Introduction
Hemorrhagic shock (HS) and subsequent resuscitation
result in an overly active systemic immuno-inflammatory response
that may produce an exaggerated physiological response, resulting
in systemic inflammatory response syndrome (SIRS). Complications of
SIRS often involve organ dysfunction and organ failure, including
acute lung injury (ALI) and multiple organ dysfunction syndrome
(MODS), with high mortality rates, ranging from 27–100% (1,2).
Although substantial progress has been made in the treatment of HS,
the incidence of SIRS following HS remains up to 43% (3), and there is no effective measure to
prevent the patients from entering the SIRS-MODS stage.
The ‘second hit’ model proposed by Moore et
al (4) is considered to be a
good standard as an empirical explanation for HS-associated
inflammation. HS, the first ‘hit’ primes the inflammatory system
and a second ‘hit’, for example through infection, aggravates the
already sensitized immune system (4). Inflammation is governed by interactions
between proinflammatory and counter-inflammatory states; the
imbalance between pro-inflammatory and anti-inflammatory responses
serves a key role in the development of ALI and MODS (5), but the exact mechanism of HS
sensitization to ALI and SIRS remains unclear. Therefore, it is of
vital importance to investigate the mechanisms involved in the
inflammatory response state following HS.
Evidence has revealed that the activation of
pulmonary vascular endothelial cells (ECs) and EC-mediated
inflammation serve important functions in organ injury following a
hemorrhage (6). The pyrin protein,
consisting of 781 amino acid residues, is expressed in neutrophils,
monocytes and macrophages, and its expression is regulated by a
variety of cytokines (7). The pyrin
protein is able to inhibit the activation of caspase-1 and inhibit
the mature release of interleukin (IL)-1β, a proinflammatory
mediator (8) that is involved in
lung inflammation (9). Caspase-1 is
also synthesized as an inactive 45 kDa protein (procaspase-1) that
undergoes autocatalytic processing following assembly of the
inflammasome in response to an appropriate stimulus (10,11). Of
the cytokines that are able to regulate the expression of pyrin,
interleukin (IL)-10 is able to induce the expression of pyrin
protein in macrophages (8). It has
been demonstrated that the immunosuppressive cytokine IL-10 may
inhibit the activation of monocytes/macrophages and decrease the
release of cytokines and chemokines induced by lipopolysaccharides
(LPS) (9). In vivo
experiments of ischemia/reperfusion have revealed that IL-10 is
able to effectively decrease structural organ damage; however, when
a IL-10 neutralizing antibody or IL-10 knockout is used, the
inflammatory reaction is aggravated (12).
Taking into consideration these diverse results, it
was hypothesized that pyrin and IL-10 are involved in the
regulation of inflammation in pulmonary vascular ECs, and that the
decrease in IL-10 expression contributes to a severe inflammatory
response.
Materials and methods
Animals
Adult female Sprague-Dawley rats (n=100; weight,
200–240 g; Zhejiang Laboratory Animal Center) were provided by the
Experimental Animal Center of the First Affiliated Hospital,
Zhejiang University School of Medicine (Zhejiang, China). The rats
were maintained in a standard cage, in a 22–24°C environment, with
a 12:12 h light:dark cycle and ad libitum access to food and
water. All experiments were performed in accordance with the
institutional guidelines for animal care and welfare. The present
study was approved by the Medical Ethics Committee of Zheijang
Hospital.
Experimental protocol
Prior to the initiation of the experiment, the rats
were fasted for 24 h with ad libitum access to water
(13,14), in order to avoid backflow and
aspiration when the trachea was intubated, and the greatest weight
loss observed was 16g (~7% of body weight). Following the induction
of anesthesia with an intraperitoneal injection of 2% sodium
pentobarbital solution (50 mg/kg; Westang Biotechenology, Co.,
Ltd.), the left femoral artery was cannulated with an external
infusion device to allow for the monitoring of mean arterial
pressure, blood sampling and resuscitation. HS was initiated by the
withdrawal of arterial blood and the mean arterial pressure reached
40±5 mmHg within 20 min. Blood was collected into a 10 ml
heparinized syringe to prevent the blood from clotting. In order to
exclude the effect of heparin on the experimental results, the
control group was administered equal amounts of heparin through
arterial catheterization. Subsequent to a hypotensive period of 1
h, autologous blood transfusion was performed and the same quantity
of lactate solution was administered (15). Resuscitation was completed within 20
min. Following resuscitation, the catheters were removed and the
artery was ligated to close the incision. The sham surgery (SM)
group underwent femoral artery catheterization without blood loss.
Following resuscitation for 2 h, the rats were administered LPS
(cat. no., L2880; Sigma-Aldrich; Merck KGaA) at a dose of 100 µg/kg
body weight (16,17), which was intratracheally injected as
the second ‘hit’ model of HS-LPS. During the entire experiment, all
rats were under anesthesia.
The experiment was divided into two parts. The first
part was used to confirm that pulmonary ECs participated in the
inflammatory process subsequent to the second ‘hit’ and confirm
whether the LPS-mediated upregulation of pyrin expression was
impaired following HS. The rats were divided into 4 groups (n=6 for
each group), as follows: i) A SM + tracheal injection of saline
(SAL) group; this was the negative control group; ii) a HS + SAL
group; iii) a SM + LPS group. with a tracheal injection of
endotoxin; and iv) a HS + LPS group. A total of 8 h after tracheal
injection, the rats were euthanized by administering an overdose of
sodium pentobarbital solution (200 mg/kg) followed by cervical
dislocation. Alveolar lavage was performed, lung tissues were
collected and stored in liquid nitrogen, and then western blot
analysis and immunofluorescence were performed.
The second part was performed to clarify the
potential mechanism of the inhibition of the LPS-mediated
upregulation of pyrin expression following HS. Rats were divided
into four groups, as follows: i) A SM + LPS group; ii) a HS + LPS
group (negative control group); ii) a HS + LPS + intratracheal
injection of saline (NS) group; and iv) a HS + LPS + IL-10, with an
intratracheal injection of recombinant IL-10 (BioLegend, Inc.)
group. A total of 8 h after tracheal injection, the rats were
euthanized by administering an overdose of sodium pentobarbital
solution (200 mg/kg) followed by cervical dislocation. Alveolar
lavage was performed, lung tissues were collected and stored in
liquid nitrogen, and then western blot analysis was performed.
Immunofluorescence
The lung tissues were fixed in 4% paraformaldehyde
at 4°C overnight. The lung tissue of each group was serially sliced
into 4-µm thick slices, and then incubated with 5% goat serum
(Beyotime Institute of Biotechnology) at 22°C for 1 h. Lung tissues
from each group were sliced into 2 sections, and each sample was
incubated for 48 h at 4°C with cluster of differentiation (CD)34
mouse primary antibodies (cat. no., 60108-1-lg) and anti-pyrin
rabbit primary antibodies (cat. no., 24280-1-AP; both 1:100;
ProteinTech Group, Inc.) respectively. CD34, a cell surface
sialomucin-like glycoprotein, is commonly used as a marker for
identifying vascular ECs (18).
Vascular ECs stained with CD34 exhibit a red color in the
cytoplasm. PBS was used as blank control instead of the primary
antibody. Subsequent to washing with PBS 3 times (3 min each), the
slides were incubated with Cy3-conjugated goat anti-mouse
immunoglobulin (Ig)G (cat. no., P0193) and FITC-conjugated goat
anti-rabbit IgG (cat. no., P0186; both 1:100; Beyotime Institute of
Biotechnology) for 1 h at room temperature. The staining method was
performed according to the manufacturer's protocol of
immunofluorescence kits (Immunol Fluorence Staining Kit with
Cy3-Labeled Goat Anti-Mouse IgG and Immunol Fluorescence Staining
Kit with FITC-Labeled Goat Anti-Rabbit IgG; both Beyotime Institute
of Biotechnology). Images were obtained using a wide-field
fluorescence microscope (Olympus X-cite 120; Olympus Corporation;
magnification, ×200).
Western blot analysis
Lung tissues of rats were homogenized on ice and
lysed in lysis buffer which was prepared as follows: 10 mM Tris (pH
7.4), 5 mM EDTA, 150 mM NaCl, 10 mM NaF, 1% Triton X-100, 1 mM
Na3VO4, 20 mM PMSF, 10 µg/ml leupeptin and 10
µg/ml aprotinin. The tissue homogenate was centrifuged at 1,000 × g
in an Eppendorf centrifuge (Eppendorf) at 4°C for 10 min, and then
the protein content in the supernatants were quantified using the
Bradford method. Proteins (30 µg/lane) for each sample were
separated using 10% SDS-PAGE gel and then transferred onto a
polyvinylidene fluoride membrane. Subsequent to being blocked by 5%
non-fat milk for 1 h at 22°C and then for 24 h at 4°C, the
membranes were incubated with the primary antibodies against pyrin
(1:1,000; cat. no., 24280-1-AP; ProteinTech Group, Inc.) and
caspase-1 (1:2,000; cat. no., ab188326; Abcam). The blots were then
washed and incubated with horseradish peroxidase (HRP)-labeled goat
anti-rabbit IgG (1:2,000; cat. no., A0208; Beyotime Institute of
Biotechnology) and HRP-labeled goat anti-mouse IgG (1:2,000; cat.
no., A0216; Beyotime Institute of Biotechnology) for 2 h at room
temperature. Protein bands were then detected by enhanced
chemiluminescence. A ChemiDoc MP System (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) was used to capture the protein bands and
then Image Lab 5.0 software (Bio-Rad Laboratories, Inc.) was used
to analyze the bands. GAPDH and pro-caspase-1 were used as loading
controls. Molecular weight standards were used from commercial
markers (Thermo Fisher Scientific Inc.).
Statistical analysis
SPSS v20.0 software (IBM Corp.) was applied for
statistical analysis and the experiments were performed and
repeated three times. Data are presented as the mean ± standard
error of the mean. Data were analyzed using one-way analysis of
variance followed by a Student-Neuman-Keuls post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HS attenuates the LPS-induced pyrin
expression in pulmonary vascular ECs
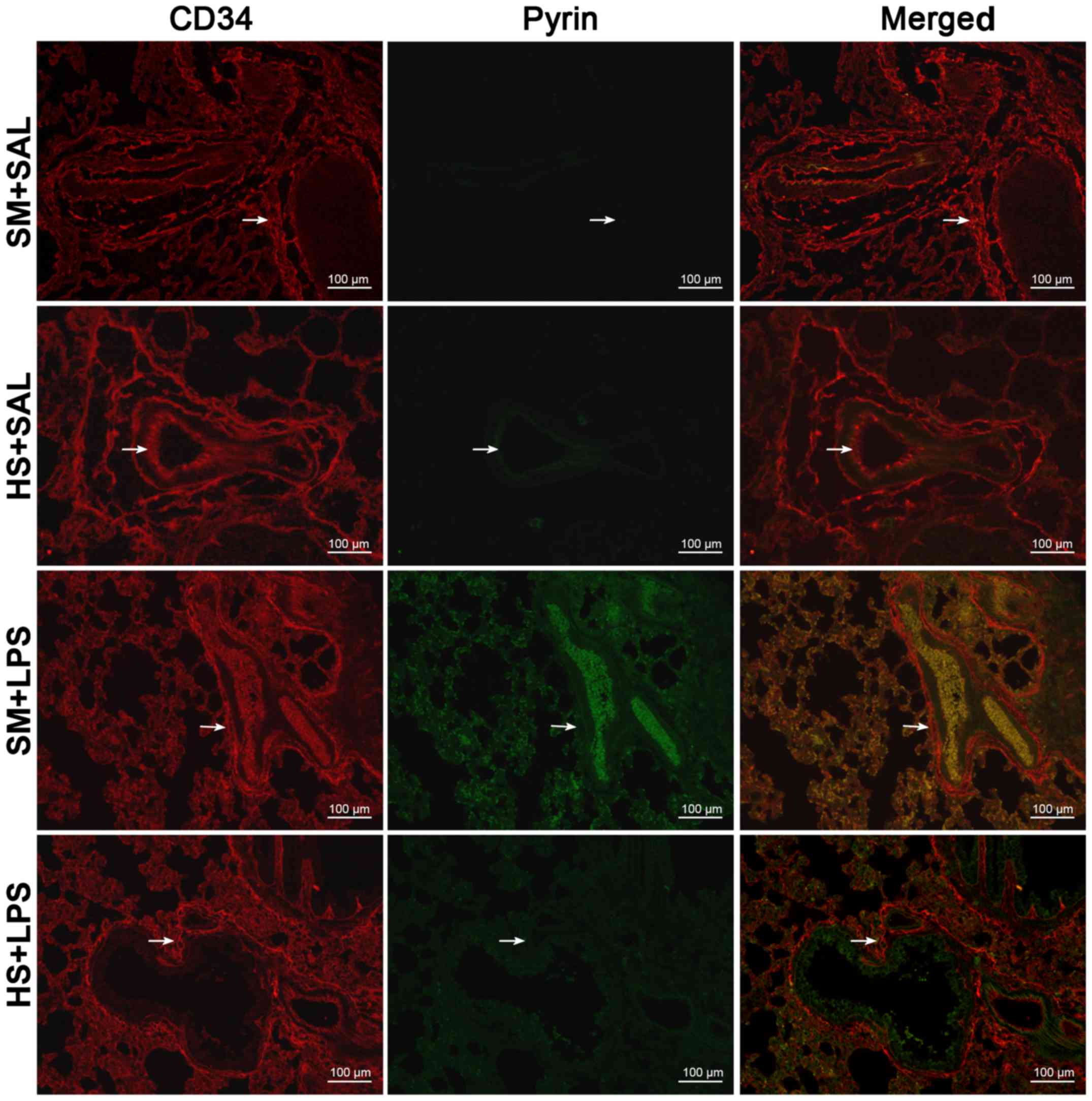
Subsequent to the removal of the majority of the
macrophages following lung lavage, an analysis of the distribution
of ECs and pyrin was performed. In immunofluorescence staining,
cells stained with CD34 (red) were considered to be pulmonary
vascular ECs. As presented in Fig.
1, these CD34-positive cells presented a typical
cobblestone-like morphology. Pyrin staining (green) demonstrated
the presence of pyrin in ECs. The second ‘hit’,
HS-LPS, substantially decreased the expression of pyrin protein in
pulmonary vascular ECs. Furthermore, the expression of pyrin was
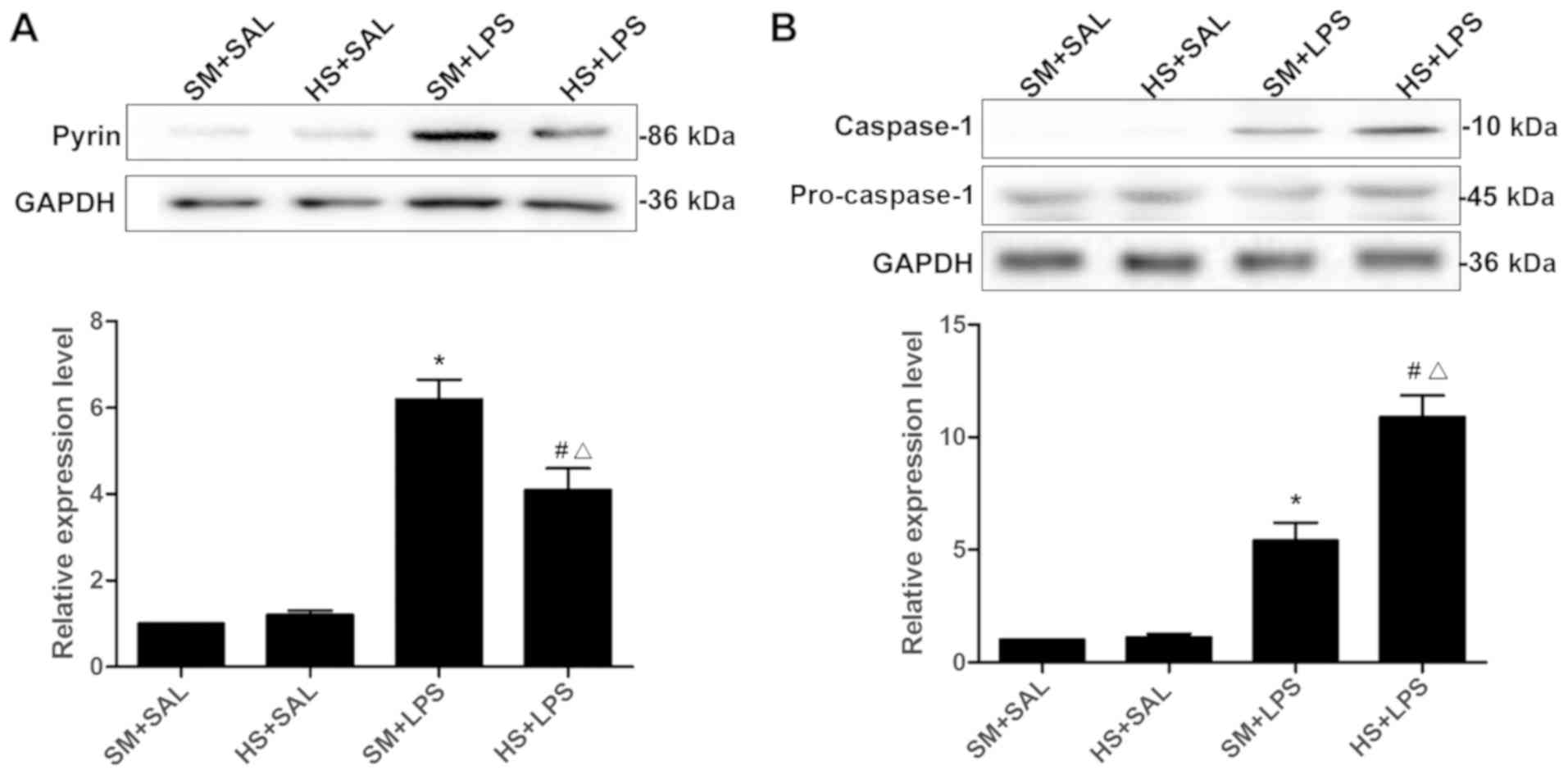
evaluated in the supernatant of the lung homogenate (Fig. 2A). No difference regarding the
expression levels of pyrin between the SM + SAL group and the HS +
SAL group was observed (P>0.05). The expression levels of pyrin
in the SM + LPS group were significantly increased in comparison
with that in the SM + SAL group (P<0.01), demonstrating that LPS
stimulation had significantly increased the pyrin protein
expression level in pulmonary vascular ECs. Additionally, the
expression of pyrin was significantly increased in the HS + LPS
group compared with the HS + SAL group (P<0.01). However, the
pyrin expression level in the HS + LPS group was significantly
decreased compared with that of the SM + LPS group (P<0.01),
indicating that HS weakened the expression of pyrin induced by
LPS.
As presented in Fig.
2B, there was no significant difference with regard to the
expression of caspase-1 in the SM + SAL and HS + SAL groups
(P>0.05). The expression of caspase-1 in the SM + LPS was
increased compared with that in the SM + SAL group (P<0.01),
demonstrating that LPS stimulation increased the expression of
caspase-1 protein in pulmonary vascular ECs. In addition, the
expression of caspase-1 in the HS + LPS group was significantly
increased compared with that in the SM + LPS group (P<0.01).
Collectively, HS augmented the LPS-induced caspase-1
expression.
Involvement of IL-10 in regulating
pyrin expression changes in pulmonary vascular ECs following the
second ‘hit’ of HS-LPS
The expression of the pyrin protein is regulated by
various cytokines, including IL-10 (8). The present study assessed the
hypothesis that HS damaged the expression of IL-10, thereby
affecting the expression of the pyrin protein, which resulted in
the activation of caspase-1 subsequent to the second
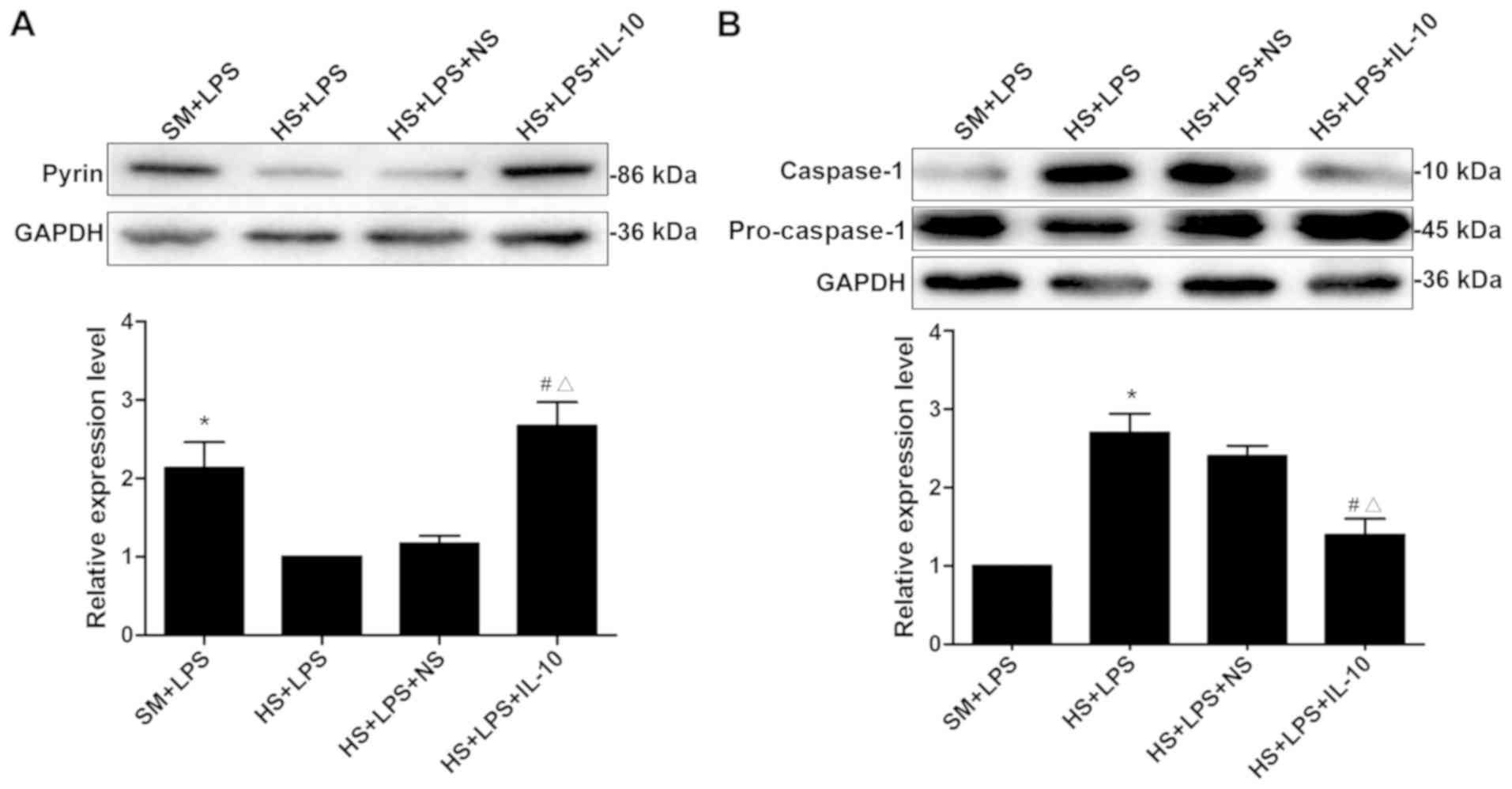
‘hit’ of HS-LPS. As presented in Fig. 3A, the expression levels of pyrin in
the SM + LPS group were significantly increased in comparison with
the HS + LPS group (P<0.01); and the expression levels of pyrin
in the HS + LPS + IL-10 group were significantly increased compared
with the HS + LPS group (P<0.01). The results demonstrated that
HS and the intratracheal injection of LPS may result in the
expression of pyrin being significantly decreased, however,
exogenous IL-10 treatment increases pyrin expression.
As presented in Fig.
3B, the expression levels of cleaved caspase-1 were
significantly different between the SM + LPS and HS + LPS groups
(P<0.01). The expression of caspase-1 was significantly
decreased subsequent to IL-10 treatment compared with those in the
HS + LPS group (P<0.01). Although HS promoted the LPS-induced
caspase-1 expression, intratracheal IL-10 treatment may decrease
the expression of caspase-1, alleviating the inflammatory reaction.
Therefore, the IL-10 treatment-inhibited caspase-1 activation
induced by LPS may occur through the upregulation of pyrin
expression in HS rats.
Discussion
Systemic inflammatory response and organ failure are
the primary factors affecting mortality in patients with HS and
trauma. How HS enhances the inflammatory response of the body
remains unclear. The activation of caspase-1 serves an important
role in systemic inflammatory responses subsequent to shock
(10). In the present study, it was
revealed that LPS induced the activation of caspase-1 and also
induced the expression of pyrin protein in pulmonary vascular ECs.
The increase of pyrin protein expression may inhibit the activation
of caspase-1 and form a negative feedback pathway. Notably, the
increased expression of IL-10 induced by LPS in lung ECs may
enhance the expression levels of pyrin in lung ECs and enhance the
negative feedback regulation of inflammation. However, HS, by
inhibiting the expression of IL-10, weakens the expression of
pyrin, thereby damaging the negative feedback regulation of the
inflammatory response and resulting in the enhancement of
inflammatory body activation and consequently increasing the
release of caspase-1.
The pyrin protein may inhibit the activation of
caspase-1, and subsequently inhibit the maturation of inflammatory
bodies. The destruction of the C terminal of the pyrin protein may
enhance the sensitivity of mice to endotoxins and increase the
activation of caspase-1, which indicates that the pyrin protein
serves an important role in the maturation of inflammatory bodies
(19). The pyrin protein interacts
with caspase-1, apoptosis-associated speck-like protein containing
a CARD, NLR family pyrin domain containing (NLRP)1, NLRP2, NLRP3
and IL-1B (precursors of inflammatory components) through its
SPIa/ryanodine receptor and pyrin structural domains, thereby
functioning as an inhibitor of the inflammatory body (20). A previous study has demonstrated that
pyrin mutations may result in the activation of the NLRP3
independent inflammatory body, causing autoimmune diseases
(21). The present study suggested
that pyrin may result in different inflammatory outcomes through
multiple different pathways.
IL-10 serves an important role in limiting
inflammation and maintaining immune homeostasis (22). In acute respiratory distress
syndrome, IL-10 has been demonstrated to inhibit proinflammatory
mediators produced by pulmonary macrophages (23). In a previous clinical study, patients
with acute respiratory distress syndrome, but with low levels of
circulating and alveolar lavage fluid IL-10 exhibited an increased
mortality rate compared with those with high IL-10 levels (24). At the gene level, a previous study
indicated that, compared with pure LPS stimulation, hemorrhagic
shock combined with LPS may significantly decrease the
transcription of IL-10 mRNA (25).
Patients that succumbed to mortality due to sepsis exhibited a
decrease in IL-10 secretion (26),
indicating that exogenous IL-10 had protective effects in sepsis or
acute pancreatitis models (26,27). A
limitation of the present study is the absence of an in
vitro model. A previous study confirmed that an in vitro
HS model inhibited the LPS-induced increase of IL-10 expression at
the gene level, which is closely associated with the progression of
HS-induced pulmonary inflammation (28). It has been suggested that pyrin may
serve an inhibitory role in casepase-1 activation and inhibit the
development of inflammation (29,30). In
the present study, it was revealed that IL-10 may inhibit the
activation of inflammatory cells by inducing the expression of
pyrin in pulmonary vascular ECs. It was also confirmed that LPS
induced the IL-10-based upregulated pyrin expression in lung ECs.
The LPS treatment that induced pyrin protein expression in
pulmonary vascular ECs was weakened by HS and LPS in the second
‘hit’ model. However, exogenous IL-10 may reverse the inhibition of
pyrin expression in HS. It was additionally confirmed that in
pulmonary vascular ECs, IL-10 was able to inhibit HS in a rat model
of LPS-induced caspase-1 activation in the lungs, and the data from
the present study revealed that in pulmonary vascular ECs, the
expression of IL-10 may induce pyrin, while decreasing the
activation of the inflammasome, thereby serving an important role
in lung injury that occurs subsequent to HS. Furthermore, the
present study had certain limitations: In addition to those
aforementioned, only exogenous IL-10 was used, and the results
obtained were used to examine our hypothesis. Due to the lack of
measurement of the other endogenous dynamic changes following HS in
this experiment, the association between pyrin and endogenous IL-10
requires additional verification.
In conclusion, it was proposed that HS attenuated
the LPS-induced pyrin expression in pulmonary vascular ECs and that
HS may also damage the expression of IL-10, thereby decreasing the
expression of the pyrin protein in pulmonary vascular ECs,
resulting in the activation of caspase-1 following the second LPS
‘hit’. Additional studies investigating the expression changes and
mechanism of pyrin in pulmonary vascular ECs are required to
identify the underlying mechanism of SIRS induced by HS and novel
targets for clinical intervention.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Provincial Natural Science Foundation of China (grant no.,
LY15H150005) and Medical Health Science and Technology Project of
Zhejiang Provincial Health Commission (grant no., 2017KY176).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ and YY performed the majority of the experiments.
XJ, YY and XL wrote the draft manuscript. XL also participated in
the experimental design and helped aquire and analyze the
experimental data. YY and PX designed and planned the
implementation of the study. YX and SZ revised the manuscript, and
agree to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Zhejiang Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Menyar A, El-Thani A, El Rasheid Z,
Ahmad Z, Tuma M, AbdulRahman H, Parchani A, Peralta R and Latifi R:
Multiple organ dysfunction syndrome (MODS): Is it preventable or
inevitable. Int J Clin Med. 3:722–730. 2012. View Article : Google Scholar
|
|
2
|
van Wessem KJP and Leenen LPH: Reduction
in mortality rates of postinjury multiple organ dysfunction
syndrome: A shifting paradigm? A prospective population-based
cohort study. Shock. 49:33–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anne Morrison C, Moran A, Patel S,
Vidaurre Mdel P, Carrick MM and Tweardy DJ: Increased apoptosis of
peripheral blood neutrophils is associated with reduced incidence
of infection in trauma patients with hemorrhagic shock. J Infect.
66:87–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore FA, Sauaia A, Moore EE, Haenel JB,
Burch JM and Lezotte DC: Postinjury multiple organ failure: A
bimodal phenomenon. J Trauma. 40:501–512. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minei JP, Cuschieri J, Sperry J, Moore EE,
West MA, Harbrecht BG, O'Keefe GE, Cohen MJ, Moldawer LL, Tompkins
RG, et al: The changing pattern and implications of multiple organ
failure after blunt injury with hemorrhagic shock. Crit Care Med.
40:1129–1135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Xiang M, Yuan Y, Xiao G, Zhang J,
Jiang Y, Vodovotz Y, Billiar TR, Wilson MA and Fan J: Hemorrhagic
shock augments lung endothelial cell activation: Role of temporal
alterations of TLR4 and TLR2. Am J Physiol Regul Integr Comp
Physiol. 297:R1670–R1680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chae JJ, Wood G, Richard K, Jaffe H,
Colburn NT, Masters SL, Gumucio DL, Shoham NG and Kastner DL: The
familial Mediterranean fever protein, pyrin, is cleaved by
caspase-1 and activates NF-kappaB through its N-terminal fragment.
Blood. 112:1794–1803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chae JJ, Komarow HD, Cheng J, Wood G,
Raben N, Liu PP and Kastner DL: Targeted disruption of pyrin, the
FMF protein, causes heightened sensitivity to endotoxin and a
defect in macrophage apoptosis. Mol Cell. 11:591–604. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He X, Qian Y, Li Z, Fan EK, Li Y, Wu L,
Billiar TR, Wilson MA, Shi X and Fan J: TLR4-upregulated IL-1β and
IL-1RI promote alveolar macrophage pyroptosis and lung inflammation
through an autocrine mechanism. Sci Rep. 6:316632016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki T, Franchi L, Toma C, Ashida H,
Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C and Nuñez G:
Differential regulation of caspase-1 activation, pyroptosis, and
autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS
Pathog. 3:e1112007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Welborn BM III, Moldawer LL, Seeger JM,
Minter RM and Huber TS: Role of endogenous interleukin-10 in local
and distant organ injury after visceral ischemia-reperfusion.
Shock. 20:35–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Molthen RC: A simple, inexpensive, and
effective light-carrying laryngoscopic blade for orotracheal
intubation of rats. J Am Assoc Lab Anim Sci. 45:88–93.
2006.PubMed/NCBI
|
|
14
|
Zhu X, Hu H, Li Z, Lin R, Mao J and Chen
L: Gua Lou Gui Zhi decoction attenuates post-stroke spasticity via
the modulation of GABAB receptors. Mol Med Rep. 12:5957–5962. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou R, Hu DY, Liu LM and Zhou XW:
Protective effects of apocynin on ‘two-hit’ injury induced by
hemorrhagic shock and lipopolysaccharide. Acta Pharmacol Sin.
23:1023–1028. 2002.PubMed/NCBI
|
|
16
|
Ulich TR, Fann MJ, Patterson PH, Williams
JH, Samal B, Del CJ, Yin S, Guo K and Renick DG: Intratracheal
injection of LPS and cytokines. V. LPS induces expression of LIF
and LIF inhibits acute inflammation. Am J Physiol. 267:L442–L446.
1994.PubMed/NCBI
|
|
17
|
Tawadros PS, Powers KA, Yang I, Becker DA,
Ginsberg MD, Szaszi K, Kapus A and Rotstein OD: Stilbazulenyl
nitrone decreases oxidative stress and reduces lung injury after
hemorrhagic shock/resuscitation and LPS. Antioxid Redox Signal.
9:1971–1977. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Testa JE, Chrastina A, Oh P, Li Y,
Witkiewicz H, Czarny M, Buss T and Schnitzer JE: Immunotargeting
and cloning of two CD34 variants exhibiting restricted expression
in adult rat endothelia in vivo. Am J Physiol Lung Cell Mol
Physiol. 297:L251–L262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feldmeyer L, Keller M, Niklaus G, Hohl D,
Werner S and Beer HD: The inflammasome mediates UVB-induced
activation and secretion of interleukin-1beta by keratinocytes.
Curr Biol. 17:1140–1145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinon F, Hofmann K and Tschopp J: The
pyrin domain: A possible member of the death domain-fold family
implicated in apoptosis and inflammation. Curr Biol. 11:R118–R120.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chae J, Cho YH, Lee GS, Cheng J, Liu PP,
Feigenbaum L, Katz SI and Kastner DL: Gain-of-function Pyrin
mutations induce NLRP3 protein-independent interleukin-1β
activation and severe autoinflammation in mice. Immunity.
34:755–768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iyer SS and Cheng G: Role of interleukin
10 transcriptional regulation in inflammation and autoimmune
disease. Crit Rev Immunol. 32:23–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lo C, Fu M and Cryer HG: Interleukin 10
inhibits alveolar macrophage production of inflammatory mediators
involved in adult respiratory distress syndrome. J Surg Res.
79:179–184. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Armstrong L and Millar AB: Relative
production of tumour necrosis factor alpha and interleukin 10 in
adult respiratory distress syndrome. Thorax. 52:442–446. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khadaroo RG, Fan J, Powers KA, Fann B,
Kapus A and Rotstein OD: Impaired induction of IL-10 expression in
the lung following hemorrhagic shock. Shock. 22:333–339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeh FL, Shen HD and Fang RH: Deficient
transforming growth factor beta and interleukin-10 responses
contribute to the septic death of burned patients. Burns.
28:631–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kusske AM, Rongione AJ, Ashley SW,
McFadden DW and Reber HA: Interleukin-10 prevents death in lethal
necrotizing pancreatitis in mice. Surgery. 120:284–289. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cruz CM, Rinna A, Forman HJ, Ventura AL,
Persechini PM and Ojcius DM: ATP activates a reactive oxygen
species-dependent oxidative stress response and secretion of
proinflammatory cytokines in macrophages. J Biol Chem.
282:2871–2879. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Halle A, Hornung V, Petzold GC, Stewart
CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ and
Golenbock DT: The NALP3 inflammasome is involved in the innate
immune response to amyloid-beta. Nat Immunol. 9:857–865. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watanabe H, Gaide O, Pétrilli V, Martinon
F, Contassot E, Roques S, Kummer JA, Tschopp J and French LE:
Activation of the IL-1beta-processing inflammasome is involved in
contact hypersensitivity. J Invest Dermatol. 127:1956–1963. 2007.
View Article : Google Scholar : PubMed/NCBI
|