Introduction
Cerebral ischemia, which results from the occlusion
of an artery in brain, is a leading cause of morbidity and
mortality in adults all over the world (1). During ischemia and the subsequent
condition, reperfusion, the decline of cerebral blood flow leads to
the deprivation of oxygen and glucose, and determines the severity
of the ischemic insult (2). An
ischemic insult is an area where there is a complete absence of
blood flow and neuronal death occurs. Around the insult is an
ischemic penumbra, in this area blood flow is reduced and function
is impaired, however metabolism remains active (3). If blood flow is not restored in a short
time, the neurons in the penumbra enter apoptosis (4). Traditionally, necrosis is considered
the major cell death pathway in cerebral ischemia, but studies in
the past decades have revealed that in the first few hours after
ischemia neurons in the ischemic penumbra transiently undergo
reversible damage and ultimately apoptosis (2,5).
Although the duration of ischemia is a crucial factor of brain
tissue damage, subsequent reperfusion also serves a prominent role
in the acceleration of irreversible damage (6). Therefore, the initial few hours of
reversible neuronal injury provides an opportunity for therapeutic
intervention (2).
MicroRNAs are small non-coding RNAs 18–25
nucleotides in length, which can bind to the 3′-untranslated region
of mRNAs to achieve downregulation of the target mRNA via
degradation or translational inhibition (7). Previous studies have demonstrated that
microRNAs served a role in the therapy of cerebral ischemia and
reperfusion injury (8–10). In response to cerebral ischemia,
microRNA expression was dynamic; among these microRNAs,
microRNA-124 was expressed at high levels in the central nervous
system (7). MicroRNA-124 protected
neurons against cerebral ischemia through multiple molecular
mechanisms (11–13) and showed promising therapeutic
potential for the treatment of central nervous system diseases
(14). In addition, microRNA-124
possessed anti-cancer effects (15–17) and
was hepatoprotective (18). To
better understand cerebral ischemia and reperfusion injury, and how
to provide effective therapeutic intervention, the present study
examined the changes in microRNA-124 expression in the brain tissue
of rats during early ischemia and reperfusion. The tissue was
examined at different time points and the association with cell
apoptosis involving STAT3 activation was investigated.
Materials and methods
Chemicals and reagents
Water was prepared from water distilled by Millipore
Milli-Q® system (EMD Millipore, Billerica, MA, USA).
Antibodies directed against apoptosis regulator Bcl-2 (Bcl-2; cat.
no. 610538) were purchased from BD Biosciences (Franklin Lakes, NJ,
USA). Anti-apoptosis regulator Bax (Bax; cat. no. AF0120),
-caspase-3 (cat. no. AF7022) and -β-tubulin (cat. no. T0023)
antibodies were obtained from Affinity Biosciences (Cincinnati, OH,
USA). The total RNA extraction kit and microRNA-124 reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) kit
were supplied by Suzhou Jima Gene Co. Ltd. (Suzhou, China).
Anti-signal transducer and activator of transcription 3 (STAT3;
cat. no. ARH4012) and -phosphorylated (p-)STAT3 (cat. no. ARE6058)
antibodies were purchased from AR Biosciences USA, Inc. (San Diego,
CA, USA).
Animals
A total of 54 male Sprague Dawley rats (250–280 g,
8.0±0.5 weeks) were supplied by the Experimental Animals Center of
North China University of Science and Technology (Tangshan, China).
The animals were housed in controlled conditions (temperature,
23±2°C; humidity, 45–60%; 12 h light/dark cycle; free access to
water and a standard diet). All animal experiments were performed
in accordance with the Guide for the Care and Use of Laboratory
Animals guidelines (National Institutes of Health, Bethesda, MD,
USA) (19), with the approval of the
Animal Care and Use Committee of North China University of Science
and Technology.
Middle cerebral artery occlusion
(MCAO)
The rats were subjected to MCAO, as previous
description (20). Briefly, the rats
were anesthetized with 10% chloral hydrate [350 mg/kg,
intraperitoneal injection (IP)]. The internal, external and right
common carotid arteries were exposed surgically. A 3600AAA
monofilament nylon suture (Guangzhou Jialing Biotech Co., Ltd.,
Guangzhou, China) was inserted into the internal carotid artery
through the external carotid artery stump and the MCA was occluded.
To ensure MCAO caused ischemia, the rats above grade 2 in
neurologic examination were considered successful models, as
previously described (21). After 2
h of MCAO, the suture was removed to restore blood flow. At certain
time points (3, 6, 12 and 24 h after reperfusion), the rats were
sacrificed under anesthesia [10% chloral hydrate (350 mg/kg, IP)]
by decapitation to get the brain tissue on ice as previously
described (22). The brain tissue
was stored under −80°C until they were analyzed. The rats in the
sham group underwent the same surgery after 3 h, but the MCA was
not occluded.
RNA extraction and RT-qPCR
analysis
The total RNA was extracted from the brain tissue by
a single-step method using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to
manufacturer's protocol. The concentration and integrity of RNA
were determined by Hairpin-it™ miRNA qPCR Quantitation kit (cat.
no. QPM-012; Suzhou GenePharma Co., Ltd., Suzhou, China) according
to the manufacturer's protocol. An RT-qPCR analysis was performed
on the iQ™5 Real-Time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) under following conditions: 95°C for 3 min, and
40 cycles of 95°C for 12 sec and 62°C for 40 sec. Primers for
microRNA-124 and U6 were supplied in the Hairpin-it™ miRNA qPCR
Quantitation kit. The microRNA-124 and U6, the internal control,
were detected using a U6 real time RT-qPCR normalization kit
(Suzhou GenePharma Co., Ltd.) according to the manufacturer's
protocol. The relative quantification value for the target gene was
calculated using the 2−ΔΔCq method (23).
Immunohistochemical analysis
At the aforementioned time points, the rats were
sacrificed by decapitation under deep anesthesia with 10% chloral
hydrate (350 mg/kg, IP), then perfused with 10% paraformaldehyde at
room temperature for 10 min and washed with saline. The whole brain
was removed and immersed in 10% paraformaldehyde for 24 h at 4°C.
The tissues were rinsed with PBS, dehydrated through gradients of
alcohol and xylene, and then embedded in paraffin. Brain sections
(5-µm-thick) were prepared on the RM2245 Semi-Automated Rotary
Microtome (Leica Microsystems GmbH, Wetzlar, Germany). The sections
was rehydrated in descending alcohol series at room temperature.
The antigen retrieval was implemented in 0.01 M citrate buffer
solution in an autoclave at 95°C for 1 min. The sections were
cooled to room temperature and washed with PBS three times. Then
the sections were incubated in 3% H2O2 for 15
min to block endogenous peroxidase and subsequently washed with
0.01 M PBS three times for 3 min each time. Then the sections were
blocked in buffer with 10% goat serum from a SPlink detection kit
(cat. no. AR100089; OriGene Technologies, Inc., Beijing, China) at
room temperature for 15 min. Caspase-3, Bcl-2 and Bax primary
antibodies (1:500 dilution) were added to the sections and
incubated overnight at 4°C. The sections were washed with 0.01 M
PBS for 3 min again. Then the goat anti-rat immunoglobulin (Ig)G
secondary antibodies conjugated to biotin from the SPlink detection
kit were applied and incubated at room temperature for 15 min
followed by the addition of an avidin-biotin-horseradish peroxidase
complex solution from the SPlink detection kit at room temperature
for another 15 min. The sections were treated with
3,3′-Diaminobenzidine tetrahydrochloride as the color substrate at
room temperature for 5 min. Then the sections were rinsed with
water and stained by hematoxylin at room temperature for 5 min. The
labeled sections were examined under an inverted optical microscope
at a magnification of ×400. With the exception of the incubation of
the primary antibodies, the procedure was the same for the control
groups.
Western blot analysis
Western blot analyses were employed to determine the
expression of cleaved caspase-3, Bcl-2, Bax, STAT3 and p-STAT3. In
short, total protein was extracted using Whole Cell Lysis Assay kit
(cat. no. KGP2100; Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) from brain tissue and quantified by a bicinchoninic acid
assay kit according to the manufacturer's protocol. Then the
samples (30 µg/lane) were denatured at 95°C for 30 min and
separated by SDS-PAGE on a 15% gel. The samples were then
transferred to polyvinylidene difluoride membranes. Following
blocking of the membranes with 5% skimmed milk at 37°C for 1 h, the
membranes were incubated overnight with anti-caspase-3, -Bcl-2,
-Bax, -STAT3, -p-STAT3 and -β-tubulin primary antibodies (1:1,000
dilution) at 4°C. The goat anti-rat IgG secondary antibodies
conjugated to horseradish peroxidase (1:1,000 dilution; cat no.
A0192; Beyotime Institute of Biotechnology, Shanghai, China) were
added and incubated at room temperature for 2 h. The results were
detected by enhance chemiluminescence substrate (cat no. P0018;
Beyotime Institute of Biotechnology) on Azure c300 Chemiluminescent
Western Blot Imaging System (Azure Biosystems, Inc., Dublin, CA,
USA). β-tubulin (1:1,000 dilution) was used as the internal
control. Densitometric analysis was performed by ImageJ software
(version 1.5; National Institutes of Health) to obtain the relative
intensities of the bands.
Statistical analysis
All results are expressed as mean ± standard
deviation. GraphPad Prism software (version 5.0; GraphPad Software,
Inc., La Jolla, CA, USA) analyzed the results. Statistical
differences were compared by one-way analysis of variance followed
by Dunnett's test for comparisons between multiple groups and
Student's t-test for comparisons between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
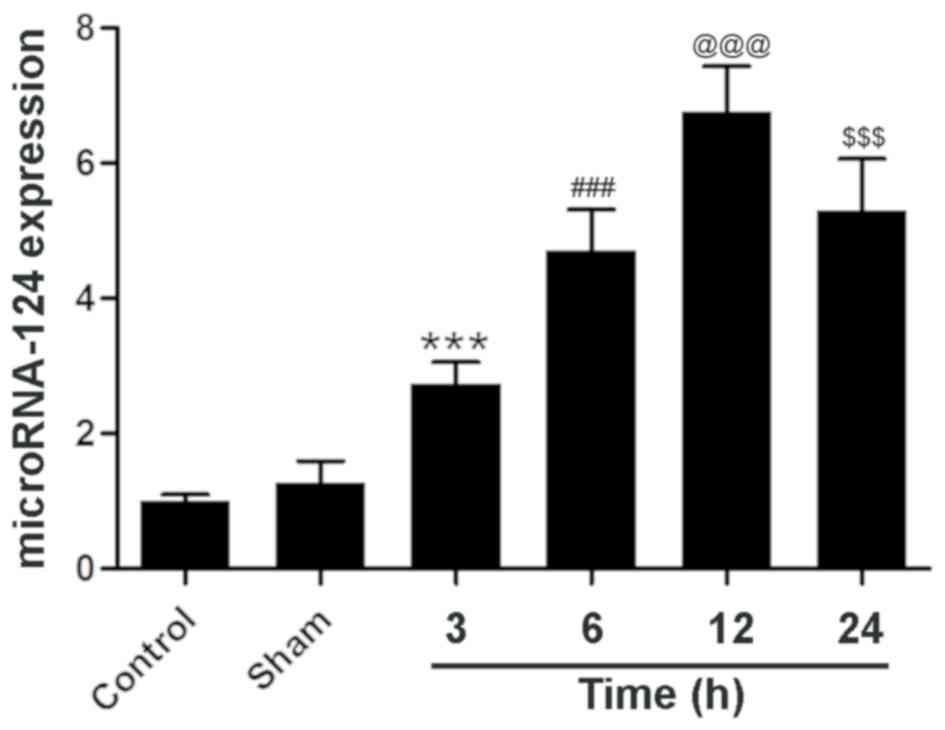
MicroRNA-124 expression increases in
ischemic brain tissue in the 12 h after reperfusion
The expression of microRNA-124 in the sham group was
similar to that of the control group (Fig. 1). However, 3 h after reperfusion,
compared with the sham group, the expression of microRNA-124 in the
brain tissue of experimental rats was significantly elevated at
2.74±0.32. Then at the 6 h time point, the expression reached
4.70±0.62, which was significant increased compared with the 3 h
treatment group. MicroRNA-124 expression at the 12 h time point was
~6 times that of the control group at 6.77±0.67, which was a
significant increase compared with the 6 h treatment group. At 24
h, the expression of microRNA-124 was 5.30±0.76, which was similar
to the 6 h and significantly reduced compared with the 12 h
treatment group. These results demonstrate that microRNA-124
expression is dynamic following reperfusion and neuronal
injury.
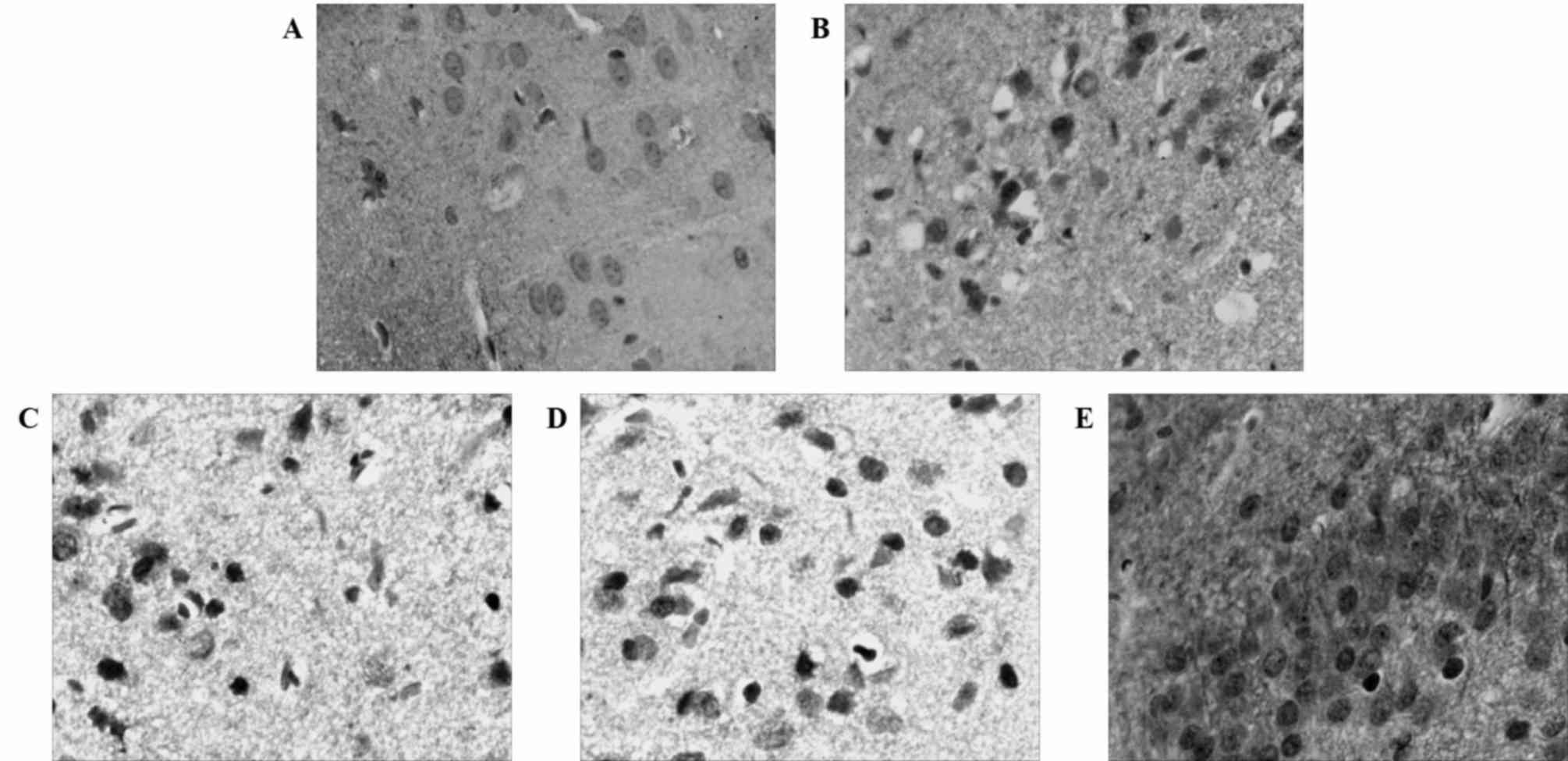
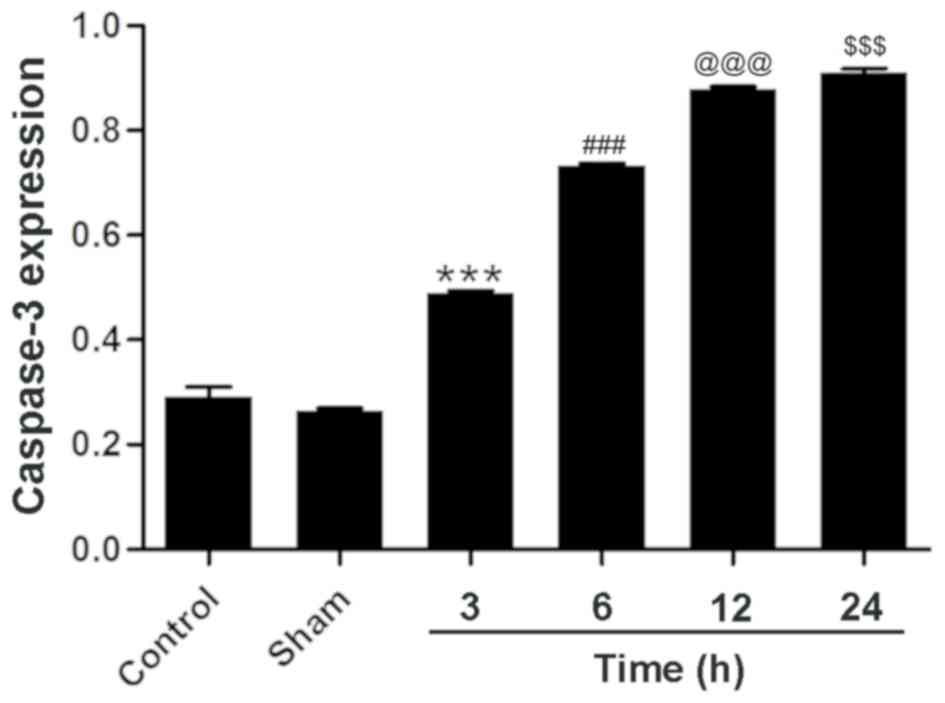
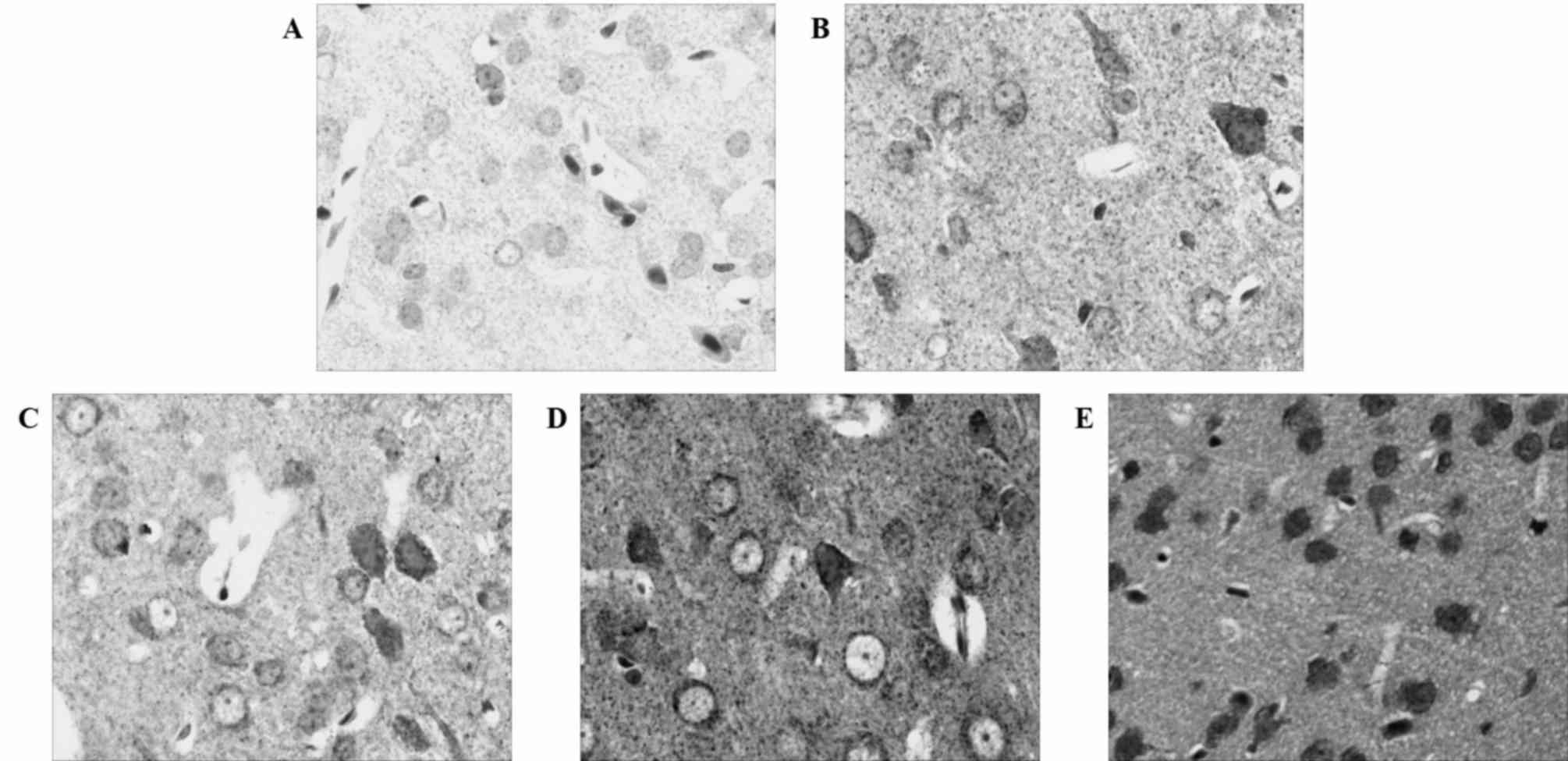
Caspase-3 expression increases in
ischemic brain tissue in the 24 h after MCAO and reperfusion
As a member of the cysteine-dependent
aspartate-directed protease family, caspase-3 is an effector enzyme
involved in cell apoptosis (24). An
immunohistochemical analysis revealed that the expression of
caspase-3 was upregulated following MCAO and reperfusion, although
the expression of caspase-3 was not evident in the control group
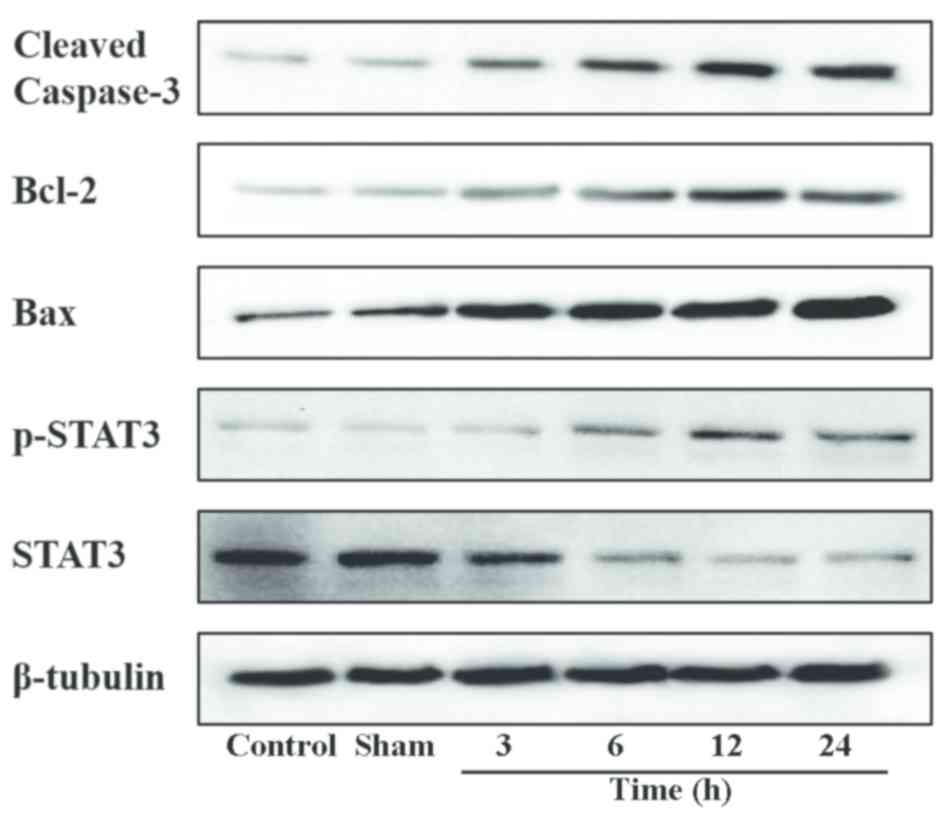
(Fig. 2). Western blotting confirmed
the aforementioned results and gave a profile of caspase-3
expression in relative levels (Fig.
3). Following a densitometric analysis, caspase-3 expression
was identified to be significantly upregulated 3 h after MCAO and
reperfusion (0.49±0.01) compared with the sham group; the
expression level of caspase-3 continued to increase significantly
24 h after reperfusion (0.91±0.01; Fig.
4) compared with the 12 h treatment group. These results
demonstrate that neuronal apoptosis follows MCAO and reperfusion,
and that the injury worsens with time at the early stage of
cerebral ischemia and reperfusion.
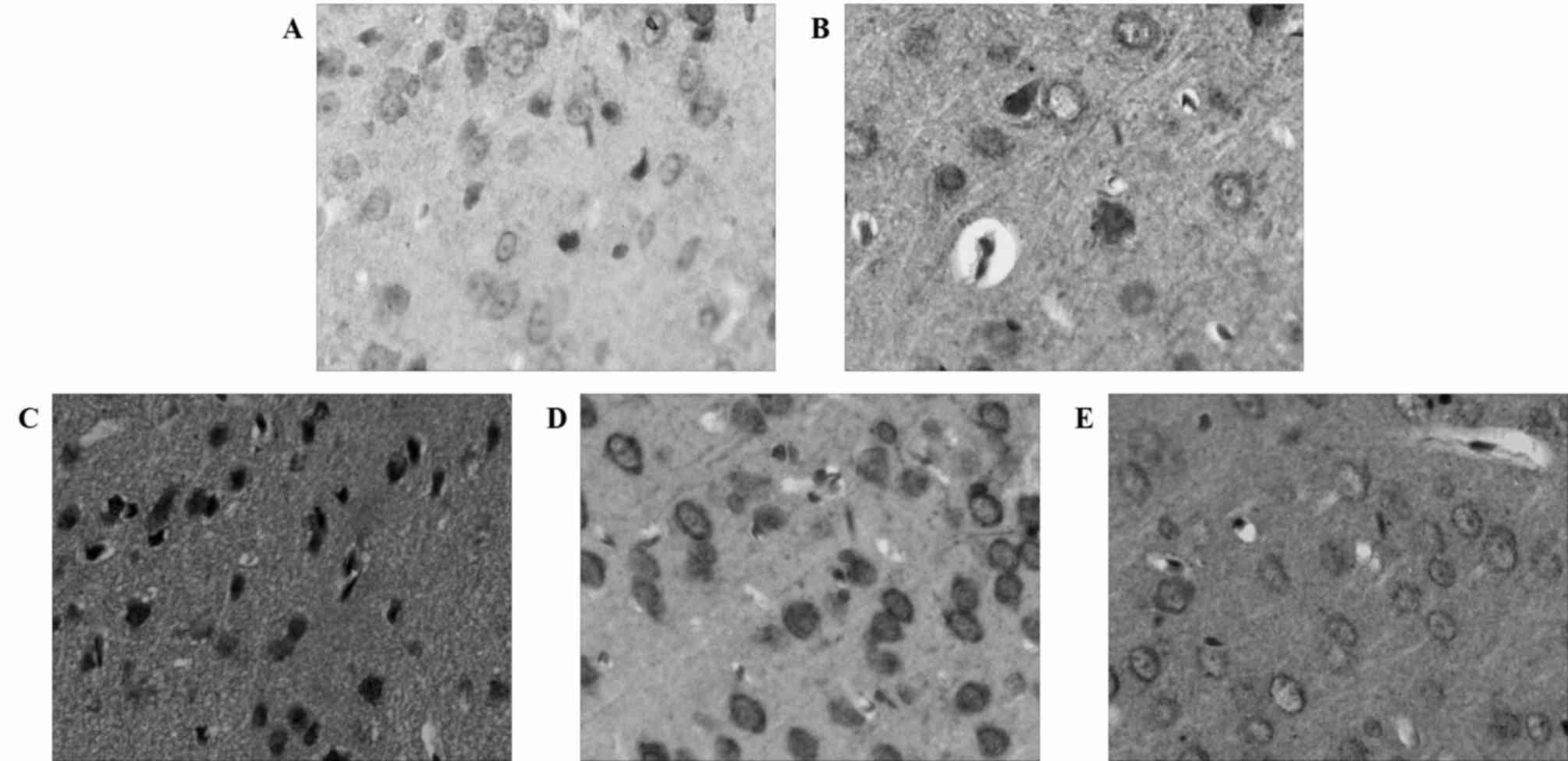
Bcl-2 and Bax exhibit similar
expression levels in ischemic brain tissue as their expression
increases following MCAO and reperfusion
An immunohistochemical analysis revealed the dynamic
expression of Bcl-2 and Bax following MCAO and reperfusion. The
expression of Bax increased similarly to caspase-3 following MCAO
and reperfusion (Fig. 5). By
contrast, Bcl-2 expression increased and peaked 12 h after MCAO and
reperfusion (Fig. 6), which was in
accordance with the dynamic changes of microRNA-124. Western
blotting confirmed these results (Fig.
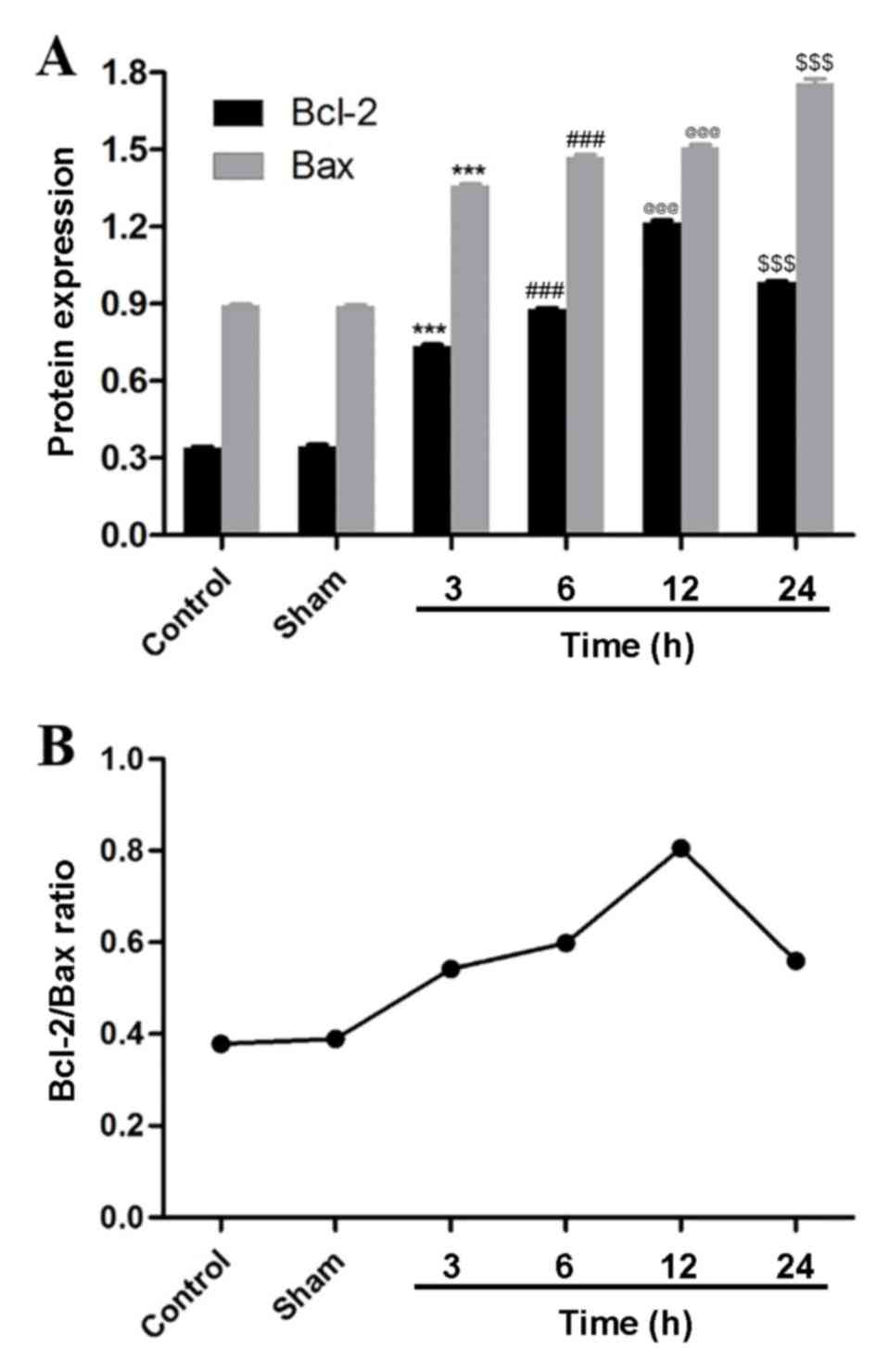
3) and the densitometric analysis of the relative levels of
Bcl-2 and Bax supported the results quantitatively (Fig. 7A). Bcl-2 and Bax were significantly
upregulated at the 3, 6, 12 and 24 h time points compared with the
sham group and, 3, 6 and 12 h treatment groups, respectively.
Additionally, the relative ratio of Bcl-2 and Bax demonstrated that
the protein expression increased following MCAO and reperfusion,
peaked at 12 h, and then decreased (Fig.
7B).
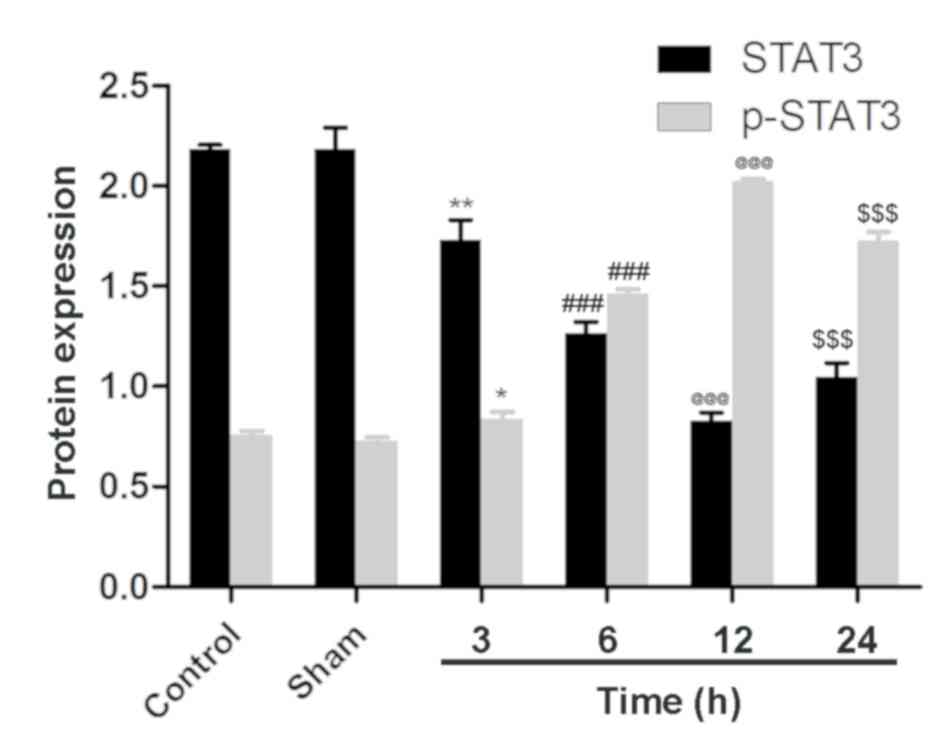
STAT3 and p-STAT3 exhibit opposing
expression profiles, with p-STAT3 expression increasing following
MCAO and reperfusion in ischemic brain tissue
To investigate the involvement of STAT3 in cerebral
ischemia and reperfusion, STAT3 and p-STAT3 were analyzed by
western blotting and western blot analysis. No significant
differences in the expression of STAT3 and p-STAT3 were identified
between the control group and the sham group, although p-STAT3
expression was low (Figs. 3 and
8). Following MCAO and reperfusion,
STAT3 was activated through phosphorylation, which led to a
significant upregulation of p-STAT3 at 3 h compared with the sham
group. Following MCAO and reperfusion, p-STAT3 expression peaked at
12 h, which was significantly greater compared with the expression
at 6 h, then significantly declined at the 24 h time point.
Accordingly, compared with the sham group, STAT3 was significantly
reduced after 3 h of MCAO and reperfusion, and after 12 h STAT3
expression reached its minimum level, which was significantly
reduced compared with the expression at 6 h. At the 24 h time
point, STAT3 expression was significantly upregulated compared with
the 12 h treatment group. These results were consistent with the
dynamic expression of Bcl-2 and microRNA-124, thus an association
between STAT3, Bcl-2 and microRNA-124 was indicated.
Discussion
Cerebral ischemia and reperfusion injury results
from a deficiency in cerebral blood flow, as well as subsequent
oxygen and glucose deprivation, and restoration (2). Apoptosis contributes largely to cell
death in cerebral ischemia and reperfusion injury (2). As a microRNA specifically expressed in
brain tissue, microRNA-124 can protect cells from apoptosis in
cerebral ischemic areas (11). The
results of the present study demonstrated that the expression of
microRNA-124 in brain tissue was dynamic during the early stage of
cerebral ischemia and reperfusion injury, and the level of
microRNA-124 expression peaked at the 12 h time point. This result
supports the hypothesis that microRNA-124 attenuates the injury
induced by cerebral ischemia and reperfusion during the early
stage.
Caspase-3 is a member of the cysteine-dependent
aspartate-directed protease family and was revealed to drive the
mitochondria- and death receptor-mediated signaling pathways in
apoptosis (25). Caspase-3 is
activated through the cleavage of pro-caspase-3, the cleaved
caspase-3 served a role in mitochondria-mediated apoptosis
(3). Bcl-2 and Bax are members of
the Bcl-2 protein family, which regulates various cellular
activities (26). Bcl-2 and Bax were
also involved in cerebral ischemia through the modulation of
mitochondria-mediated apoptosis (2).
Bcl-2 exhibits an anti-apoptotic effect and inhibits the activation
of caspase-3 while Bax demonstrates a pro-apoptotic effect
(27). In addition, Bax can
dimerize, dimerized Bax initiates the activation of terminal
caspases, which results in the promotion of apoptosis (27). Conversely, the heterodimer of
Bcl-2/Bax prevents certain downstream events in apoptosis (26). In the present study, the expression
of caspase-3 and Bax increased during the early stage of cerebral
ischemia and reperfusion, which indicated that the injury was being
exacerbated. On the contrary, the expression of Bcl-2 increased
prior to the injury and reached peak expression at the 12 h time
point, following by a decrease. Accordingly, the ratio of Bcl-2/Bax
was highest 12 h after MCAO and reperfusion. These results were
consistent with the dynamic expression of microRNA-124, which
implies that Bcl-2 is involved in the protection of the brain
against cerebral ischemia and reperfusion injury, and that
caspase-3 and Bax are promoters of that injury.
STAT3 serves an important role in the regulation of
cell apoptosis. STAT3, an inactive protein, can be activated
through phosphorylation upon neural injury to exert a protective
effect (28). p-STAT3 is involved in
the tyrosine-protein kinase JAK (JAK)2/STAT3 signaling pathway to
promote cell viability (29) and
protect the brain against cerebral ischemia injury (30). As the substrate of STAT3, Bcl-2 was
regulated by p-STAT3 during cerebral ischemia (31). The present study determined that the
expression of p-STAT3 was upregulated and peaked 12 h after MCAO
and reperfusion, and then the expression decreased. Accordingly,
the expression of STAT3 was downregulated and its lowest level was
at the 12 h time point. This trend mirrored microRNA-124 and Bcl-2
expression, which suggests that STAT3 is associated with Bcl-2.
In conclusion, the present study elucidated the
dynamic expression of microRNA-124 during the early stage of
cerebral ischemia and reperfusion. Anti-apoptotic proteins,
including Bcl-2, STAT3 and p-STAT3 exhibit similar changes in their
expression, synchronous with microRNA-124, protecting brain tissue
from injury. Pro-apoptotic proteins, including caspase-3 and Bax,
were consistently upregulated following reperfusion. This present
study reveals that the protective effect of microRNA-124 is
associated with Bcl-2 and involved in the JAK2/STAT3 signaling
pathway. This gives insight into the possible diagnosis of and
therapy for cerebral ischemia and reperfusion injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ designed the study, performed the collection,
analysis and interpretation of data, and wrote the manuscript. AM
designed the study, performed the analysis and interpretation of
data, and revised this manuscript.
Ethics approval and consent to
participate
The current study was approved by the Animal Care
and Use Committee of North China University of Science and
Technology (Tangshan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Flynn RW, MacWalter RS and Doney AS: The
cost of cerebral ischemia. Neuropharmacology. 55:250–256. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mehta SL, Manhas N and Raghubir R:
Molecular targets in cerebral ischemia for developing novel
therapeutics. Brain Res Rev. 54:34–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji HJ, Wang DM, Hu JF, Sun MN, Li G, Li
ZP, Wu DH, Liu G and Chen NH: IMM-H004, a novel courmarin
derivative, protects against oxygen- and
glucose-deprivation/restoration-induced apoptosis in PC12cells. Eur
J Pharmacol. 723:259–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Endres M, Engelhardt B, Koistinaho J,
Lindvall O, Meairs S, Mohr JP, Planas A, Rothwell N, Schwaninger M,
Schwab ME, et al: Improving outcome after stroke: Overcoming the
translational roadblock. Cerebrovasc Dis. 25:268–278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu DM, Wang ZH, Liu L, Zhang XM and Lou
FL: Acetylpuerarin increases cell viability and reduces apoptosis
in rat hippocampal neurons following oxygen-glucose
deprivation/reperfusion. Mol Med Rep. 8:1453–1459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li D, Shao Z, Vanden Hoek TL and Brorson
JR: Reperfusion accelerates acute neuronal death induced by
simulated ischemia. Exp Neurol. 206:280–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Y, Lei Y, Yu F, Changfeng F, Song W and
Xuming M: MicroRNAs expression and function in cerebral ischemia
reperfusion injury. J Mol Neurosci. 53:242–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Y, Deng H, Xu S and Zhang J: MicroRNAs
regulate mitochondrial function in cerebral ischemia-reperfusion
injury. Int J Mol Sci. 16:24895–24917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Min XL, Wang TY, Cao Y, Liu J, Li JT and
Wang TH: MicroRNAs: A novel promising therapeutic target for
cerebral ischemia/reperfusion injury. Neural Regen Res.
10:1799–1808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fasanaro P, Greco S, Ivan M, Capogrossi MC
and Martelli F: MicroRNA: Emerging therapeutic targets in acute
ischemic diseases. Pharmacol Therape. 125:92–104. 2010. View Article : Google Scholar
|
|
11
|
Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan
JL and Liu X: MicroRNA-124 protects neurons against apoptosis in
cerebral ischemic stroke. CNS Neurosci Ther. 19:813–819.
2013.PubMed/NCBI
|
|
12
|
Liu X, Li F, Zhao S, Luo Y, Kang J, Zhao
H, Yan F, Li S and Ji X: MicroRNA-124-mediated regulation of
inhibitory member of apoptosis-stimulating protein of p53 family in
experimental stroke. Stroke. 44:1973–1980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doeppner TR, Doehring M, Bretschneider E,
Zechariah A, Kaltwasser B, Müller B, Koch JC, Bähr M, Hermann DM
and Michel U: MicroRNA-124 protects against focal cerebral ischemia
via mechanisms involving Usp14-dependent REST degradation. Acta
Neuropathol. 126:251–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Y, Luo ZM, Guo XM, Su DF and Liu X: An
updated role of microRNA-124 in central nervous system disorders: A
review. Front Cellular Neurosci. 9:1932015. View Article : Google Scholar
|
|
15
|
Taniguchi K, Sugito N, Kumazaki M,
Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama
K, et al: MicroRNA-124 inhibits cancer cell growth through
PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett.
363:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y, Ai X, Shen S and Lu S:
NF-κB-mediated miR-124 suppresses metastasis of non-small-cell lung
cancer by targeting MYO10. Oncotarget. 6:8244–8254. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xi ZW, Xin SY, Zhou LQ, Yuan HX, Wang Q
and Chen KX: Downregulation of rho-associated protein kinase 1 by
miR-124 in colorectal cancer. World J Gastroenterol. 21:5454–5464.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Yi S, Deng Y, Cheng J, Wu X, Liu W,
Tai Y, Chen G, Zhang Q and Yang Y: MiR-124 protects human hepatic
L02 cells from H2O2-induced apoptosis by
targeting Rab38 gene. Biochem Biophys Res Commun. 450:148–153.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwiatkowski K, Piotrowska A, Rojewska E,
Makuch W and Mika J: The RS504393 influences the level of
nociceptive factors and enhances opioid analgesic potency in
neuropathic rats. J Neuroimmune Pharmacol. 12:402–419. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia D, Han B, Yang S and Zhao J: Anemonin
alleviates nerve injury after cerebral ischemia and reperfusion
(I/R) in rats by improving antioxidant activities and inhibiting
apoptosis pathway. J Mol Neurosci. 53:271–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu JR, Tao YF, Lou S and Wu ZM:
Protective effects of ginsenoside Rb3 on oxygen and glucose
deprivation-induced ischemic injury in PC12 cells. Acta Pharmacol
Sin. 31:273–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murphy KM, Ranganathan V, Farnsworth ML,
Kavallaris M and Lock RB: Bcl-2 inhibits Bax translocation from
cytosol to mitochondria during drug-induced apoptosis of human
tumor cells. Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou H, Zhang Z, Wei H, Wang F, Guo F, Gao
Z, Marsicano G, Wang Q and Xiong L: Activation of STAT3 is involved
in neuroprotection by electroacupuncture pretreatment via
cannabinoid CB1 receptors in rats. Brain Res. 1529:154–164. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin Y, Cai B, Xue XH, Fang L, Wu ZY and
Wang N: TAT-mediated delivery of neuroglobin attenuates apoptosis
induced by oxygen–glucose deprivation via the Jak2/Stat3 pathway in
vitro. Neurol Res. 37:531–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Li H and Li M: Curcumin protects
against cerebral ischemia-reperfusion injury by activating
JAK2/STAT3 signaling pathway in rats. Int J Clin Exp Med.
8:14985–14991. 2015.PubMed/NCBI
|
|
31
|
Shyu WC, Lin SZ, Chiang MF, Chen DC, Su
CY, Wang HJ, Liu RS, Tsai CH and Li H: Secretoneurin promotes
neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway
in murine models of stroke. J Clin Invest. 118:133–148. 2008.
View Article : Google Scholar : PubMed/NCBI
|