Introduction
Gastric ischemia-reperfusion injury (GI-RI) is
caused by the restoration of a blood supply following short-term
stomach blood supply deficiency (1).
It is commonly observed in critically ill patients (1). GI-RI is closely associated with the
mechanism of stress-related mucosal disease (SRMD) due to severe
trauma, burns, shock, major surgery and critical illness (1). Additionally, SRMD may further result in
prolonged hospitalization, mental pain and heavy economic burden.
It may also significantly increase mortality if complicated with
stress ulcer bleeding and enterogenous infection, which has led to
the consumption of lots of medical resources (2). Preventing and relieving SRMD and
maintaining the whole physiological status has been the focus of
emergency and critical care medicine and nursing (3).
Prostaglandin (PG) E2 is a PG with complex
biological activity, which is involved in GI-RI inflammation
(4). PGE2 synthase is the last key
enzyme in the synthesis of PGE2. PGE2 is the PG with the highest
abundance and greatest distribution in the human body (5). It serves a major role in inflammation
as a pain and fever mediator during inflammation. Additionally, it
may induce vasodilation and microvascular leakage (5). As a type of unsaturated fatty acid,
PGE2 is predominantly composed of 20 carbon atoms (6). It has the basic structure of one
five-carbon ring and two side chains. Arachidonic acid is
synthesized into PGE2 under the catalysis of cyclooxygenase (COX)
and PG synthase (6). PGE2 escapes
through facilitated diffusion and binds to E-prostanoid 1–4 in an
autocrine or paracrine manner. In this way, it may alter the levels
of intracellular second messengers, and send signals to cells,
causing a series of physiological or pathophysiological changes
(4).
Cyclooxygenase prevents the conversion of
arachidonic acid into PGE2 (7).
Therefore, it may quickly alleviate active substance-induced
inflammation. As a result, it may reduce the excitability of the
peripheral and central pain sensing conduction system (7). Thus, COX-2 serves anti-inflammatory and
analgesic functions. PGs may promote the secretion of gastric fluid
and bicarbonate. In this manner, they are able to protect the
gastric mucosal barrier, promote the renewal of gastric mucosal
cells and improve mucosal blood flow (8). Furthermore, COX-2 may stimulate the
active transport process of cells, activate adenylate cyclase and
stabilize the lysosome (8). It may
also maintain the level of mucosal thiol compounds and stimulate
surface-active phospholipids in gastric mucosa (8). Previous study has revealed that the
role of COX in the protection of gastric mucosa is not only
associated with the PGs that it produces, but is more related to a
variety of growth factors that it secretes, which act on
fibroblasts (8). Additionally, COX-2
may stimulate the synthesis and secretion of hepatocyte growth
factors and vascular endothelial growth factors, and promote the
repair of tissue injury (8,9).
Positive acceleration adaptive training (PAAT) is a
training method utilized by fighter pilots. Improving the load
resistance protective capability of high-performance fighter pilots
is a primary focus of aviation medicine research (10). In the field of aerospace medicine,
the alleviation of PAAT damage to pilot systemic organs has been a
topic of extensive research (11).
Previous studies have demonstrated that pre-adaptive training
mitigates damage to gastric mucosa of PAAT exposed rats (10,11).
However, the precise mechanism of action is yet to be elucidated.
Therefore, the aim of the present study was to determine the
effects of positive acceleration adaptive training (PAAT) on GI-RI
in a rat model.
Materials and methods
Models of ischemia/reperfusion
(I/R)-induced gastric injury
A total of 24 Male Sprague-Dawley rats (200–230 g,
8–10 weeks) were supplied by the Experimental Animal Center at
Peking University (Beijing, China). Rats were housed in 22–24°C at
a relative humidity of 60±10% under 12-h light/dark cycles. Rats
were sustained on standard rat chow and tap water ad
libitum. All experimental procedures were performed under the
Care of Laboratory Animals and approved by the Animal Care and Use
Committee of People's Liberation Army (Beijing, China) (12). The rats were randomly divided into
three groups (n=8/group): Sham operation, GI-RI model (gastric
model) and PAAT (gastric I/R injury) groups. In the PAAT group, the
rats were trained using PAAT for 7 days. Firstly, the rat was fixed
with a set-up box at a centrifuge with a radius of 1 m and the head
of the rat was orientated towards the axis of the centrifuge at 5 ×
g/day for 7 days (13).
Subsequently, gastric I/R injury was performed. The rats were
injected with 400 mg/kg chloral hydrate (intraperitoneal;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and the abdominal
cavity was opened. Celiac arterial trunk tissues were cautiously
isolated and clamped with a small vascular clamp for 1 h. This
procedure was also performed in the GI-RI model group, without
prior PAAT. In the sham operation group, the rats were injected
with 400 mg/kg chloral hydrate and the abdominal cavity was only
opened.
Histopathological measurements
The rats were sacrificed and the stomach was
removed, washed with PBS three times and fixed with 4%
paraformaldehyde for 24 h at room temperature. Tissue samples were
embedded in paraffin, sliced to 5-mm thickness and stained with
hematoxylin and eosin for 5 min at room temperature. All samples
were imaged using a laser scanning confocal microscope
(magnification, ×20; TCS SP5; Leica Microsystems GmbH, Wetzlar,
Germany).
Measurement of inflammation and
caspase-3 levels using ELISA kits
Blood was collected and centrifuged at 3,000 × g for
10 min at 4°C. Serum was collected and used to determine the levels
of interleukin (IL)-1β (cat. no. H002), IL-6 (cat. no. H007), tumor
necrosis factor-α (TNF-α; cat. no. H052) and nuclear factor-κB
(NF-κB, H202) using ELISA kits (Nanjing Jiancheng Biology
Engineering Institute, Nanjing, China). Total protein was extracted
from gastric tissues using radioimmunoprecipitation assay (RIPA;
Beyotime Institute of Biotechnology, Haimen, China) buffer and
quantified using a bicinchoninic acid (BCA) assay. A total of 10 µg
protein was used to measure the caspase-3 levels using an ELISA
kit.
Western blot assay
Total protein was extracted from gastric tissues
using lysis buffer (RIPA) and quantified using a BCA assay.
Proteins (50 µg) were separated by 8–12% SDS-PAGE, transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA), and blocked with 5% dried skimmed-milk in Tris-buffered
saline and 0.1% Tween 20 (Sangon Biotech Co., Ltd., Shanghai,
China) for 1 h at 37°C. Subsequently, membranes were incubated
overnight at 4°C with TNF-α (1:500; sc-8301), tumor necrosis factor
receptor 1 (TNFR1; 1:500; sc-7895), tumor necrosis factor-related
apoptosis inducing ligand (TRAIL; 1:500; sc-7877), death receptor
(DR)4 (1:500; sc-7863), DR5 (1:500; sc-166624), COX-2 (1:500;
sc-7951), COX-1 (1:500; sc-7950), PGE2 (1:500; sc-20676) and GAPDH
(1:500; sc-25778), all purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Following this, the membranes were
individually incubated for 2 h at room temperature with Horseradish
peroxidase conjugated goat anti-rabbit Immunoglobulin G (1:5,000,
cat. no. sc-2004; Santa Cruz Biotechnology, Inc.). Protein bands
were visualized using an enhanced chemiluminescence system
(Beyotime Institute of Biotechnology) and imaged using Quantity One
software version 4.62 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
All data were expressed as the mean ± standard
deviation using SPSS 21.0 (IBM Corp., Armonk, NY, USA). Statistical
analysis of the quantitative data for multiple group comparisons
was performed using one-way analysis of variance followed by
Duncan's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
PAAT alleviates GI-RI
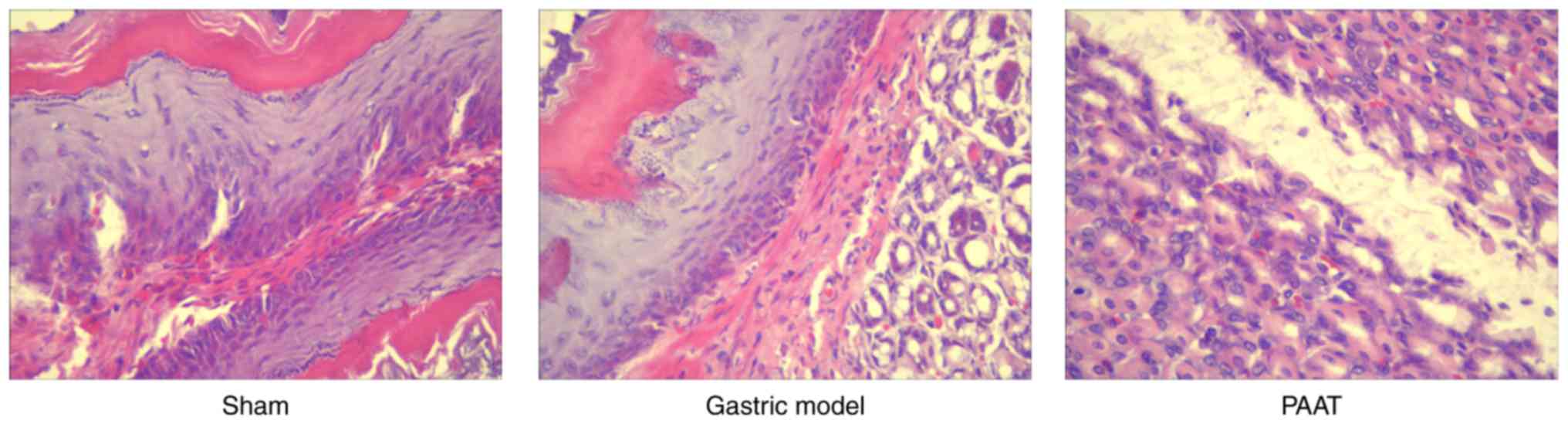
Compared with the sham group, stomach cells appeared
damaged in GI-RI model group. In addition, GI-RI was successfully
established in the model and it was demonstrated that PAAT
alleviated this injury (Fig. 1).
PAAT also effectively alleviated GI-RI compared with the GI-RI
model (Fig. 1). These results
demonstrated that PAAT prevented GI-RI; however, its mechanism is
yet to be elucidated.
PAAT reduces inflammatory factors in
rats with GI-RI
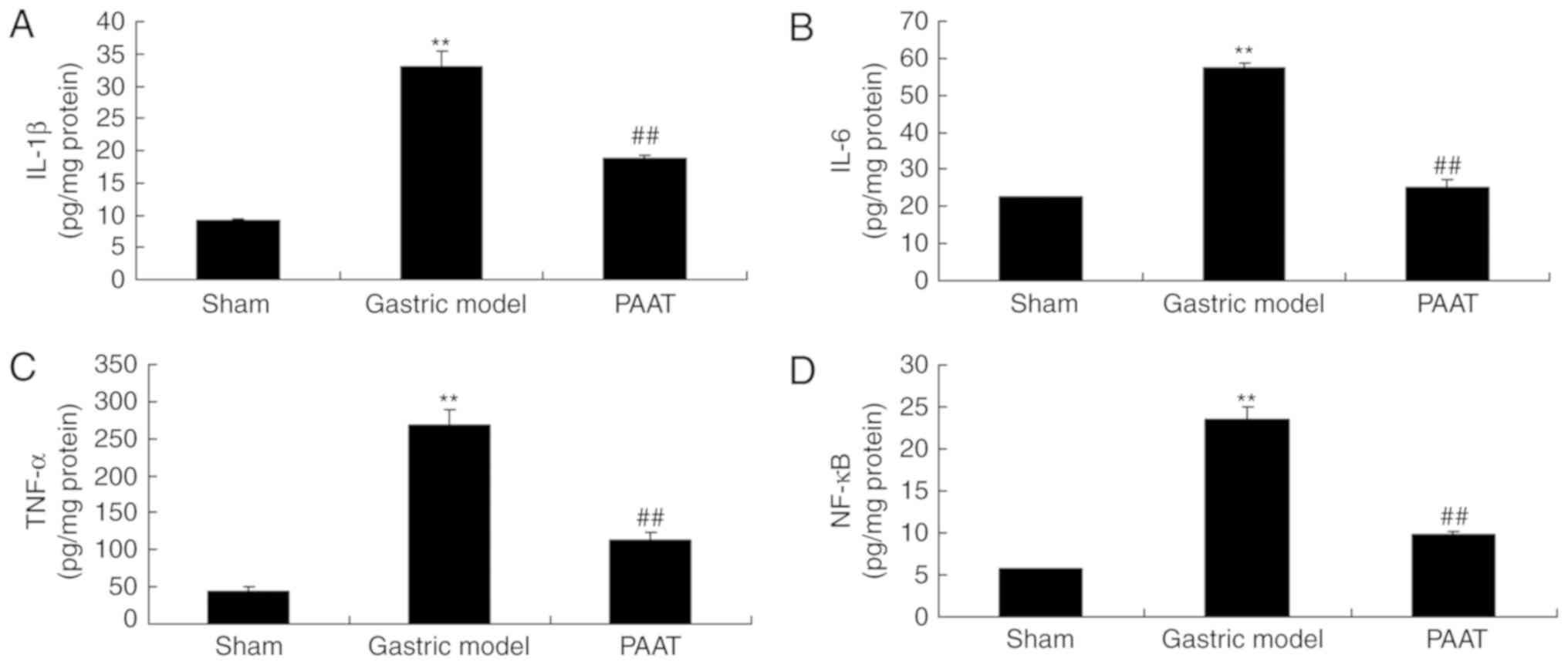
The levels of inflammatory factors were measured and
the results demonstrated that there were significant increases of
IL-1β, IL-6, TNF-α and NF-κB serum levels in the GI-RI model
compared with the levels in the sham group (Fig. 2). PAAT significantly alleviated
IL-1β, IL-6, TNF-α and NF-κB serum levels in rats with GI-RI
compared with the levels in the GI-RI model (Fig. 2). Therefore, these results
demonstrated that PAAT reduced inflammation in GI-RI.
PAAT suppresses caspase-3 activity in
rats with GI-RI
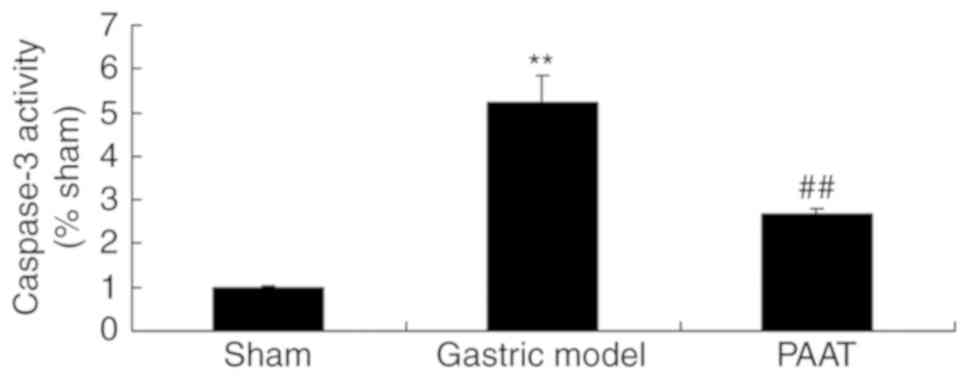
As demonstrated in Fig.
3, the caspase-3 activity in rats with GI-RI was significantly
increased compared with the level in the sham group. However, PAAT
significantly suppressed the caspase-3 activity in rats with GI-RI
compared with that in the GI-RI model group (Fig. 3). These results indicate that PAAT
may reduce GI-RI-induced apoptosis in a rat model.
PAAT suppresses TNF-α and TNFR1
protein expression levels in rats with GI-RI
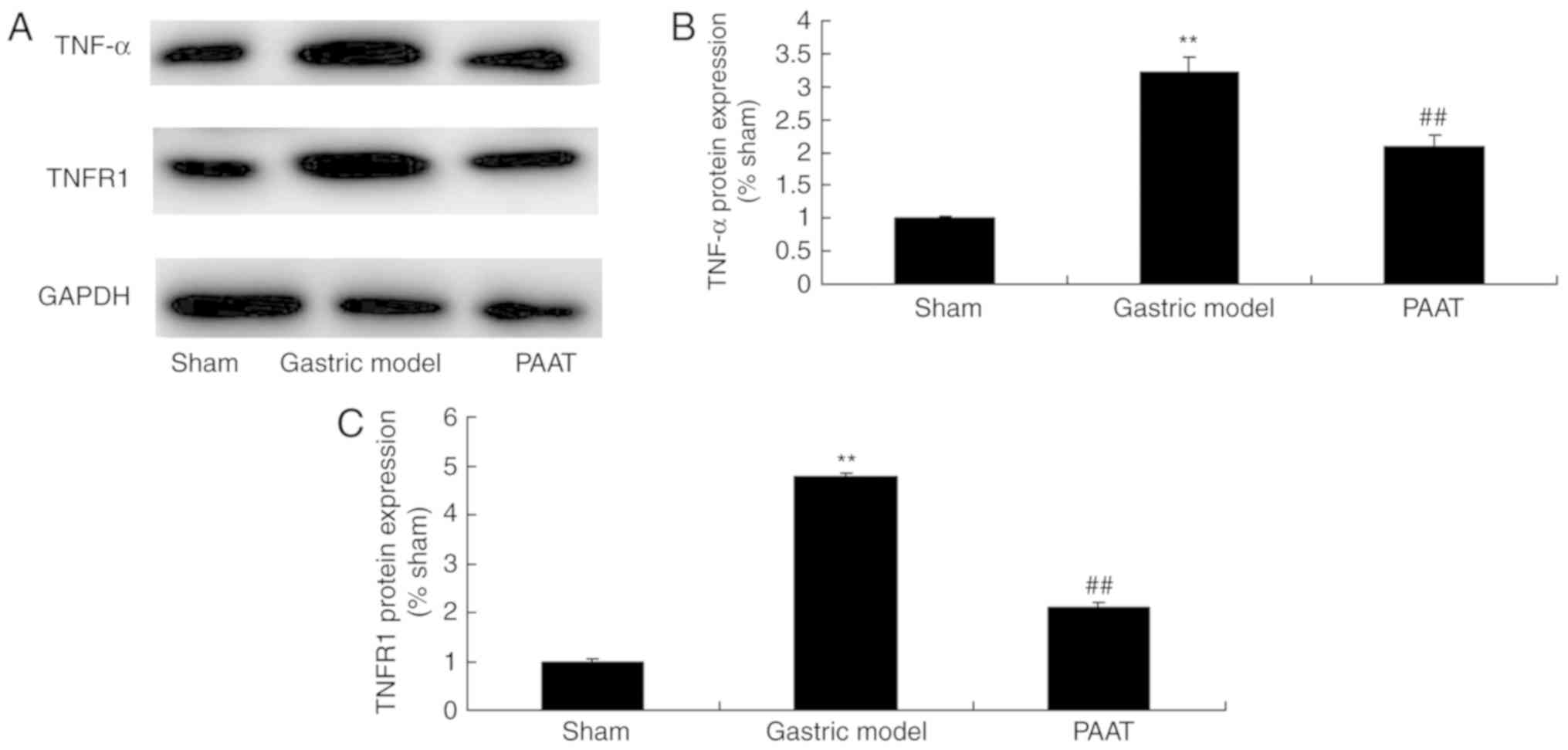
The protein expression levels of TNF-α and TNFR1 in
rats with GI-RI were significantly higher than those of the sham
group (Fig. 4). PAAT significantly
suppressed TNF-α and TNFR1 protein expression levels in rats with
GI-RI compared with the levels in the GI-RI model group (Fig. 4). These results indicated that PAAT
significantly suppressed TNF-α and TNFR1 protein expression levels,
thus reducing inflammation in GI-RI.
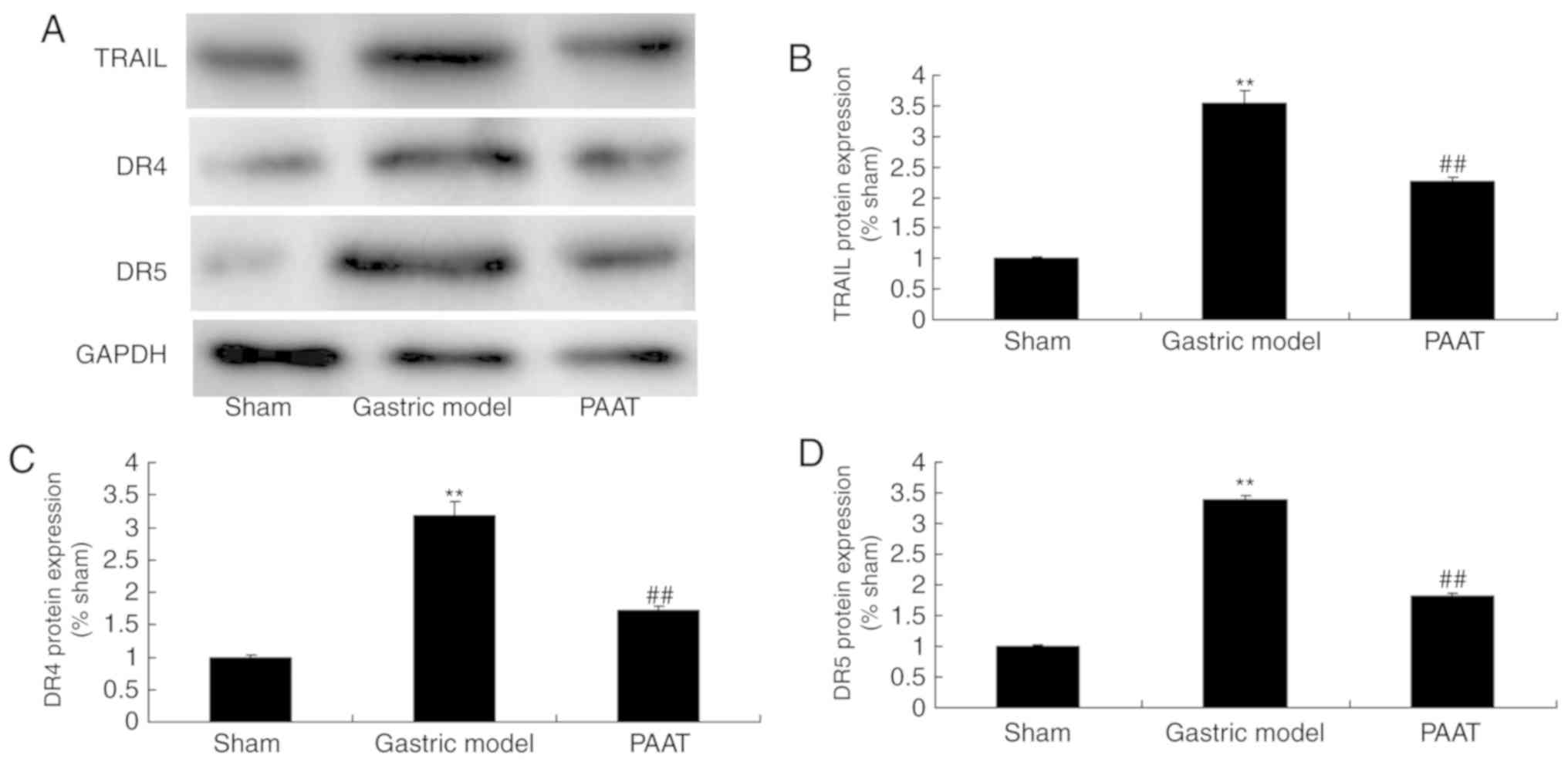
PAAT suppresses TRAIL, DR4 and DR5
protein expression in rats with GI-RI
As demonstrated in Fig.
5, TRAIL, DR4 and DR5 protein expression levels in rats with
GI-RI were significantly induced compared with the levels in the
sham group. Furthermore, PAAT significantly suppressed TRAIL, DR4
and DR5 protein expression levels in rats with GI-RI compared with
the levels in the GI-RI model group (Fig. 5). These data demonstrated that PAAT
reduced inflammation in GI-RI via the regulation of TRAIL, DR4 and
DR5 expression.
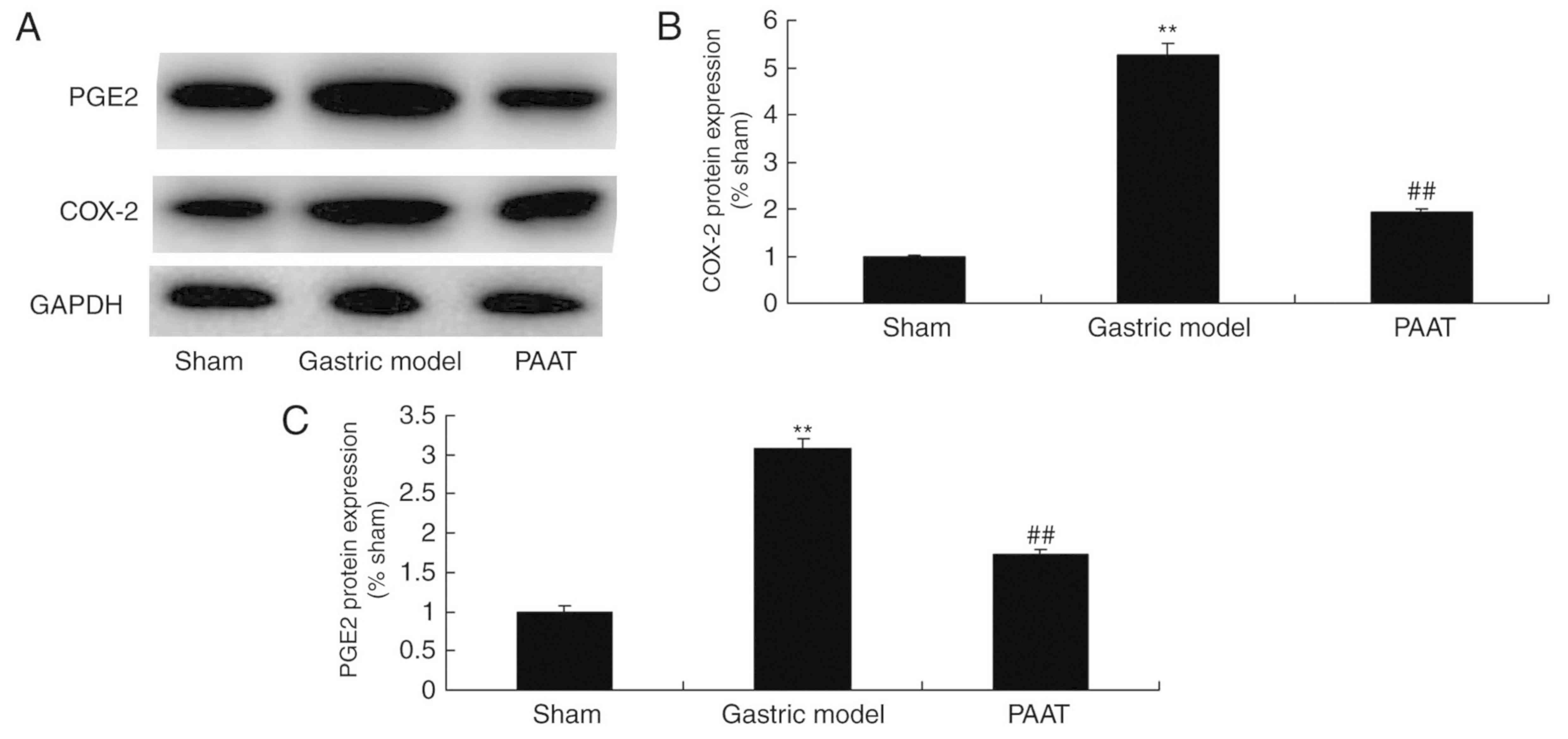
PAAT suppresses COX-2 and PGE2 protein
expression levels in rats with GI-RI
As demonstrated in Fig.
6, COX-2 and PGE2 protein expression levels in rats with GI-RI
were higher than those of the sham group. Additionally, PAAT
significantly suppressed COX-2 and PGE2 protein expression levels.
(Fig. 6). The results demonstrated
that PAAT suppresses COX-2 and PGE2 protein expression levels to
inhibit inflammation in GI-RI.
Discussion
GI-RI is common in critically ill patients (10). Neuroendocrine system dysfunction
occurs under the stress of injury, burns, shock, major surgery and
critical diseases. This results in increased catecholamine
production, vasoconstriction, reduced blood volume, decreased
cardiac output and the release of pro-inflammatory factors
(14). Therefore, the blood may flow
into the heart, brain and muscles from the gastrointestinal tract
and skin (7). In this manner, GI-RI
may ensure a sufficient blood supply for vital organs.
Gastrointestinal blood flow is reduced with disease progression.
This leads to an insufficient oxygen supply and decreased
bicarbonate secretion, thus damaging the gastric mucosa (12). Hydrogen ion reflux and pepsin erosion
may further injure the mucosal epithelial layer in the presence of
damage to the gastric mucosal layer (12). Slowing down the blood flow may affect
the healing of gastric mucosa, accompanied with splanchnic
perfusion deficiency and decreased gastrointestinal motion
(1). Additionally, elimination of
acids and other irritants in the stomach may be slowed down
(6). Thus, the damaged gastric
mucosa may be exposed to gastric acid for an increased time, which
thereby increases the risk of ulcer formation (12). Nitric oxide (NO) synthase levels
increase with reperfusion following prolonged ischemia, resulting
in congestion and cell death, and promotion of the inflammatory
response (2).
Numerous cytokines (including TNF-α, NO, IL-1 and
IL-8) are involved in GI-RI. Amongst them, TNF-α is the first and
core endogenous inflammatory cytokine released from the
inflammatory response. TNF-α is also the major cause of the
inflammatory cascade reaction (6).
Normal TNF-α levels serve an important role in the natural immune
and host defense. However, excessive production and release of
TNF-α may destroy the body's immunological equilibrium, which may
cause a variety of pathological damages, along with other
inflammatory factors (13). The main
biological function of TNF-α is to induce the inflammatory response
by upregulating gene transcription (15). It is involved in numerous
physiological and pathological responses via its receptor, TNFR1.
Furthermore, it serves a vital role in inflammation, proliferation,
differentiation and other pathophysiological processes (16). PAAT is known to reduce the
inflammatory response (17). The
present study indicated that PAAT significantly alleviated IL-1β,
IL-6, TNF-α and NF-κB serum levels in rats with GI-RI.
The association between pathogenesis and the
development of GI-RI apoptosis has not yet been fully elucidated.
Nonetheless, the mechanism of apoptosis has always been a research
hotspot and a source of debate. A previous study revealed that
apoptosis has far-reaching significance and broad application
prospects in the etiology and treatment of GI-RI science (7,18).
However, scholars have also identified another TNF family of TRAIL
(7,18).
TRAIL has been revealed in a previous study to lead
to selective apoptosis of virus-infected tissues or cells and
transformed cells (19). However, it
may not affect the growth and differentiation of normal cells
(19). FAS/FAS ligand- and
TNFR1/TNF-α-induced apoptosis regulates the DR signaling pathway
(18). In addition, the
corresponding DRs mainly include DR3, DR4 and DR5 (18). Amongst them, FAS and TNF-α belong to
the TNF superfamily, which have been revealed to be closely
associated with the incidence and development of GI-RI (20). Consistently, the present study
demonstrated that PAAT significantly suppresses TNF-α and TNFR1
protein expression and reduces TRAIL, DR4 and DR5 protein
expression levels in rats with GI-RI.
PG2 is a PG with complex biological activity, which
is involved in GI-RI inflammation (19). The levels of PGE2, TNF-α, IL-1β and
leukotriene B4 have been demonstrated to increase in a rat model of
Escherichia coli lipopolysaccharide-induced GI-RI (19). PGE2, which is a pro-inflammatory
mediator, serves a regulatory role in lung injury (21). Furthermore, important cellular
mechanisms of GI-RI include the recruitment and activation of
neutrophils in the lungs (19).
Additionally, the release of oxygen free radicals, protease,
inflammatory mediators and macrophages, as well as capillary
endothelial cell involvement, is also involved in GI-RI (22). The data in the present study
indicated that PAAT significantly suppressed PGE2 protein
expression levels in rats with GI-RI.
COX is a membrane-bound protein that mainly exists
in microsomal membrane. The human COX-1 gene is ~22.5 kb in length
(7). Transcriptional regulation is
the major regulation mode of COX-2 expression (7). Furthermore, the promoter and its
activity are of great significance to the regulation of COX-2
transcription (23). The COX-2
promoter is 1 kb in length. However, the conserved sequence of
COX-1 is not evident, with a promoter of 2.4 kb in length (21). Various activator molecules, including
cytokines, serum or protein kinase C, regulate the expression of
two enzymes independently (21).
Transcription factor sequences containing COX-2 promoters include
GATA-1, NF-κB and IL-6 (21). The
mechanism of COX-2 expression and regulation is complex, and varies
between different cell types and divergent inducers (24). Macrophages are the main source of
PGE2 induction and lead to the generation of the inflammatory
response (6). COX-2 only exists in
macrophages and fibroblasts in patients with gastric ulcers between
necrosis and granuloma tissues (23). COX-2 is mainly responsible for the
regulation of inflammation in GI-RI (23). The results of the present study
revealed that PAAT significantly suppressed COX-2 protein
expression levels and did not notably affect COX-1 protein
expression in rats with GI-RI. The present study also demonstrated
that PAAT is able to inhibit inflammation; however, its specific
mechanism requires further investigation.
In summary, PAAT attenuated GI-RI and reduced
inflammation via the downregulation of COX-2 and PGE2 expression.
The results indicate that suppression of COX-2 and PGE2, but not
COX-1, is involved in the alleviation of GI-RI by PAAT, which
inhibits inflammation.
Acknowledgements
The present study was financially supported by
Supported by the 12th Five-Year Major Scientific Research Program
of General Logistics Department of PLA (grant no. AKJ11J004).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ward MG, Warner B, Unsworth N, Chuah SW,
Brownclarke C, Shieh S, Parkes M, Sanderson JD, Arkir Z, Reynolds
J, et al: Infliximab and adalimumab drug levels in Crohn's disease:
Contrasting associations with disease activity and influencing
factors. Aliment Pharmacol Ther. 46:150–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bukowczan J, Warzecha Z, Ceranowicz P,
Kusnierz-Cabala B and Tomaszewska R: Obestatin accelerates the
recovery in the course of ischemia/reperfusion-induced acute
pancreatitis in rats. PLoS One. 10:e01343802015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Broekman MM, Coenen MJ, Wanten GJ, van
Marrewijk CJ, Klungel OH, Verbeek AL, Hooymans PM, Guchelaar HJ,
Scheffer H, Derijks LJ, et al: Risk factors for thiopurine-induced
myelosuppression and infections in inflammatory bowel disease
patients with a normal TPMT genotype. Aliment Pharmacol Ther.
46:953–963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Del Carmen S, de Moreno de, LeBlanc A and
LeBlanc JG: Development of a potential probiotic yoghurt using
selected anti-inflammatory lactic acid bacteria for prevention of
colitis and carcinogenesis in mice. J Appl Microbiol. 121:821–830.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Negroni A, Prete E, Vitali R, Cesi V, Aloi
M, Civitelli F, Cucchiara S and Stronati L: Endoplasmic reticulum
stress and unfolded protein response are involved in paediatric
inflammatory bowel disease. Dig Liver Dis. 46:788–794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roblin X, Boschetti G, Williet N, Nancey
S, Marotte H, Berger A, Phelip JM, Peyrin-Biroulet L, Colombel JF
and Del Tedesco E: Azathioprine dose reduction in inflammatory
bowel disease patients on combination therapy: An open-label,
prospective and randomised clinical trial. Aliment Pharmacol Ther.
46:142–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brzozowski T, Konturek PC, Konturek SJ,
Sliwowski Z, Drozdowicz D, Stachura J, Pajdo R and Hahn EG: Role of
prostaglandins generated by cyclooxygenase-1 and cyclooxygenase-2
in healing of ischemia-reperfusion-induced gastric lesions. Eur J
Pharmacol. 385:47–61. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tlaskalová-Hogenová H, Stěpánková R,
Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř
T, Kverka M, Zákostelská Z, et al: The role of gut microbiota
(commensal bacteria) and the mucosal barrier in the pathogenesis of
inflammatory and autoimmune diseases and cancer: Contribution of
germ-free and gnotobiotic animal models of human diseases. Cell Mol
Immunol. 8:110–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akahoshi T, Tanigawa T, Sarfeh IJ, Chiou
SK, Hashizume M, Maehara Y and Jones MK: Selective cyclooxygenase
(COX) inhibition causes damage to portal hypertensive gastric
mucosa: Roles of nitric oxide and NF-kappaB. FASEB J. 19:1163–1165.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Tang HL, Chen Y, Fan Q, Shao YT, Jia
M, Wang JC and Yang CM: Malondialdehyde and SOD-induced changes of
gastric tissues in acute gastric mucosal injury under positive
acceleration. Genet Mol Res. 14:4361–4368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sarosiek I, Bashashati M, Alvarez A, Hall
M, Shankar N, Gomez Y, McCallum RW and Sarosiek J: Lubiprostone
accelerates intestinal transit and alleviates small intestinal
bacterial overgrowth in patients with chronic constipation. Am J
Med Sci. 352:231–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Sandhu HK, Zhi F, Hua F, Wu M and
Xia Y: Effects of hypoxia and ischemia on microRNAs in the brain.
Curr Med Chem. 22:1292–1301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gezginci-Oktayoglu S, Orhan N and Bolkent
S: Prostaglandin-E1 has a protective effect on renal
ischemia/reperfusion-induced oxidative stress and inflammation
mediated gastric damage in rats. Int Immunopharmacol. 36:142–150.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen T, Kim CY, Kaur A, Lamothe L, Shaikh
M, Keshavarzian A and Hamaker BR: Dietary fibre-based SCFA mixtures
promote both protection and repair of intestinal epithelial barrier
function in a Caco-2 cell model. Food Funct. 8:1166–1173. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bibi S, Kang Y, Du M and Zhu MJ: Dietary
red raspberries attenuate dextran sulfate sodium-induced acute
colitis. J Nutr Biochem. 51:40–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XZ, Jiang WD, Feng L, Wu P, Liu Y,
Zeng YY, Jiang J, Kuang SY, Tang L, Tang WN and Zhou XQ: Low or
excess levels of dietary cholesterol impaired immunity and
aggravated inflammation response in young grass carp
(Ctenopharyngodon idella). Fish Shellfish Immunol.
78:202–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Devkota S and Chang EB: Interactions
between diet, Bile acid metabolism, gut microbiota, and
inflammatory bowel diseases. Dig Dis. 33:351–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CC, Lin WN, Cho RL, Wang CY, Hsiao LD
and Yang CM: TNF-α-induced cPLA2 expression via NADPH
oxidase/reactive oxygen species-dependent NF-κB cascade on human
pulmonary alveolar epithelial cells. Front Pharmacol. 7:4472016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang F, Xu C, Ning L, Hu F, Shan G, Chen
H, Yang M, Chen W, Yu J and Xu G: Exploration of serum proteomic
profiling and diagnostic model that differentiate Crohn's disease
and intestinal tuberculosis. PLos One. 11:e01671092016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng L, Jin H, Qiang Y, Wu S, Yan C, Han
M, Xiao T, Yan N, An H, Zhou X, et al: High fat diet exacerbates
dextran sulfate sodium induced colitis through disturbing mucosal
dendritic cell homeostasis. Int Immunopharmacol. 40:1–10. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Battison AL, Després BM and Greenwood SJ:
Ulcerative enteritis in Homarus americanus: Case report and
molecular characterization of intestinal aerobic bacteria of
apparently healthy lobsters in live storage. J Invertebr Pathol.
99:129–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ichimasa K, Kudo SE, Mori Y, Misawa M,
Matsudaira S, Kouyama Y, Baba T, Hidaka E, Wakamura K, Hayashi T,
et al: Artificial intelligence may help in predicting the need for
additional surgery after endoscopic resection of T1 colorectal
cancer. Endoscopy. 50:C22018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watson H, Cockbain AJ, Spencer J, Race A,
Volpato M, Loadman PM, Toogood GJ and Hull MA: Measurement of red
blood cell eicosapentaenoic acid (EPA) levels in a randomised trial
of EPA in patients with colorectal cancer liver metastases.
Prostaglandins Leukot Essent Fatty Acids. 115:60–66. 2016.
View Article : Google Scholar : PubMed/NCBI
|