Quick (Sysmex,
Kobe, Japan) according to the manufacturer's instructions. We
counted the invading cells in four representative fields using
light microscopy at magnification, ×200. Moreover, we evaluated
mean ± standard deviation (SD) from three independent experiments.
Furthermore, the cells plated on the 96-well plate were assessed
using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide assay to determine the metabolically active cells. Of note,
the number of invading cells was adjusted accordingly.
Statistical analysis
In this study, we used the Wilcoxon-Mann-Whitney
two-tailed exact test (Statcel 3; OMS Publishing, Tokorozawa,
Japan) to assess the statistical significance of the differences in
the wound closure, protein expression, and invasion studies. Data
are presented as mean ± SD from experiments that were repeated at
least three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
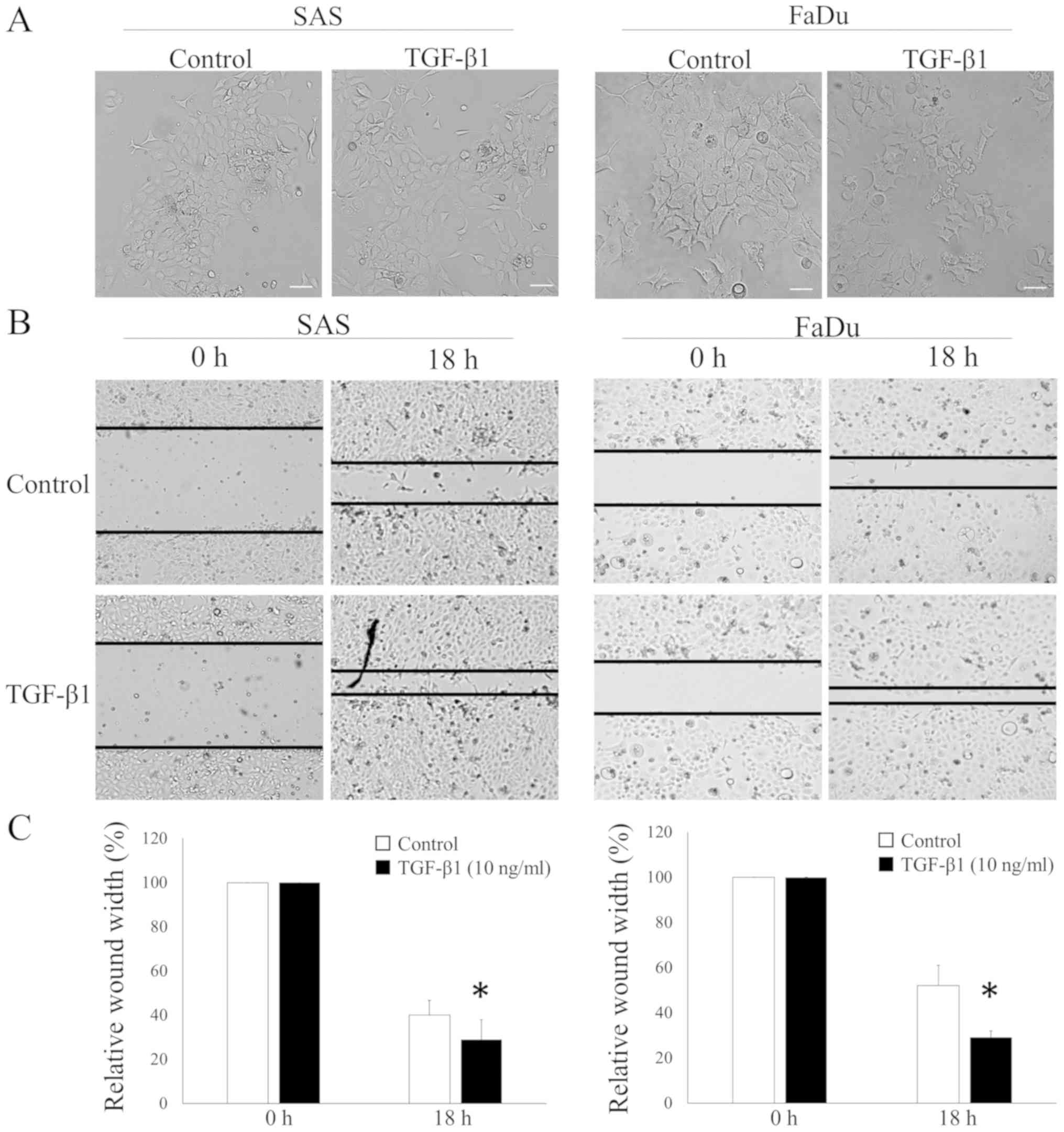
Induction of the morphological change
and the cell migration of HNSCC cells by TGF-β1
We cultured two HNSCC cell lines, SAS and FaDu
cells, with TGF-β1 to investigate whether TGF-β1 induced the
EMT-related phenomena in HNSCC. We examined the morphological
change using phase-contrast microscopy. Both cell lines exhibited a
cobblestone appearance and clustered growth. However, cells lost
adhesiveness and displayed a spindle-shaped morphology with TGF-β1
stimulation (Fig. 1A). In addition,
we assessed the effects of the exogenous TGF-β1 treatment on the
ability of HNSCC cells to induce wound closure to determine the
biological relevance of the EMT-induced tumorigenicity in HNSCC
cells. HNSCC cells migrated to an artificially produced wound in
the culture dish to a markedly greater extent in the presence of
TGF-β1 in both HNSCC cell lines (Fig. 1B
and C).
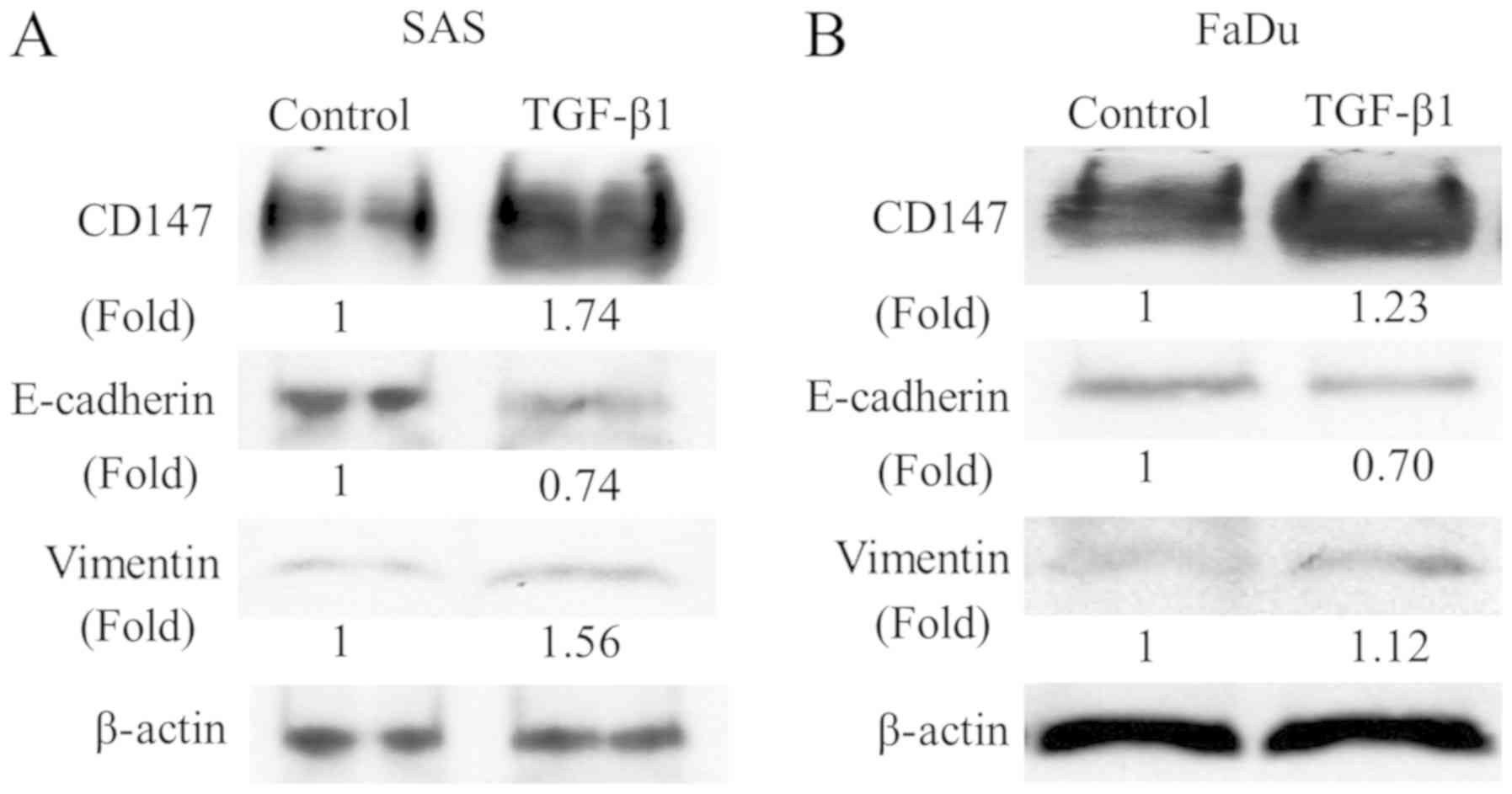
Induction of CD147 expression during
TGF-β1-induced EMT in HNSCC cells
In addition, we assessed the expression of EMT
markers in two HNSCC cell lines cultured with TGF-β1 to investigate
whether these morphological changes and cell migration are the
result of the EMT. We examined the expression of EMT markers,
E-cadherin, and vimentin using western blot analysis. Although
E-cadherin was downregulated, vimentin was upregulated with the
TGF-β1 treatment in both cell lines. These findings indicated that
the EMT was induced in HNSCC cells by the TGF-β1 treatment resulted
in these morphological changes and cell migration. Interestingly,
the CD147 expression was also upregulated by the TGF-β1 stimulation
(Fig. 2), suggesting that CD147 is
correlated with the TGF-β1-induced EMT in HNSCC cells.
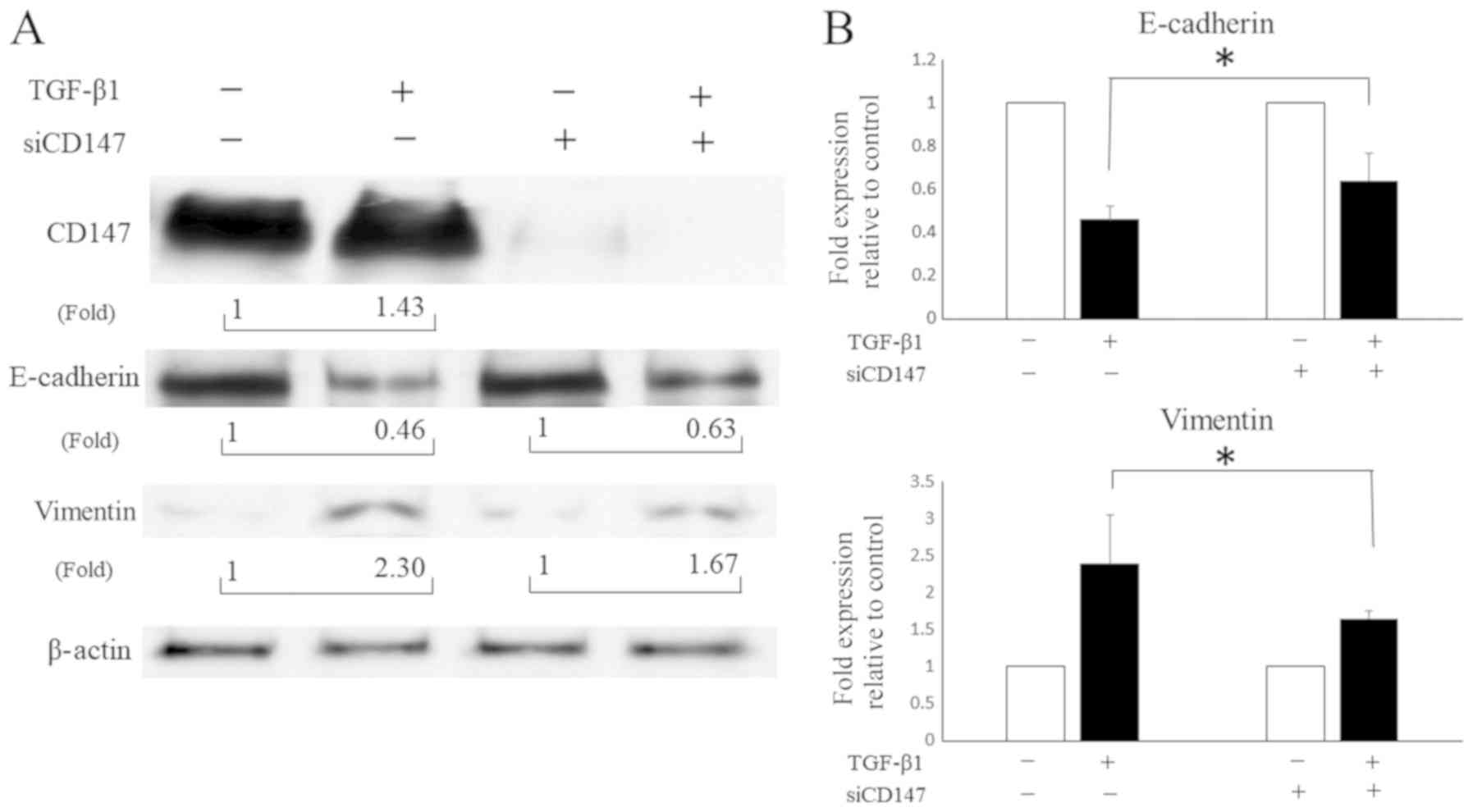
CD147 mediates the TGF-β1-induced EMT
and the following cell invasion of SCC cells of the tongue
The results suggest that TGF-β1 induced the EMT in
HNSCC cells (Fig. 1). In addition,
the CD147 expression was upregulated during this TGF-β1 induced
EMT, suggesting that CD147 plays an important role in the
TGF-β1-induced EMT in HNSCC. Although CD147 is known to mediate the
EMT in some solid tumors, it remains unclear whether CD147 mediates
the EMT in HNSCC cells. Thus, we used siRNA on CD147 before
culturing cells in the presence or absence of TGF-β1 with SAS to
validate whether CD147 mediates the EMT in HNSCC cells. The
downregulation of E-cadherin and upregulation of vimentin by TGF-β1
were attenuated when CD147 was silenced by siRNA (Fig. 3), indicating that CD147 partly
contributed to the TGF-β1-induced EMT in HNSCC cells.
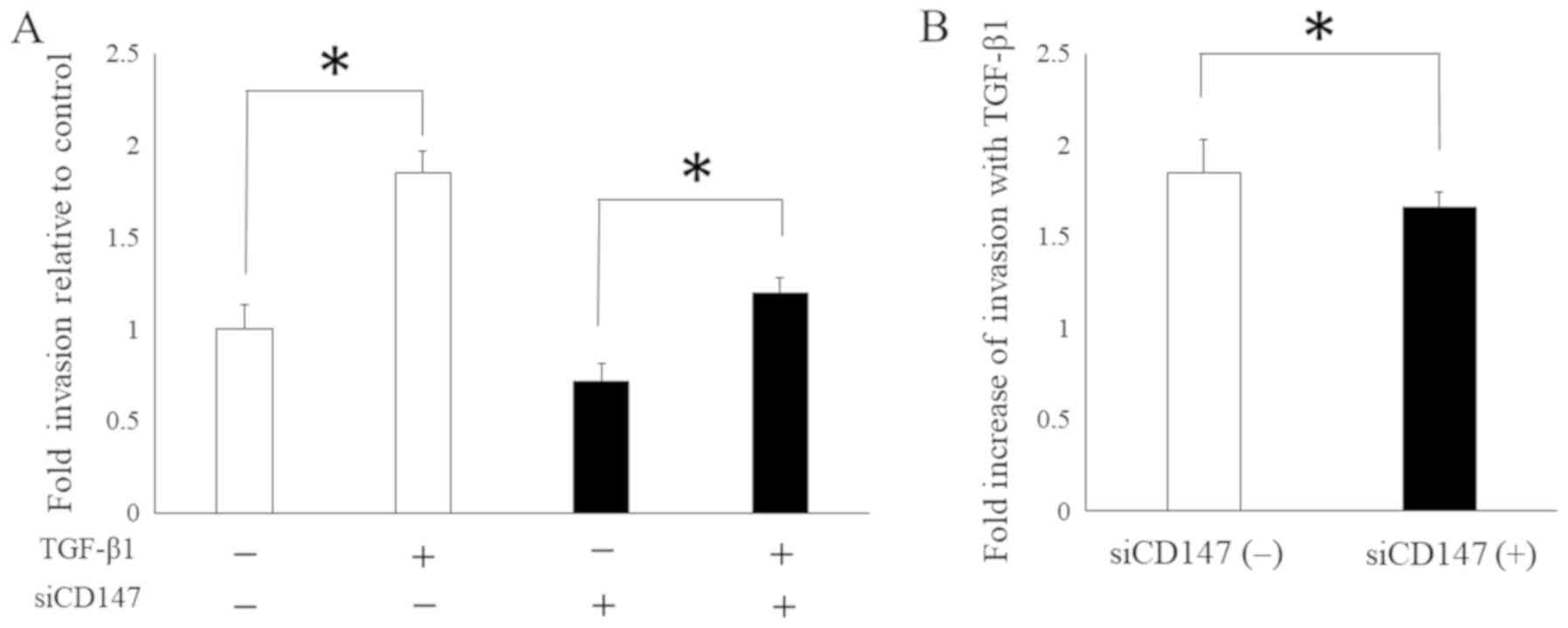
Furthermore, on the basis of these findings, we
hypothesized that CD147 mediates EMT-related malignant phenotypes
in HNSCC. Thus, we analyzed the cell invasiveness of SAS in the
presence or absence of siRNA-targeting CD147, with or without
TGF-β1, in the cell culture media to investigate the significance
of CD147 in the tumorigenic process which induced by EMT. The
findings revealed a trend for SAS to increase invasion with TGF-β1
in either the presence or absence of CD147 knockdown (Fig. 4A). However, the fold increase in
invasion was markedly lower in the presence of CD147 knockdown
(Fig. 4B), suggesting that CD147
plays a vital role in cell invasion induced by the EMT in SAS.
Discussion
The present study confirmed that TGF-β1 induces EMT
following migration and CD147 is upregulated during TGF-β1-induced
EMT in HNSCC cell lines. In addition, CD147 knockdown attenuated
this TGF-β1-induced EMT and invasion in SCC of the tongue. These
findings suggest that CD147 partly promotes EMT and cell invasion
induced by the TGF-β1 stimulation in individuals with HNSCC and
indicated the need for further assessment of the mechanism
underlying CD147 targeting HNSCC cells that express increased
levels of such proteins. EMT is a crucial developmental process
that often initiates during cancer invasion and metastasis
(2). The molecular characteristics
of EMT include the downregulation of epithelial cell markers and
the upregulation of mesenchymal markers (14). Although various growth factors and
cytokines can induce EMT, TGF-β has a vital role in EMT (15); therefore, the mechanisms underlying
the TGF-β-induced EMT must be further investigated to help identify
novel, effective therapeutic strategies for cancer, including
HNSCC.
The significance of CD147 in tumor progression and
its correlation with poor prognosis have extensively been observed
in individuals with solid tumors, which include those with HNSCC.
Moreover, several studies have suggested that CD147 is a negative
prognostic factor of malignant tumors (16). Previously, we have reported that the
CD147-CD147 hemophilic interaction or CD147-cyclophilin A
interaction has a vital role in matrix metalloproteinase expression
and activation as well as invasion and migration (8,11). In
addition, other studies have shown a correlation between CD147 and
EMT in several types of cancer (9,13).
However, data on the role of CD147 in the EMT in HNSCC are limited.
The present study revealed that CD147 mediates the TGF-β1-induced
EMT in SCC of the tongue, which indicates that CD147 is an
essential therapeutic target in HNSCC. Furthermore, studies have
reported that CD147 has an antitumor effect against HNSCC (17). Additionally, we have previously
reported that the inhibition of CD147 combined with the epidermal
growth factor receptor increases the effect of growth prevention
and migration of HNSCC cells (18).
Therefore, more detailed knowledge regarding the role of CD147 in
HNSCC progression might elucidate the prognosis of patients.
Recently, oncology and immunotherapy have been a
topic of interest. Moreover, anti-PD-L1 therapies for recurrent or
metastatic HNSCC have shown promising results, and cumulative
studies regarding immunotherapy in patients with HNSCC have been
conducted (19). Conversely,
immunotherapy resistance is frequently observed in several patients
with malignant tumors, including HNSCC (20). A recent study has reported that
patients with PD-1-therapy-resistant melanoma presented with
distinct signatures of upregulated genes involved in
immunosuppression and EMT (21).
Moreover, EMT contributes to immunoescape and immunosuppression in
individuals with solid tumors (22).
Therefore, targeting these pathways, including EMT, via
immunotherapy might help prevent resistance. In the present study,
CD147 was found to mediate EMT in individuals with SCC of the
tongue. CD147, which regulates EMT, might have an important role in
improving the therapeutic effect of immunotherapy in individuals
with HNSCC. However, our result cannot be generalized, and further
studies must be conducted to validate the mechanisms underlying
immunotherapy resistance in individuals with HNSCC.
In conclusion, CD147 plays a role in EMT and related
tumorigenicity in SCC of the tongue. Furthermore, targeting CD147
may improve the prognoses of individuals with HNSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Grant-in-Aid for
Scientific Research (C) (grant no. 15K10796) from the Ministry of
Education, Culture, Sports, Science and Technology, Japan.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS conceived and designed the experiments. SS, ST,
TT and YK performed the experiments. SS analyzed data and
contributed to writing of the manuscript. TY performed experiments,
and data analysis and interpretation. All authors reviewed the
final manuscript.
Ethics approval and consent to
publication
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EMMPRIN
|
extracellular matrix metalloproteinase
inducer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
RNA
|
ribonucleic acid
|
|
SCC
|
squamous cell carcinoma
|
|
SD
|
standard deviation
|
References
|
1
|
Cho J, Johnson DE and Grandis JR:
Therapeutic implications of the genetic landscape of head and neck
cancer. Semin Radiat Oncol. 28:2–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Futur Oncol. 5:1145–1168. 2009. View Article : Google Scholar
|
|
4
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miettinen PJ, Ebner R, Lopez AR and
Derynck R: TGF-beta induced transdifferentiation of mammary
epithelial cells to mesenchymal cells: Involvement of type I
receptors. J Cell Biol. 127:2021–2036. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith A, Teknos TN and Pan Q: Epithelial
to mesenchymal transition in head and neck squamous cell carcinoma.
Oral Oncol. 49:287–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caudroy S, Polette M, Tournier JM, Burlet
H, Toole B, Zucker S and Birembaut P: Expression of the
extracellular matrix metalloproteinase inducer (EMMPRIN) and the
matrix metalloproteinase-2 in bronchopulmonary and breast lesions.
J Histochem Cytochem. 47:1575–1580. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki S, Sato M, Senoo H and Ishikawa K:
Direct cell-cell interaction enhances pro-MMP-2 production and
activation in co-culture of laryngeal cancer cells and fibroblast:
Involvement of EMMPRIN and MT1-MMP. Exp Cell Res. 293:259–266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu T, Zhou M, Peng L, Kong S, Miao R, Shi
Y, Sheng H and Li L: Upregulation of CD147 promotes cell invasion,
epithelial-to-mesenchymal transition and activates MAPK/ERK
signaling pathway in c. Int J Clin Exp Pathol. 7:7432–7441.
2014.PubMed/NCBI
|
|
10
|
Hibino T, Sakaguchi M, Miyamoto S,
Yamamoto M, Motoyama A, Hosoi J, Shimokata T, Ito T, Tsuboi R and
Huh NH: S100A9 is a novel ligand of EMMPRIN that promotes melanoma
metastasis. Cancer Res. 73:172–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi M, Suzuki S and Ishikawa K:
Cyclophilin A-EMMPRIN interaction induces invasion of head and neck
squamous cell carcinoma. Oncol Rep. 27:198–203. 2012.PubMed/NCBI
|
|
12
|
Suzuki S, Honda K, Nanjo H, Iikawa N,
Tsuji T, Kawasaki Y, Yamazaki K, Sato T, Saito H, Shiina K and
Ishikawa K: CD147 expression correlates with lymph node metastasis
in T1-T2 squamous cell carcinoma of the tongue. Oncol Lett.
14:4670–4676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ru NY, Wu J, Chen ZN and Bian H:
HAb18G/CD147 is involved in TGF-β-induced epithelial-mesenchymal
transition and hepatocellular carcinoma invasion. Cell Biol Int.
39:44–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu F, Gu LN, Shan BE, Geng CZ and Sang
MX: Biomarkers for EMT and MET in breast cancer: An update. Oncol
Lett. 12:4869–4876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu C, Pan Y, He B, Wang B, Xu Y, Qu L,
Bao Q, Tian F and Wang S: Inhibition of CD147 gene expression via
RNA interference reduces tumor cell invasion, tumorigenicity and
increases chemosensitivity to cisplatin in laryngeal carcinoma Hep2
cells. Oncol Rep. 25:425–432. 2011.PubMed/NCBI
|
|
17
|
Ma C, Wang J, Fan L and Guo Y: Inhibition
of CD147 expression promotes chemosensitivity in HNSCC cells by
deactivating MAPK/ERK signaling pathway. Exp Mol Pathol. 102:59–64.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki S and Ishikawa K: Combined
inhibition of EMMPRIN and epidermal growth factor receptor prevents
the growth and migration of head and neck squamous cell carcinoma
cells. Int J Oncol. 44:912–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE,
Even C, et al: Nivolumab vs. investigator's choice in recurrent or
metastatic squamous cell carcinoma of the head and neck: 2-year
long-term survival update of CheckMate 141 with analyses by tumor
PD-L1 expression. Oral Oncol. 81:45–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelderman S, Schumacher TN and Haanen JB:
Acquired and intrinsic resistance in cancer immunotherapy. Mol
Oncol. 8:1132–1139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bu X, Mahoney KM and Freeman GJ: Learning
from PD-1 resistance: New combination strategies. Trends Mol Med.
22:448–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Terry S, Savagner P, Ortiz-Cuaran S,
Mahjoubi L, Saintigny P, Thiery JP and Chouaib S: New insights into
the role of EMT in tumor immune escape. Mol Oncol. 11:824–846.
2017. View Article : Google Scholar : PubMed/NCBI
|