Introduction
Vitiligo is a common acquired skin disease
characterised by a loss of skin color that may occur at any age but
most commonly occurs in young adults (1). Vitiligo can arise in any area of the
skin, but mostly presents in exposed regions and those where
rubbing occurs (2). This disease
does not cause significant physical disorder of the affected skin;
however, severe effects on the physical and mental health of
patients may occur, hindering their ability to work and communicate
socially (3). The pathogenesis of
vitiligo remains unclear, which makes its clinical treatment more
challenging. Since hair follicle cells are considered to be immune
privileged (4), melanocyte precusors
that are stored in this area may be protected from disease. It is
therefore critical to induce perifollicular repigmentation in
vitiligo lesions of hair-covered skin. It has been demonstrated
that the rate of ineffectiveness in patients with vitiligo may be
as high as 50% (5,6), which may be associated with the
possibility that clinicians cannot assess the presence of
potentially mature melanocytes in vitiligo hair follicles prior to
treatment.
If melanocyte linkage-specific genes are identified
in vitiligo lesions, the appropriate treament measure may be
implemented for melanocytes. Tyrosinase (Tyr), tyrosine related
protein (TRP)-1 and TRP-2 are important enzymes for melanin
synthesis, with Tyr being the most vital (7) Tyr possesses tyrosine hydroxylase and
dopa oxidase activities and functions to promote the synthesis of
dopaquinone from L-tyrosine (8). Tyr
activity is positively associated with melanin synthesis in
melanocytes and the regulation of Tyr activity mediates melanin
production (9). In addition, Tyr
activity in vitiligo lesions has been determined to be lower than
that of normal skin (8).
Furthermore, Tyr is only expressed in differentiated mature
melanocytes (9). TRP-2, also known
as Dopachrome tautomerase (Dct), is a recognized biomarker for
melanocyte stem cells (10). The
current study aimed to assess the expression of melanocyte-specific
biomarker genes (Tyr and Dct) and proteins in needle biopsies of
vitiligo lesions using reverse transcription (RT)-semi-quantitative
polymerase chain reaction (PCR), the results of which may help
establish an accurate and minimally invasive method for the
prediction of perifollicular repigmentation in vitiligo
lesions.
Materials and methods
Clinical data
The present study was approved by the Medical Ethics
Committee of Wuhan Third Hospital and Tongren Hospital of Wuhan
University (Wuhan, China). A total of 4 healthy volunteers (2 males
and 2 females; age range, 24–50 years old) and 6 patients with
static stage vitiligo (3 males and 3 females; age range, 21–65
years old) diagnosed by the Department of Dermatology at Wuhan
Third Hospital were enrolled from January 2012 to December 2017.
Written informed consent was obtained from all participants. The
inclusion criteria were as follows: Patients with vitiligo in
stationary phases, aged 18–70. The exclusion criteria were as
follows: Patients with a family history of vitiligo or autoimmune
diseases, or patients who had undergone early ultraviolet
treatment.
Needle biopsy
The skin of the affected area was disinfected with
conventional active iodine solution and deiodinated using 75%
ethanol. The vitiligo lesion and surrounding normal skin were
exposed under surgical towel and biopsies were collected from the
center of the whitened area, the edge of the whitened area and the
surrounding normal skin. A total of three skin tissue specimens
were taken per patient; 18 skin specimens were obtained from six
patients with vitiligo. A total of 0.5 ml 2% lidocaine solution was
drawn into a 1-ml syringe and subcutenanously injected to form a
skin pimple, which was then gently lifted using a needle tip. The
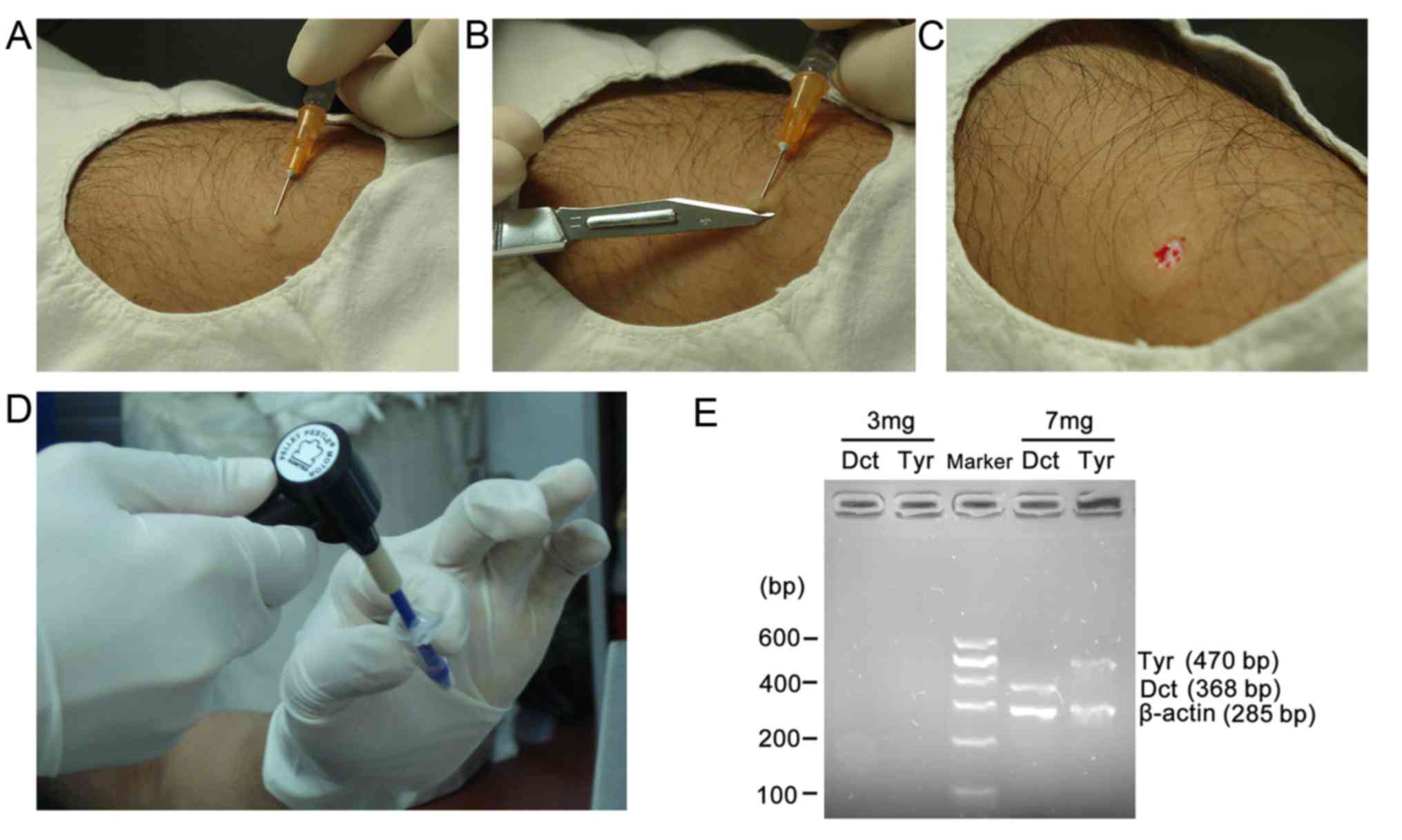
epidermis (~3 or 7 mg samples) of the pimple was cut with a scalpel
parallel to the surface of the skin (Fig. 1A-D). If point bleeding occurred, the
area was pressed with a sterilized gauze. Skin tissue specimens
were then placed directly into an eppendorf (EP) tube with 50 µl of
cold TRIzol reagent (Invitogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and weighed using an analytical balance and then
homogenized by micro tissue grinders (Kimble Chase Life Science and
Research Products LLC, Rockwood, TN, USA; Fig. 1E). To avoid genetic contamination,
all tools (syringe, scalpel and gauze) were changed for each
specimen.
RT-semi-quantitative PCR
Skin specimens were homogenized in the EP tube using
a Pellet Pestle Cordless Motor (Kimble Chase Life Science and
Research Products LLC). Total RNA was extracted using Trizol
reagent following the manufacturer's protocol. To increase the
efficiency of RNA extraction, all reagents and equipment were RNAse
free the RNA concentration was measured at 260 nm to calculate the
A260/A280. First strand cDNA was synthesized using a M-MLV Reverse
Transcription kit at 42°C for 40 min (Invitrogen; Thermo Fisher
Scientific, Inc.). PCR was performed using DreamTaq DNA polymerase
(cat. no. EP0702; Thermo Fisher Scientific, Inc.) with the
following thermocycling conditions: Initial denaturation at 94°C
for 2 min; denaturation for 35 cycles of 94°C for 30 sec; annealing
at 55°C for 30 sec and extension at 72°C for 30 sec; and extension
at 72°C for 2 min. The product was then separated on a 2% agarose
gel. The bands were visualized using ethidium bromide. The specific
primers utilized for PCR were as follows: β-actin (internal
control), forward 5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse
5′-GGGCACGAAGGCTCATCATT-3′, with a PCR product of 285 bp; Dct,
forward 5′-AGTGATTCGGCAGAACATCC-3′ and reverse
5′-AGTTCCAGTAGGGCAAAGCA-3′, with a PCR product of 368 bp; Tyr,
forward 5′-CAGCTTTCAGGCAGAGGTTC-3′ and reverse
5′-GCTTCATGGGCAAAATCAAT-3′, with a PCR product of 470 bp.
Immunostaining
Tyr and Dct protein expression from tissue obtained
from the biopsy was assessed using immunohistochemistry. The
tissues were fixed with 4% formalin for 24 h at room temperature,
dehydrated in different concentrations (50, 70, 80, 95 and 100%) of
ethanol, paraffin-embedded and cut into 5-µm-thick sections. To
deparaffinized the tissue sections, they were immersed in xylene
two times (5 min each), then they were immersed in 100, 95, 75 and
50% ethanol in turn (3 min each), washed in distilled water for 10
min, washed in PBS two times (5 min each), and treated with 3%
H2O2 for 30 min at room temperature. Antigen
retrieval for Tyr and Dct was performed by boiling the sections in
citrate buffer (0.01 mol/l; pH 6.0) for 10 min at 118°C. Sections
were incubated with 5% bovine serum albumin for 30 min at room
temperature, and then incubated with rabbit anti-Tyr (cat. no.
251315; 1:100; Abbiotec, Inc., San Diego, CA, USA) and anti-Dct
(cat. no. ab74073; 1:100; Abcam, Cambridge, UK) antibodies
overnight at 4°C. Sections were then washed with PBS and incubated
with horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin (Ig)G (cat. no. ab6721; 1:100; Abcam) for 1 h at
room temperature. Following washing, peroxidase activity was
visualized using DAB staining (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) for 1 min at room temperature and sections
were counter-stained with haematoxylin for 30 sec at room
temperature. Images were captured using a light microscope at a
magnification of ×200. Negative controls were constructed by
omitting the primary antibody or replacing it with normal rabbit
IgG (cat. no. 2729; 1:100; Cell Signaling Technology, Inc.,
Danvers, MA, USA). Positive controls were constructed using the
skin tissue from the healthy volunteers.
Light treatment and skin
autografts
Vitiligo lesions were treated with 308 nm eximer
light radiation eight times with TheraBeam UV308 equipment (USHIO,
Inc., Tokyo, Japan) at 200 mJ/cm2, each lasting 2–3 min.
Skin autografts were peformed by an orthopedist, skin samples were
taken from the inner thigh and transplanted to the forehead.
Perifollicular repigmentation was evaluated by measurement the
vilitigo lesion area.
Follow-up period
A total of 10 participants were followed up for 3
months. The participants came back to Wuhan Third Hospital once a
week in the first month, but only the patients came back every 2
weeks during the second month. The follow up period was once per
month after 2 months. One patient with vilitigo (male, 27 years
old) was followed up for 5 months. The perifollicular
repigmentation was observed by measuring the diameter and the area
of vitiligo skin lesions.
Results
Needle biopsy method
Fig. 1A shows that
0.5 ml 2% lidocaine was injected into the skin. The skin was then
cut (Fig. 1B) and removed (Fig. 1C), and skin tissue (3 and 7 mg)
retrieved via needle biopsy (Fig.
1D). The results demonstrated that Dct, Tyr and β-actin mRNA
was detected in 7 mg needle biopsy specimens, but not in the 3 mg
or epidermis specimens obtained via needle biopsy respectively
(Fig. 1E).
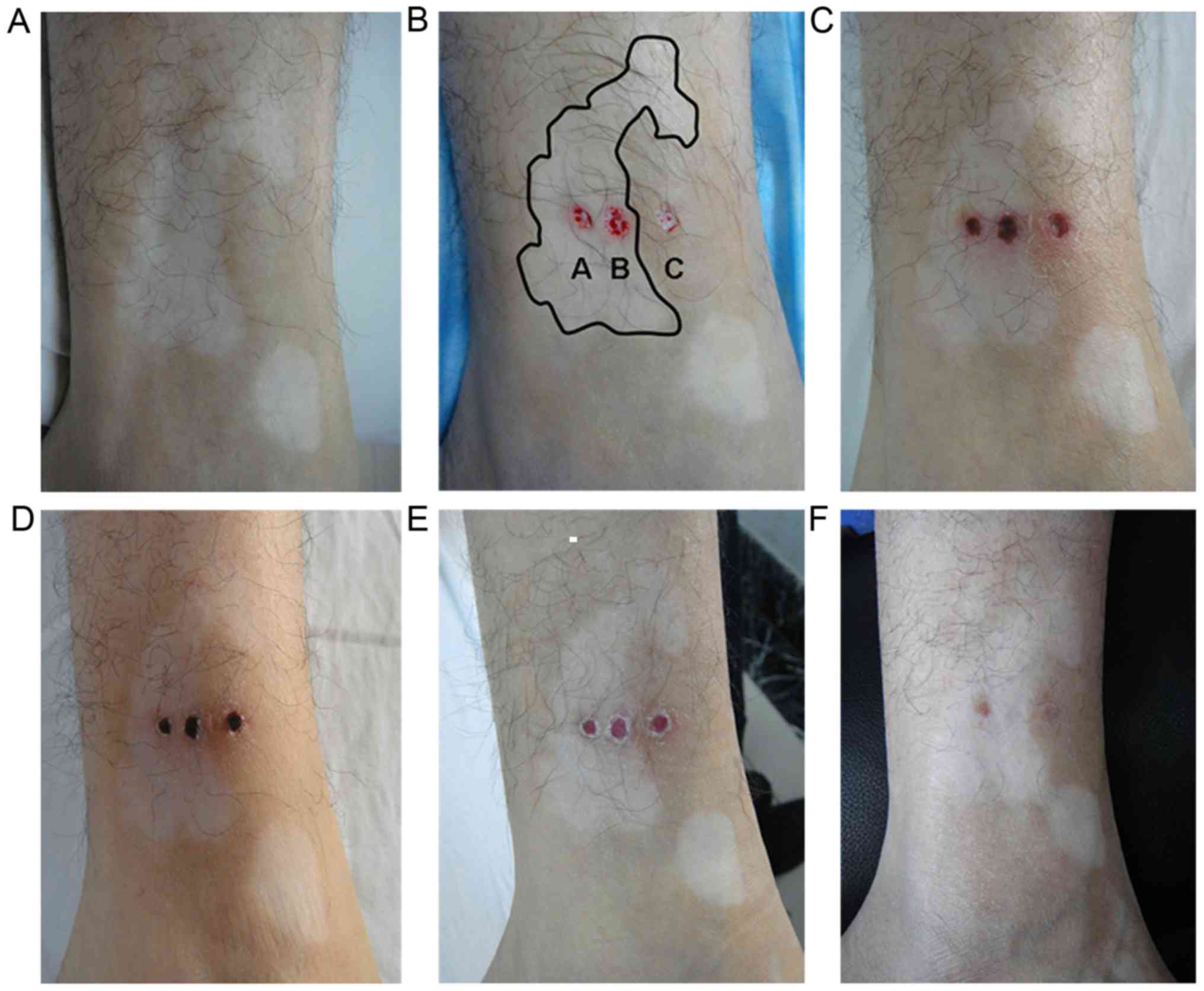
Needle biopsy wound healing
The healing of the wounds created following needle
biopsy in the 10 participants was followed up for 3–5 months.
Following one month, the wound was nearly healed without the
formation of scar (Fig. 2).
Detection of melanocyte
lineage-specific genes in vitiligo lesion needle biopsies
The expression of Dct, Tyr and β-actin was detected
in 18 skin specimens obtained from six patients with vitiligo
(three specimens per patient). As needle biopsies only retrieve a
small quantity of skin tissue, the specimens were homogenized using
a pellet pestle cordless motor, which markedly increased the
efficiency of RNA extraction (data not shown). However, 1–10 µg of
RNA were retrieved from 1 mg of skin sample using TRIzol.
Furthermore, the proportion of melanocytes and melanocyte stem
cells was small in skin tissue, ~10% of melanocytes appearing in
the basal layer (data not shown).
In the centre of the vitiligo lesion, three patterns
of Dct, Tyr and β-actin expression were detected: One case of
Dct+Tyr- β-actin (ACTB)+ (Fig. 3A);
four cases of Dct-Tyr-ACTB+; and one case of Dct+Tyr+ACTB+
(Fig. 3C). Healthy volunteers
presented with Dct+Tyr+ACTB+ (Fig.
3B). Measuring Dct and Tyr protein expression in the centre of
the vitiligo lesion revealed Dct+ staining (Fig. 3D), Tyr staining was negative
(Fig. 3E), no staining as the
section was treated with PBS (Fig.
3F), the Dct positive control (Fig.
3G) and the Tyr positive control (Fig. 3H).
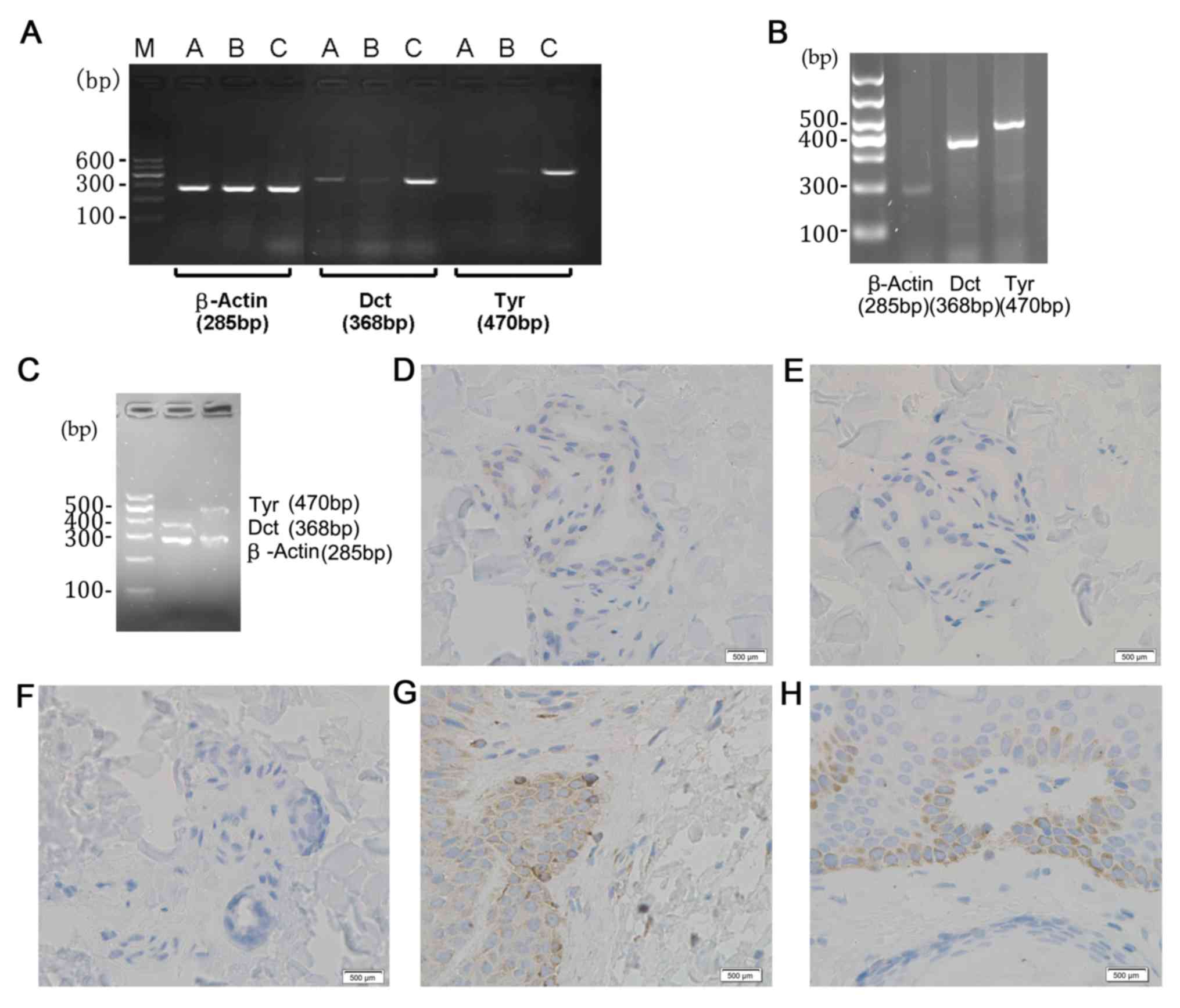 | Figure 3.Expression of Dct, Tyr and β-actin in
a patient with vitiligo as determined by reverse transcription-semi
quantitative PCR. (A) PCR result (Dct+Tyr-ACTB+) of one patient
(male, 27 years old) with vitiligo. Lanes were labelled
accordingly: A, the centre of lesion; B, the edge of lesion; C,
surrounding normal skin. (B) PCR results obtained from one healthy
volunteer (male, 25 years old). (C) PCR results (Dct+Tyr+ACTB+) of
a patient (female, 32 years old) with vilitigo. Immunostaning
results were (D) Dct+ (male, 27 years old), (E) Tyr- (male, 27
years old), (F) negative control (male, 27 years old). (G) Dct+
(healthy male, 36 years old) and (H) Tyr+ (healthy male, 36 years
old) were positive. The blue staining represents nuclei and brown
staining represents positive protein staining. Scale bar=500 µm.
Dct, dopachrome tautomerase; Tyr, tyrosinase; PCR, polymerase chain
reaction; ACTB, β-actin; M, DNA markers. |
Light treatment and skin
autografts
The results of light treatment in one patient with
Dct+Tyr-ACTB+ was followed-up for 5 months. Following two
treatments with 200 mJ/cm2 using TheraBeam UV308
equipment after the needle biopsy, perifollicular repigmentation
was observed within the vitiligo lesion area after 3 months
(Fig. 4A and B). In a second patient
with Dct+Tyr+ACTB+, who was treated the same as aforementioned, but
with eight treatments, perifollicular repigmentation was observed
within the vitiligo lesion area following treatment (Fig. 4C and D). In 3 patients with
Dct-Tyr-ACTB+, perifollicular repigmentation was also observed
following skin treatment autograft (Fig.
4E and F; representative image of 1 patient). In 1 patient with
Dct-Tyr-ACTB+, no perifollicular repigmentation was detected
following treatment with 200 mJ/cm2 eight times
(Fig. 4G and H).
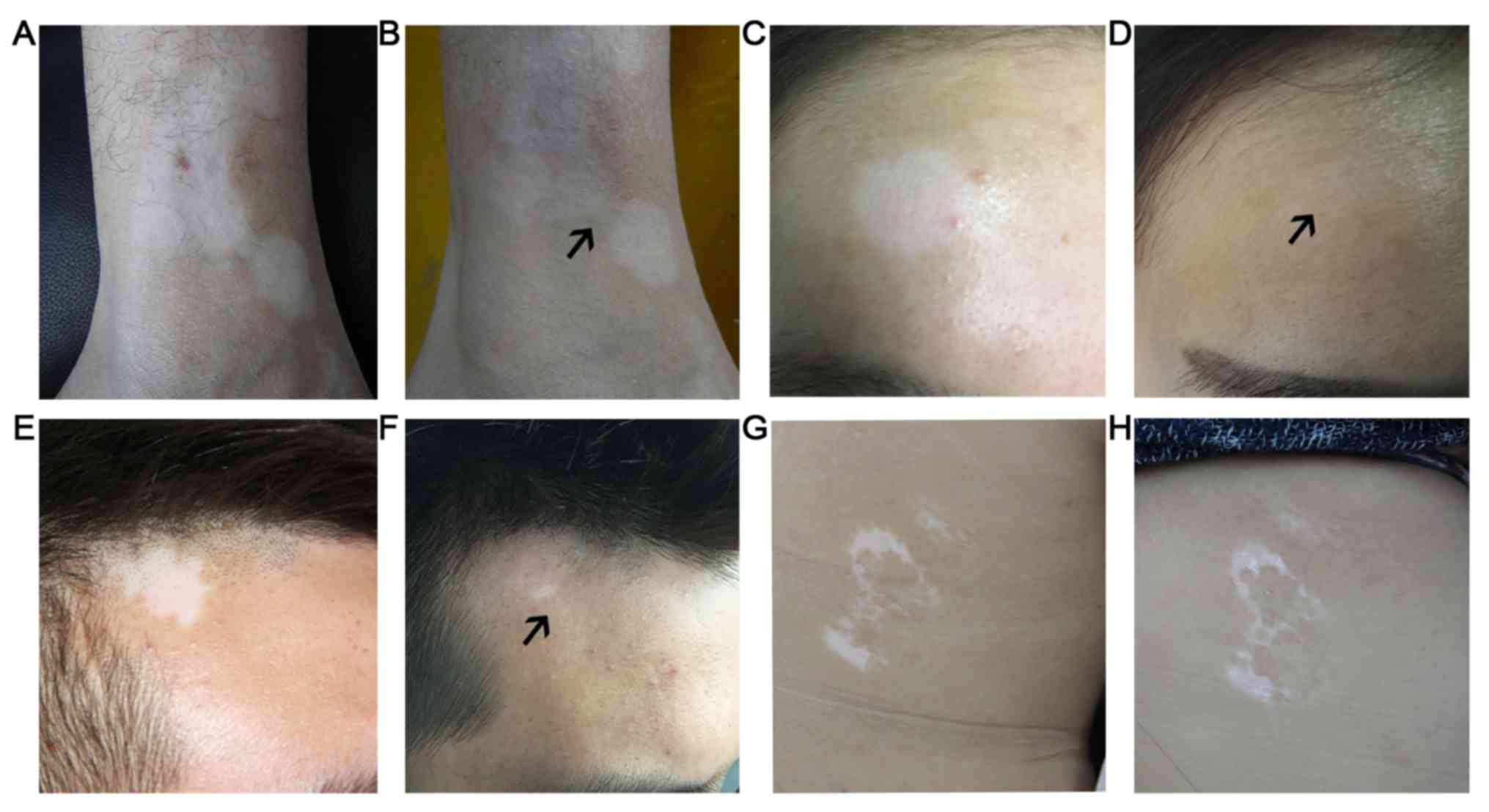 | Figure 4.Several patients received different
treatments to induce perifollicular repigmentation. The patient
(male, 27 years old) was treated twice using TheraBeam UV308
equipment at 200 mJ/cm2 (2–3 mins each. Lesion (A) prior
to and (B) following light treatment. A second patient (female, 32
years old) with a Dct+Tyr+ACTB+ expression profile was assessed.
Following eight treatments at 200 mJ/cm2 using TheraBeam
UV308 equipment, Hair follicle re-coloring was observed in the
vitiligo lesion area. Lesion (C) prior to and (D) following light
treatment. In a patient (male, 35 years old) exhibiting
Dct-Tyr-ACTB+, perifollicular repigmentation was observed in the
vitiligo lesion following a skin autograft. Lesion (E) prior to and
(F) following autograft. In one patient (female, 42 years old) with
the Dct-Tyr-ACTB+ expression profile, no perifollicular
repigmentation was observed following eight treatments at 200
mJ/cm2 using TheraBeam UV308. Lesions (G) prior to and
(H) following light treatment. Black arrows indicate perifollicular
repigmentation. Dct, dopachrome tautomerase; Tyr, tyrosinase; ACTB,
β-actin. |
Discussion
The pathogenesis of vitiligo is complex and its
clinical treatments are diverse, with patients recieving local
topical or oral corticosteroids, immunomodulators, phototherapy and
surgery (11). The re-coloring
mechanism of vitiligo has not yet been fully elucidated, but a
previous study have demonstrated that the most important mechanism
includes re-coloring that expands outward from the center of the
hair follicle (12). For the
effective non-dermatoplastic treatment of vitiligo, most theapies
require live melanocytes around hair follicles, as live melanocytes
are the source of the melanin (13).
Melanocyte stem cells are stored in the hair follicle and funtion
to proliferate, migrate towards the epidermis and synthesize
melanin following stimulation (13).
Melanin is then transported to keratinocytes to induce vitiligo
lesion re-coloring (13). Therefore,
it is critical to detect the presence of melanocyte stem cells
around the hair follicle, which will help determine the possibility
of hair follicle re-coloring in vitiligo lesions. However, a
non-invasive method for the clinical detection of melanocyte
presence has yet to be elucidated. The current study therefore
aimed to establish an effective method for the detection of
melanocyte linkage-specific gene expression in order to determine
the presence of melanocyte stem cells.
Previous studies revealed that Dct mutations
severely affect the maturation and development of late melanin
bodies (14,15). Therefore, the present study also
aimed to assess the presence of melanocyte stem cells or
melanocytes through the expression of Tyr and Dct in vitiligo
lesions.
To detect melanocyte gene expression in vitiligo
lesions, it is necessary to establish a minimally invasive and
effective method for the collection of skin specimens. Suction
blistering is a common method for skin sampling in dermatology and
is often used in autologous skin tranplantations to treat patients
with vitiligo (5,16,17). To
collect dermal skin tissue, the current study utilized a needle
biopsy to ensure that sampling occurred where melanocyte stem cells
were located (the hair follicle area). This was then used to detect
the expression of the melanocyte-specific gene, Dct, in retrieved
tissues. The results demonstrated that the expression of
melanocyte-specific genes was detected in 7 mg of normal skin
tissues. Furthermore, the dynamic changes of wound healing were
followed up following needle biopsy. Wound suppuration and scar
formation were not exhibited, indicating that this method was safe
and effective.
Since needle biopsy only retrieves a small quantity
of skin tissue, a conventional homogenizer cannot homogenize the
tissue completely and small quantities of homogenization solution
may stick to the wall of tube, resulting in false-negative results.
Therefore, all specimens retrieved by needle biopsy in the present
study were homogenized using a pellet pestle cordless motor. To
increase the sensitivity of screening for melanocyte-specific
genes, it is critical to ensure the quality of sampling. In the
present study, the differences in gene expression between 3 and 7
mg normal skin samples from healthy volunteers were assessed. The
results revealed that the expression of melanocyte-specific genes
was not detected in 3 mg specimens, but was observed in 7 mg skin
tissues, the latter of which meets experimental requirements.
Therefore, 7 mg skin specimens were determined to be the optimal
size of skin tissue samples.
The current study further assessed the expression of
Dct and Tyr in vitiligo lesion specimens. A previous study on hair
growth in postnatal mice have determined that there are three types
of melanocytes: i) Melanocytes that are located in the hair
follicle area, only express Dct and are usually considered to be
stem cells with the potential to proliferate and differentiate; ii)
cell populations that are located outside the root sheath, which
express Dct and Tyr, possess weak TRP-1 expression, exhibit
proliferative activity and are deemed differentiated melanocytes;
and iii) cell populations located in the hair matrix, close to the
layer of dermal papilla, which express Dct, TRP-1 and Tyr genes,
exhibit no proliferative activity but are able to melanin (18). According to the localization of
melanin genes, Benzekri et al (19) proposed phase criteria for the
assessment of vitiligo lesions, comprising three phases: Phase I, a
partial loss of epidermal melanocytes with a possibility of
re-coloring; phase II, complete loss of epidermal melanocytes, with
the possibility of re-colouring via melanocytes stored in the hair
follicle; and phase III, complete loss of melanocytes in the hair
follicle with the impossibility of vitiligo lesion re-coloration
without melanocyte transplantation. Based on the above criteria and
the data of the current study, three possible results for the
detection of melanocyte-specific genes in vitiligo lesions were
determined: i) Dct+Tyr-. Dct is a recognized biomarker for
melanocyte stem cells (10), but
there is no Tyr gene expression in melanocyte stem cells. Dct and
Tyr are both expressed in mature melanocyte (18). The Tyr- results indicated that there
were no mature melanocytes in vitiligo lesions. The Dct+Tyr-
results indicated a high possibility of melanocyte stem cell
presence in vitiligo lesions and that treatment may activate the
proliferation and differentiation of melanocyte stem cells, thus
leading to hair follicle re-coloring. ii) Dct+Tyr+. Epidermal
melanocyte cells and bulb portion melanocyte cells express both Tyr
and Dct (18). This indicates the
presence of melanocytes in vitiligo lesions; however, the loss of
skin color means melanocytes may be dysfunctional. iii) Dct-Tyr- or
Dct-Tyr+. These indicate the destruction of melanocytes and their
stem cells or that there were no melanocytes in the vitiligo
lesion, and a low possibility of hair follicle re-coloring
(10,20). In one case of Dct+Tyr-, hair follicle
re-coloring occurred followng treatment with TheraBeam UV308
equipment. These results further support that the re-coloring of
hair follicles requires the presence of melanocyte stem cells.
This current study established a method for the
detection of vitiligo lesion-associated gene expression: Skin
tissue samples were obtained by needle biopsies and melanocyte
lineage-specific genes in vitiligo were detected. However, the
number of patients was limited and an increased sample size should
be utilized to verify the reliability of this method. Furthermore,
future studies that assess hair follicle re-coloring in patients
with vitiligo and the expression of melanocyte stem cell-specific
genes are necessary to predict the significance of the results of
the present study. The Dct gene is a biomarker of melanocyte stem
cells and is highly expressed in adult melanocyte stem cells and
mature melanocytes (10). Therefore,
the detection of Dct gene expression will be helpful to assess the
possibility of hair follicle re-coloring in vitiligo lesions. The
proliferation and differentiation of melanocyte stem cells is
regulated by Dct and other genes, including
mdicrophthalmia-associated transcription factor (MITF) and paired
box 3 (PAX3) (21). MITF is one of
the earliest biomarkers for the development of melanoid
differentiation into melanocytes (22). It regulates the expression of
tyrosine family genes and participates in the regulation of
melanogenesis, determining whether melanocytes are successfully
differentiated or malignantly transformed to melanoma (22,23).
MITF-M, a ‘melanocyte-specific’ isoform, is selectively expressed
in melanocyte lines (24), whereas
the PAX3 gene is associated with undifferentiated melanocytes
(21). As the current study isolated
RNA using TRIzol reagent from 7 mg skin specimens for
RT-semi-quantitative PCR, the concentration of extracted RNA was
unknown, serving as a limitation. Future studies should therefore
measure the concentration of RNA and use the same quantity of RNA
for RT-semi quantitative PCR analysis. Additionally, RT-semi
quantitative PCR was utilized to detect gene transcription levels;
however, this method is not sensitive and requires more RNA input
compared with quantitative PCR, which is widely used to detect gene
transcription. The purpose of the present study was to
qualitatively detect gene expression. In order to ensure the
accuracy of the test results, the skin sample must be large enough
(more than 7 mg for each patient) for the RNA to be evaluated.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Health and
Family Planning Commission of Wuhan municipality (grant no.
WX16D08).
Availability of data and materials
The datasets created during and/or analysed during
the current study available from the corresponding author on
reasonable request.
Authors' contributions
WX contributed to conception and design,
acquistition of data, and was a major contribution in drafting the
manuscript. XW acquired and analyzed the patient data, and detected
the tissue RNA. Both authors read and approve the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Wuhan Third Hospital & Tongren Hospital of Wuhan
University (Wuhan, China). Written informed consent was obtained
from all participants.
Patient consent for publication
All patients and healthy volunteers consented to the
publication of their images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Manga P, Elbuluk N and Orlow SJ: Recent
advances in understanding vitiligo. F1000Res. 5:F10002016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacigalupi RM, Postolova A and Davis RS:
Evidence-based, non-surgical treatments for vitiligo: A review. Am
J Clin Dermatol. 13:217–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai YC, Yew YW, Kennedy C and Schwartz RA:
Vitiligo and depression: A systematic review and meta-analysis of
observational studies. Br J Dermatol. 177:708–718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boniface K, Seneschal J, Taïeb A and
Merched A: Vitiligo therapy: Restoring immune privilege? Exp
Dermatol. 26:635–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel NS, Paghdal KV and Cohen GF:
Advanced treatment modalities for vitiligo. Dermatol Surg.
38:381–391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghafourian A, Ghafourian S, Sadeghifard N,
Mohebi R, Shokoohini Y, Nezamoleslami S and Hamat RA: Vitiligo:
Symptoms, pathogenesis and treatment. Int J Immunopathol Pharmacol.
27:485–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsatmali M, Ancans J and Thody AJ:
Melanocyte function and its control by melanocortin peptides. J
Histochem Cytochem. 50:125–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eskandani M, Golchai J, Pirooznia N and
Hasannia S: Oxidative stress level and tyrosinase activity in
vitiligo patients. Indian J Dermatol. 55:15–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu PY, Yin WH, Wang MR, Dang YY and Ye
XY: Andrographolide suppresses melanin synthesis through
Akt/GSK3β/β-catenin signal pathway. J Dermatol Sci. 79:74–83. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimura EK, Jordan SA, Oshima H, Yoshida
H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y and
Nishikawa S: Dominant role of the niche in melanocyte stem-cell
fate determination. Nature. 416:854–860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodrigues M, Ezzedine K, Hamzavi I, Pandya
AG and Harris JE; Vitiligo Working Group, : Current and emerging
treatments for vitiligo. J Am Acad Dermatol. 77:17–29. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parsad D, Pandhi R, Dogra S and Kumar B:
Clinical study of repigmentation patterns with different treatment
modalities and their correlation with speed and stability of
repigmentation in 352 vitiliginous patches. J Am Acad Dermatol.
50:63–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arrunátegui A, Arroyo C, Garcia L, Covelli
C, Escobar C, Carrascal E and Falabella R: Melanocyte reservoir in
vitiligo. Int J Dermatol. 33:484–487. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XM, Zhou Q, Xu SZ, Wakamatsu K and Lei
TC: Maintenance of immune hyporesponsiveness to melanosomal
proteins by DHICA-mediated antioxidation: Possible implications for
autoimmune vitiligo. Free Radic Biol Med. 50:1177–1185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang S, Liu XM, Dai X, Zhou Q, Lei TC,
Beermann F, Wakamatsu K and Xu SZ: Regulation of DHICA-mediated
antioxidation by dopachrome tautomerase: Implication for skin
photoprotection against UVA radiation. Free Radic Biol Med.
48:1144–1151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Budania A, Parsad D, Kanwar AJ and Dogra
S: Comparison between autologous noncultured epidermal cell
suspension and suction blister epidermal grafting in stable
vitiligo: A randomized study. Br J Dermatol. 167:1295–1301. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maleki M, Banihashemi M and Sanjari V:
Efficacy of suction blister epidermal graft without phototherapy
for locally stable and resistant vitiligo. Indian J Dermatol.
57:282–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Botchkareva NV, Botchkarev VA and
Gilchrest BA: Fate of melanocytes during development of the hair
follicle pigmentary unit. J Investig Dermatol Symp Proc. 8:76–79.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benzekri L, Ezzedine K and Gauthier Y:
Vitiligo potential repigmentation index: A simple clinical score
that might predict the ability of vitiligo lesions to repigment
under therapy. Br J Dermatol. 168:1143–1146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldstein NB, Koster MI, Hoaglin LG,
Spoelstra NS, Kechris KJ, Robinson SE, Robinson WA, Roop DR, Norris
DA and Birlea SA: Narrow band ultraviolet B treatment for human
vitiligo is associated with proliferation, migration, and
differentiation of melanocyte precursors. J Invest Dermatol.
135:2068–2076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mou Y, Jiang X, Du Y and Xue L:
Intelligent bioengineering in vitiligo treatment: Transdermal
protein transduction of melanocyte-lineage-specific genes. Med
Hypotheses. 79:786–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawakami A and Fisher DE: The master role
of microphthalmia-associated transcription factor in melanocyte and
melanoma biology. Lab Invest. 97:649–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo J, Zhang JF, Wang WM, Cheung FW, Lu
YF, Ng CF, Kung HF and Liu WK: MicroRNA-218 inhibits melanogenesis
by directly suppressing microphthalmia-associated transcription
factor expression. RNA Biol. 11:732–741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cichorek M, Wachulska M, Stasiewicz A and
Tymińska A: Skin melanocytes: Biology and development. Postepy
Dermatol Alergol. 30:30–41. 2013. View Article : Google Scholar : PubMed/NCBI
|