Introduction
Portal vein thrombosis (PVT) is a complication that
frequently occurs during the course of liver cirrhosis, especially
in advanced stages. Although it is considered a rare event, PVT has
been increasingly identified due to the increased frequency of
liver imaging techniques used in patients with cirrhosis (1,2). The
prevalence of PVT is ~1% in the general population and
investigators have demonstrated in different series that it
develops in 5–20% of cirrhosis patients. However, there are
differences in the reported prevalence of PVT (3–5).
There are various etiological factors that are
considered to predispose liver cirrhosis patients to the formation
of PVT, including inflammation, neoplasms, coagulation disorders,
trauma and abdominal surgery. In a study of patients with
cirrhosis, it was demonstrated that prothrombotic states were more
frequent in patients with PVT compared with patients without PVT
(6). While the mechanism of the
occurrence of PVT in cirrhosis patients is not still clear, studies
have demonstrated that there is a close association between PVT and
advanced liver disease (7). In this
regard, several risk factors have been proposed for this
complication (8). The development of
PVT is a multifactorial process. Frequently, more than one risk
factor is identified, whereas a single factor is rarely identified
in cirrhosis patients (9). Hepatic
structural derangement secondary to a slowing of portal vein blood
flow is primarily responsible for PVT; damage to the vessel wall
and hypercoagulability serve important roles as well. Inherited and
acquired thrombotic risk factors also serve a role (10). The presence of PVT in cirrhosis
patients is very important, as it can lead to technical
difficulties in liver transplantation and active variceal bleeding
(11,12).
The purpose of the study was to determine the
clinical features of PVT in patients with liver cirrhosis attending
a tertiary support hospital in Malatya, Turkey and to recognize the
biochemical factors associated with PVT.
Materials and methods
Guidelines and data availability
Ethics committee approval is not required for
retrospective studies in Inonu University Medical Faculty (Malatya,
Turkey). From January 2009 to March 2015, overall 978 patients with
liver cirrhosis (605 men, 373 women) were enrolled in the database
of the Gastroenterology Unit of Turgut Ozal Medical Center, a
tertiary care hospital in Malatya, Turkey.
Study selection
Liver cirrhosis was diagnosed if the patient had a
positive liver biopsy on record, by imaging techniques or clinical
signs including evidence of portal hypertension, ascites and
encephalopathy. However, the seriousness of the liver disease was
classified in accordance with Child-Pugh class A to C and model for
end-stage liver disease (MELD) (13). The exclusion criteria for the
patients were as follows: Liver tumor and other malignant tumors,
liver transplantation, Budd-Chiari syndrome, splenectomy, non-liver
disease complicated (contraceptive use, previous venous thrombosis,
familial thrombophilia, abdominal surgery, abdominal trauma or
sepsis or overt myeloproliferative disorders) by PVT and usage of
anticoagulant or anti-platelet medications. Patients with
hepatocellular carcinoma were eliminated by abdominal ultrasound,
spiral abdominal computed tomography and α-fetoprotein values. The
number of individuals was determined based on power analysis given
below and their characteristics are provided in Table I.
 | Table I.The clinical features and the
descriptive statistics of the groups. |
Table I.
The clinical features and the
descriptive statistics of the groups.
| Factors | PVT (n=98) | Non-PVT
(n=101) | P-value |
|---|
| Agea | 56 (6-83) | 53 (16-87) | <0.05 |
| Genderb |
|
| >0.05 |
|
Male | 64 (65.3) | 72 (71.3) |
|
|
Female | 34 (34.7) | 29 (28.7) |
|
|
Etiologyb |
|
|
|
|
HBV | 37 (37.7) | 48 (47.5) | >0.05 |
|
HCV | 18 (18.3) | 10 (9.9) | >0.05 |
|
Alcohol | 9 (9.2) | 4 (3.96) | >0.05 |
|
Criptogenic | 25 (25.5) | 27 (26.7) | >0.05 |
|
Mixed | 4 (4.0) | 11 (10.9) | >0.05 |
| Child-Pugh
classb |
|
|
|
| A | 13 (13.3) | 17 (16.9) | >0.05 |
| B | 37 (37.8) | 31 (30.7) | >0.05 |
| C | 48 (48.9) | 53 (52.4) | >0.05 |
| MELD score | 16.18 (90) | 15.25 (92) | >0.05 |
|
Hematocrita | 34.32
(18.8–53.2) | 38.4
(20.4–51.6) | <0.001 |
|
Plateleta | 95 (13-1254) | 123 (25-441) | >0.05 |
| INRa | 1.415
(0.92–4.1) | 1.32 (0.9–6.4) | <0.05 |
|
Albumina | 2.97 (1.3–5.1) | 3.2 (1.4–5.2) | <0.05 |
|
Bilirubina | 2.805
(0.56–36.2) | 1.6
(0.3–42.38) | <0.001 |
| Aspartate
aminotransferasea | 58.5 (18-2375) | 60 (13-3924) | >0.05 |
| Alanine
aminotransferasea | 48 (9-1131) | 43 (8-2420) | >0.05 |
| Sodiuma | 135 (122-143) | 137 (118-145) | >0.05 |
|
Creatininea | 0.775
(0.37–5.3) | 0.8 (0.46-9) | >0.05 |
|
Glucosea | 119.5 (47-552) | 98 (63-381) | <0.001 |
Creation of groups
A total of 98 cirrhosis patients with PVT were
identified from the retrospective patient records. In order to
recognize factors associated with PVT, 101 consecutive cirrhosis
patients without PVT from 880 patients remaining were chosen
randomly to make up the control group. The median ages were 56
years (range, 6–83) in the PVT group and 53 years (range, 16–87) in
the non-PVT group. A total of two groups were formed: The patient
(PVT) and control (non-PVT) groups. PVT was categorized as complete
or partial if thrombus was concluded as nonexistent or if lessening
of blood flow occurred in the main portal trunk, left and right
branches of the portal vein, superior mesenteric vein and splenic
vein; the existence of a portal cavernoma was appraised. The
diagnosis of PVT was made by Doppler flow imaging, as is routine.
When diagnosis of PVT was uncertain, it was verified by computed
tomography or magnetic resonance imaging. In addition to imaging,
patients who had not yet developed the characteristics of chronic
PVT, including collateral circulation (cavernous portal
transformation) or portal hypertension were deemed to have acute
PVT. Acute PVT was exhibited in 40% of all patients with PVT.
Patient clinical features and the prevalence of patient risk
factors are listed in Tables I and
II. The records of the cirrhosis
patients were appraised to attain data regarding demographic
characteristics (age, gender) and hematocrit (HTC), platelet (PLT),
international normalized ratio (INR), albumin (ALB), bilirubin
(BLB), aspartate aminotransferase (AST), alanine aminotransferase
(ALT), sodium (Na), creatinine (CR), and glucose values obtained at
baseline. The variables were measured and the groups were compared
statistically.
 | Table II.Prevalence of thrombophilic risk
factors in patients with and without portal vein thrombosis. |
Table II.
Prevalence of thrombophilic risk
factors in patients with and without portal vein thrombosis.
| Thrombophilic risk
factors | PVT n (%) | Control groups n
(%) | P-value |
|---|
| FVL | 12 (12.2) | 5 (4.9) | 0.003 |
| PTHR 20210 | 15 (15.3) | 11 (10.8) | 0.18 |
| MTHFR TT677 | 16 (16.3) | 13 (12.8) | 0.24 |
| Homocysteine | 17 (17.3) | 21 (20.7) | 0.13 |
| Protein C | 73 (74.4) | 70 (69.3) | 0.20 |
| Protein S | 71 (72.4) | 67 (66.3) | 0.23 |
| Antithrombin
III | 63 (64.2) | 59 (58.4) | 0.36 |
All the databases were screened for thrombophilia
panel including protein C, S, antithrombin III, fasting plasma
homocysteine, the mutation of factor V Leiden (FVL), prothrombin
gene (PG) and the methylenetetrahydrofolate reductase (MTHFR)
enzyme. Due to the limitations of a retrospective study, the
thrombophilic risk factors in the control group had not been
previously studied before the beginning of the present study. The
aforementioned factors were measured and the groups were compared
statistically with respect to the factors after the start of the
present study. In addition, the medical guideline of the
STrengthening the Reporting of OBservational studies in
Epidemiology (STROBE) was completed, which evaluates the quality of
observational studies.
Sample size
The minimum sample size required to detect a
significant difference of HCT values between the groups should be
at least 88 in each group, (176 in total), considering a type I
error (α) of 0.05, power (1-β) of 0.8, effect size of 0.43 and
two-sided alternative hypothesis (H1). To increase the power of
this study, 199 individuals (98 in PVT group; 101 in non-PVT group)
were included in the present study.
Statistical analysis
Categorical variables were given as frequencies with
percentages and continuous variables were presented as median
(min-max) based on the distribution of variables. Normality was
confirmed with the Shapiro-Wilk test. Pearson's chi-square test was
used for categorical variables and the Mann-Whitney U test was used
to determine non-normally distributed variables. P<0.05 was
considered to indicate a statistically significant difference.
Backward stepwise multiple logistic regression models were
conducted to predict associations between the predictor variables
(age, gender, etiology, HTC, PLT, INR, ALB, BLB, AST, ALT, Na, CR,
glucose, Child-Pugh class, MELD score and thrombophilia panel) and
dependent variable (PVT/Non-PVT groups). The results of the
predicted model were assessed with the Hosmer-Lemeshow test and
Omnibus test of the model coefficient. Mortality rates were
analyzed for all patients by the Kaplan-Meier method and
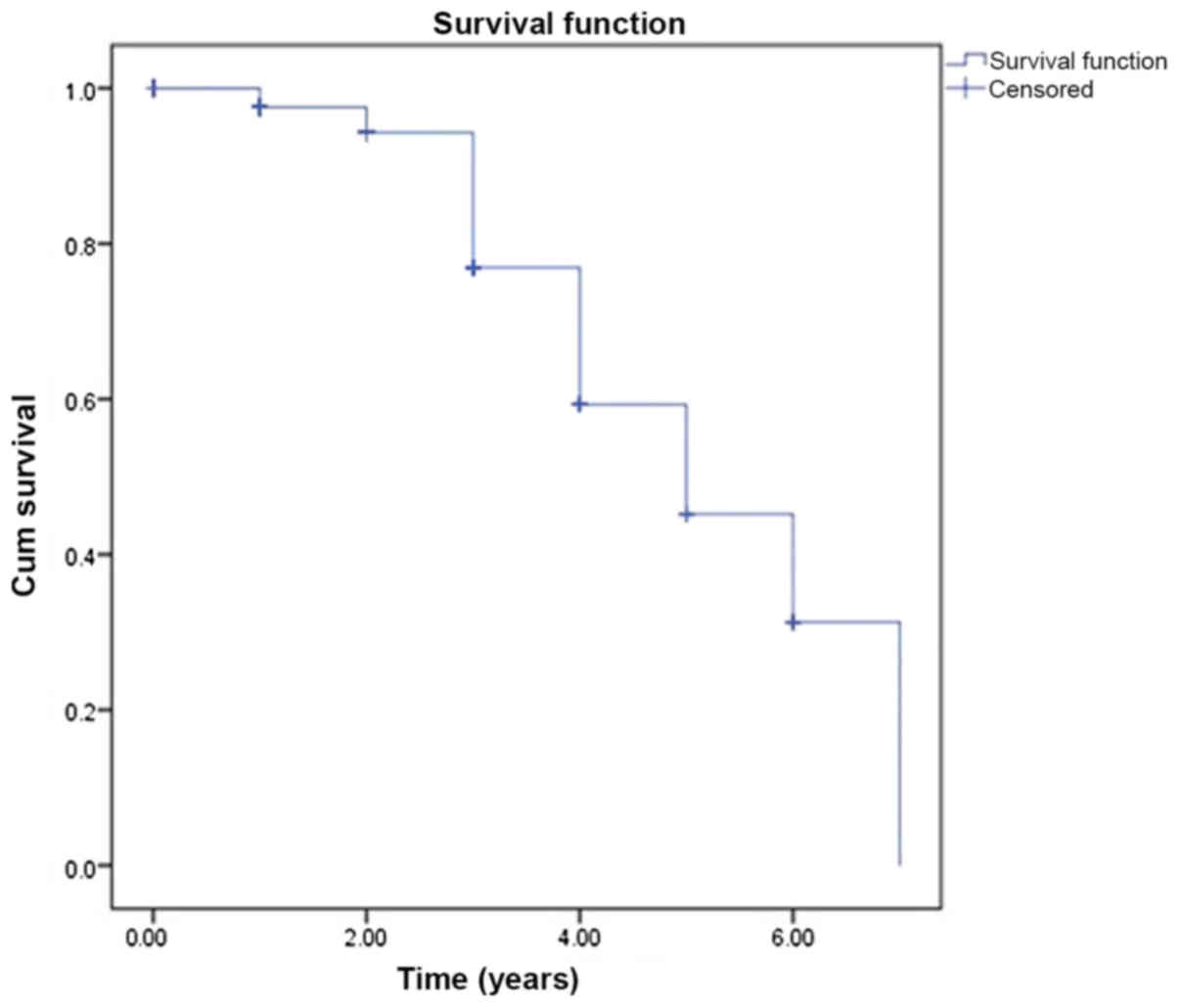
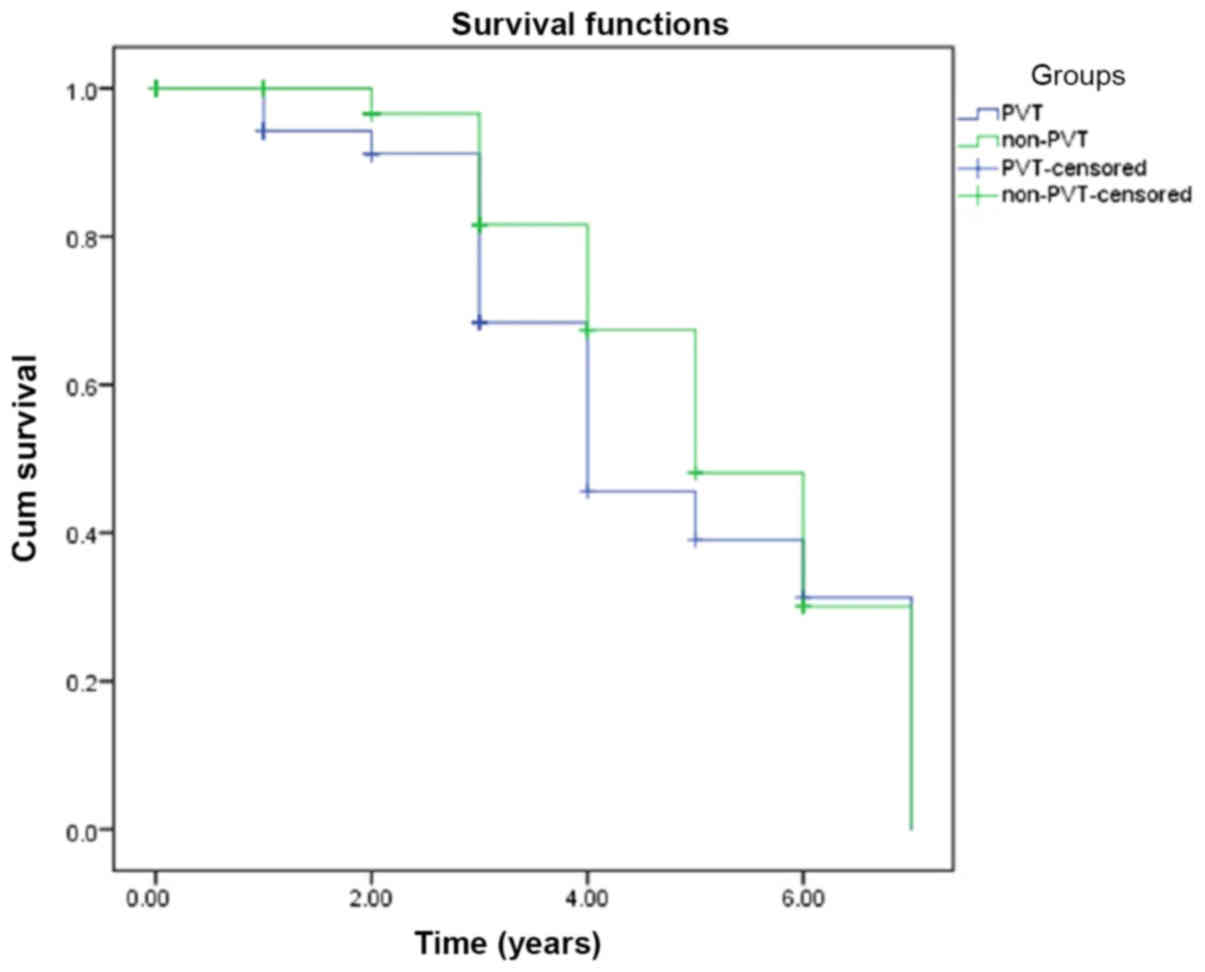
statistical differences were defined by the log-rank test.
Kaplan-Meier survival curves were formed from the survival time of
patient according to being dead and alive. All analyses were
conducted using IBM SPSS Statistics 23.0 software (IBM Corporation,
Armonk, NY).
Results
Study and patient characteristics
This study consisted of 199 individuals: 98 (49.2%)
in the PVT group and 101 (50.8%) in the non-PVT group. The
descriptive statistics of the groups are provided in Table I.
Parameter analysis
Significant differences in HTC, INR, ALB, BLB and
glucose were identified among the groups (P<0.05). There were no
significant differences in the other variables between the two
groups (P>0.05). Among the patients with PVT, 13 (13.3%) were in
Child-Pugh class A, 37 (37.8%) in class B and 48 (48.9%) in class
C. There were no significant differences in Child-Pugh classes
among the groups. Among the thrombophilic risk factors in patients
with PVT, FVL (12.2%), PTHR 20210 (15.3%) and MTHFR (16.3%) were
identified. Furthermore, a significant difference between the
groups was identified (P<0.05). It was also revealed that the
FVL mutation was more frequent in cirrhotic patients with PVT
compared with control groups (Table
II).
Statistical results
The results of backward stepwise multiple logistic
regression modeling are presented in Table III. According to these results, age
and HTC were significantly associated with PVT (P<0.05). The
Hosmer-Lemeshow test indicated that the backward stepwise multiple
logistic regression fit well to the data of interest (P=0.568;
Table III). The Omnibus tests of
model coefficients were statistically significant (P<0.001;
Table III).
 | Table III.The results of backward stepwise
multiple logistic regression modeling. |
Table III.
The results of backward stepwise
multiple logistic regression modeling.
|
|
|
|
|
| 95% C.I. for
OR |
|---|
|
|
|
|
|
|
|
|---|
| Factors | B | S.E. | P | OR | Lower | Upper |
|---|
| Age | 0.020 | 0.011 | 0.059 | 1.020 | 0.999 | 1.042 |
| HTC | −0.102 | 0.025 | 0.000 | 0.903 | 0.860 | 0.949 |
| Constant | 2.496 | 0.991 | 0.012 | 12.134 |
|
|
The Kaplan-Meier analysis of patient survival time
(years) was performed among groups and was demonstrated in Figs. 1 and 2. The survival time of the patients was
compared and the different was not significant (P>0.05).
Discussion
PVT was identified in 10% of the patients in this
study, confirming the increased occurrence of PVT in advanced
cirrhosis patients. This result supports those of a previous study
indicating that PVT is seen in advanced cirrhosis cases (14). Among the patients in the two groups,
hepatitis B virus (HBV) was identified to be the most frequent
cause of cirrhosis patients. This result supports a previous study
indicating that HBV is the major risk factor for PVT (15).
Different laboratory markers were analyzed in
several previous studies (1,3). In the present study, HCT, PLT, INR,
ALB, BLB, AST, ALT, Na and CR values were examined. However, only a
few of the various laboratory markers studied appeared to affect
the risk of developing PVT.
HTC levels were demonstrated to be a risk factor for
PVT in the present study. In fact, with splenomegaly, HTC levels
decrease as they are affected by hypersplenism and it is already
known that hypersplenism is a serious risk factor for PVT (16,17). In
a different previous study, the authors demonstrated that the
spleen was significantly larger in PVT patients (12). This condition was demonstrated in
another previous study as well (18). A significant association between
decreased PLT count and PVT is typically expected, as they are
associated with the same causes in cirrhosis patients. However, in
the comparison conducted in this study, no significant differences
in PLT were identified between the groups (19–21). The
results of other studies support the results of the present study
(7,22). In a previous study, it was
demonstrated that platelet counts were not associated with venous
thromboembolism (VTE) risk of cirrhosis (23).
The liver has a number of hemostatic roles including
the production of most coagulation factors and inhibitors, as well
as fibrinolytic factors (24). Under
normal physiological conditions, there is a stability among
procoagulant and anticoagulant factors (25). This delicate hemostatic balance
between the two groups works to avoid excessive thrombin generation
(3). This stability is disrupted by
the effects of advanced liver disease in the form of decreased
synthesis of coagulation factors, inhibitors, abnormal clotting
factors, abnormalities of fibrinolytic activity, disseminated
intravascular coagulation and platelet function defects (24). It is known that reduced levels of the
natural anticoagulant antithrombin III (ATIII), protein C (PC) and
protein S (PS) in liver cirrhosis are strong indicators of the
disorder of hepatocyte synthetic function. This finding is
corroborated further by the positive correlations between the
levels of these parameters and other synthetic liver products,
including ALB, fibrinogen, and plasminogen in liver cirrhosis
(26). The stability between
procoagulant and anticoagulant factors is disrupted in association
with the severity of liver damage. However, the reduction in
natural anticoagulant level is greater compared with other factors
(27). In a previous study, the
authors demonstrated that plasma levels of anticoagulant proteins,
including PC, PS and ATIII, were lower during follow-up in
cirrhosis patients who developed PVT compared with those without
PVT (24). In a previous
retrospective cohort study, the authors demonstrated that a higher
INR level did not translate into a decreased risk of thrombosis in
cirrhosis patients (28). Therefore,
rising INR levels are probably caused by PVT. The results obtained
in the present study agree with this hypothesis.
The Child-Turcotte-Pugh (CTP) scoring system is used
as a prognostic tool to measure the severity of cirrhosis in
patients. In the present study, no significant differences in CTP
classes were identified between the groups, which is similar to the
results of a previous study (3).
Several laboratory markers are used in the CTP scoring system, two
of which are BLB and ALB. As such, it is known that increased BLB
and low ALB levels parallel the stages of the disease (29). In chronic liver disease, the slowing
of portal blood flow associated with the degree of portal
hypertension is a condition that creates a tendency toward
thrombosis (30). It is well known
that portal stream speed is inversely associated with CTP score
(31); therefore, the incidence of
PVT is increased in cirrhosis patients with CTP class C. In a
previous study, the authors demonstrated that chronic liver disease
patients with thrombosis had increased BLB levels compared with
controls (32). In the present
study, elevated BLB levels were associated with a higher incidence
of thrombosis in cirrhosis patients. This situation is probably
associated with deterioration in portal and hepatic venous stream,
which may predispose the patient to PVT. Therefore, BLB and ALB are
the two independent risk factors. It is well known that low ALB
level may be a reflection of overall hepatic function and reserve.
In the current study, serum ALB level was identified to be
associated with PVT.
The associated analyses in the present study
suggested that glucose levels may be the only risk factor
associated with PVT. Diabetes mellitus, which has previously been
considered to be a risk factor for VTE and arterial thrombosis
(33,34), may be complicated by microvascular
occlusive disease and systemic effects on inflammation,
coagulation, and fibrinolysis. This situation has been suggested to
cause VTE among patients with diabetes (35). The association between glucose levels
and PVT is probably due to the same adverse influences on the
vascular wall.
No significant differences in AST or ALT were
demonstrated in the comparisons between the groups. The MELD
scoring system includes INR, serum BLB and Cr levels. In a previous
study, it was demonstrated that a reduction in the level of
antithrombotic proteins is strongly associated with the severity of
liver cirrhosis in accordance with the MELD system (14). No differences in blood levels of Na
or Cr, which is a member of the MELD, were demonstrated in the
comparisons between the groups.
The thrombophilia risk factors that are contained at
least a portion (17.6%) of patients with PVT were effect absolutely
on the formation of PVT. The comparison between groups could be
performed. It was demonstrated that there was a difference in FVL
mutation levels when the groups were compared. These results were
demonstrated that was similar to previous studies (1,36,37).
Previous studies have demonstrated that certain thrombophilic
genotypes, including FVL mutation, may be more frequent in
cirrhotic patients with PVT compared with cirrhotic patients
without PVT (7).
In a previous study, it was demonstrated that
mortality rates were increased in the patients with PVT (11). However, in this study, no differences
in survival time were identified in the comparisons between the
groups. This situation can explain by the fact that the majority of
the patients without PVT in the advanced stage.
As a result, in the present study, it has been
demonstrated that patients with hyperglycemia, hypoalbuminemia,
anemia, hyperbilirubinemia and elevated INR levels are
statistically more likely to have thromboses compared with control
group patients. The results of the present study are similar to
those of previously published studies of cirrhosis patients
(38,39).
The current study had certain limitations as a
result of its retrospective design and all the data were obtained
from a single center. Therefore, it was a relatively small number
of patients. Therefore, larger samples and prospective randomized
controlled trials are required to better understand the
characteristics of PVT.
Several different risk factors serve a role in the
development of PVT in liver cirrhosis and it is important to
identify the early development of PVT in cirrhosis patients
(40,41). PVT should certainly not be ignored,
as it may increase the bleeding risk of varices and cause
difficulties associated with liver transplantation in cirrhosis
patients (42,43). PVT is mostly asymptomatic, but when
patients start to exhibit symptoms the prognosis becomes poor
(1). PVT is also associated with
increased length of hospital stay and increased costs, leading to
an increased health care burden. Therefore, there is a need for
parameters to predict PVT. These results remind us that PVT should
be considered when evaluating cirrhosis patients. However,
treatment against PVT should be considered very cautiously in the
absence of absolute contraindications in all these patients.
Although these parameters have been studied previously, sample size
increases statistical value.
The present study indicates that there is an
association between the formation of PVT and several laboratory
variables, including HCT, INR, ALB, BLB, glucose and FVL. However,
further research efforts are required to better identify and
confirm additional risk factors in a larger population.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets and materials used during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YFC conceived and wrote the manuscript. FF and AKA
collected the data. CC statistically analyzed the results. YB, IB,
OY, MAE, YS and MH designed and interpreted the current study and
contributed to further drafts. YFC is the guarantor.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of
interest regarding the publication of this paper.
References
|
1
|
Amitrano L, Guardascione MA, Brancaccio V,
Margaglione M, Manguso F, Iannaccone L, Grandone E and Balzano A:
Risk factors and clinical presentation of portal vein thrombosis in
patients with liver cirrhosis. J Hepatol. 40:736–741. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nery F, Chevret S, Condat B, de Raucourt
E, Boudaoud L, Rautou PE, Plessier A, Roulot D, Chaffaut C,
Bourcier V, et al: Causes and consequences of portal vein
thrombosis in 1,243 patients with cirrhosis: Results of a
longitudinal study. Hepatology. 61:660–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen H, Trilok G, Wang F, Qi X, Xiao J and
Yang C: A single hospital study on portal vein thrombosis in
cirrhotic patients-clinical characteristics & risk factors.
Indian J Med Res. 139:260–266. 2014.PubMed/NCBI
|
|
4
|
Fimognari FL and Violi F: Portal vein
thrombosis in liver cirrhosis. Intern Emerg Med. 3:213–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ogren M, Bergqvist D, Björck M, Acosta S,
Eriksson H and Sternby NH: Portal vein thrombosis: Prevalence,
patient characteristics and lifetime risk: A population study based
on 23796 consecutive autopsies. World J Gastroenterol.
12:2115–2119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amitrano L, Brancaccio V, Guardascione MA,
Margaglione M, Iannaccone L, D'Andrea G, Marmo R, Ames PR and
Balzano A: Inherited coagulation disorders in cirrhotic patients
with portal vein thrombosis. Hepatology. 31:345–348. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Francoz C, Valla D and Durand F: Portal
vein thrombosis, cirrhosis, and liver transplantation. J Hepatol.
57:203–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tripodi A, Anstee QM, Sogaard KK,
Primignani M and Valla DC: Hypercoagulability in cirrhosis: Causes
and consequences. J Thromb Haemost. 9:1713–1723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar A, Sharma P and Arora A: Review
article: Portal vein obstruction-epidemiology, pathogenesis,
natural history, prognosis and treatment. Aliment Pharmacol Ther.
41:276–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shetty S and Ghosh K: Thrombophilic
dimension of Budd chiari syndrome and portal venous thrombosis-a
concise review. Thromb Res. 127:505–512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Englesbe MJ, Kubus J, Muhammad W,
Sonnenday CJ, Welling T, Punch JD, Lynch RJ, Marrero JA and
Pelletier SJ: Portal vein thrombosis and survival in patients with
cirrhosis. Liver Transpl. 16:83–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raja K, Jacob M and Asthana S: Portal vein
thrombosis in cirrhosis. J Clin Exp Hepatol. 4:320–331. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Durand F and Valla D: Assessment of the
prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 42
(Suppl 1):S100–S107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ponziani FR, Zocco MA, Garcovich M,
D'Aversa F, Roccarina D and Gasbarrini A: What we should know about
portal vein thrombosis in cirrhotic patients: A changing
perspective. World J Gastroenterol. 18:5014–5020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lertpipopmetha K and Auewarakul CU: High
incidence of hepatitis B infection-associated cirrhosis and
hepatocellular carcinoma in the Southeast Asian patients with
portal vein thrombosis. BMC Gastroenterol. 11:662011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawanaka H, Akahoshi T, Kinjo N, Konishi
K, Yoshida D, Anegawa G, Yamaguchi S, Uehara H, Hashimoto N,
Tsutsumi N, et al: Impact of antithrombin III concentrates on
portal vein thrombosis after splenectomy in patients with liver
cirrhosis and hypersplenism. Ann Surg. 251:76–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Condat B and Valla D: Nonmalignant portal
vein thrombosis in adults. Nat Clin Pract Gastroenterol Hepatol.
3:505–515. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ushitora Y, Tashiro H, Takahashi S, Amano
H, Oshita A, Kobayashi T, Chayama K and Ohdan H: Splenectomy in
chronic hepatic disorders: Portal vein thrombosis and improvement
of liver function. Dig Surg. 28:9–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi X, Zhang C, Han G, Zhang W, He C, Yin
Z, Liu Z, Bai W, Li R, Bai M, et al: Prevalence of the JAK2V617F
mutation in Chinese patients with Budd-Chiari syndrome and portal
vein thrombosis: A prospective study. J Gastroenterol Hepatol.
27:1036–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang D, Hao J and Yang N: Protein C and
D-dimer are related to portal vein thrombosis in patients with
liver cirrhosis. J Gastroenterol Hepatol. 25:116–121. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Wen TF, Yan LN, Yang HJ, Deng XF,
Li C, Wang C and Liang GL: Preoperative predictors of portal vein
thrombosis after splenectomy with periesophagogastric
devascularization. World J Gastroenterol. 18:1834–1839. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lisman T and Porte RJ: The role of
platelets in liver inflammation and regeneration. Semin Thromb
Hemost. 36:170–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Northup PG, McMahon MM, Ruhl AP,
Altschuler SE, Volk-Bednarz A, Caldwell SH and Berg CL:
Coagulopathy does not fully protect hospitalized cirrhosis patients
from peripheral venous thromboembolism. Am J Gastroenterol.
101:1524–1528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zocco MA, Di Stasio E, De Cristofaro R,
Novi M, Ainora ME, Ponziani F, Riccardi L, Lancellotti S,
Santoliquido A, Flore R, et al: Thrombotic risk factors in patients
with liver cirrhosis: Correlation with MELD scoring system and
portal vein thrombosis development. J Hepatol. 51:682–689. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts LN, Patel RK and Arya R:
Haemostasis and thrombosis in liver disease. Br J Haematol.
148:507–521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al Ghumlas AK, Abdel Gader AG and Al Faleh
FZ: Haemostatic abnormalities in liver disease: Could some
haemostatic tests be useful as liver function tests? Blood Coagul
Fibrinolysis. 16:329–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vukovich T, Teufelsbauer H, Fritzer M,
Kreuzer S and Knoflach P: Hemostasis activation in patients with
liver cirrhosis. Thromb Res. 77:271–278. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dabbagh O, Oza A, Prakash S, Sunna R and
Saettele TM: Coagulopathy does not protect against venous
thromboembolism in hospitalized patients with chronic liver
disease. Chest. 137:1145–1149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nicoll A: Surgical risk in patients with
cirrhosis. J Gastroenterol Hepatol. 27:1569–1575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fimognari FL, De Santis A, Piccheri C,
Moscatelli R, Gigliotti F, Vestri A, Attili A and Violi F:
Evaluation of D-dimer and factor VIII in cirrhotic patients with
asymptomatic portal venous thrombosis. J Lab Clin Med. 146:238–243.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zironi G, Gaiani S, Fenyves D, Rigamonti
A, Bolondi L and Barbara L: Value of measurement of mean portal
flow velocity by Doppler flowmetry in the diagnosis of portal
hypertension. J Hepatol. 16:298–303. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anthony Lizarraga W, Dalia S, Reinert SE
and Schiffman FJ: Venous thrombosis in patients with chronic liver
disease. Blood Coagul Fibrinolysis. 21:431–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Piazza G, Goldhaber SZ, Kroll A, Goldberg
RJ, Emery C and Spencer FA: Venous thromboembolism in patients with
diabetes mellitus. Am J Med. 125:709–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Movahed MR, Hashemzadeh M and Jamal MM:
The prevalence of pulmonary embolism and pulmonary hypertension in
patients with type II diabetes mellitus. Chest. 128:3568–3571.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lowe GD: Common risk factors for both
arterial and venous thrombosis. Br J Haematol. 140:488–495. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Egesel T, Büyükasik Y, Dündar SV, Gürgey
A, Kirazli S and Bayraktar Y: The role of natural anticoagulant
deficiencies and factor V Leiden in the development of idiopathic
portal vein thrombosis. J Clin Gastroenterol. 30:66–71. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qi X, Ren W, De Stefano V and Fan D:
Associations of coagulation factor V Leiden and prothrombin G20210A
mutations with Budd-Chiari syndrome and portal vein thrombosis: A
systematic review and meta-analysis. Clin Gastroenterol Hepatol.
12:1801–1812.e7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weber A, Krebs S, Lenhardt C, Wagenpfeil
S, Schmid RM and Schulte-Frohlinde E: Correlation of routinely used
coagulation parameters and presence of portal vein thrombosis in
patients with liver cirrhosis. Hepatol Res. 39:882–887. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen H, Qi X, He C, Yin Z, Fan D and Han
G: Coagulation imbalance may not contribute to the development of
portal vein thrombosis in patients with cirrhosis. Thromb Res.
131:173–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mantaka A, Augoustaki A, Kouroumalis EA
and Samonakis DN: Portal vein thrombosis in cirrhosis: Diagnosis,
natural history, and therapeutic challenges. Ann Gastroenterol.
31:315–329. 2018.PubMed/NCBI
|
|
41
|
Denninger MH, Chaït Y, Casadevall N,
Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Briere J and Valla
D: Cause of portal or hepatic venous thrombosis in adults: The role
of multiple concurrent factors. Hepatology. 31:587–591. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stine JG, Pelletier SJ, Schmitt TM, Porte
RJ and Northup PG: Pre-transplant portal vein thrombosis is an
independent risk factor for graft loss due to hepatic artery
thrombosis in liver transplant recipients. HPB (Oxford).
18:279–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Englesbe MJ, Schaubel DE, Cai S, Guidinger
MK and Merion RM: Portal vein thrombosis and liver transplant
survival benefit. Liver Transpl. 16:999–1005. 2010. View Article : Google Scholar : PubMed/NCBI
|