Introduction
As the economy develops and vehicles become more and
more common, the incidence of car accidents is constantly
increasing, and that of traumatic brain injury (TBI) is also
increasing. Severe traumatic brain injury (STBI) is an acute and
severe disease that is common, with rapid progression and
complicated conditions. After the patient is injured, there will be
energy metabolism disturbance, hypoxia, apoptosis and massive
release of oxygen free radicals in the body, which cause different
necrosis and degeneration of the tissue (1,2). Studies
have shown that (3,4) the progression of STBI is related to
vascular permeability and inflammatory factor release. There is a
study showing that (5) patients with
STBI produce a large number of inflammatory mediators under
long-term pathological stress, thereby leading the body into an
inflammatory state and further aggravating the condition.
Cerebrospinal fluid (CSF) is a rich medium in which inflammation is
rapidly transmitted to the central nervous system through a series
of events when an intracranial infection occurs. Intracranial
infection has been treated in recent years by lumbar cistern
drainage and broad-spectrum antimicrobial drugs. Vancomycin has
been widely used in the treatment of intracranial infection
(6) and is highly sensitive to
Gram-positive bacteria, such as staphylococcus aureus and
staphylococcus epidermidis. Meropenem is one of the semi-synthetic
carbapenem antibacterial agents, the sensitivity of which to
Gram-negative bacteria is up to 95%. The treatment safety and
efficacy of intracranial infection has been confirmed (7). Intravenous injection is difficult to
pass the blood-brain barrier, which results in severe intracranial
infection, eventually causing the treatment to be more difficult
with unsatisfactory effects. Therefore, other routes of
administration are often selected clinically to reduce intravenous
administration. Intrathecal injection is a treatment that is
administered directly to the patient's CSF through lumbar puncture.
Whether it can improve the efficacy and safety has also attracted
the attention of scholars. In this study, the efficacy and safety
of intrathecal meropenem and vancomycin in the treatment of
postoperative intracranial infection in patients with STBI were
investigated, in order to provide a reference for future clinical
treatment.
Materials and methods
General information
A retrospective analysis was performed on 86
patients with intracranial infections after cranial trauma
operation in Tai'an Traditional Chinese Medicine Hospital and
Affiliated Hospital of Taishan Medical University (Tai'an, China)
from May 2004 to June 2017. The patients were divided into the
control group (43 patients) and the experimental group (43
patients) according to the treatment. The experimental group
consisted of 22 males and 21 females with an age range of
30.01±9.04 years. The control group consisted of 24 males and 19
females with an age range of 29.73±10.37 years. There were no
statistically significant differences in the sex, age, body weight
and cause of injury of patients between the two groups (P>0.05),
which were comparable (Table I).
 | Table I.General information of patients in the
two groups [n (%)]. |
Table I.
General information of patients in the
two groups [n (%)].
| Group | Experimental group
(n=43) | Control group
(n=43) | χ2
test | P-value |
|---|
| Sex |
| Male | 22 (51.16) | 24 (55.81) | 0.187 | 0.666 |
|
Female | 21 (48.84) | 19 (44.19) |
|
|
| Age (years) |
|
<30 | 17 (39.53) | 16 (37.21) | 0.049 | 0.825 |
| ≥30 | 26 (60.47) | 27 (62.79) |
|
|
| Body weight |
|
<55 | 15 (34.88) | 13 (30.23) | 0.212 | 0.645 |
| ≥55 | 28 (65.12) | 30 (69.77) |
|
|
| Cause of injury |
| Falling
down | 11 (25.58) | 9 (20.93) | 1.408 | 0.704 |
| Traffic
accident | 8 (18.60) | 12 (27.91) |
|
|
|
Dropping | 14 (32.56) | 11 (25.58) |
|
|
|
Industrial accident | 10 (23.26) | 11 (25.58) |
|
|
Methods
Patients in the two groups were routinely treated
with antibiotics to prevent infection after craniotomy. After being
diagnosed with intracranial infection, lumbar cistern drainage was
performed on patients in the control group for the release of CSF,
and then the patients were given vancomycin hydrochloride injection
(Zhejiang Pharmaceutical Co., Ltd.; Xinchang Pharmaceutical
Factory, Zhejiang, China; guoyaozhunzi: H20033366) 1.0 g, ivgtt,
Q12H and intravenously dripped meropenem (Sumitomo Pharmaceutical
Suzhou Co., Ltd., Suzhou, China; guoyaozhunzi: J20140169) 2.0 g,
ivgtt, Q8H. Lumbar cistern drainage was performed on patients in
the experimental group for the release of CSF, and then the
patients were slowly given 20 mg of vancomycin hydrochloride
injection. After the tube was flushed with 2 ml of 0.9% sodium
chloride injection, the patients in the experimental group were
given meropenem intrathecal injection 20 mg, bid. Patients in both
groups were treated for 2 weeks.
Inclusion and exclusion criteria
Inclusion criteria were: Patients who were diagnosed
with STBI after admission; patients with Glasgow Coma Scale (GCS)
scores between 3 and 8 points; patients with mydriasis. Exclusion
criteria were: Patients with contraindications for vancomycin and
meropenem; patients with uncertain conditions; patients with shock
and fractures.
The study was approved by the Ethics Committee of
Tai'an Traditional Chinese Medicine Hospital and Affiliated
Hospital of Taishan Medical University. Patients and their families
were informed in advance of the study, and signed an informed
consent form.
Observation indicators and outcome
measures
Observation indicators: The clinical efficacy, cure
time, treatment cost, adverse reactions and sequelae after 6 months
of treatment of patients in the two groups.
Outcome measures were: Recovered: Symptoms and signs
disappeared and CSF examination was normal; markedly effective: the
observation indicators were not fully recovered but the condition
was improved; invalid: The condition deteriorated or was not
improved after 72 h of treatment. Response rate (RR) = (number of
recovered cases + number of markedly effective cases)/total number
of cases ×100%.
Statistical analysis
SPSS 17.0 (Beijing Boyi Zhixun Information
Technology Co., Ltd., Beijing, China) software system was used for
statistical analysis. Enumeration data were expressed as [n (%)],
and χ2 test was used for comparison between the two
groups. Students' t-test was used for comparison between the two
groups for continuous data. At P<0.05, the difference was
considered statistically significant.
Results
Clinical efficacy of patients in the
two groups
There were 22 recovered patients, 19 markedly
effective patients and 2 invalid patients in the experimental
group, with an RR of 95.35%. There were 15 recovered patients, 16
markedly effective patients and 12 invalid patients in the control
group, with an RR of 72.09%. The RR of patients in the experimental
group was significantly higher than that in the control group, with
a statistically significant difference (P<0.05) (Table II).
 | Table II.The clinical efficacy of patients in
the two groups [n (%)]. |
Table II.
The clinical efficacy of patients in
the two groups [n (%)].
| Group | Recovered | Markedly
effective | Invalid | RR |
|---|
| Experimental group
(n=43) | 22 (51.16) | 19 (44.19) | 2 (4.65) | 41 (95.35) |
| Control group
(n=43) | 15 (34.88) | 16 (37.21) | 12 (27.91) | 31 (72.09) |
| χ2
test | – | – | – | 8.532 |
| P-value | – | – | – | 0.004 |
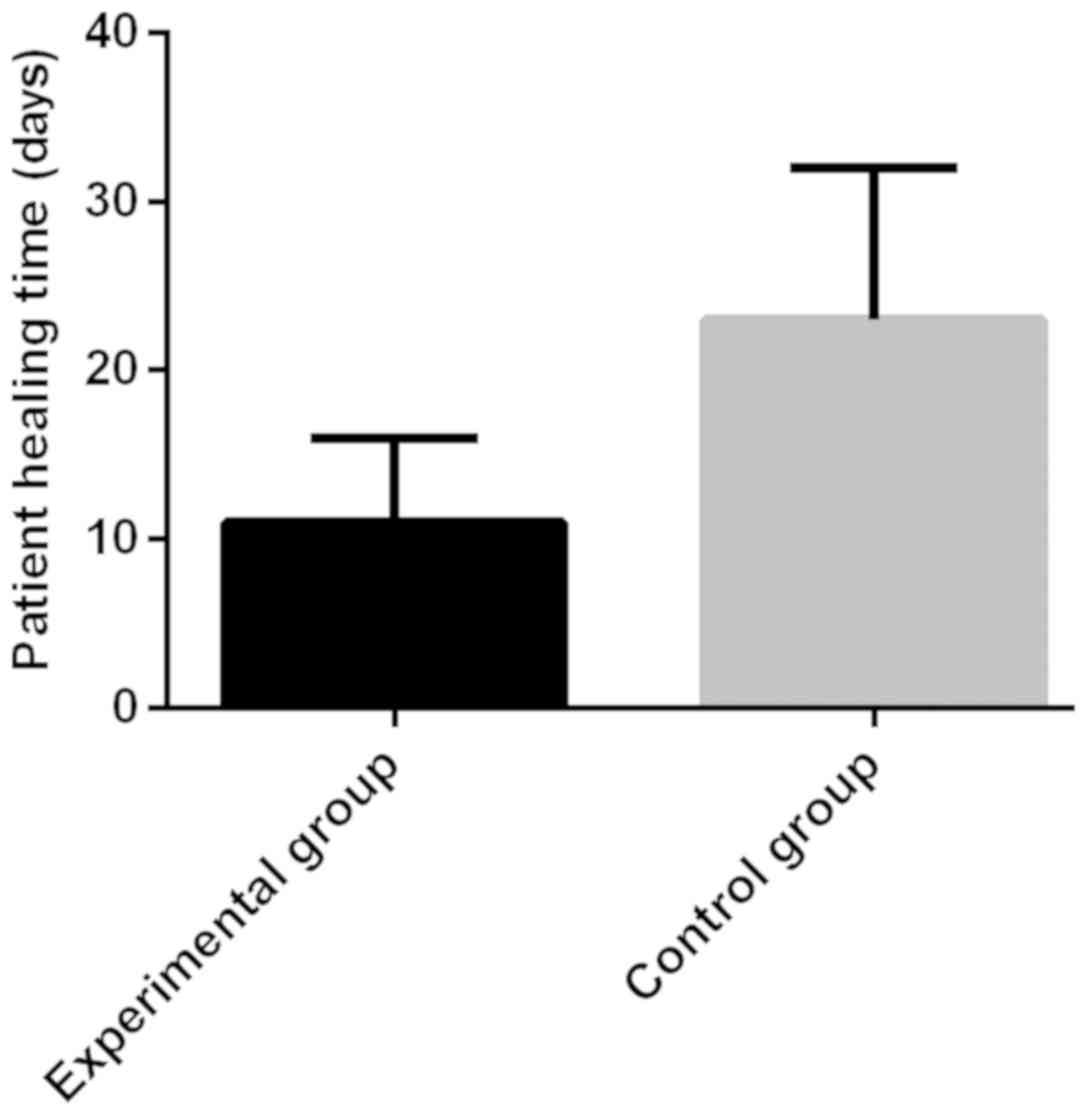
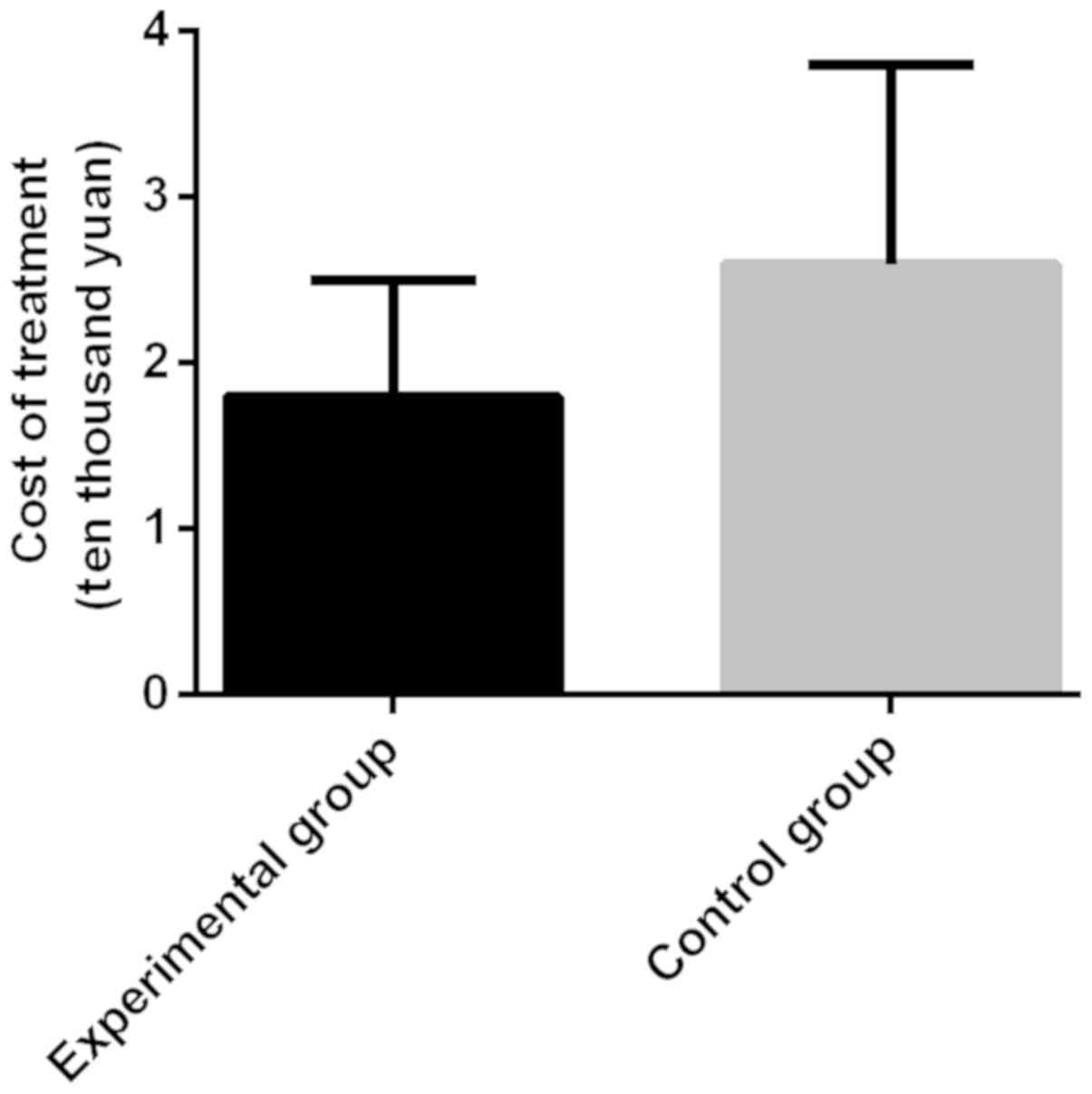
The cure time and treatment cost of
patients in the two groups
The cure time and treatment cost of patients in the
control group were 23±9 days and 2.6±1.2 yuan, respectively. Those
of patients in the experimental group were 11±5 days and 1.8±0.7
yuan, respectively. The time to cure of patients in the
experimental group was significantly shorter than that in the
control group, with a statistically significant difference
(t=7.643, P<0.001). The treatment cost of patients in the
experimental group was significantly lower than that in the control
group, with a statistically significant difference (t=3.776,
P<0.001) (Figs. 1 and 2).
Adverse reaction records of patients
in the two groups
There was 1 patient with diarrhea, 1 patient with
swelling and pain in the injection site and 1 patient with nausea
in the experimental group, with an incidence of 6.98%. There were 2
patients with diarrhea, 4 patients with swelling and pain in the
injection site and 4 patients with nausea in the control group,
with the incidence of 23.26%. The incidence of adverse reactions of
patients in the experimental group was significantly lower than
that in the control group, with a statistically significant
difference (P<0.05) (Table
III).
 | Table III.Adverse reaction records of patients
in the two groups [n (%)]. |
Table III.
Adverse reaction records of patients
in the two groups [n (%)].
| Group | Diarrhea | Swelling and pain in
the injection site | Nausea | Incidence |
|---|
| Experimental group
(n=43) | 1 (2.33) | 1 (2.33) | 1 (2.33) | 3 (6.98) |
| Control group
(n=43) | 2 (4.65) | 4 (9.30) | 4 (9.30) | 10 (23.26) |
| χ2
test | – | – | – | 4.440 |
| P-value | – | – | – | 0.035 |
Sequelae of patients after 6 months of
treatment in the two groups
After 6 months of treatment, there was no patient
with auditory nerve abnormalities, 1 patient with motor nerve
abnormalities and no patient with epilepsy in the experimental
group, with the incidence of sequelae of 2.33%. There were 3
patients with auditory nerve abnormalities, 4 patients with motor
nerve abnormalities and 2 patients with epilepsy in the control
group, with the incidence of sequelae of 20.93%. The incidence of
sequelae of patients in the experimental group was significantly
lower than that in the control group, with a statistically
significant difference (P<0.05) (Table IV).
 | Table IV.Sequelae of patients after 6 months of
treatment in the two groups [n (%)]. |
Table IV.
Sequelae of patients after 6 months of
treatment in the two groups [n (%)].
| Group | Auditory nerve
abnormalities | Motor nerve
abnormalities | Epilepsy | Total incidence |
|---|
| Experimental group
(n=43) | 0 (0) | 1 (2.33) | 0 (0) | 1 (2.33) |
| Control group
(n=43) | 3 (6.98) | 4 (9.30) | 2 (4.65) | 9 (20.93) |
| χ2
test | – | – | – | 7.242 |
| P-value | – | – | – | 0.007 |
Discussion
TBI refers to a common trauma and is divided into
light, moderate, heavy and extra-heavy traumas according to
severity. Often accompanied by epilepsy, infection and cerebral
infarction, which even causes coma in patients with serious TBI
(8,9). The mortality of TBI has been
significantly reduced in recent years with the improvement of
medical technology. However, due to the nerve function injury of
patients, its disability rate remains high (10,11).
Patients with STBI are critically ill and often affected by
increased intracranial pressure and toxic metabolites, resulting in
sustained brain injury (12,13). Intracranial infection, one of the
complications of cranial operation, is treated with the complete
elimination of surgical infected wounds, with antibiotic,
symptomatic and supportive treatments (14). Intracranial infection usually occurs
within 3–7 days after cranial operation (15), accompanied by fever, disturbance of
consciousness and other clinical manifestations. Postoperative
intracranial infection poses a serious threat to the patient's life
and requires timely action (16).
Intracranial infection refers to a disease caused by viruses and
bacteria, especially for patients undergoing neurosurgery
operation. It has a 10–25% infection rate, and a high mortality and
disability rate (17–19). Therefore, optimizing STBI is of great
significance. It is necessary to improve the prognosis of the
patient, as well as reduce the cure time, hospitalization cost and
incidence of complications in order to improve the quality of life
and prognosis of the patient.
The efficacy was first compared in this experiment.
The results showed that the RR of patients in the experimental
group was significantly higher than that in the control group, with
a statistically significant difference (P<0.05). There is a
study showing that (20) intravenous
meropenem and vancomycin has a long cure time and high treatment
cost, which also affects the liver and kidney function of the
patient. At present, the use of intrathecal drugs has attracted
much attention (21). Studies have
confirmed (22,23) that intrathecal vancomycin and
meropenem has good clinical efficacy. This is similar to the
results of this study. Then, the cure time and treatment cost of
patients were compared between the two groups. The results showed
that the cure time of patients in the experimental group was
significantly lower than that in the control group, with a
statistically significant difference (P<0.001). The treatment
cost of patients in the experimental group was significantly lower
than that in the control group, with a statistically significant
difference (P<0.001). A study shows that (24) intrathecal meropenem and vancomycin is
more effective than intravenous meropenem and vancomycin in the
treatment of postoperative infection, which shortens the treatment
time and reduces the treatment cost. Next, the adverse reactions of
patients in the two groups were compared. The results showed that
the incidence of adverse reactions of patients in the experimental
group was significantly lower than that in the control group, with
a statistically significant difference (P<0.05). Finally, the
sequelae of patients after 6 months of treatment were compared. The
results showed that the incidence of sequelae of patients in the
experimental group was significantly lower than that in the control
group, with a statistically significant difference (P<0.05).
There is a study suggesting (25)
that continuous lumbar drainage allows the CSF to drain
continuously and slowly through the drainage tube, as well as
reduces pathogenic microorganisms and inflammatory factors in the
subarachnoid space so as to replace the CSF. In addition,
intrathecal administration through the lumbar cistern reduces the
incidence of adverse reactions and complications. The present study
is an excellent complement to the results of that paper.
The number of patients included in this study is not
large enough for conclusive statistics, so certain limitations
exist and further investigations are required.
In summary, intrathecal meropenem and vancomycin is
more effective than intravenous injection in the treatment of
intracranial infection after craniotomy. It can shorten the
treatment time and reduce the treatment cost, and is safer and has
less adverse reactions and fewer complications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ wrote the manuscript. QZ and HC were responsible
for observation indicators. CZ collected the patient data. FC
analyzed and interpreted the patient general data with severe
traumatic brain injury. SS and NL contributed to outcome measures.
QZ and WZ helped with statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Tai'an Traditional Chinese Medicine Hospital and Affiliated
Hospital of Taishan Medical University (Tai'an, China). Patients
who participated in this study had complete clinical data. Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gupta MK, Mondkar JA and Hegde D:
Paradoxical reaction to midazolam in preterm neonates: A case
series. Indian J Crit Care Med. 22:300–302. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dey S and Kumar M: Comparison of
pretreatment with dexmedetomidine with midazolam for prevention of
etomidate-induced myoclonus and attenuation of stress response at
intubation: A randomized controlled study. J Anaesthesiol Clin
Pharmacol. 34:94–98. 2018.PubMed/NCBI
|
|
3
|
Paleti S, Prasad PK and Lakshmi BS: A
randomized clinical trial of intrathecal magnesium sulfate versus
midazolam with epidural administration of 0.75% ropivacaine for
patients with preeclampsia scheduled for elective cesarean section.
J Anaesthesiol Clin Pharmacol. 34:23–28. 2018.PubMed/NCBI
|
|
4
|
Azeem TM, Yosif NE, Alansary AM, Esmat IM
and Mohamed AK: Dexmedetomidine vs morphine and midazolam in the
prevention and treatment of delirium after adult cardiac surgery; a
randomized, double-blinded clinical trial. Saudi J Anaesth.
12:190–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mazzeo AT, Filippini C, Rosato R, Fanelli
V, Assenzio B, Piper I, Howells T, Mastromauro I, Berardino M,
Ducati A, et al: Multivariate projection method to investigate
inflammation associated with secondary insults and outcome after
human traumatic brain injury: A pilot study. J Neuroinflammation.
13:1572016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldschmidt E, Rasmussen J, Chabot JD,
Gandhoke G, Luzzi E, Merlotti L, Proni R, Loresi M, Hamilton DK,
Okonkwo DO, et al: The effect of vancomycin powder on human dural
fibroblast culture and its implications for dural repair during
spine surgery. J Neurosurg Spine. 25:665–670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blassmann U, Roehr AC, Frey OR,
Vetter-Kerkhoff C, Thon N, Hope W, Briegel J and Huge V:
Cerebrospinal fluid penetration of meropenem in neurocritical care
patients with proven or suspected ventriculitis: a prospective
observational study. Crit Care. 20:3432016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caughlin S, Maheshwari S, Agca Y, Agca C,
Harris AJ, Jurcic K, Yeung KK, Cechetto DF and Whitehead SN:
Membrane-lipid homeostasis in a prodromal rat model of Alzheimers
disease: Characteristic profiles in ganglioside distributions
during aging detected using MALDI imaging mass spectrometry.
Biochim Biophys Acta, Gen Subj. 1862:1327–1338. 2018. View Article : Google Scholar
|
|
9
|
Benktander J, Barone A, Johansson MM and
Teneberg S: Helicobacter pylori SabA binding gangliosides of
human stomach. Virulence. 9:738–751. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasaki N, Itakura Y and Toyoda M:
Ganglioside GM1 contributes to extracellular/intracellular
regulation of insulin resistance, impairment of insulin signaling
and down-stream eNOS activation, in human aortic endothelial cells
after short- or long-term exposure to TNFα. Oncotarget.
9:5562–5577. 2017.PubMed/NCBI
|
|
11
|
Iwasawa T, Zhang P, Ohkawa Y, Momota H,
Wakabayashi T, Ohmi Y, Bhuiyan RH and Furukawa K and Furukawa K:
Enhancement of malignant properties of human glioma cells by
ganglioside GD3/GD2. Int J Oncol. 52:1255–1266. 2018.PubMed/NCBI
|
|
12
|
Patet C, Suys T, Carteron L and Oddo M:
Cerebral lactate metabolism after traumatic brain injury. Curr
Neurol Neurosci Rep. 16:312016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hackenberg K and Unterberg A: Traumatic
brain injury. Nervenarzt. 87:203–214; quiz 215–216, 2016 (In
German). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan M, Wang D, Wang J, Xiao X, Han L and
Zhang F: A case report of intracranial infection caused by
Shewanella putrefaciens. Neurol Sci. 36:625–629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cook AM, Arora S, Davis J and Pittman T:
Augmented renal clearance of vancomycin and levetiracetam in a
traumatic brain injury patient. Neurocrit Care. 19:210–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huttner HB, Nagel S, Tognoni E, Köhrmann
M, Jüttler E, Orakcioglu B, Schellinger PD, Schwab S and Bardutzky
J: Intracerebral hemorrhage with severe ventricular involvement:
Lumbar drainage for communicating hydrocephalus. Stroke.
38:183–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wattanathum A, Chaoprasong C, Nunthapisud
P, Chantaratchada S, Limpairojn N, Jatakanon A and Chanthadisai N:
Community-acquired pneumonia in southeast Asia: The microbial
differences between ambulatory and hospitalized patients. Chest.
123:1512–1519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El Sayed Zaki M and Goda T:
Clinico-pathological study of atypical pathogens in
community-acquired pneumonia: A prospective study. J Infect Dev
Ctries. 3:199–205. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaillat J, Flahault A, deBarbeyrac B,
Orfila J, Portier H, Ducroix JP, Bébéar C and Mayaud C: Community
epidemiology of Chlamydia and Mycoplasma pneumoniae
in LRTI in France over 29 months. Eur J Epidemiol. 20:643–651.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doder R, Canak G, Vukadinov J, Turkulov V
and Sević S: Antibiotics in the treatment of bacterial infections
of the central nervous system. Med Pregl. 63 (Suppl 1):22–26.
2010.PubMed/NCBI
|
|
21
|
Kneen R, Solomon T and Appleton R: The
role of lumbar puncture in suspected CNS infection - a disappearing
skill? Arch Dis Child. 87:181–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bao Y, Qiu B, Zeng H, Mo Y, Zhang N and Qi
S: Combined intravenous and intrathecal vancomycin in treatment of
patients with intracranial infections after craniotomy. Zhonghua
Wei Zhong Bing Ji Jiu Yi Xue. 28:169–172. 2016.(In Chinese).
PubMed/NCBI
|
|
23
|
Lee K, Rho M, Yu M, Kwak J, Hong S, Kim J,
Kim Y and Pai H: A case of recurrent meningitis caused by
Rhodococcus species successfully treated with antibiotic
treatment and intrathecal injection of vancomycin through an Ommaya
reservoir. Infect Chemother. 47:183–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen K, Wu Y, Wang Q, Wang J, Li X, Zhao Z
and Zhou J: The methodology and pharmacokinetics study of
intraventricular administration of vancomycin in patients with
intracranial infections after craniotomy. J Crit Care.
30:218.e1–218.e5. 2015. View Article : Google Scholar
|
|
25
|
Chen QH, Lin D, Yu QG and Zhou J: Efficacy
of lumbar cistern drainage combined with intrathecal antibiotherapy
for the treatment of ventriculo-subarachnoid infections following
surgery for hypertensive intracerebral hemorrhage. Neurochirurgie.
63:13–16. 2017. View Article : Google Scholar : PubMed/NCBI
|