Introduction
Pulpitis is an inflammation of the dental pulp and
the most common type of pathological disease to affect the pulp
tissue; it is characterized by pain, which can be severe (1). The diagnosis and treatment of pulpitis
are difficult as there are multiple causes, which can involve
various different microbes. Therefore, pulpitis has become a focus
in clinical research. Multiple factors are responsible for the
occurrence of pulpitis, including bacterial infection, physical and
chemical stimulation and the immune response, although bacterial
infection is the primary cause (2,3).
Obligate anaerobes and facultative anaerobes are the primary
pathogens that cause pulpitis (4).
Following lysis, Gram-negative bacteria release bacterial
endotoxin, which has strong cytotoxicity and antigenicity (5). Endotoxin may directly destroy the local
tissues and induce an inflammatory reaction (6). It has been previously reported that
endotoxin induces the release of prostaglandins, interleukin (IL),
leukotrienes, transforming growth factor and tumor necrosis factor
(TNF) by macrophages and pulp cells (7). IL-6 is a key cytokine in inflammatory
processes and has been previously reported to serve an important
role in the pathogenesis of pulpitis (8,9).
However, IL-6 only exerts its biological effects after binding with
the IL-6 receptor (IL-6R) to form an IL-6/IL-6R complex, which is
subsequently combined with gp130 to form a high-affinity complex
(10). However, the regulatory
effects of IL-6R in pulpitis and the regulatory mechanism of the
upstream genes of IL-6R have not been entirely identified.
MicroRNAs (miRNAs or miRs) are a type of non-coding,
small RNA molecule made up of 18–22 nucleotides, which regulate the
expression of proteins at mRNA level in eukaryotes (11–13). The
pathogenesis of pulpitis is accompanied by changes in the
expression of a variety of miRNA molecules and proteins, suggesting
that miRNA may serve important roles in the regulation of proteins
associated with the disease (14–16). In
the present study, the expression of IL-6R mRNA and protein were
examined in pulp tissues, blood and saliva from patients with
pulpitis. The association between IL-6R and miR-30b was also
investigated as well as their effects on pulpitis.
Patients and methods
Patients
A total of 28 patients with pulpitis (12 male and 16
female; age range, 20–51 years; median age, 37.6 years) who
underwent tooth extraction at Nanjing Stomatological Hospital
(Nanjing, China) and The First Affiliated Hospital of Nanjing
Medical University (Nanjing, China) between June 2014 and December
2016 were recruited into the experimental group. A total of 16
subjects who had no pulpitis but also underwent a tooth extraction
operation (6 male and 10 female; age range, 19–52 years; median
age, 36.9 years) were recruited into the control group. The
exclusion criteria were: Intake of non-steroidal drugs, use of
antibiotics and drinking alcohol or smoking within two weeks prior
to diagnosis. Pulp tissues were collected from all subjects under
sterile conditions, washed with saline and stored in liquid
nitrogen (−196°C) prior to use. Fasting peripheral blood was
collected from all subjects on the morning of the tooth extraction
and plasma was separated from venous blood (30 ml) by
centrifugation at 1,000 × g at 4°C for 10 min. Saliva was collected
from all subjects prior to the operation on the day of tooth
extraction and stored under −80°C prior to its further
investigation. All procedures were approved by the Ethics Committee
of Nanjing University (Nanjing, China) and written informed consent
was obtained from all patients or their families prior to their
inclusion within the study.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Pulp tissues (100 mg) were ground into a powder in
liquid nitrogen and lysed using 1 ml TRIzol reagent (Thermo Fisher
Scientific, Waltham, MA, USA) according to the manufacturer's
protocol. Plasma (100 µl) or saliva (100 µl) were directly lysed
using 1 ml TRIzol reagent. Total RNA was extracted using the phenol
chloroform method (17). RNA (1 µg)
was reverse-transcribed into cDNA at 42°C for 60 min and stored at
−20°C prior to further experimentation. The TIANScript II cDNA
First Strand Synthesis Kit (Tiangen Biotech Co., Ltd., Beijing,
China) was used for RT of mRNA and the miRcute miRNA First-strand
cDNA Synthesis kit (Tiangen Biotech Co., Ltd.) was used for the
reverse transcription of miRNA.
To detect IL-6R mRNA expression the SuperReal PreMix
(SYBR-Green) RT-qPCR kit (Tiangen Biotech Co., Ltd.) was used and
GAPDH was used as the internal reference gene. The primer sequences
of IL-6R were forward, 5′-TGGTGGATGTTCCCCCCGAG-3′ and reverse,
5′-TCCTGGGAATACTGGCACGG-3′. The sequences of GAPDH were forward,
5′-AAGGCTGTGGGCAAGG-3′ and reverse, 5′-TGGAGGAGTGGGTGTCG-3′. The
reaction system (20 µl) was composed of RT-qPCR-Mix (10 µl),
upstream primer (0.5 µl), downstream primer (0.5 µl), cDNA (2 µl)
and ddH2O (7 µl). The PCR protocol was as follows:
Initial denaturation at 95°C for 30 sec; denaturation at 95°C for 5
sec and 39 cycles for elongation at 60°C for 20 sec (iQ5; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The 2−ΔΔCq
method (18) was used to calculate
the relative expression of IL-6R mRNA against GAPDH. Each sample
was examined in triplicate.
The miRcute miRNA RT-PCR kit (Tiangen Biotech Co.,
Ltd.) was used to detect the expression of miR-30b and U6 was used
as the internal reference. The sequences of the miR-30b primers
were forward, 5′-CGCGCTGTAAACATCCTACAC-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′. The sequences of the U6 primers were
forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The reaction system (20 µl) was the
same as above. The reaction protocol was as follows: Initial
denaturation at 95°C for 5 min; 95°C for 10 sec and 40 cycles at
60°C for 20 sec; and elongation at 72°C for 10 sec. The
2−ΔΔCq method was used to calculate the relative
expression of miR-30b against U6. Each sample was tested in
triplicate.
Western blot analysis
Tissue samples in each group were lysed with 600 µl
precooled radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China). Following lysis for 30
min on ice, the mixture was centrifuged at 1,200 × g for 10 min at
4°C. Protein concentration was determined from the supernatant
using a bicinchoninic acid determination kit (cat. no. RTP7102;
Real-Times Biotechnology Co., Ltd., Beijing, China). The protein
samples were subsequently mixed with 5X SDS loading buffer.
Following denaturation in boiling water for 5 min, the 20 µg
samples were loaded onto 10% SDS-PAGE for electrophoresis. The
resolved proteins were transferred to polyvinylidene difluoride
membranes on ice (100 V for 2 h) and blocked with 5% skimmed milk
at room temperature for 1 h. The membranes were incubated with
rabbit anti-human IL-6R (1:1,000; cat. no. ab128008) and β-actin
(1:5,000; cat. no. ab8227) polyclonal primary antibodies (Abcam,
Cambridge, UK) at 4°C overnight. Following washing 5 times (5 min
each time) with PBS with Tween-20 (PBST), the membranes were
incubated with goat anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:3,000; cat. no. ab6721;
Abcam) for 1 h at room temperature prior to washing again with PBST
5 times (5 min each time). The membranes were subsequently
developed using an enhanced chemiluminescence detection kit (cat.
no. 11520709001; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for
imaging. Image Lab v3.0 software (Bio-Rad Laboratories, Inc.) was
used to acquire and analyze the imaging signals. The relative
content of IL-6R protein was expressed against β-actin.
ELISA
An IL-6R ELISA kit (Shanghai Joe Feather Biological
Science and Technology Co., Ltd., Shanghai, China) was used to
determine the concentration of IL-6R in plasma and saliva samples.
In 96-well microplates, the standards (50 µl) and samples (10 µl
liquid samples and 40 µl diluent) were added into the predefined
wells, while the blank wells were left empty. In the wells for
standards and samples, HRP-labeled conjugates (100 µl) were added
prior to sealing the plates for incubation at 37°C for 1 h.
Following washing of the plates 5 times, the substrates A (50 µl)
and B (50 µl) were added into each well. After incubation at 37°C
for 15 min, the stop solution (50 µl) was added into each well and
the absorbance of each well was measured at 450 nm within 15
min.
Dual luciferase reporter assay
Bioinformatics prediction is a powerful tool used to
study the various functions of miRNAs. To understand the regulatory
mechanism of IL-6R miRanda (microrna.org/microrna/home.do), TargetScan (targetscan.org), PiTa (genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (bibiserv.techfak.uni-bielefeld.de/rnahybrid/) and
PICTA (pictar.mdc-berlin.de/) were used to
predict the miRNA molecules that may regulate IL-6R. The databases
revealed that miR-30b may potentially regulate IL-6R.
Based on the bioinformatics results, wild-type (WT)
and mutant seed regions of miR-30b in the 3′-untranslated region
(UTR) of the IL-6R gene were chemically synthesized in vitro
by Sangon Biotech Co., Ltd., Shanghai, China). The two ends were
subsequently attached using the Spe1 and HindIII
restriction sites and cloned into pMIR-REPORT luciferase reporter
plasmids (Ambion) using Lipofectamine 2000 (both Thermo Fisher
Scientific, Inc.). Plasmids (0.8 µg) with either the negative
control (NC), WT or mutant 3′-UTR DNA sequences were co-transfected
with agomiR-30b (100 nM; Sangon Biotech Co., Ltd.) into 293T cells.
Following incubation at 37°C for 24 h, the cells were lysed using a
dual luciferase reporter assay kit (Promega Corporation, Fitchburg,
WI, USA) according to the manufacturer's manual. Fluorescence
intensity was measured using a GloMax 20/20 luminometer (Promega
Corporation). The fluorescence values of each group of cells were
measured using Renilla fluorescence activity as the internal
reference.
Statistical analysis
The results were analyzed using SPSS 18.0
statistical software (SPSS, Inc., Chicago, IL, USA). The data were
expressed as the mean ± standard deviation. The data were tested
for normality and multi-group data were analyzed using one-way
analysis of variance. In cases of homogeneity of variance the Least
Significant Difference and Student-Newman-Keuls post hoc methods
were used; in cases of heterogeneity of variance the Tamhane's T2
or Dunnett's T3 post hoc methods were used. Comparison between two
groups was performed using a Student's t-test. Each test was
repeated at least 3 times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of IL-6R mRNA is
significantly elevated in pulp tissues, plasma and saliva from
patients with pulpitis
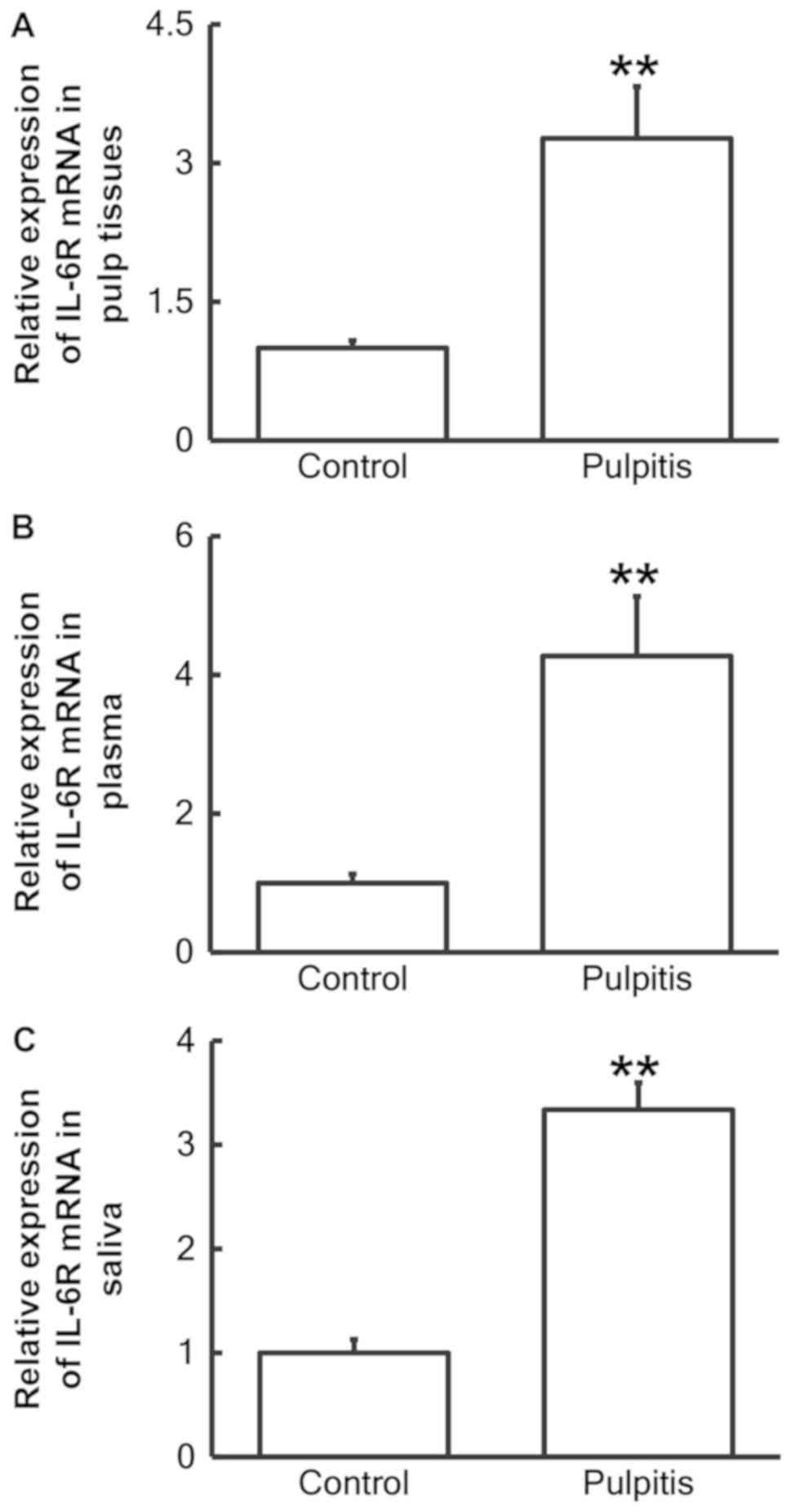
RT-qPCR was performed to measure the expression of
IL-6R mRNA in pulp tissue, plasma and saliva. The data revealed
that the IL-6R mRNA levels in the pulp tissue (Fig. 1A), plasma (Fig. 1B) and saliva (Fig. 1C) from patients with pulpitis were
significantly increased compared with the control groups (P<0.01
for all).
Protein expression of IL-6R is
significantly elevated in pulp tissues from patients with
pulpitis
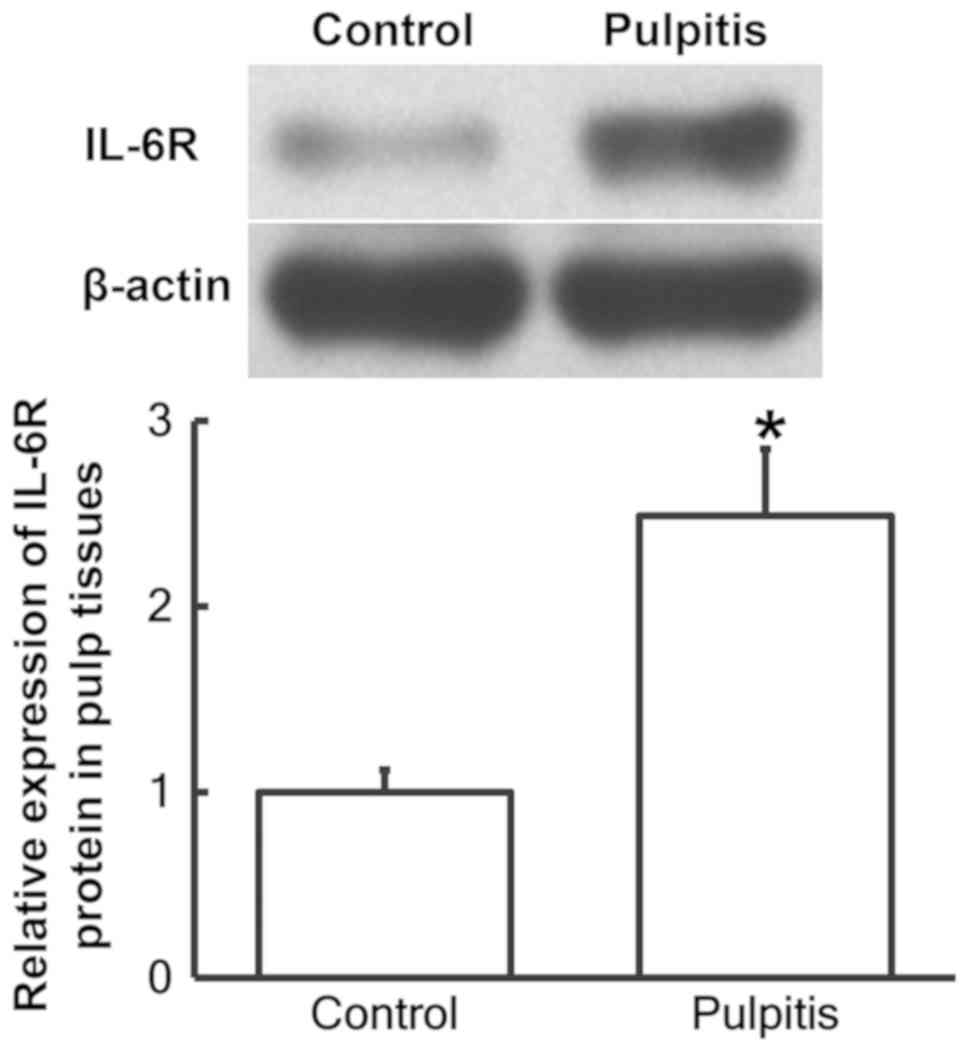
To determine the protein expression of IL-6R in the
pulp tissues, western blot analysis was performed. The results
demonstrated that IL-6R protein expression was significantly
increased in pulp tissues from patients with pulpitis compared with
the control group (P<0.05; Fig.
2).
IL-6R protein is significantly
increased in the plasma and saliva from patients with pulpitis
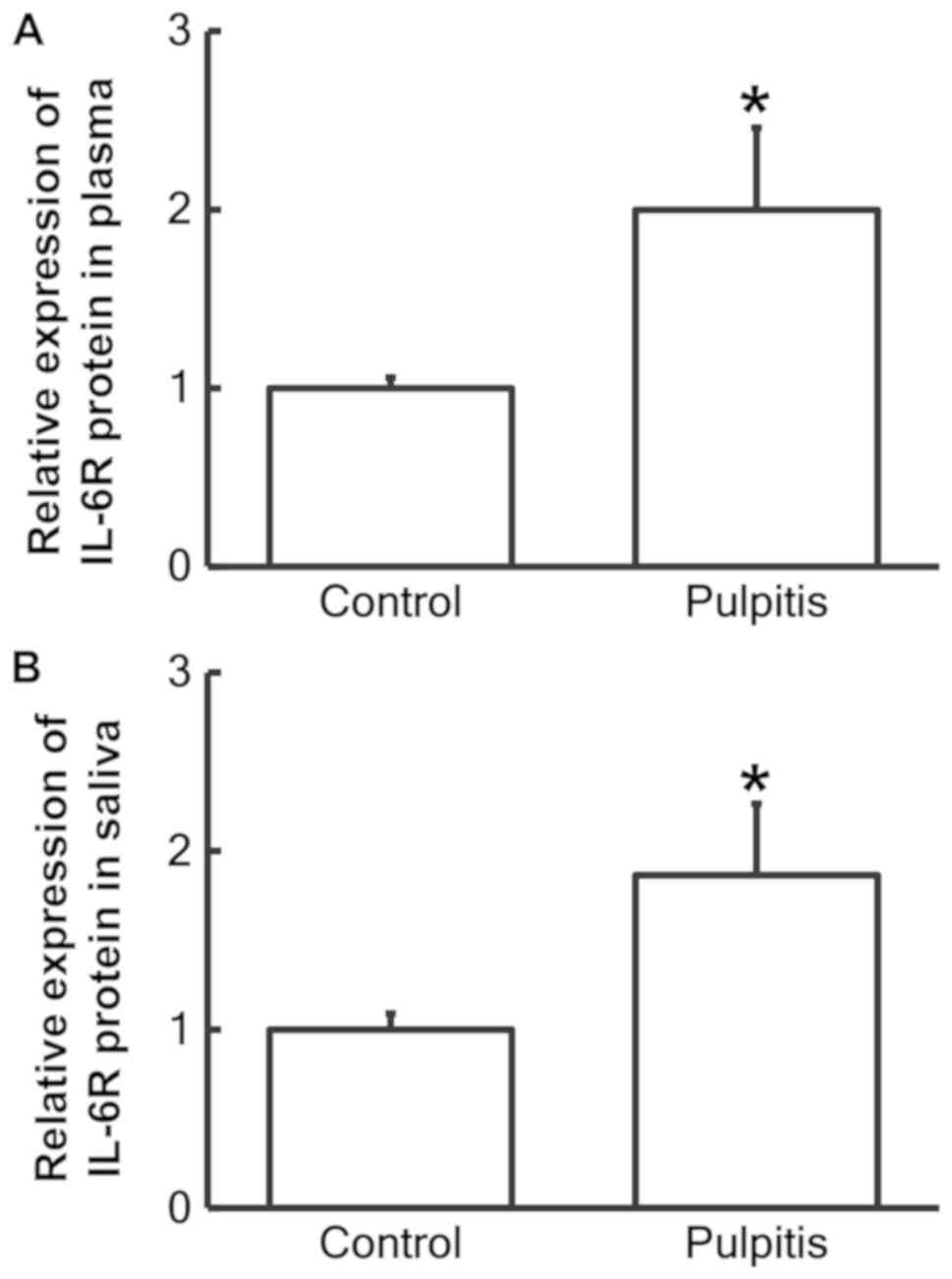
ELISA was used to examine the level of IL-6R protein
in the plasma and saliva. The results revealed that the IL-6R
protein content in the plasma and saliva of patients with pulpitis
was significantly increased compared with the control group (both
P<0.05; Fig. 3).
Levels of miR-30b are reduced in the
pulp tissue, plasma and saliva of patients with pulpitis
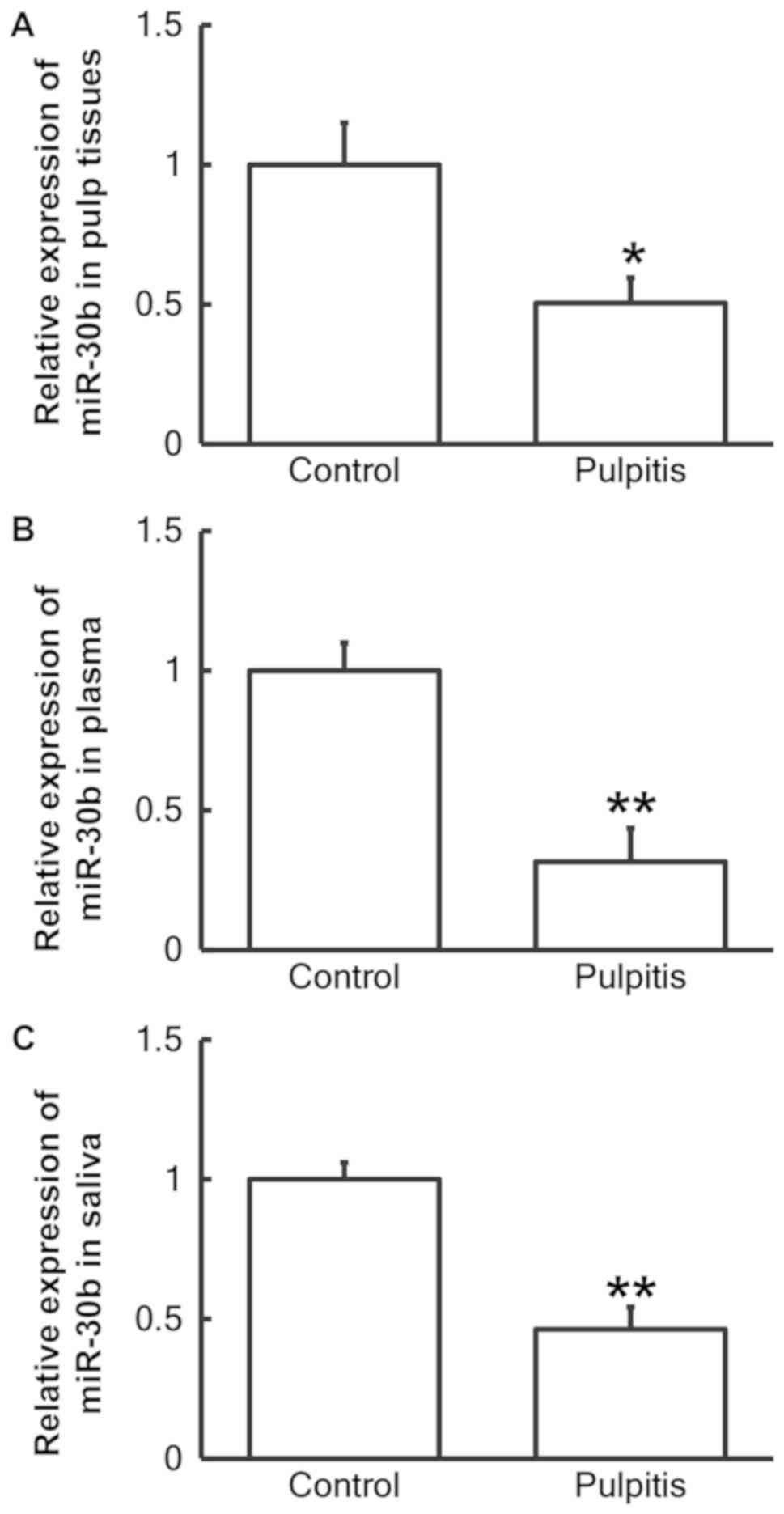
RT-qPCR was used to measure the expression of
miR-30b in the three sample types. The results demonstrated that
the level of miR-30b in the pulp tissue (P<0.05; Fig. 4A), plasma (P<0.01; Fig. 4B) and saliva (P<0.01; Fig. 4C) of patients with pulpitis was
significantly reduced compared with normal individuals.
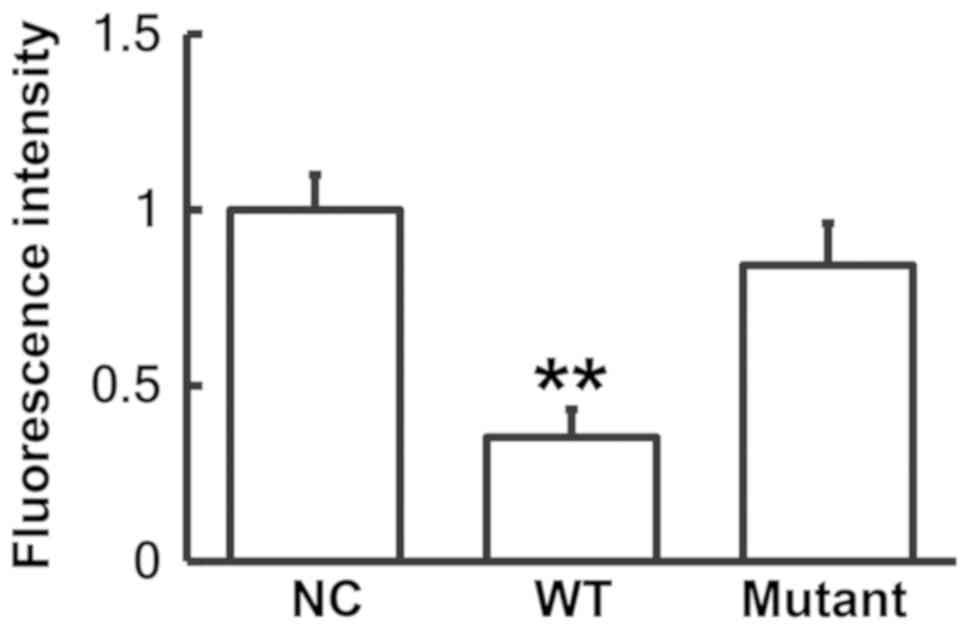
miR-30b may bind with the 3′-UTR seed
region of IL-6R mRNA to regulate its expression
A dual luciferase reporter assay was performed to
identify any interactions between miR-30b and the 3′-UTR of IL-6R
mRNA as predicted by bioinformatics. The fluorescence value of the
cells co-transfected with the agomiR-30b and pMIR-REPORT-WT
luciferase reporter plasmids was significantly reduced compared
with the NC group (P<0.01; Fig.
5). By contrast, the fluorescence value of the cells
co-transfected with the agomiR-30b and pMIR-REPORT-mutant
luciferase reporter plasmids was not significantly different from
the NC group. These results indicate that miR-30b binds with the
3′-UTR seed region of IL-6R mRNA to regulate its expression.
Discussion
IL-6 is a cytokine with a wide variety of functions
and its expression is typically elevated as part of the immune
response (19). The production of
IL-6 is stimulated by foreign bodies, including bacteria, endotoxin
and dust particles (20). Elevated
IL-6 expression in the body can lead to inflammatory diseases,
including rheumatoid arthritis and Crohn's disease (21). In rheumatoid arthritis IL-6
stimulates the secretion of inflammatory mediators, including IL-1,
by T lymphocytes and B lymphocytes, promotes the maturation and
differentiation of B lymphocytes and enhances the effects of IL-1β
and TNF-α (22). It has been
reported that IL-6 has a chemotaxis effect on other inflammatory
cells during inflammatory responses, including neutral lymphocytes
and mononuclear macrophages (23).
IL-6 also induces the body to produce C-reactive protein and
fibrinogen during inflammation and may promote thrombosis (24). In addition, IL-6 also serves an
important role in the occurrence and development of cell
differentiation, coagulation and various types of cancer (25). The expression of IL-6 is
significantly elevated during inflammatory responses, which are
caused by injury, trauma, stress and infection. To have an effect
in cells, IL-6 must first bind with the membrane receptor IL-6R,
which is only expressed in hepatocytes, mononuclear cells,
macrophages and certain lymphocytes (26). This receptor-ligand complex then
combines with gp130 to form a dimer, which initiates intracellular
signals (27). Therefore, IL-6R is
an important regulator for the biological functions of IL-6.
In the present study, it was revealed that the
expression of IL-6R mRNA and protein was significantly increased in
the pulp tissues, plasma and saliva of patients with pulpitis
compared with the control group. This suggests that there is
inflammation associated with pulpitis and upregulation of IL-6R is
caused by the activation of mononuclear cells and lymphocytes,
which secrete abundant IL-6 to produce antigenic immune responses.
This is consistent with immune responses observed in other areas of
the body (28).
The regulation of mRNA transcription and expression
is a complex process, which is affected by multiple factors
(29). The present study focused on
miRNA molecules as the upstream regulatory factors of IL-6R.
Bioinformatics was used to predict the upstream genes that regulate
IL-6R. Literature searches suggested that miR-30b is an upstream
regulator of IL-6R. Notably, miRNA molecules exert negative
feedback effects on their target mRNA molecules by cutting the mRNA
and serving as a translation restraint. Therefore, miRNA molecules
are important regulatory factors in normal development, physiology
and disease. A number of miRNA molecules have been identified as
biomarkers of specific diseases (30,31).
miR-30b is a member of the miR-30 family, which includes miR-30a,
miR-30b, miR-30c, miR-30d and miR-30e (32). These five miRNA molecules are encoded
by genes located on different chromosomes; however, they have
similar seed sequences (33). It has
been previously reported that miR-30b may be involved in a number
of processes, including inflammatory responses, malignant tumor
development and epithelial mesenchymal transition (34–37), and
may have positive effects on nerve repair, the inhibition of
apoptosis and blood vessel regeneration (34–37). A
previous study has demonstrated that miR-30b is associated with the
occurrence and development of human neural tube cells and their
tumors; its expression is also associated with schizophrenia
(38). In melanoma miR-30b regulates
the expression of acetylglucosamine transferase, which promotes
tumor metastasis (39). In lung
cancer, trastuzumab inhibits the growth of cancer cells by
upregulating miR-30b (40). In
addition, miR-30b affects the development and degeneration of the
mammary gland (41) and regulates
the receptivity of the human endometrium (42). Therefore, miR-30b is associated with
the growth, development, differentiation and migration of cells.
The results of the present study were consistent with these
previous findings as they reveal that miR-30b was significantly
downregulated and IL-6R was significantly upregulated in the pulp
tissues of patients with pulpitis. This suggests that the immune
system may negatively regulate the cutting effect of miR-30b on
IL-6R through the downregulation of miR-30b and also promote the
expression of IL-6R, which participates in the immune response. The
results of the expression of miR-30b and IL-6R in the plasma and
saliva were similar to the results observed in the pulp tissues.
This indicates that the changes to miR-30b and IL-6R levels may
reflect the status of the inflammatory response and tissue damage
in pulpitis. The present study has some limitations, including the
small sample size and the patients primarily being Chinese.
In conclusion, the present study demonstrates in
patients with pulpitis the decreased expression of miR-30b in pulp
tissues, plasma, and saliva regulates the expression of IL-6R,
which serves a crucial biological role in the occurrence and
development of the disease. The gene assessed in the present study
may be used as a genetic marker for pulpitis detection in the
future. However, further studies are required to fully assess its
use in the diagnosis and treatment of the disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation (grant no. 81570952), the Natural
Science Foundation of Jiangsu Province (grant no. BK20161114), the
Program on Key Basic Research Project of Jiangsu Province (grant
no. BE2016623), the Doctoral Fund of Science and Technology
Commission Foundation of Nanjing (grant no. 201605043) and the
Medical Scientific Research and Development Project of Nanjing
(grant no. YKK15113).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
NZ, WY and LM conceived and designed the study. NZ,
QZ, NW, SW, JG, XL and JW performed experiments. NZ and QZ wrote
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nanjing University (Nanjing, China) and written
informed consent was obtained from all patients or their families
prior to their inclusion within the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dabuleanu M: Pulpitis
(reversible/irreversible). J Can Dent Assoc. 79:d902013.PubMed/NCBI
|
|
2
|
Hui T, Wang C, Chen D, Zheng L, Huang D
and Ye L: Epigenetic regulation in dental pulp inflammation. Oral
Dis. 23:22–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cooper PR, Holder MJ and Smith AJ:
Inflammation and regeneration in the dentin-pulp complex: A
double-edged sword. J Endod. 40 (4 Suppl):S46–S51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pourhajibagher M, Ghorbanzadeh R, Parker
S, Chiniforush N and Bahador A: The evaluation of cultivable
microbiota profile in patients with secondary endodontic infection
before and after photo-activated disinfection. Photodiagnosis
Photodyn Ther. 18:198–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lakshminarayanan R, Tan WX, Aung TT, Goh
ET, Muruganantham N, Li J, Chang JY, Dikshit N, Saraswathi P, Lim
RR, et al: Branched peptide, B2088, disrupts the supramolecular
organization of lipopolysaccharides and sensitizes the
gram-negative bacteria. Sci Rep. 6:259052016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jacinto RC, Gomes BP, Shah HN, Ferraz CC,
Zaia AA and Souza-Filho FJ: Quantification of endotoxins in
necrotic root canals from symptomatic and asymptomatic teeth. J Med
Microbiol. 54:777–783. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakanishi T, Matsuo T and Ebisu S:
Quantitative analysis of immunoglobulins and inflammatory factors
in human pulpal blood from exposed pulps. J Endod. 21:131–136.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Renard E, Gaudin A, Bienvenu G, Amiaud J,
Farges JC, Cuturi MC, Moreau A and Alliot-Licht B: Immune cells and
molecular networks in experimentally induced pulpitis. J Dent Res.
95:196–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elsalhy M, Azizieh F and Raghupathy R:
Cytokines as diagnostic markers of pulpal inflammation. Int Endod
J. 46:573–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolf J, Waetzig GH, Chalaris A, Reinheimer
TM, Wege H, Rose-John S and Garbers C: Different soluble forms of
the interleukin-6 family signal transducer gp130 fine-tune the
blockade of interleukin-6 trans-signaling. J Biol Chem.
291:16186–16196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia W, Wu Y, Zhang Q, Gao GE, Zhang C and
Xiang Y: Expression profile of circulating microRNAs as a promising
fingerprint for cervical cancer diagnosis and monitoring. Mol Clin
Oncol. 3:851–858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Graziano A, Lo Monte G, Piva I, Caserta D,
Karner M, Engl B and Marci R: Diagnostic findings in adenomyosis: A
pictorial review on the major concerns. Eur Rev Med Pharmacol Sci.
19:1146–1154. 2015.PubMed/NCBI
|
|
13
|
Jiang XI, Luo Y, Zhao S, Chen Q, Jiang C,
Dai Y, Chen Y and Cao Z: Clinical significance and expression of
microRNA in diabetic patients with erectile dysfunction. Exp Ther
Med. 10:213–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sehic A, Tulek A, Khuu C, Nirvani M, Sand
LP and Utheim TP: Regulatory roles of microRNAs in human dental
tissues. Gene. 596:9–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu L, Shu S, Cheung GS and Wei X: Effect
of miR-146a/bFGF/PEG-PEI nanoparticles on inflammation response and
tissue regeneration of human dental pulp cells. Biomed Res Int.
2016:38926852016.PubMed/NCBI
|
|
16
|
Kong Q, Liu L, Huang Y, Zhang F, Wei X and
Ling J: The effect of octamer-binding transcription factor 4B1 on
microRNA signals in human dental pulp cells with inflammatory
response. J Endod. 40:101–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vijayalakshmy K, Kumar P, Virmani M,
Pawaria S, Lalaji NS, Sharma P, Rajendran R, Yadav PS and Kumar D:
A novel combination of silane-coated silica colloid with hybrid RNA
extraction protocol and RNA enrichment for downstream applications
of spermatozoal RNA. Andrologia. 14:e130302018. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andersen BL, Goyal NG, Weiss DM, Westbrook
TD, Maddocks KJ, Byrd JC and Johnson AJ: Cells, cytokines,
chemokines, and cancer stress: A biobehavioral study of patients
with chronic lymphocytic leukemia. Cancer. 2018.(Epub ahead of
print). View Article : Google Scholar
|
|
20
|
Badding MA, Schwegler-Berry D, Park JH,
Fix NR, Cummings KJ and Leonard SS: Sintered indium-tin oxide
particles induce pro-inflammatory responses in vitro, in part
through inflammasome activation. PLoS One. 10:e01243682015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tone M, Powell MJ, Tone Y, Thompson SA and
Waldmann H: IL-10 gene expression is controlled by the
transcription factors Sp1 and Sp3. J Immunol. 165:286–291. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim WH, An HJ, Kim JY, Gwon MG, Gu H, Lee
SJ, Park JY, Park KD, Han SM, Kim MK and Park KK: Apamin inhibits
TNF-α- and IFN-γ-induced inflammatory cytokines and chemokines via
suppressions of NF-κB signaling pathway and STAT in human
keratinocytes. Pharmacol Rep. 69:1030–1035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo Q, Ma X, Wahl SM, Bieker JJ, Crossley
M and Montaner LJ: Activation and repression of interleukin-12 p40
transcription by erythroid Kruppel-like factor in macrophages. J
Biol Chem. 279:18451–18456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baeuerle PA and Henkel T: Function and
activation of NF-kappa B in the immune system. Annu Rev Immunol.
12:141–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andreesen R, Scheibenbogen C, Brugger W,
Krause S, Meerpohl HG, Leser HG, Engler H and Löhr GW: Adoptive
transfer of tumor cytotoxic macrophages generated in vitro from
circulating blood monocytes: A new approach to cancer
immunotherapy. Cancer Res. 50:7450–7466. 1990.PubMed/NCBI
|
|
26
|
Garbers C, Heink S, Korn T and Rose-John
S: Interleukin-6: Designing specific therapeutics for a complex
cytokine. Nat Rev Drug Discov. 17:395–412. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heo TH, Wahler J and Suh N: Potential
therapeutic implications of IL-6/IL-6R/gp130-targeting agents in
breast cancer. Oncotarget. 7:15460–15473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jordan SC, Choi J, Kim I, Wu G, Toyoda M,
Shin B and Vo A: Interleukin-6, A cytokine critical to mediation of
inflammation, autoimmunity and allograft rejection: Therapeutic
implications of IL-6 receptor blockade. Transplantation. 101:32–44.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Y, Yao X and Wang G: ‘Mediatoring’
messenger RNA processing. Wiley Interdiscip Rev RNA. 6:257–269.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Varshney J and Subramanian S: MicroRNAs as
potential target in human bone and soft tissue sarcoma
therapeutics. Front Mol Biosci. 2:312015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ouzounova M, Vuong T, Ancey PB, Ferrand M,
Durand G, Le-Calvez Kelm F, Croce C, Matar C, Herceg Z and
Hernandez-Vargas H: MicroRNA miR-30 family regulates non-attachment
growth of breast cancer cells. BMC Genomics. 14:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang SJ, Yang SY, Wang DD, Chen X, Shen
HY, Zhang XH, Zhong SL, Tang JH and Zhao JH: The miR-30 family:
Versatile players in breast cancer. Tumour Biol.
39:10104283176922042017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Donate PB, Fornari TA, Macedo C, Cunha TM,
Nascimento DC, Sakamoto-Hojo ET, Donadi EA, Cunha FQ and Passos GA:
T cell post-transcriptional miRNA-mRNA interaction networks
identify targets associated with susceptibility/resistance to
collagen-induced arthritis. PLoS One. 8:e548032013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He J, Jiang S, Li FL, Zhao XJ, Chu EF, Sun
MN, Chen MZ and Li H: MicroRNA-30b-5p is involved in the regulation
of cardiac hypertrophy by targeting CaMKIIdelta. J Investig Med.
61:604–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Braun J, Hoang-Vu C, Dralle H and
Huttelmaier S: Downregulation of microRNAs directs the EMT and
invasive potential of anaplastic thyroid carcinomas. Oncogene.
29:4237–4244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu F, Deng H, Yao H, Liu Q, Su F and Song
E: Mir-30 reduction maintains self-renewal and inhibits apoptosis
in breast tumor-initiating cells. Oncogene. 29:4194–4204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song PP, Hu Y, Liu CM, Yan MJ, Song G, Cui
Y, Xia HF and Ma X: Embryonic ectoderm development protein is
regulated by microRNAs in human neural tube defects. Am J Obstet
Gynecol. 204:544.e9–17. 2011. View Article : Google Scholar
|
|
39
|
Gaziel-Sovran A, Segura MF, Di Micco R,
Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert
JF, Shang S, Kerbel RS, et al: miR-30b/30d regulation of GalNAc
transferases enhances invasion and immunosuppression during
metastasis. Cancer Cell. 20:104–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ichikawa T, Sato F, Terasawa K, Tsuchiya
S, Toi M, Tsujimoto G and Shimizu K: Trastuzumab produces
therapeutic actions by upregulating miR-26a and miR-30b in breast
cancer cells. PLoS One. 7:e314222012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Le Guillou S, Sdassi N, Laubier J, Passet
B, Vilotte M, Castille J, Laloë D, Polyte J, Bouet S and Jaffrézic
F: Overexpression of miR-30b in the developing mouse mammary gland
causes a lactation defect and delays involution. PLoS One.
7:e457272012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Altmae S, Martinez-Conejero JA, Esteban
FJ, Ruiz-Alonso M, Stavreus-Evers A, Horcajadas JA and Salumets A:
MicroRNAs miR-30b, miR-30d, and miR-494 regulate human endometrial
receptivity. Reprod Sci. 20:308–317. 2013. View Article : Google Scholar : PubMed/NCBI
|