Introduction
Acute respiratory distress syndrome (ARDS) is a
disorder comprising pulmonary inflammation induced by direct or
indirect damage to the lungs (1). To
date, management of ARDS has failed to decrease the mortality rate
of ARDS to <30%, primarily due to an insufficient understanding
of the pathophysiology of ARDS. The stimulation of platelets in the
lung microvasculature and the sequestration of leukocytes,
particularly neutrophils, appear to have critical roles in the
pathophysiology of ARDS, resulting in the formation of
microthrombus and blockage of blood circulation (2–4).
ARDS is common in critically ill patients. Of those
patients receiving mechanical ventilation in the intensive care
unit (ICU), 19% suffer from ARDS, which is associated with
refractory hypoxemia and an elevated level of extravascular lung
water (EVLW), and has a mortality rate ranging from 32 to 65%
(5). In the early stage of ARDS,
lung ultrasound (LUS) is performed to locate pulmonary edema, and
‘B-lines’ in LUS indicate a reduced level of lung aeration, likely
triggered by an elevated level of EVLW (6). Based on the pulmonary ultrasonography
in patients with ARDS, certain studies have recommended the use of
LUS to measure EVLW semi-quantitatively (7). It has been demonstrated that, as a
user-friendly, simple, cost-efficient and non-invasive bedside
test, LUS was closely associated with the prognosis and mortality
risk of ARDS (8), and may thus be
used to determine the best treatment scheme for ARDS patients
(8).
The level of platelet-activating factors (PAF), a
family of pro-inflammatory lipids present in newborns with an
immature immune system, is increased in infants affected by
inflammatory conditions, including necrotizing enterocolitis
(9). Earlier studies in rodents and
humans revealed that PAF acetylhydrolase (PAFAH), which is present
in breast milk and is able to degrade PAF, probably has a pivotal
role in inhibiting the inflammatory effect of PAF (10).
Pro-inflammatory PAF is one of the strongest
neutrophil activators and functions via a specific PAF receptor,
which belongs to the family of seven transmembrane
G-protein-coupled receptors (11).
Deletion of the acetyl group at the sn-2 site of PAF by PAFAH
results in the inactivation and degradation of PAF (12). Circulating in the blood, PAFAH binds
to and degrades oxidized phospholipids and PAF that are
biologically active (13). Earlier
studies demonstrated that critically ill patients with sepsis had a
reduced activity of plasma PAFAH when compared with that in healthy
subjects (14). In addition, changes
in the activity of plasma PAFAH may affect the severity of asthma
(15). Furthermore, a correlation
between plasma PAFAH activity, allelic alterations and the severity
of ARDS has been demonstrated (11).
A point mutation at the nucleotide site 994 in exon
9 [single-nucleotide polymorphism (SNP) ID, rs16874954] leads to a
G994→T mutation in the PAFAH gene, resulting in a Val279→Phe
substitution in the mature protein (V279F), which is located in the
catalytic domain of the enzyme, thus reducing its catalytic
activity. In Japanese families with a deficiency in plasma PAFAH
activity, the enzymatic activity of PAFAH is completely abolished
in subjects harbouring the TT homozygotes, whereas a decreased
PAFAH activity is observed in subjects harbouring the GT
homozygotes (16–18).
Since the LUS score and an SNP in PAFAH have been
demonstrated to be associated with the prognosis of ARDS (16–18), the
present study evaluated the potential of assessing different PAFAH
variants in conjunction with the LUS score to predict the prognosis
of ARDS.
Materials and methods
Patients and samples
Blood samples were collected from 112 patients
diagnosed with ARDS. All subjects were recruited at Wenzhou
People's Hospital (Wenzhou, China) within 72 h of their ARDS
diagnosis and none of them suffered from left ventricular failure.
Baseline PAFAH measurements were performed using blood samples
collected prior to any treatment. The partial oxygen pressure
(PaO2)/fraction of inspired oxygen (FiO2),
positive end-expiratory pressure (PEEP) and lactic acid levels were
recorded for each patient. The protocol of the present study was
approved by the Ethics Committee of Wenzhou People's Hospital
(Wenzhou, China) and written informed consent was obtained from all
participants prior to the study. The study was performed according
to the Declaration of Helsinki.
Genotyping
Genomic DNA was extracted from blood samples and
subjected to amplification by polymerase chain reaction (PCR).
Electrophoresis on 2.5% agarose gels was performed to analyse the
PCR products and a restriction analysis was performed to determine
the PAFAH G994T genotypes. The TaqMan method (Roche, Basel,
Switzerland) was utilized to analyse the SNPs (19).
PAFAH activity measurement
A Human Platelet-activating Factor Acetylhydrolase
ELISA kit (cat. no. E0699Hu; Shanghai Korain Biotech, Co., Ltd.,
Shanghai, China) was utilized to determine PAFAH activity,
including total PAHAH activity, low-density lipoprotein-PAFAH
(L-PAFAH) and high-density lipoprotein-PAFAH (H-PAFAH). Blood
samples collected from patients with ARDS within 72 h of their
diagnosis were utilized.
APACHE II, clinical pulmonary
infection score (CPIS) and sequential organ failure assessment
(SOFA) scoring
The APACHE II (20),
CPIS (21) and SOFA (22) score were evaluated as described
previously.
LUS scoring
An M-Turbo ultrasound machine (FUJIFILM SonoSite,
Bothell, WA, USA) equipped with a 2- to 5-MHz curved array probe
was used to perform all tests. Prone position lung ultrasound
examination was performed immediately after the prone positioning
was started (H0), as well as at 3 and 6 h after the initialization
of prone positioning (H3 and H6, respectively). The points of
examination were located on the posterior axillary line, the
scapular line and the paravertebral line. These three lines served
as body markers to divide one side of the back into three regions,
and each region was subsequently divided into 3 areas of equal
size. Therefore, a total of 9 examination areas were obtained on
each subject, and 8 points (excluding the point covered by the
scapular bone) were analysed on each side of the back, with a total
of 16 points being analysed on each subject. Sonographic signs of
lung aeration were divided into four groups as follows: i)
Consolidation (C) indicated by a tissue pattern characterized as
dynamic air bronchograms; ii) severe loss of lung aeration (B2)
indicated by coalescent B lines; iii) moderate loss of lung
aeration indicated by multiple spaced B lines; iv) normal pattern
(N) indicated by lung sliding with isolated B lines (<3) or A
lines. During the analysis, the points in a particular region were
scored based on the worst ultrasound pattern, and the scores of all
points were added. By definition, the aeration score variation was
the difference between scores at various time-points (H6 vs. H3 and
H3 vs. H0). All LUS video recordings were scored and evaluated by
two independent ICU physicians in a blinded manner.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Independent-samples t-tests were utilized to evaluate
differences between the groups. χ2 analyses were
performed to evaluate the deviations of PAFAH G994T genotype
distributions from the Hardy-Weinberg equilibrium, and to calculate
the frequencies of genotypes or alleles among different groups.
Logistic regression and χ2 analysis were performed to
calculate 95% confidence intervals and odds ratios, so as to
determine the risk associated with the T allele. Kaplan-Meier plots
and the log-rank test were performed to analyse the association
between PAFAH G994T polymorphism and the outcome of ARDS patient
mortality. A Pearson's analysis was performed to assess correlation
between PAFAH G994T genotype and the activity of PAFAH. P<0.05
was considered to indicate a statistically significant difference.
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was used to
perform all statistical analyses.
Results
The LUS score is correlated with the
partial oxygen pressure (PaO2)/fraction of inspired
oxygen (FiO2), positive end-expiratory pressure (PEEP)
and lactic acid levels
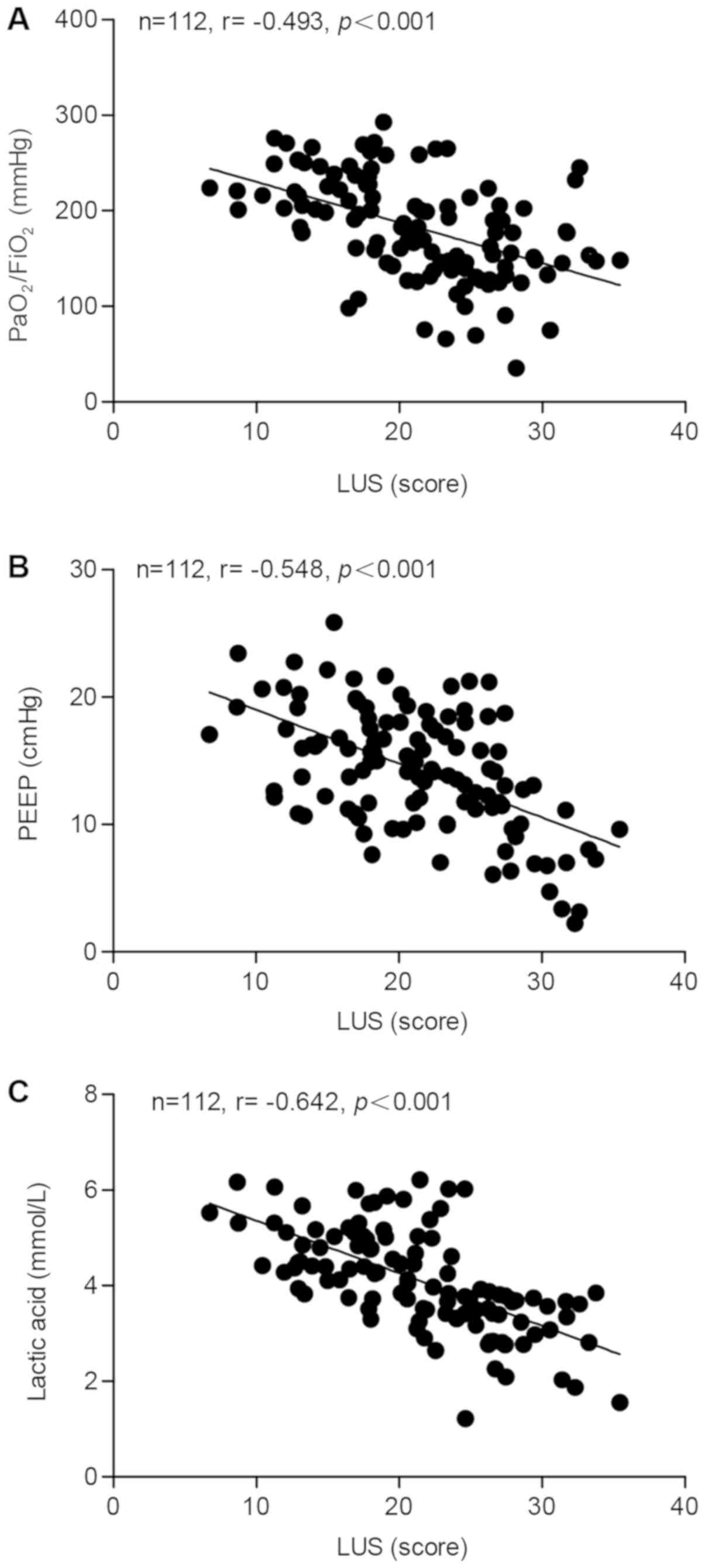
The correlation between the LUS score and the
PaO2/FiO2 ratio, PEEP and lactic acid was
determined using blood samples collected from 112 ARDS patients. A
significant negative correlation was identified between the LUS
score and the PaO2/FiO2 ratio (Fig. 1A), PEEP (Fig. 1B) and lactic acid (Fig. 1C), with correlation coefficients of
−0.493, −0.548 and −0.642, respectively. Furthermore, the
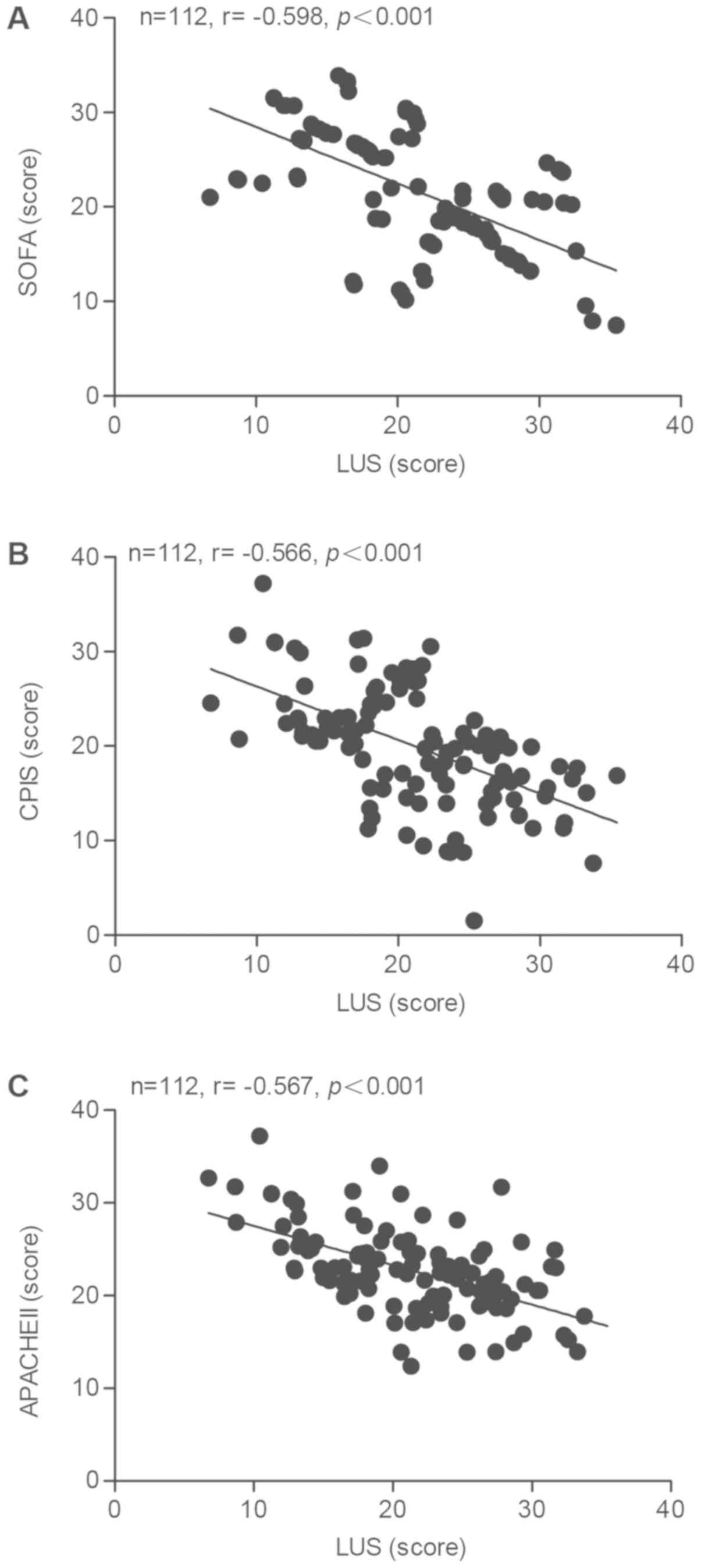
correlation between the LUS and the SOFA, CPIS or APACHE II score
was calculated. As presented in Fig.
2, negative correlation coefficients of −0.598, −0.566 and
−0.567 were determined for the correlation between the LUS and the
SOFA (Fig. 2A), the CPIS (Fig. 2B) and the APACHE II score (Fig. 2C), respectively.
Association between serum PAFAH
activity and PAHAF G994T genotype
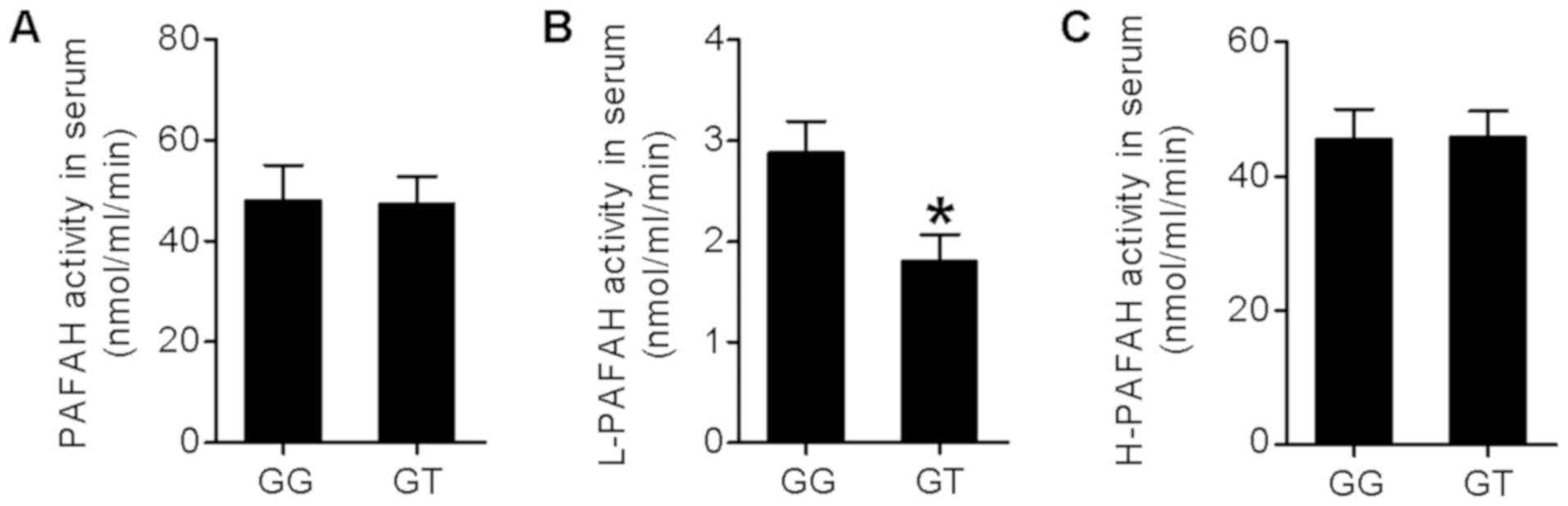
In the next step, the subjects were genotyped for
the PAHAF G994T polymorphism, with 90 subjects being genotyped as
GG and 22 subjects being genotyped as GT (none of the subjects was
genotyped as TT). PAFAH activities, including total activity of
PAHAH, low-density L-PAFAH and H-PAFAH, were measured using the
blood samples collected from ARDS subjects within 72 h of their
diagnosis. As presented in Fig. 3,
the activity of total PAFAH and H-PAFAH in the serum exhibited no
difference between subjects of the GG or GT genotype, whereas the
L-PAFAH activity in the serum of GG subjects was significantly
higher than that in the GT subjects (P<0,05).
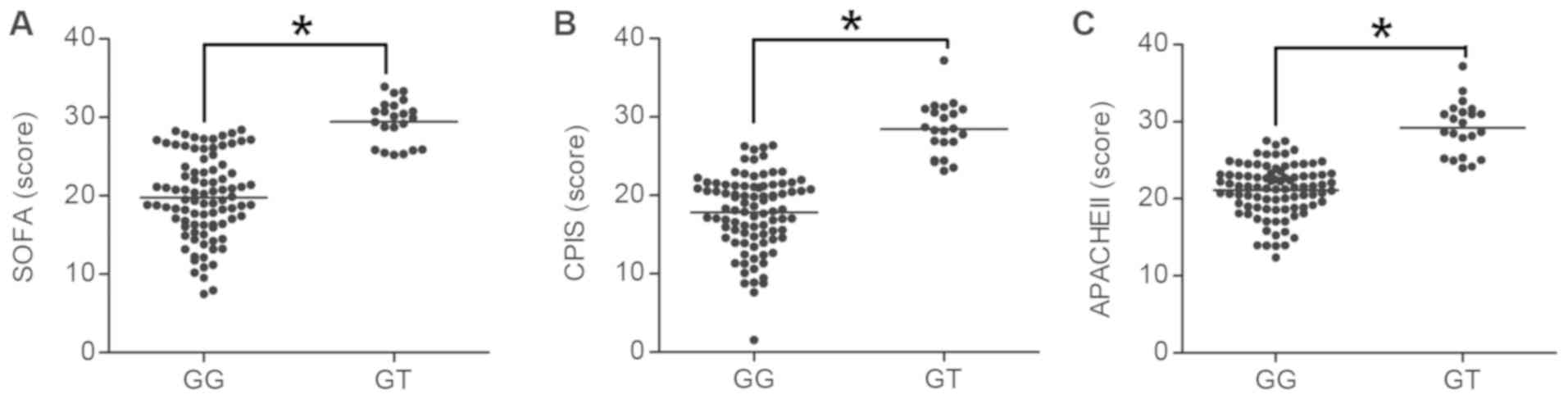
PAFAH G994T polymorphism is associated
with the clinical features of ARDS
PAF has been reported to be associated with the
severity of ARDS and sepsis (14,17). In
addition, since the pro-inflammatory effect of PAF is abrogated by
PAFAH, PAFAH activity has also been reported to affect the severity
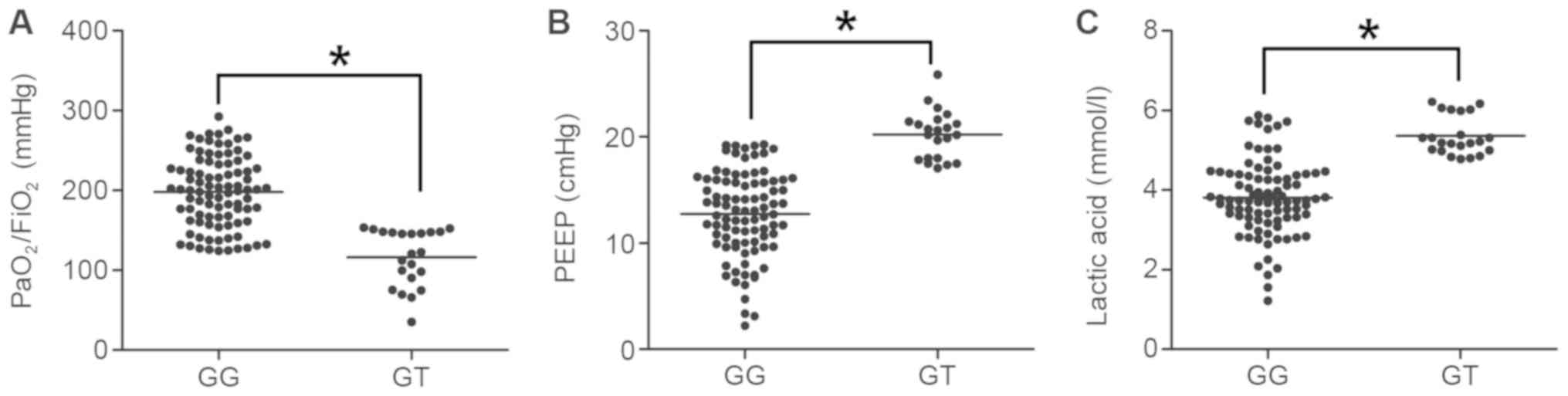
of ARDS. In the present study, the association between the
polymorphism of PAFAH G994T and clinical features of ARDS,
including the PaO2/FiO2 ratio, PEEP and
lactic acid levels, as well as the SOFA, CPIS and APACHE II scores,
was determined. As presented in Fig.
4, the PaO2/FiO2 ratio in GG subjects was
significantly higher than that in GT subjects (P<0.05; Fig. 4A), whereas the PEEP (Fig. 4B) and lactic acid levels (Fig. 4C) in GG subjects were significantly
lower than those in the GT subjects (P<0.05). In addition, the
SOFA (Fig. 5A), CPIS (Fig. 5B) and APACHE II scores (Fig. 5C) in GG subjects were lower as
compared with those in GT subjects (P<0.05).
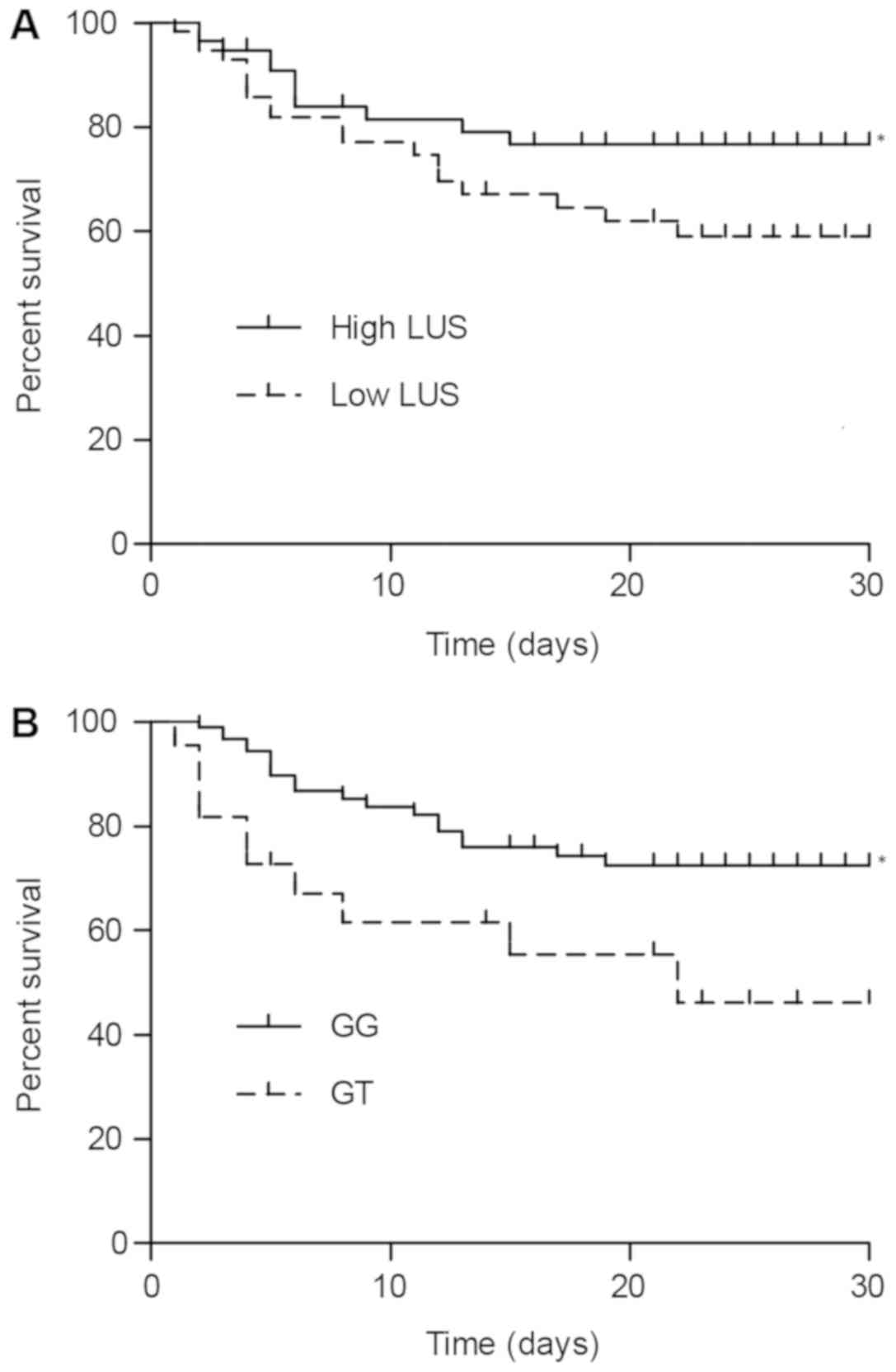
Effect of PAFAH G994T polymorphism on
the prognosis of ARDS
The effect of the PAFAH G994T polymorphism on the
survival rate of ARDS patients within the first 30 days following
diagnosis was determined using Kaplan-Meier plots and the log-rank
test. The 112 ARDS subjects were divided into two groups according
to their LUS scores, with 56 subjects assigned to the group of high
LUS scores (above the median) and the other 56 subjects assigned to
the group of low LUS scores (below the median). The survival rates
in the two groups were determined and compared. As presented in
Fig. 6A, the subjects with a high
LUS score exhibited a significantly higher rate of survival than
those with a low LUS score (P<0.05). In addition, the 112
subjects were divided into two groups based on their genotype: GG
subjects (n=90) and GT subjects (n=22). As presented in Fig. 6B, the GG genotype was associated with
a significantly lower risk of mortality than the GT genotype
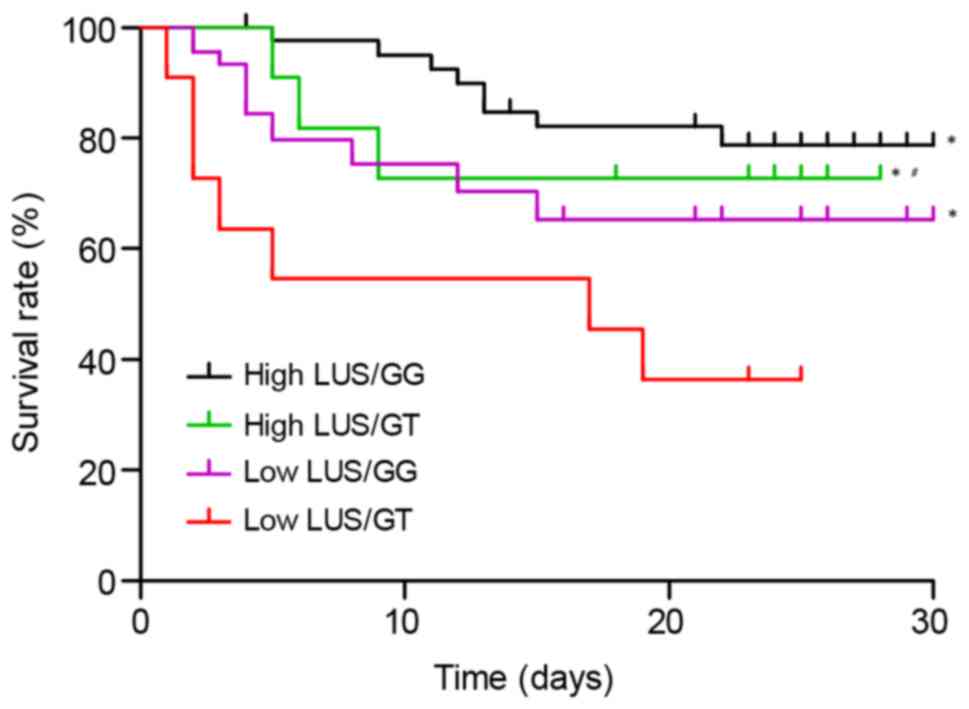
(P<0.05). Subsequently, the 112 subjects were divided into four
groups according to the combination of their genotypes and LUS
scores: GG genotype and a high LUS score (n=45), GT genotype and a
high LUS score (n=11), GG genotype and a low LUS score (n=45) and
GT genotype and a low LUS score (n=11). The prognosis of the
subjects was compared between these four groups. As presented in
Fig. 7, compared with the GT
subjects with a low LUS score who presented the highest mortality
risk, the GG subjects with a high LUS score were had the lowest
risk of mortality. In addition, the survival rates of GT subjects
with a high LUS score and GG subjects with a low LUS score were
significantly higher than that of GT subjects with a low LUS score.
Furthermore, the survival rate among GT subjects with a high LUS
score was significantly higher than that among GG subjects with a
low LUS score (P<0.05).
Discussion
Tsubo et al (23) have used the LUS score to evaluate
PEEP-induced changes in lung function. Other studies have reported
that the LUS score accurately reflects changes in patients with
heart diseases, as well as in pigs with oleic acid-induced acute
lung injury (24,25). In a study on ARDS patients, Bataille
et al (26) determined that
the B-line score of lung ultrasound (with a total score of 20
points) was able to predict the severity of the disease, with a
score of 5 indicating mild disease, a score of 9 indicating
moderate disease and a score of 11 indicating severe disease.
Leblanc et al (27) reported
that the LUS score was able to predict the incidence of ARDS in
patients with pulmonary contusion with a sensitivity and
specificity of 58 and 96%, respectively. In addition, the LUS score
in conjunction with the clinical features was able to predict the
prognosis of ARDS with a higher accuracy than X-ray alone.
Therefore, in the present study, LUS was performed to record 12
sections in the lung, with a total LUS score of 36, to evaluate
pulmonary fluid and pulmonary ventilation. The application of the
LUS score in assessing the incidence of respiratory distress was
first performed by Soummer et al (28), and their results indicated that the
risk of extubation failure was low in patients with a LUS score of
<13 at the end of the spontaneous breathing trial, whereas the
risk of extubation failure was high in patients with a LUS score of
>17. Caltabeloti et al (29) indicated that the bedside LUS score
may be used to assess the change in pulmonary ventilation during
ARDS and early septic shock, which occurred prior to the change in
the oxygenation index. In the present study, negative correlations
between the LUS score and the PaO2/FiO2, PEEP
and lactic acid were identified, with correlation coefficients of
−0.493, −0.548 and −0.642, respectively. In addition, a negative
correlation between the LUS and the SOFA, CPIS and APACHE II score
was determined, with the correlation coefficients being −0.598,
−0.566 and −0.567, respectively.
As a Ca2+-independent catalyst of
serine-dependent phospholipid hydrolysis, PAFAH belongs to the
phospholipase A2 (PLA2) superfamily (30). Also known as lipoprotein-associated
PLA2, the plasma form of PAFAH is a single 45-kilodalton
polypeptide of 441 amino acids encoded by the PLA2G7 gene (31). Since PAFAH oxidizes phospholipids and
hydrolyses PAF with a modified short fatty acyl chain esterified at
the site of sn-2, it has a critical role in numerous physiological
disorders, including ARDS, rheumatic diseases and severe
anaphylaxis (32–35). It has been demonstrated that the
activity of plasma PAFAH was significantly lower in ARDS patients
carrying the His92 allele (16).
This was surprising, given that the kinetics of the His92 mutant
enzyme were not different from those of the wild-type PAFAH,
although recombinant PAFAH proteins Val379 and Thr198 exhibited a
decreased substrate affinity (i.e., a higher Michaelis Menten
constant) (36). In addition, it has
been indicated that non-survivors of ARDS had a significantly lower
level of PAFAH circulating in their blood within 72 h of diagnosis
(16,37). Multiple studies have also indicated a
correlation between decreased levels of PAFAH and an increased
severity of inflammatory disorders. For instance, a decreased level
of plasma PAFAH was reported to be associated with an enhanced
severity of paediatric asthma (38),
while a variation in the activity of plasma PAFAH at the onset of
ARDS was demonstrated to be associated with a genetic alteration at
the PAFAH locus (16–18).
Stafforini et al (15) identified the molecular structure of
the human PAFAH gene, and a loss of its enzymatic activity was
attributed to the G994T single-point mutation in its exon 9. The
enzymatic activity of PAFAH is decreased in patients harbouring the
heterozygous mutation, while it is fully abrogated in patients
harbouring the homozygous mutation. Earlier studies have also
identified that the polymorphism in the PAFAH gene was associated
with the severity of certain atherosclerotic diseases, including
peripheral artery occlusive disease, stroke, nephrotic syndrome in
children and asthma attack (16–18). In
the present study, the association between serum PAFAH activity and
the PAHAF G994T SNP was investigated, and it was revealed that the
activity of PAFAH and H-PAFAH in GG subjects was similar, whereas a
higher L-PAFAH activity was observed in GG subjects. In addition,
it was identified that the level of PEEP and lactic acid, as well
as the SOFA, CPIS and APACHE II scores, in GG subjects was
significantly lower than that in GT subjects, whereas the
PaO2/FiO2 in GG subjects was significantly
higher. In line with these results, it was also revealed that the
genotype of the PAFAH G994T polymorphism and the LUS score were
associated with the clinical outcomes of ARDS patients. The
observation that the G994T polymorphism only influenced the L-PAFAH
activity is different from the result of a previous study, which
reported that the polymorphism influenced the PAHAF, H-PAHAF and
D-PAHAF (16). This discrepancy may
be attributed to the fact that differences in disease background,
as the participants enrolled were either patients with polycystic
ovary syndrome or healthy subjects. Further large-scale studies are
required to confirm the results of the present study. The current
study is however limited by a relatively small sample size. Further
study with a larger sample size using more sophisticated
statistical analysis is therefore warranted.
In conclusion, the present study was the first, to
the best of our knowledge, to report that the G994T polymorphism in
the PAFAH gene is associated with the clinical outcome of ARDS.
Since this polymorphism was indicated to be associated with the
activity of PAFAH and the prognosis of ARDS, which may contribute
to the pathogenesis of ARDS, its functions should be further
explored in order to develop novel therapeutic approaches to treat
ARDS.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by The
funding of Medical Research of Wenzhou (grant no. 2017B04 from
2017).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL and RG designed the current study. SW, LW and ZW
collected the literature and data, and analysed the data. YJ and XC
interpreted and visualized the data. WL, SW and YJ prepared the
manuscript. RG revised the final manuscript. All authors read and
approved the final manuscript.
Ethical approval and consent to
participate
The protocol of the present study was approved by
the Ethics Committee of Wenzhou People's Hospital (Wenzhou, China)
and written informed consent was obtained from all participants
prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hogg JC: Neutrophil kinetics and lung
injury. Physiol Rev. 67:1249–1295. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshida K, Kondo R, Wang Q and Doerschuk
CM: Neutrophil cytoskeletal rearrangements during capillary
sequestration in bacterial pneumonia in rats. Am J Respir Crit Care
Med. 174:689–698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Irish Critical Care Trials Group, . Acute
lung injury and the acute respiratory distress syndrome in Ireland:
A prospective audit of epidemiology and management. Crit Care.
12:R302008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Volpicelli G: Lung sonography. J
Ultrasound Med. 32:165–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Volpicelli G, Skurzak S, Boero E,
Carpinteri G, Tengattini M, Stefanone V, Luberto L, Anile A,
Cerutti E, Radeschi G and Frascisco MF: Lung ultrasound predicts
well extravascular lung water but is of limited usefulness in the
prediction of wedge pressure. Anesthesiology. 121:320–327. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Z, Jiang L, Xi X, Jiang Q, Zhu B,
Wang M, Xing J and Zhang D: Prognostic value of extravascular lung
water assessed with lung ultrasound score by chest sonography in
patients with acute respiratory distress syndrome. BMC Pulm Med.
15:982015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walker A: Breast milk as the gold standard
for protective nutrients. J Pediatr. 156 (2 Suppl):S3–S7. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caplan MS, Sun XM, Hseuh W and Hageman JR:
Role of platelet activating factor and tumor necrosis factor-alpha
in neonatal necrotizing enterocolitis. J Pediatr. 116:960–964.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu T, Honda Z, Nakamura M, Bito H and
Izumi T: Platelet-activating factor receptor and signal
transduction. Biochem Pharmacol. 44:1001–1008. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stafforini DM, McIntyre TM, Zimmerman GA
and Prescott SM: Platelet-activating factor acetylhydrolases. J
Biol Chem. 272:17895–17898. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stafforini DM, Carter ME, Zimmerman GA,
McIntyre TM and Prescott SM: Lipoproteins alter the catalytic
behavior of the platelet-activating factor acetylhydrolase in human
plasma. Proc Natl Acad Sci USA. 86:2393–2397. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Graham RM, Stephens CJ, Silvester W, Leong
LL, Sturm MJ and Taylor RR: Plasma degradation of
platelet-activating factor in severely ill patients with clinical
sepsis. Crit Care Med. 22:204–212. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stafforini DM, Satoh K, Atkinson DL,
Tjoelker LW, Eberhardt C, Yoshida H, Imaizumi T, Takamatsu S,
Zimmerman GA, McIntyre TM, et al: Platelet-activating factor
acetylhydrolase deficiency. A missense mutation near the active
site of an anti-inflammatory phospholipase. J Clin Invest.
97:2784–2791. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan P, Liu HW, Wang XS, Zhang F, Song Q,
Li Q, Wu HM and Bai H: Identification of the G994T polymorphism in
exon 9 of plasma platelet-activating factor acetylhydrolase gene as
a risk factor for polycystic ovary syndrome. Hum Reprod.
25:1288–1294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li S, Stuart L, Zhang Y, Meduri GU,
Umberger R and Yates CR: Inter-individual variability of plasma
PAF-acetylhydrolase activity in ARDS patients and PAFAH genotype. J
Clin Pharm Ther. 34:447–455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Yang Q, Li L, Guan J, Liu Z, Han J,
Chao Y, Wang Z and Yu X: The value of lung ultrasound score on
evaluating clinical severity and prognosis in patients with acute
respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi
Xue. 27:579–584. 2015.(In Chinese). PubMed/NCBI
|
|
19
|
Shen GQ, Abdullah KG and Wang QK: The
TaqMan method for SNP genotyping. Methods Mol Biol. 578:293–306.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knaus WA, Draper EA, Wagner DP and
Zimmerman JE: APACHE II: A severity of disease classification
system. Crit Care Med. 13:818–829. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sachdev A, Chugh K, Sethi M, Gupta D,
Wattal C and Menon G: Clinical pulmonary infection score to
diagnose ventilator-associated pneumonia in children. Indian
Pediatr. 48:939–954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vincent JL, Moreno R, Takala J, Willatts
S, De Mendonça A, Bruining H, Reinhart CK, Suter PM and Thijs LG:
The SOFA (Sepsis-related Organ Failure Assessment) score to
describe organ dysfunction/failure. On behalf of the working group
on sepsis-related problems of the European society of intensive
care medicine. Intensive Care Med. 22:707–710. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsubo T, Sakai I, Suzuki A, Okawa H,
Ishihara H and Matsuki A: Density detection in dependent left lung
region using transesophageal echocardiography. Anesthesiology.
94:793–798. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agricola E, Picano E, Oppizzi M, Pisani M,
Meris A, Fragasso G and Margonato A: Assessment of stress-induced
pulmonary interstitial edema by chest ultrasound during exercise
echocardiography and its correlation with left ventricular
function. J Am Soc Echocardiogr. 19:457–463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gargani L, Lionetti V, Di Cristofano C,
Bevilacqua G, Recchia FA and Picano E: Early detection of acute
lung injury uncoupled to hypoxemia in pigs using ultrasound lung
comets. Crit Care Med. 35:2769–2774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bataille B, Rao G, Cocquet P, Mora M,
Masson B, Ginot J, Silva S and Moussot PE: Accuracy of ultrasound
B-lines score and E/Ea ratio to estimate extravascular lung water
and its variations in patients with acute respiratory distress
syndrome. J Clin Monit Comput. 29:169–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leblanc D, Bouvet C, Degiovanni F, Nedelcu
C, Bouhours G, Rineau E, Ridereau-Zins C, Beydon L and Lasocki S:
Early lung ultrasonography predicts the occurrence of acute
respiratory distress syndrome in blunt trauma patients. Intensive
Care Med. 40:1468–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soummer A, Perbet S, Brisson H, Arbelot C,
Constantin JM, Lu Q and Rouby JJ; Lung Ultrasound Study Group, :
Ultrasound assessment of lung aeration loss during a successful
weaning trial predicts postextubation distress*. Crit Care Med.
40:2064–2072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caltabeloti F, Monsel A, Arbelot C,
Brisson H, Lu Q, Gu WJ, Zhou GJ, Auler JO and Rouby JJ: Early fluid
loading in acute respiratory distress syndrome with septic shock
deteriorates lung aeration without impairing arterial oxygenation:
A lung ultrasound observational study. Crit Care. 18:R912014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dennis EA: The growing phospholipase A2
superfamily of signal transduction enzymes. Trends Biochem Sci.
22:1–2. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Derewenda ZS and Ho YS:
PAF-acetylhydrolases. Biochim Biophys Acta. 1441:229–236. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arai H: Platelet-activating factor
acetylhydrolase. Prostaglandins Other Lipid Mediat. 68-69:83–94.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vadas P, Gold M, Perelman B, Liss GM, Lack
G, Blyth T, Simons FE, Simons KJ, Cass D and Yeung J:
Platelet-activating factor, PAF acetylhydrolase, and severe
anaphylaxis. N Engl J Med. 358:28–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vergne P, Praloran V, Treves R and Denizot
Y: Decreased levels of serum platelet-activating factor
acetylhydrolase in patients with rheumatic diseases. Mediators
Inflamm. 6:241–242. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakos G, Kitsiouli E, Hatzidaki E,
Koulouras V, Touqui L and Lekka ME: Phospholipases A2 and
platelet-activating-factor acetylhydrolase in patients with acute
respiratory distress syndrome. Crit Care Med. 33:772–779. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kruse S, Mao XQ, Heinzmann A, Blattmann S,
Roberts MH, Braun S, Gao PS, Forster J, Kuehr J, Hopkin JM, et al:
The Ile198Thr and Ala379Val variants of plasmatic
PAF-acetylhydrolase impair catalytical activities and are
associated with atopy and asthma. Am J Hum Genet. 66:1522–1530.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Claus RA, Russwurm S, Dohrn B, Bauer M and
Lösche W: Plasma platelet-activating factor acetylhydrolase
activity in critically ill patients. Crit Care Med. 33:1416–1419.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miwa M, Miyake T, Yamanaka T, Sugatani J,
Suzuki Y, Sakata S, Araki Y and Matsumoto M: Characterization of
serum platelet-activating factor (PAF) acetylhydrolase. Correlation
between deficiency of serum PAF acetylhydrolase and respiratory
symptoms in asthmatic children. J Clin Invest. 82:1983–1991. 1988.
View Article : Google Scholar : PubMed/NCBI
|