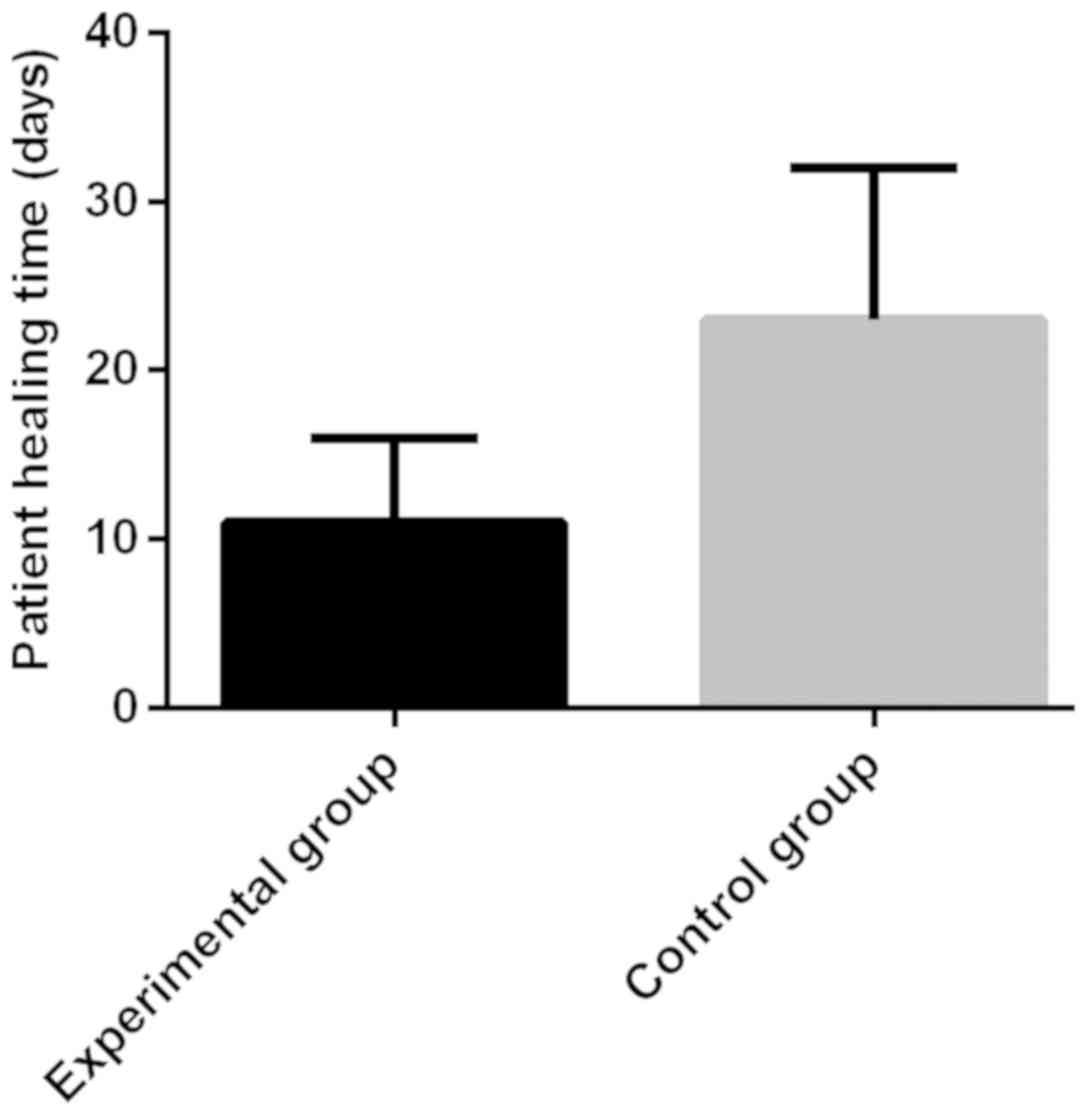
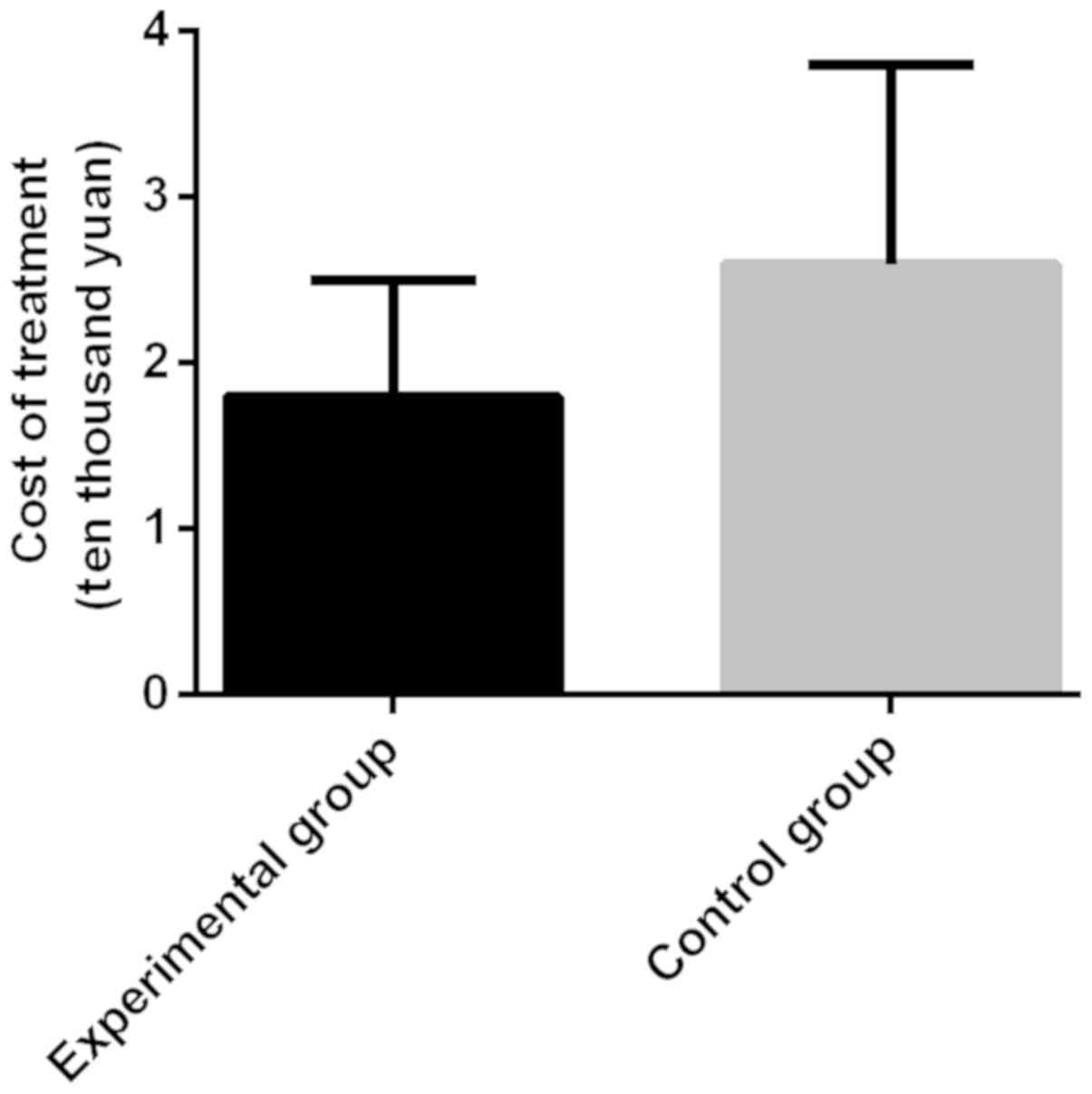
|
1
|
Gupta MK, Mondkar JA and Hegde D:
Paradoxical reaction to midazolam in preterm neonates: A case
series. Indian J Crit Care Med. 22:300–302. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dey S and Kumar M: Comparison of
pretreatment with dexmedetomidine with midazolam for prevention of
etomidate-induced myoclonus and attenuation of stress response at
intubation: A randomized controlled study. J Anaesthesiol Clin
Pharmacol. 34:94–98. 2018.PubMed/NCBI
|
|
3
|
Paleti S, Prasad PK and Lakshmi BS: A
randomized clinical trial of intrathecal magnesium sulfate versus
midazolam with epidural administration of 0.75% ropivacaine for
patients with preeclampsia scheduled for elective cesarean section.
J Anaesthesiol Clin Pharmacol. 34:23–28. 2018.PubMed/NCBI
|
|
4
|
Azeem TM, Yosif NE, Alansary AM, Esmat IM
and Mohamed AK: Dexmedetomidine vs morphine and midazolam in the
prevention and treatment of delirium after adult cardiac surgery; a
randomized, double-blinded clinical trial. Saudi J Anaesth.
12:190–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mazzeo AT, Filippini C, Rosato R, Fanelli
V, Assenzio B, Piper I, Howells T, Mastromauro I, Berardino M,
Ducati A, et al: Multivariate projection method to investigate
inflammation associated with secondary insults and outcome after
human traumatic brain injury: A pilot study. J Neuroinflammation.
13:1572016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldschmidt E, Rasmussen J, Chabot JD,
Gandhoke G, Luzzi E, Merlotti L, Proni R, Loresi M, Hamilton DK,
Okonkwo DO, et al: The effect of vancomycin powder on human dural
fibroblast culture and its implications for dural repair during
spine surgery. J Neurosurg Spine. 25:665–670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blassmann U, Roehr AC, Frey OR,
Vetter-Kerkhoff C, Thon N, Hope W, Briegel J and Huge V:
Cerebrospinal fluid penetration of meropenem in neurocritical care
patients with proven or suspected ventriculitis: a prospective
observational study. Crit Care. 20:3432016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caughlin S, Maheshwari S, Agca Y, Agca C,
Harris AJ, Jurcic K, Yeung KK, Cechetto DF and Whitehead SN:
Membrane-lipid homeostasis in a prodromal rat model of Alzheimers
disease: Characteristic profiles in ganglioside distributions
during aging detected using MALDI imaging mass spectrometry.
Biochim Biophys Acta, Gen Subj. 1862:1327–1338. 2018. View Article : Google Scholar
|
|
9
|
Benktander J, Barone A, Johansson MM and
Teneberg S: Helicobacter pylori SabA binding gangliosides of
human stomach. Virulence. 9:738–751. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasaki N, Itakura Y and Toyoda M:
Ganglioside GM1 contributes to extracellular/intracellular
regulation of insulin resistance, impairment of insulin signaling
and down-stream eNOS activation, in human aortic endothelial cells
after short- or long-term exposure to TNFα. Oncotarget.
9:5562–5577. 2017.PubMed/NCBI
|
|
11
|
Iwasawa T, Zhang P, Ohkawa Y, Momota H,
Wakabayashi T, Ohmi Y, Bhuiyan RH and Furukawa K and Furukawa K:
Enhancement of malignant properties of human glioma cells by
ganglioside GD3/GD2. Int J Oncol. 52:1255–1266. 2018.PubMed/NCBI
|
|
12
|
Patet C, Suys T, Carteron L and Oddo M:
Cerebral lactate metabolism after traumatic brain injury. Curr
Neurol Neurosci Rep. 16:312016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hackenberg K and Unterberg A: Traumatic
brain injury. Nervenarzt. 87:203–214; quiz 215–216, 2016 (In
German). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan M, Wang D, Wang J, Xiao X, Han L and
Zhang F: A case report of intracranial infection caused by
Shewanella putrefaciens. Neurol Sci. 36:625–629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cook AM, Arora S, Davis J and Pittman T:
Augmented renal clearance of vancomycin and levetiracetam in a
traumatic brain injury patient. Neurocrit Care. 19:210–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huttner HB, Nagel S, Tognoni E, Köhrmann
M, Jüttler E, Orakcioglu B, Schellinger PD, Schwab S and Bardutzky
J: Intracerebral hemorrhage with severe ventricular involvement:
Lumbar drainage for communicating hydrocephalus. Stroke.
38:183–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wattanathum A, Chaoprasong C, Nunthapisud
P, Chantaratchada S, Limpairojn N, Jatakanon A and Chanthadisai N:
Community-acquired pneumonia in southeast Asia: The microbial
differences between ambulatory and hospitalized patients. Chest.
123:1512–1519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El Sayed Zaki M and Goda T:
Clinico-pathological study of atypical pathogens in
community-acquired pneumonia: A prospective study. J Infect Dev
Ctries. 3:199–205. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaillat J, Flahault A, deBarbeyrac B,
Orfila J, Portier H, Ducroix JP, Bébéar C and Mayaud C: Community
epidemiology of Chlamydia and Mycoplasma pneumoniae
in LRTI in France over 29 months. Eur J Epidemiol. 20:643–651.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doder R, Canak G, Vukadinov J, Turkulov V
and Sević S: Antibiotics in the treatment of bacterial infections
of the central nervous system. Med Pregl. 63 (Suppl 1):22–26.
2010.PubMed/NCBI
|
|
21
|
Kneen R, Solomon T and Appleton R: The
role of lumbar puncture in suspected CNS infection - a disappearing
skill? Arch Dis Child. 87:181–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bao Y, Qiu B, Zeng H, Mo Y, Zhang N and Qi
S: Combined intravenous and intrathecal vancomycin in treatment of
patients with intracranial infections after craniotomy. Zhonghua
Wei Zhong Bing Ji Jiu Yi Xue. 28:169–172. 2016.(In Chinese).
PubMed/NCBI
|
|
23
|
Lee K, Rho M, Yu M, Kwak J, Hong S, Kim J,
Kim Y and Pai H: A case of recurrent meningitis caused by
Rhodococcus species successfully treated with antibiotic
treatment and intrathecal injection of vancomycin through an Ommaya
reservoir. Infect Chemother. 47:183–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen K, Wu Y, Wang Q, Wang J, Li X, Zhao Z
and Zhou J: The methodology and pharmacokinetics study of
intraventricular administration of vancomycin in patients with
intracranial infections after craniotomy. J Crit Care.
30:218.e1–218.e5. 2015. View Article : Google Scholar
|
|
25
|
Chen QH, Lin D, Yu QG and Zhou J: Efficacy
of lumbar cistern drainage combined with intrathecal antibiotherapy
for the treatment of ventriculo-subarachnoid infections following
surgery for hypertensive intracerebral hemorrhage. Neurochirurgie.
63:13–16. 2017. View Article : Google Scholar : PubMed/NCBI
|