Introduction
Chronic hepatitis B (CHB) is a common disease in
clinical medicine. According to the World Health Organization,
about 1 million people die each year from liver failure, liver
cirrhosis and primary hepatocellular carcinoma caused by HBV
infection, which seriously endanger human health (1,2).
Although some oral antiviral drugs and interferon have been widely
used in clinic in recent years, the trend that CHB develops into
cirrhosis, severe hepatitis has not been effectively curbed.
Reducing the incidence of end-stage complications such as
cirrhosis, effectively prolonging the life cycle of patients and
improving the quality of life has become the key to be solved in
the current medical field. Transition from CHB to liver cirrhosis
involves many factors in key roles, such as changes in the ratio of
T-helper 17 cells (Th17) to Treg cells, changes in the levels of
alanine aminotransferase (ALT) and aspartic transaminase (AST) in
liver function as well as the expression of various
inflammation-associated factors (3–5).
In this study, with CHB patients and liver cirrhosis
patients as subjects, we detected the changes in Th17/Treg ratio,
variations in ALT and AST levels in liver function, and the
expression of inflammation-associated factors [including
interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and
nuclear factor κB (NF-κB) in liver functions] in liver tissues of
all the groups to find the differences in expression during the
transition from CHB to liver cirrhosis, and explore the
correlations of Th17/Treg ratio with the liver function and
inflammation in this process, aiming to provide new ideas and
orientation for genetic diagnosis and treatment of CHB and liver
cirrhosis.
Patients and methods
Sample collection
Patients
There were 35 CHB patients and 40 post-hepatitis
liver cirrhosis patients who were admitted to Yantai Infectious
Disease Hospital (Yantai, China) between May 2010 and July 2015,
included in this study. Samples were collected from tissues
resected during surgeries, fixed in 10% formaldehyde and embedded
in paraffin. A total of 35 paraffin samples were collected from
patients who underwent resection surgery in the hospital and were
diagnosed as CHB through postoperative histopathological
examination: There were 18 males and 17 females aged from 30 to 62
years. Additionally, 40 paraffin samples were collected from those
who also underwent surgical resection in the hospital and were
diagnosed as post-hepatitis liver cirrhosis: There were 27 males
and 13 females aged from 36 to 69 years. In addition, 20 samples of
normal liver tissues were collected from the liver transplantation
center of the hospital as the control group, including 14 males and
6 females aged from 30 to 48 years.
The study was approved by the Ethics Committee of
Yantai Infectious Disease Hospital and informed consents were
signed by the patients or the guardians.
Major reagents
Roswell Park Memorial Institute (RPMI)-1640 medium
and fetal bovine serum (FBS) were purchased from Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA); detection kits of AST
and ALT were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China); bicinchoninic acid (BCA) detection kit
of protein concentration and BeyoECL Plus kit were purchased from
Beyotime Institute of Biotechnology (Haimen, China); extraction kit
of tissue protein was purchased from Nanjing KeyGen Biotech Co.,
Ltd. (Nanjing, China); RNAiso Plus, PrimeScript® RT
reagent kit with gDNA Eraser, SYBR®Premix Ex Taq™ II
(Tli RNaseH Plus) was purchased from Takara Biotechnology Co., Ltd.
(Dalian, China); primary, secondary and fluorescent secondary
antibodies of glyceraldehyde-phosphate dehydrogenase (GAPDH) and
NF-κB were purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA).
Experimental methods
Detection of Th17/Treg cell frequency in peripheral
blood. Peripheral venous blood (5 ml) was extracted from patients
of the control, CHB and liver cirrhosis groups for isolating the
peripheral blood mononuclear cells (PBMCs) in density of
2×106/ml through Ficoll density gradient centrifugation.
The results were analyzed to identify the difference in frequencies
of Th17 and Treg among the groups, and thus explore the
correlations of Th17/Treg ratio with the liver function and
inflammation.
Measurement of liver functions
According to the instructions of the kit, we assayed
the enzymatic activity of AST and ALT in serum to evaluate the
damage to liver of patients in three groups.
Hematoxylin and eosin (H&E)
staining
In all the groups, samples of liver tissues were
embedded in paraffin to prepare the paraffin blocks, which were
later sliced into 5 µm sections as the blank sections. Thereafter,
according to the regular method of histopathology, H&E staining
was performed for sections that were later placed under a
microscope (×200, Olympus Corporation, Tokyo, Japan) for
histopathological analysis.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Liver tissues in appropriate amount in the control,
CHB and liver cirrhosis groups were transferred rapidly into the
reagent supplemented with 1 ml TRIzol to sufficiently grind the
tissues into homogenate. Homogenate was placed at room temperature
for 5 min RIPA lysis buffer, and then 12,000 × g for 5 min at 4°C.
Supernatant was collected, mixed well with chloroform and placed at
room temperature for 5 min, followed by 12,000 × g for 15 min at
4°C. The same volume of isopropanol was added in the supernatant,
and the solution was placed at room temperature for 10 min,
followed by 12,000 × g for 10 min at 4°C. Sediment was collected,
and mixed with 75% ethanol to rinse the sediment of RNA.
Subsequently, RNase-free water was added to resolve the sediment,
and the optical density (OD) was determined to calculate the
OD260/OD280 ratio for measurement of RNA
concentration. Under the manufacturer's instructions, stepwise
amplification was performed for the primer sequences (Table I), and the product was collected for
RT-PCR analysis.
 | Table I.Primer sequences in RT-PCR. |
Table I.
Primer sequences in RT-PCR.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| Human
GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
| Human
IL-1β |
CTGAGCACCTTCTTTCCCTTCA |
TGGACCAGACATCACCAAGCT |
| Human
IL-6 |
TGGCTGAAAAAGATGGATGCT |
TCTGCACAGCTCTGGCTTGT |
| Human
TNF-α |
TGTAGCCCATGTTGTAGCAAACC |
GAGGACCTGGGAGTAGATGAGGTA |
Immunofluorescent assay
After being fixed in 10% formaldehyde for 48 h,
liver tissues in the three groups were embedded in paraffin
regularly to prepare the sections (5 µm). Paraffin sections, after
being dewaxed in xylene and dehydrated in ethanol of gradient
concentrations, underwent antigen retrieval, followed by rinsing
with 0.01 M phosphate-buffered saline (PBS; pH 7.4) three times (5
min/time). Thereafter, sections were blocked in a wet box
containing 10% bovine serum albumin (BSA) at 37°C for 30 min,
followed by incubation with appropriately diluted (1:70) mouse
anti-human GAPDH and NF-κB monoclonal antibodies (cat. nos.
sc-365062 and sc-515045; Santa Cruz Biotechnology, Inc.) on a
section at 4°C overnight. The section were rinsed with PBS (pH 7.4)
three times (5 min/time), fluorescent rabbit anti-mouse secondary
polyclonal antibody (diluted at 1:100; cat. no. sc-2010; Santa Cruz
Biotechnology, Inc.) were added on the sections in the dark for
incubation in a wet box at 37°C for 2 h. The sections were then
rinsed using PBS (pH 7.4) three times (5 min/time),
4′,6-diamidino-2-phenylindole (DAPI) was added in the dark for
staining for 15 min, and the sections were mounted with the 100%
glycerol. Finally, with an upright fluorescent microscope (Olympus
Corporation), the sections were observed and images were
captured.
Western blot analysis
After being rinsed with ice-cold normal saline,
samples of liver tissue in three groups were used for protein
extraction in accordance with instructions of the extraction kit
for total protein. Samples were ground sufficiently on ice with
immunoprecipitation lysis containing phenylmethanesulfonyl fluoride
(PMSF) and protease inhibitor. Tissues were then centrifuged at
12,000 × g for 10 min at 4°C for sufficient homogenization, and the
supernatant was again centrifuged 12,000 × g at 4°C for 20 min.
After protein quantification with some of the supernatant, samples
containing the same volume of total protein in the rest of
supernatant were loaded in the wells for isolation through sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under
a constant voltage of 220 V until the bromophenol blue reached the
bottom of the gel. According to molecular weight of the target
proteins, gel was cut, and the proteins on the gel were transferred
onto the polyvinylidene fluoride (PVDF) membrane. Then, PVDF
membrane with protein was blocked in 5% skimmed milk on a shaker at
room temperature for 3 h, and incubated with corresponding primary
antibody (1:1,000) at 4°C overnight. Thereafter, membrane was
rinsed with Tween-20 and Tris-buffer saline (TTBS) three times (10
min/time), and incubated with secondary antibody (1:2,000) for 1 h
at room temperature. After the membrane was rinsed with TTBS three
times (10 min/time), enhanced chemiluminescence (ECL) reagent was
added for color development and photographing (ImageJ software;
National Institutes of Health, Bethesda, MD, USA). The gray value
of theband was scanned by Quantity One software (Bio-Rad
Laboratories, Hercules, CA, USA).
Statistical analysis
Experiment data were presented as mean ± standard
deviation, and the results were analyzed with SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). A t-test was carried out for mean
comparison between two groups, while one-way analysis of variance
with LSD post hoc test for comparison of means of samples among
groups, and p-test for pairwise comparison. P<0.05 suggested
that the difference had statistical significance.
Results
Results of changes in Th17/Treg
ratio
As shown in Table
II, frequency of Treg cells in the CHB and liver cirrhosis
groups were significantly higher than that in the control group,
and similar phenomena were also observed in a comparison of Th17
cell frequencies among these groups. In the CHB and liver cirrhosis
groups, increases in Th17 cells were more evident than those in
Treg cells, and the Th17/Treg ratio was also higher than that in
control group.
 | Table II.Changes in Th17/Treg ratio in control
group, CHB group and liver cirrhosis group. |
Table II.
Changes in Th17/Treg ratio in control
group, CHB group and liver cirrhosis group.
| Index | Control group | CHB group | Liver cirrhosis
group |
|---|
| Thl7 (%) | 0.21±0.18 | 0.22±0.21 | 0.24±0.19 |
| Treg (%) | 6.02±1.12 | 6.73±1.68 | 6.94±2.13 |
| Thl7/Treg ratio | 35.21±13.18 | 65.22±34.32 | 69.25±43.64 |
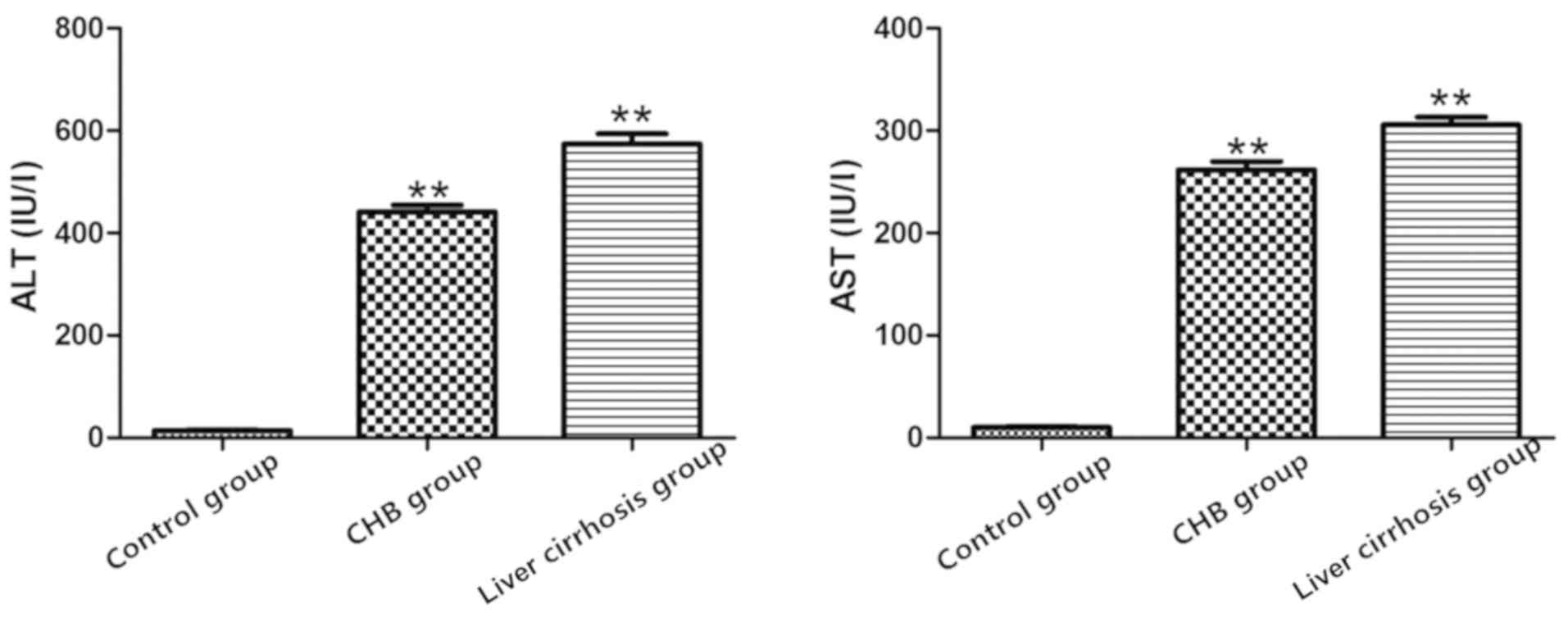
Liver functions in the three
groups
The results showed that compared with control group,
increases in levels of AST and ALT in the CHB and liver cirrhosis
groups were more evident (P<0.01), suggesting a decline in liver
functions in CHB group and liver cirrhosis group, and severe damage
to liver (Fig. 1).
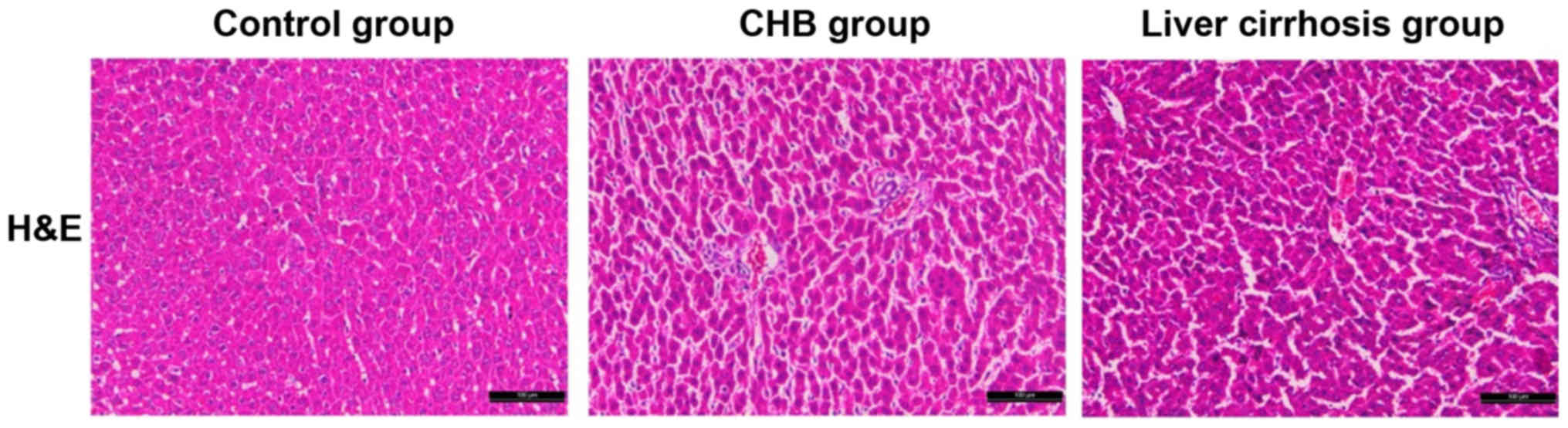
Observation of pathological features
through H&E staining
With the H&E staining section in the control,
CHB and liver cirrhosis groups, we aimed to identify the
differences in pathological features of samples. Compared with the
sections in control group, structures of liver tissues in sections
of the CHB and liver cirrhosis groups were destroyed with
hyperplasia of fibrous connective tissue, severe steatosis
surrounding the central veins of liver, diversified size, hydropic
degeneration and cloudy swelling of peripheral hepatocytes. In some
massive necrosis area of hepatocytes, infiltration of inflammatory
cells was also identified. As shown in Fig. 2, there were significant differences
in the histopathological features of liver tissues among the three
groups.
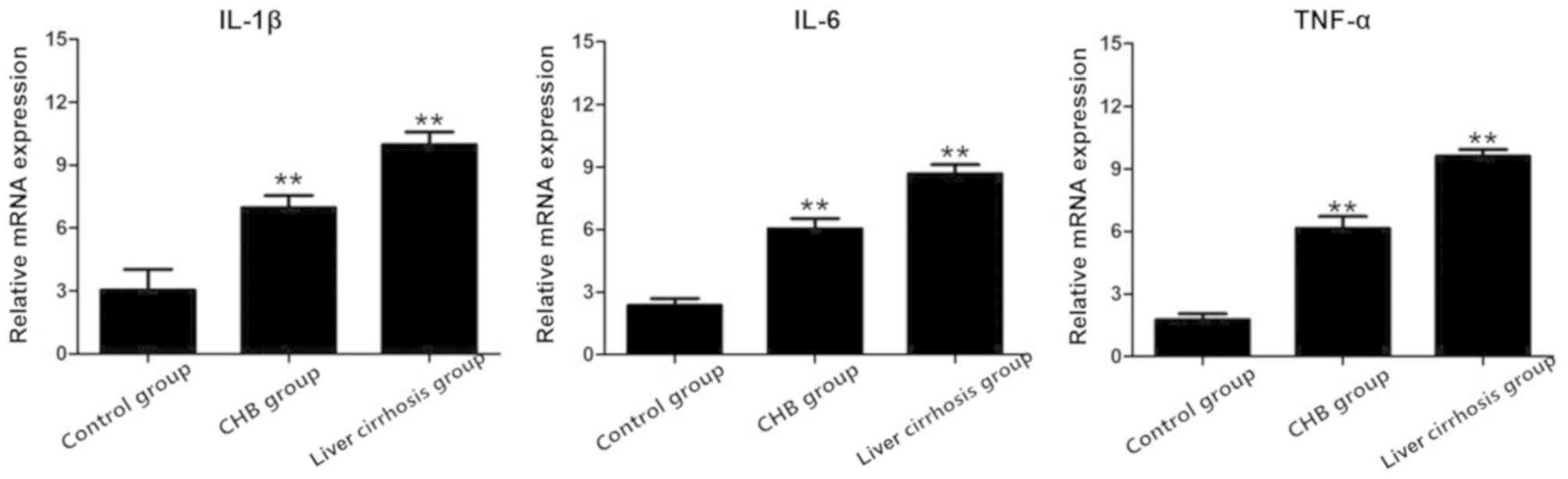
Results of RT-PCR
After RT-PCR, total RNA extracted from the three
groups was used to detect the mRNA expression of IL-1β, IL-6 and
TNF-α, and the results showed that the mRNA expression of these
indexes in the CHB and liver cirrhosis groups were obviously higher
than those in the control group (Fig.
3).
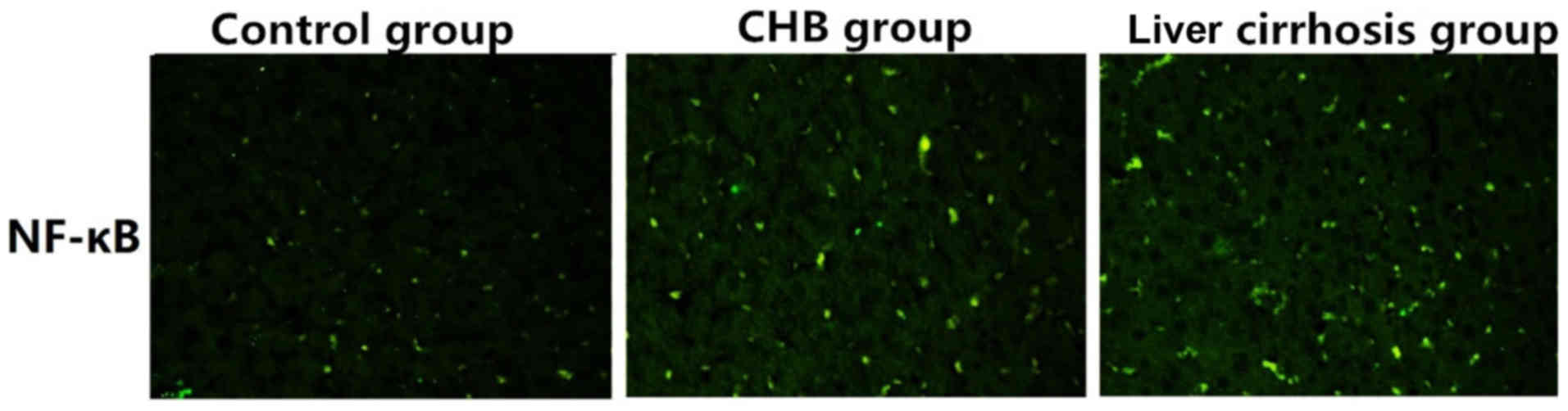
Detection of the expression of NF-κB
in control group, CHB group and liver cirrhosis group via
immunofluorescence
As shown in Fig. 4,
the expression of NF-κB in control group was significantly lower
than those in the CHB and liver cirrhosis groupd, revealing that
NF-κB is critical to the development and progression in CHB, or
even liver cirrhosis.
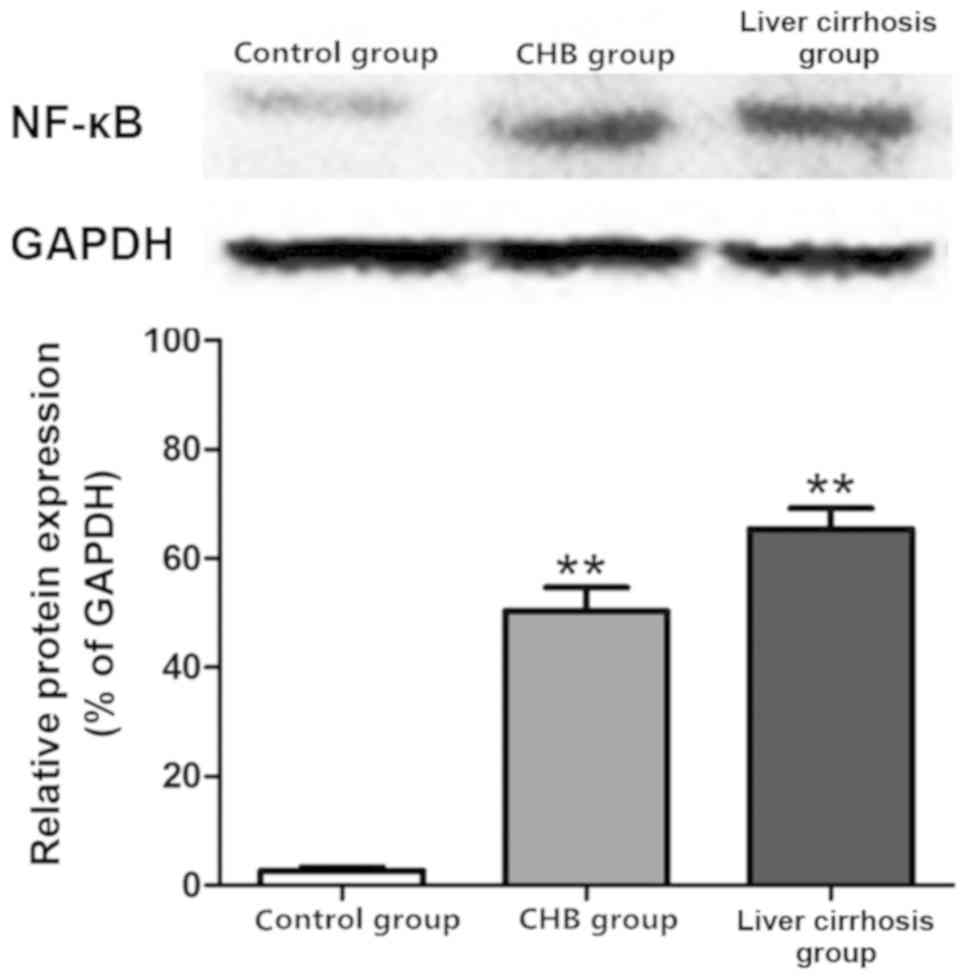
Protein expression of NF-κB in the
control, CHB and liver cirrhosis groups
Results of the western blot analysis revealed the
protein expression of NF-κB in the three groups. As shown in
Fig. 5, the protein expression of
NF-κB in liver tissues of the CHB and liver cirrhosis groups was
far higher than that in control group.
Discussion
Liver is one of the major organs with strong
defensive ability and regeneration ability in the body. Damage to
liver affects the normal functions of body severely, and a variety
of common liver diseases in clinic, including viral hepatitis,
hepatic fibrosis, liver cirrhosis and liver cancer, seriously
threaten the life and health of human beings (6–8). In the
transition of CHB to liver cirrhosis, multiple factors are
involved, including inflammation, oxidative stress, apoptosis and
autophagy (9–11). Without effective treatment, it will
evolve into liver cancer, leading to severe threat to life and
health of human (12–14). Therefore, prophylaxis and treatment
of liver diseases remain global problems, and it is imperative to
develop an effective and safe treatment method for liver disease.
Through years of studies, we have identified the genes that closely
correlate with the disease, and appropriate intervention on these
genes is expected to be a new orientation for treatment.
Inflammation is a complicated defensive response of
living tissues with vascular system to destructive factors
(15–17). Inflammatory factors mainly refer to
the cytokines involved in inflammatory responses, and can be
classified into several types: TNF-α, IL-1β, IL-6 and TGF-β. The
roles of inflammatory factors are mainly to induce the
proliferation and differentiation of T lymphocytes in the body
(18). Among various
inflammation-associated cytokines, TNF-α, IL-1β and IL-6 are
considered as the inflammatory factors with major effects (19,20).
In this study, 20 healthy subjects (control group),
35 CHB patients (CHB group) and 40 post-hepatitis liver cirrhosis
patients (liver cirrhosis group) were enrolled into this study. In
addition, liver functions were measured through the levels of ALT
and AST with corresponding kits, and evident increases in levels of
ALT and AST were identified in the CHB and liver cirrhosis groups
in comparison with the levels in control group, suggestive of
severe liver injury in the CHB and liver cirrhosis groups.
Furthermore, H&E staining results reflected directly the
differences in histopathological features in the three groups, and
RT-PCR analysis showed the significant differences in mRNA
expression of inflammation-associated factors, including IL-1β,
IL-6 and TNF-α. Moreover, IL-1β, IL-6 and TNF-α were highly
expressed in the CHB and liver cirrhosis groups, and expressed at
low level in control group. The results of immunofluorescent assay
and western blot assay showed that the expression of NF-κB in CHB
group and liver cirrhosis group was significantly higher than that
in control group.
In conclusion, transition from CHB to liver
cirrhosis comes with significant changes in Th17/Treg ratio, which
is correlated with the decline in liver function, and also closely
associated with the development and progression of inflammation. In
future, a new treatment method is expected to protect the liver
functions through control of the deterioration of inflammation to
protect the liver and reverse the liver disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM wrote the manuscript. HM and SW performed PCR and
western blot analysis. GZ and JW were responsible for
immunofluorescent assay and H&E staining analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yantai Infectious Disease Hospital (Yantai, China) and informed
consents were signed by the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zou SS, Yang W, Yan HX, Yu LX, Li YQ, Wu
FQ, Tang L, Lin Y, Guo LN, Zhou HB, et al: Role of β-catenin in
regulating the balance between TNF-α- and Fas-induced acute liver
injury. Cancer Lett. 335:160–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeh CH, Yang JJ, Yang ML, Li YC and Kuan
YH: Rutin decreases lipopolysaccharide-induced acute lung injury
via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free
Radic Biol Med. 69:249–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jawan B, Kao YH, Goto S, Pan MC, Lin YC,
Hsu LW, Nakano T, Lai CY, Sun CK and Cheng YF: Propofol
pretreatment attenuates LPS-induced granulocyte-macrophage
colony-stimulating factor production in cultured hepatocytes by
suppressing MAPK/ERK activity and NF-kappaB translocation. Toxicol
Appl Pharmacol. 229:362–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantena SK, King AL, Andringa KK,
Eccleston HB and Bailey SM: Mitochondrial dysfunction and oxidative
stress in the pathogenesis of alcohol- and obesity-induced fatty
liver diseases. Free Radic Biol Med. 44:1259–1272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Canbay A, Friedman S and Gores GJ:
Apoptosis: The nexus of liver injury and fibrosis. Hepatology.
39:273–278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferreira DM, Castro RE, Machado MV,
Evangelista T, Silvestre A, Costa A, Coutinho J, Carepa F,
Cortez-Pinto H and Rodrigues CM: Apoptosis and insulin resistance
in liver and peripheral tissues of morbidly obese patients is
associated with different stages of non-alcoholic fatty liver
disease. Diabetologia. 54:1788–1798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malhi H and Gores GJ: Molecular mechanisms
of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver
Dis. 28:360–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsuchida T, Shiraishi M, Ohta T, Sakai K
and Ishii S: Ursodeoxycholic acid improves insulin sensitivity and
hepatic steatosis by inducing the excretion of hepatic lipids in
high-fat diet-fed KK-Ay mice. Metabolism. 61:944–953. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rana M, Reddy SS, Maurya P, Singh V,
Chaturvedi S, Kaur K, Agarwal H, Ahmad H, Naqvi A, Dwivedi AK, et
al: Turmerone enriched standardized Curcuma longa extract
alleviates LPS induced inflammation and cytokine production by
regulating TLR4-IRAK1-ROS-MAPK-NFκB axis. J Funct Foods.
16:152–163. 2015. View Article : Google Scholar
|
|
10
|
Olleros ML, Vesin D, Fotio AL,
Santiago-Raber ML, Tauzin S, Szymkowski DE and Garcia I: Soluble
TNF, but not membrane TNF, is critical in LPS-induced hepatitis. J
Hepatol. 53:1059–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gandhi A, Guo T, Shah P, Moorthy B and
Ghose R: Chlorpromazine-induced hepatotoxicity during inflammation
is mediated by TIRAP-dependent signaling pathway in mice. Toxicol
Appl Pharmacol. 266:430–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Tu Q, Yan W, Xiao D, Zeng Z,
Ouyang Y, Huang L, Cai J, Zeng X, Chen YJ and Liu A: CXC195
suppresses proliferation and inflammatory response in LPS-induced
human hepatocellular carcinoma cells via regulating
TLR4-MyD88-TAK1-mediated NF-κB and MAPK pathway. Biochem Biophys
Res Commun. 456:373–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin WH, Jeon MT, Leem E, Won SY, Jeong
KH, Park SJ, McLean C, Lee SJ, Jin BK, Jung UJ and Kim SR:
Induction of microglial toll-like receptor 4 by prothrombin
kringle-2: A potential pathogenic mechanism in Parkinson's disease.
Sci Rep. 5:147642015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gandoura S, Weiss E, Rautou PE, Fasseu M,
Gustot T, Lemoine F, Hurtado-Nedelec M, Hego C, Vadrot N, Elkrief
L, et al: Gene- and exon-expression profiling reveals an extensive
LPS-induced response in immune cells in patients with cirrhosis. J
Hepatol. 58:936–948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu Q, Guan H, Shi Q, Zhang Y and Yang H:
Curcumin attenuated acute Propionibacterium acnes-induced
liver injury through inhibition of HMGB1 expression in mice. Int
Immunopharmacol. 24:159–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao A, Song J, Lan T, Xu X, Kvietys P, Kao
R, Martin C and Rui T: Cardiomyocyte-fibroblast interaction
contributes to diabetic cardiomyopathy in mice: Role of
HMGB1/TLR4/IL-33 axis. Biochim Biophys Acta 1852 (10 Pt A).
2075–2085. 2015.
|
|
17
|
Ramm S and Mally A: Role of
drug-independent stress factors in liver injury associated with
diclofenac intake. Toxicology. 312:83–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang C, Song S, Zhang Y, Ge Y, Fang X,
Huang T, Du J and Gao J: Inhibition of the Rho/Rho kinase pathway
prevents lipopolysaccharide-induced hyperalgesia and the release of
TNF-α and IL-1β in the mouse spinal cord. Sci Rep. 5:145532015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arrese M and Trauner M: Molecular aspects
of bile formation and cholestasis. Trends Mol Med. 9:558–564. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma X, Wang J, He X, Zhao Y, Wang J, Zhang
P, Zhu Y, Zhong L, Zheng Q and Xiao X: Large dosage of chishao in
formulae for cholestatic hepatitis: A systematic review and
meta-analysis. Evid Based Complement Alternat Med. 2014:3281522014.
View Article : Google Scholar : PubMed/NCBI
|